Here we introduce a novel method for performing functional magnetic resonance imaging in rats without the need of any anesthesia. The method enables animals to readily participate in scans with minimal movement and associated stress, and acclimation can be accomplished quickly over a few days. The paper comprehensively describes the animal training and the setup for performing such experiments. The method has general applicability to the field of animal neuroimaging and should facilitate better translational research.
Keywords: awake rat, functional MRI
Abstract
Functional magnetic resonance imaging (fMRI) in rodents holds great promise for advancing our knowledge about human brain function. However, the use of anesthetics to immobilize rodents during fMRI experiments has restricted the type of questions that can be addressed using this technique. Here we describe an innovative procedure to train rats to be constrained without the need of any anesthesia during the whole procedure. We show that with 8–10 days of acclimation rats can be conscious and remain still during fMRI experiments under minimal stress. In addition, we provide fMRI results of conscious rodents in a variety of commonly used fMRI experimental paradigms, and we demonstrate the improved quality of these scans by comparing results when the same rodents were scanned under anesthesia. We confirm that the awake scanning procedure permits an improved evaluation of brain networks and brain response to external stimuli with minimal movement artifact. The present study further advances the field of fMRI in awake rodents, which provide more direct, forward and reverse, translational opportunities regarding brain functional correspondences between human and rodent research.
NEW & NOTEWORTHY
Here we introduce a novel method for performing functional magnetic resonance imaging in rats without the need of any anesthesia. The method enables animals to readily participate in scans with minimal movement and associated stress, and acclimation can be accomplished quickly over a few days. The paper comprehensively describes the animal training and the setup for performing such experiments. The method has general applicability to the field of animal neuroimaging and should facilitate better translational research.
functional magnetic resonance imaging (fMRI) is widely used to gain understanding about brain function during cognitive tasks, sensory processing, and rest (Baliki et al. 2006; Bantick et al. 2002; LaBar et al. 1998; Wager et al. 2004), and its use in humans has provided a wealth of information about brain organization and information processing. With laboratory rodents, fMRI holds great promise because research techniques that are only available in the animal (e.g., optogenetics, genetic manipulation, etc.) can be supplemented with fMRI to explore a variety of basic neuroscience questions and, ultimately, will provide a more comprehensive mechanistic picture of human brain function. Thus development of fMRI in rodent is essential to establishing a translational link between animal and human research.
Despite the potential of rodent fMRI in basic science and preclinical research, the optimal technique has remained elusive to date. Traditionally, imaging in the rodent requires the use of anesthetics to ensure it remains still inside the scanner. Some aspects of neural activity appear to be preserved across anesthesia and awake conditions (Niell and Stryker 2010), and animal experiments in anesthetized preparations have led to valuable insights in neuroscience. Nevertheless, as anesthesia makes otherwise stressful imaging procedures possible by rendering the animal incapable of sensing and/or reacting to an unfamiliar and uncomfortable environment, it also obstructs brain function and obscures how the brain consciously processes information. Moreover, the characteristics of the hemodynamic readout, which constitute the basics of the fMRI signal, are affected by the type and concentration of anesthetic exerted on vascular reactivity and neurovascular coupling (Arthurs and Boniface 2002; Martin et al. 2006; Masamoto et al. 2009; Schlegel et al. 2015). For example, α-chloralose can reduces sensitivity of the cerebrovascular response (Sandor et al. 1977), and volatile anesthetics (e.g., isoflurane) can increase baseline cerebral blood flow (Hansen et al. 1988). While anesthesia does maximize experimental control, it also affects cognition, behavior, and fMRI signal dynamics. Thus scanning awake animals is necessary to gain a more realistic picture of the working brain.
Performing fMRI in conscious rodents has presented a number of serious technical challenges. It is generally believed that, unlike primates, rodents are far more difficult to be trained to stay still in the fMRI scanner. For this reason, many awake rodent protocols call for an initial anesthesia to restrain the animal in the beginning preparation of an experiment, with the aim to facilitate restraint and minimize movement after it has regained consciousness (Becerra et al. 2011; King et al. 2005; Liang et al. 2015; Upadhyay et al. 2011). However, it is difficult to know in such acclimation approaches where animals have a limited ability to escape unpleasant restraint situations whether rats learn not to move or simply learn to be helpless. The presence of learned helplessness/hopelessness could underlie reduced head displacements after acclimation. Also, it is not clearly understood how long it takes for the brain to fully recover from lingering effects of anesthesia. Apparatuses and procedures for electrophysiological recording in behaving rodents have been well-developed and do not require an initial anesthesia. Such head-post fixation approach has been implemented in an awake mice fMRI study (Desai et al. 2011). As rats are bigger and stronger than mice, additional novel procedural and technological components must be introduced while satisfying constraints of the fMRI magnet environment. Primary among these is the fact that the rodent must be habituated to a noisy and confined environment. Otherwise, the rodent may feel distress and generate severe, frequent movements.
Here we describe an innovative approach to fMRI in conscious rats by which we have successfully overcome these technical challenges. Our technique allows for the recording of the rodent brain without the need for any anesthetics or causing great distress (and movement) in the animal. Through a short acclimation procedure, we were able to train rats to freely enter and comfortably stay in our scanning apparatus during scanning with gentle body restraint and head-post fixation. To demonstrate the quality of our awake fMRI data, we also compare with fMRI data obtained in the same animals while anesthetized.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN; 325–400 g) were used in the present study. Ten animals were used for fMRI experiments in both awake and anesthetized conditions. An additional six rats were used to evaluate plasma corticosterone level in awake fMRI. Rodents were housed on soft bedding in groups of two per cage on a 12:12-h light-dark cycle in a temperature-controlled environment (21 ± 2°C) with food and water available ad libitum. All animal handling and testing were performed during the light period. All of the experimental procedures were approved by Institutional Animal Care and Use Committee of Northwestern University.
Head-post surgical preparation.
During surgery, rodents were first anesthetized with 3.5% isoflurane mixed with oxygen and nitrogen. The rodents were transferred to a stereotaxic device. Anesthesia was continued using isoflurane in oxygen/nitrogen mix. Concentration of isoflurane was adjusted such that pain reflexes such as tail movement after pinch was blocked. The head fur was shaved, and the eyes were covered with ointment to prevent drying out and infection of the cornea. After disinfection of the skin overlying the skull, the rodent's scalp was incised longitudinally, and the skin was retracted from the cranium. A head post was cemented into place with orthodontic resin (CandB-Metabond, Parkell; grip cement power and liquid, Dentsply International, Milford, DE). The head-post was a square nut made from Ultem PEI polyetherimide. The head-post was designed to be short to minimize the interference with rodents' daily activities when they were in their home cages. The square shape of the head-post also prevented the rodents' head from rotating when set in the cradle. The head-post was placed at the midpoint of bregma and lambda and fixed to the skull using CandB-Metabond. The rest of the exposed skull was then covered with a thin and smooth layer of grip cement (the thickness of cement, including the layer of CandB-Metabond, was about 1/16 to 1/8 in.) to minimize image distortion caused by susceptibility mismatch in fMRI. Care was taken to ensure there were no bubbles present in either the CandB-Metabond or grip cement, and that the set cement contained no sharp edges that could irritate the skin. The skin wound was treated with antibiotics (Triple Antibiotic, Walgreens). The rodents were released from the stereotaxic. Rodents were given at least 1 wk of rest to recover from the surgical preparation prior to the acclimation procedure.
Equipment for awake rodent fMRI.
In the present study, we devised a head-fixed system for measuring fMRI brain activity in awake rodents (Fig. 1). The instrument (including MRI holder, cradle, head plate, fastener, head-post, air-puff injector, mock scanner box) was designed using SolidWorks software. Multiple copies of cradles, head plates, and air-puff injectors were three-dimensional printed (ProofX, IL) using a semitransparent, medical grade material (PolyJet photopolymer MED610, Stratasys) that we had previously tested for magnetic susceptibility. Screws (Small Parts) and fastener were made with Ultem PEI polyetherimide.
Fig. 1.
Experimental apparatus. A: photograph of a rat implemented with a nut to allow affixing fastener and head plate to the head. B: a rat in a custom-made snuggle sack with openings that provide access to head nut, hindpaw, and tail. C: head plate with rectangular groove at bottom surface to fix to the head nut and circular groove upon the upper surface for mounting surface coil. D: photograph of a rat with air-puff injector positioned adjacent to its paw. The puff of air comes out of the injector via a small outlet. E: illustration of assembled apparatus with the key components.
We chose the air-puff stimulus because it provides a natural stimulation, at least more so than electrical stimuli. Von Frey filament could be another option for natural stimulation. But as Von Frey filament is sharp and may lead to quick neural adaption, it would not be ideal for block-design fMRI paradigm.
Custom-made “snuggle sacks” were designed to tailor for rodents weighting 300–400 g. Given that rodents experience red light as darkness, soft red water-resistant fleece was chosen as the inner layer, and a cotton sheet was affixed to the outer layer to maintain the shape of the snuggle sack. Snuggle sacks included openings that provided access to the rodent's hindpaw and head-post. The design of these snuggle sack allows for the face and tail to be free from obstruction, making possible conducting a range of behavioral or sensory stimulation protocols. Snuggle sacks are washable. Odor from feces and urine can be removed with warm water and by hanging them up to dry.
Acclimation procedure.
Rats were habituated to the head-fixed system using a short and systematic graded training procedure. The acclimation included the following: 1) independently entering a snuggle sack by themselves and settling in a comfortable, natural posture (Supplemental Video S1; Supplemental material for this article is available online at the journal website); 2) immobilization of their head with the implanted head-post; 3) air-puff stimulation to their paw; and 4) exposure to a highly loud noise from fMRI scan sequence. The procedures for acclimation were carried out 30 min/day for 8–10 days within 2 wk prior to collection of imaging data. To help disseminate this new method to other research groups, a detailed description of acclimation procedure is provided below.
Prior to acclimation and head-post surgery, rodents were handled daily for at least 3 days. During handling, rats were removed from their cage and placed on the experimenter's arm, allowing them to move freely and explore and interact with the experimenter. The procedure helps rats establish familiarity both with being held and with the experimenter, which reduces stress and enhances speed of acclimation.
During acclimation, rodents were first introduced to snuggle sacks. A snuggle sack is used as swaddling for an effective yet comfortable restraint. To get rodents to enter snuggle sacks, they were first held by the experimenter until they were calm and then placed in front of snuggle sacks. As rodents felt secure in a dark, enclosed environment, they began to voluntarily walk into snuggle sacks, stopping to move forward when their shoulders were around the narrower portion of snuggle sack. Subsequently, snuggle sacks were adjusted around the rodents for a snug fit (Supplemental Video S1). When an animal was recalcitrant, the rat, along with the snuggle sack, would be placed into his home cage and then reacclimated again at a later time.
Once acclimated to their snuggle sacks, the rodents were transferred to cradles. First air-puff injectors on the cradle were adjusted around the paw, and then the animal's body was secured to cradles with Velcro straps. The head plate was affixed to the head and subsequently secured to sidewalls mounted on the cradle. Early on we observed that rats do not easily adapt to head-immobilization. Therefore, rodents were acclimated to head restraint with a graded training procedure. In the initial phase, the head plate was loosely strapped onto the cradle with Velcro straps. In addition, a conventional wooden dental stick trimmed to 0.5 in. long was temporarily used as a fastener and provided an effective holding tool to guide the animal for the head position. When a rodent had large head movement, the stick would break or fall off from the head-post. Therefore, the amount of force on the head-post was sufficient to constrain head movement, but not so forceful as to prevent the rodents from releasing themselves from head-immobilization. Rodents could terminate a head fix constraint without having their head-post damaged. With repeats of acclimation, the rodent's head was gradually held firmly to the head-plate that was securely mounted on the cradle.
To reduce the stress induced by MRI experimental environment, rodents were exposed to digital recording of the sounds generated by gradient switching in the magnet during fMRI. The audio recording included a few 1-min blink sounds so rodents were familiar with loud and sudden noise that mimics a typical MRI session. In addition, rodents were also exposed to about 1-min air-puff stimulation to both hindpaws via air-puff stimulator. If novel tasks are required in fMRI studies, rats may need to be conditioned to new tasks that are different from those that would be used in the fMRI experiments, so rats would not be nervous with novel tasks during the scan.
Rats were acclimated to restraint and the MRI environment in 30-min sessions/day for 8–10 days within a 2-wk period. They were first acclimated in the mock scanner box that simulated the MRI scanner environment and, during later times, directly in the MRI scanner for at least 2 sessions prior to actual MRI experiment. Each session was performed at approximately the same time of day, around 2:00 PM to minimize the influence of circadian rhythm. Rodents were rewarded with treats at the end of each session.
Besides acclimation for awake rodent imaging, we also habituated the rodent to sudden exposures to isoflurane to perform fMRI in anesthetized condition following the awake condition without creating an additional stressful event. This was done by using a standard Q-tip dipped into isoflurane and placed close to the nose for 30 s. Signs of discomfort (e.g., struggling to withdraw head away from isoflurane-tipped Q-tips) disappeared after a few days of such training.
fMRI experiments.
fMRI data were collected in 10 rodents, both awake and anesthetized with isoflurane. fMRI scans were first acquired when the rats were awake, immediately followed by repeat scans under isoflurane anesthesia. To induce anesthesia when rats were in the scanner without causing discomfort to rodents, we did not muzzle rats in the anesthesia nose cone with their incisors on the bite bar as in a traditional setup. Instead, 3% isoflurane was delivered through a nose cone positioned about 2 cm in front where the rats nose was located, and the bite bar was not used.
fMRI scans in resting, blocks of stimulation, and random persistent stimulated conditions were performed while rats 1) rested quietly, 2) passively received periodic stimuli, and 3) passively received randomly-timed stimuli. Resting-state fMRI was acquired when rodents were resting in the scanner for 8 min with no external stimulation. The periodic-stimulus scans had six repetitive stimulus blocks for 5 min. Each block consisted of stimulation alternated with 2 s off/12 s on/36 s off. Random-stimulus scans, which included random 2-s air-puff stimulation with 0- to 6-s intertrial intervals for 8 min, were used to stimulate a clinical condition of random persistent pain sensation in the patients with chronic pain. While the periodic-stimulus scans were used to examine the brain mapping of the stimulus, resting-state fMRI and random-stimulus fMRI were used to reveal functional connectivity during resting and stimulus-related mental state in the context of continuous sensation for extend periods of time, respectively.
During all fMRI experiments, respiratory rates were monitored using respiration pads (model 1025; SA Instruments, Stony Brook, NY). Respiratory waves were recorded during image acquisition with a sample rate of 225 samples/s.
Isoflurane anesthesia.
Isoflurane gas tubes were attached to a custom-made holder. The opening of the gas tube was about 2 cm in front where the rodent's nose was located. The air was delivered to the rodent at the beginning of the fMRI experiments. Immediately after fMRI experiments in awake condition were completed, anesthesia for rodents was induced with isoflurane of 4% mixed with air and maintained during the experiments with isoflurane around 3%. A high level of isoflurane (3%) was used for two reasons. 1) Because the nose cone was not directly over the nose, the effective dose was likely less than 3% and we wanted to ensure that the rats would stay asleep. 2) Even though conventional dosage is 1–2%, we also wanted to ensure minimal movement of the animal to provide the best head movement comparison to our awake scans. Functional images in the anesthetized condition were collected after respiratory rate remained stable around 60–80 beats/min for about 5 min. In all, rats were under anesthesia for about 30 min.
Stimuli.
Stimulation of the side surface of the right hindpaw was administered via a custom-made air-puff injector. Innocuous aversive air-puff stimuli (force about 5 g) were used in this study as stimulation to examine brain activity associated with stimulation-evoked sensations. If a rodent's paw was found moved out of the air-puff injector by the end of the scan, all of the scan data from the rodent were discarded.
Corticosterone quantification.
To assess the scanner-induced stress of awake animals, a separate set of six rodents had their corticosterone level measured immediately after 30-min resting-state fMRI. Plasma corticosterone level was accessed once before acclimation as baseline, and immediately after the first, fourth, and eighth behavioral training session, as well as the 30 min resting-state fMRI experiment in the awake condition. Blood samples were obtained on unanesthetized rodents via lateral tail vein using 23-gauge needle, collected into a heparin-coated plastic tube (Microvette CB 300, Sarstedt), centrifuged at 1,500 g for 15 min to obtain plasma, subsequently divided into aliquot samples, and stored at −80°C until analysis. Plasma corticosterone levels were evaluated by using the Corticosterone EIA Kit (Enzo Life Sciences). Corticosterone levels were measured in duplicate in accordance with the manufacturer's handbook.
MRI acquisition.
All magnetic resonance experiments were carried out on a Bruker 7 T Clinscan horizontal magnet with 8-cm inner diameter insert volume coil. A two-channel volume resonator was used for radiofrequency transmission, and a 2-cm diameter surface coil was used for signal detection.
Blood oxygen level-dependent (BOLD) contrast-sensitive T2*-weighted echo-planar images were acquired for functional images with the following parameters: gradient-echo, 16 oblique transverse slices, repetition time (TR) = 2,000 ms, echo time (TE) = 18 ms, in-plane resolution = 0.38 mm × 0.38 mm, slice thickness = 0.5 mm, interslice distance = 0, field of view = 28 mm × 34 mm, matrix dimension = 74 × 92, phase encoding direction = anterior-posterior, and number of repetitions (NR) is 150 (periodic-stimulus fMRI) or 240 (resting-state and random-stimulus fMRI). The posterior end of the olfactory bulb was chosen as a landmark for positioning slices for functional image acquisition.
To optimize good registration of functional images into a standard space, many images supplementary to functional images were also acquired. First, a functional image with the same spatial dimension and distortion as in functional images that contained better signal-to-noise ratio (TR = 2,000 ms, TE = 18 ms, NR = 1, number of average = 32) was obtained and used in registration as a functional reference image and was also the target of motion correction. Moreover, a T2-weighted anatomical image, having identical spatial dimension as in the functional images, was acquired as a main anatomical reference. An additional T2-weighted anatomical image with full-brain coverage was also obtained. Furthermore, field maps with short TE = 4 ms and long TE = 5 ms were collected. These field map images were used to reduce distortions in echo-planar image-based functional images.
Whole brain motion evaluation.
Head motion was evaluated with standard procedures using FSL's MCFLIRT routine. For each rodent, rigid head motion was estimated from the relative root-mean-square (RMS) displacement on fMRI data prior to image preprocessing. This estimation derived a motion transformation matrix for each time point, with each transformation consisting of three translations and three rotations. These parameter time series were condensed to a single vector representing the motion time series of all brain voxels.
Motion during scanning could also cause spurious changes in signal intensity. This motion-induced data variance was quantified using the DVARS metric, which was calculated as the temporal derivative of RMS variance over all brain voxels prior to image preprocessing (Power et al. 2012). DVARS measures how much the intensity of a brain image changes from one time point to the previous. Outlier points were those that fell outside 1.5 times the interquartile range (0.25–0.75 quartile).
Image preprocessing.
Images were preprocessed with FSL 5.1 (FMRIB's Software Library, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). All images were first skull stripped to remove nonbrain voxels. Functional images were corrected for image distortion from the field map and imaging parameters. The functional images were then preprocessed with correction of slice-timing and motions, spatially smoothed with a Gaussian kernel of 0.7-mm full width at half maximum (FWHM), and high-pass filtered with a cutoff of 100 s to mitigate scanner drift artifact.
Volumes from functional images were registered to a standard space with a four-step process. Functional images were first aligned to the functional reference image and then registered to the main anatomical reference using a boundary-based registration cost function, followed by alignment with the individual's full-brain anatomical image, and then coregistered to a full-brain standard space.
Average time courses from all of the voxels inside the whole brain and white matter and cerebrospinal fluid were extracted from fMRI data. The time points in an fMRI dataset that were corrupted by large motion were detected using FSL's fsl_motion_outliers routine using DVARS. Voxel-wise regressors for physiological noise, which was based on respiratory recording, was generated using the FSL tool PNM. Nuisance regressors that modeled six motion parameters (translations and rotations), global whole brain, white matter and cerebrospinal fluid signals, motion spikes, and physiological noise were removed from fMRI data through linear regression.
Statistical analysis for brain response to touch-evoked sensations.
General linear modeling (GLM) approach was performed on individual rodents to identify brain regions in which the time course of the BOLD signal was significantly related to the stimulation paradigm. After preprocessing, the time series statistical analysis was carried out with FILM with local autocorrelation correction. The GLM model was convolved with gamma hemodynamic response function with 0-s phase, 3-s standard deviation, and 6-s lag to peak along its temporal derivative.
Group-level mixed-effects group analyses were performed for each contrast by using FSL's FLAME (FMRIB's Local Analysis of Mixed Effects) module with two stages (1 + 2). The analyses were performed only within the gray matter mask. Z-statistic images were corrected for multiple comparisons by voxel-wise cluster-forming threshold of Z > 2.3 and cluster-wise significance using threshold of P < 0.05 for family-wise error (FWE). The signal change of time points in a voxel was calculated by signal in a voxel at each time point divided by the mean of the voxel signal time course and expressed as percent changes relative to the prestimulus time points.
Functional connectivity.
After preprocessing, functional connectivity during resting and random, continuous sensation was evaluated using a seed-based correlational approach. Based on brain response to periods of air-puff stimulation in the awake condition, a sphere seed with 0.5-mm radius was selected based on the peak of activation in the hindlimb S1 region (coordination: mediolateral: +2.3 mm; dorsovental: −0.8 mm; anterioposterior: −1.6 mm, relative to bregma). Average time course from all of the voxels inside the seed region was correlated with every other voxel time course of the brain. Correlation coefficients (r) were converted to a normal distribution using Fisher's z-transform [z(r)]. Voxel-wised group-level statistical analysis of functional connectivity was assessed using FSL's ordinary least squares module. The analyses were performed only within the gray matter mask.
Functional connectivity matrix.
After preprocessing, resting-state and stimulus related whole-brain functional connectivity matrices were constructed. The rodent brain was segmented using a standard rodent atlas (Schwarz et al. 2006). Region of interest (ROI) in the standard rodent atlas space that had less than 27 voxels (∼0.20 mm3) shared across the rodents were excluded from analyses, resulting in 264 anatomical ROIs (132 ROIs in each hemisphere, see Table 1). The time course of BOLD signal across all voxels within a given brain region was averaged to generate the time course for the ROI. Functional connectivity was then computed using Pearson correlation coefficients between the time courses of each pair of ROIs. Correlation coefficients (i.e., r values) were transformed to z-scores by using Fisher's z-transformation, resulting in a 264 × 264 matrix of normalized correlation coefficients for each rodent.
Table 1.
List of ROI for functional connectivity network analysis
| ROI (R, L) | Anatomical Full Name |
|---|---|
| 1,2 | Olfactory tubercle, granular layer |
| 3,4 | Olfactory tubercle, layer 2 |
| 5,6 | Olfactory tubercle, polymorph layer |
| 7,8 | Olfactory tubercle |
| 9,10 | Piriform layer region, external |
| 11,12 | Piriform layer |
| 13,14 | Piriform layer region, internal |
| 15,16 | Piriform cortex |
| 17,18 | Dorsal endopiriform nucleus |
| 19,20 | Ventral endopiriform nucleus |
| 21,22 | Claustrum |
| 23,24 | Lateral orbital cortex |
| 25,26 | Ventral orbital cortex |
| 27,28 | Infralimbic cortex |
| 29,30 | Dorsal peduncular cortex |
| 31,32 | Prelimbic cortex |
| 33,34 | Cingulate cortex area 1 |
| 35,36 | Cingulate cortex area 2 |
| 37,38 | Retrosplenial agranular cortex |
| 39,40 | Retrosplenial granular A cortex |
| 41,42 | Retrosplenial granular B cortex |
| 43,44 | Lateral septal nucleus, dorsal part |
| 45,46 | Lateral septal nucleus, intermediate part |
| 47,48 | Lateral septal nucleus, ventral part |
| 49,50 | Nucleus of the horizontal limb of the diagonal band |
| 51,52 | Accumbens nucleus, core |
| 53,54 | Accumbens nucleus, shell |
| 55,56 | Lateral accumbens shell |
| 57,58 | Lateral stripe of the striatum |
| 59,60 | Caudate putamen striatum |
| 61,62 | Lateral globus pallidus |
| 63,64 | Ventral pallidum |
| 65,66 | Substantia innominata |
| 67,68 | Substantia innominata, basal part |
| 69,70 | Substantia innominata, dorsal part |
| 71,72 | Agranular insular cortex, dorsal part |
| 73,74 | Agranular insular cortex, posterior part |
| 75,76 | Agranular insular cortex, ventral part |
| 77,78 | Dysgranular insular cortex |
| 79,80 | Granular insular cortex |
| 81,82 | Primary motor cortex |
| 83,84 | Secondary motor cortex |
| 85,86 | Parietal association cortex |
| 87,88 | Primary somatosensory cortex |
| 89,90 | Primary somatosensory cortex, barrel field |
| 91,92 | Primary somatosensory cortex, dysgranular region |
| 93,94 | Primary somatosensory cortex, forelimb region |
| 95,96 | Primary somatosensory cortex, hindlimb region |
| 97,98 | Primary somatosensory cortex, jaw region |
| 99,100 | Primary somatosensory cortex, jaw region oral surface |
| 101,102 | Primary somatosensory cortex, trunk region |
| 103,104 | Primary somatosensory cortex, upper lip region |
| 105,106 | Secondary somatosensory cortex |
| 107,108 | Amygdala intermediate tissue |
| 109,110 | Anterior amygdaloid area, dorsal part |
| 111,112 | Anterior amygdaloid area, ventral part |
| 113,114 | Anterior cortical amygdaloid nucleus |
| 115,116 | Amygdalostriatal transition area |
| 117,118 | Basolateral amygdaloid nucleus, anterior part |
| 119,120 | Basomedial amygdaloid nucleus, anterior part |
| 121,122 | Central amygdaloid nucleus, capsular part |
| 123,124 | Central amygdaloid nucleus, medial division |
| 125,126 | Lateral amygdaloid nucleus, dorsolateral part |
| 127,128 | Lateral amygdaloid nucleus, ventromedial part |
| 129,130 | Medial amygdaloid nucleus, anterior dorsal part |
| 131,132 | Medial amygdaloid nucleus, posterodorsal part |
| 133,134 | Anteromedial thalamic nucleus |
| 135, 136 | Anteroventral thalamic nucleus, dorsomedial part |
| 137, 138 | Anteroventral thalamic nucleus, ventrolateral part |
| 139, 140 | Centrolateral thalamic nucleus |
| 141, 142 | Central medial thalamic nucleus |
| 143, 144 | Laterodorsal thalamic nucleus, dorsomedial part |
| 145, 146 | Laterodorsal thalamic nucleus, ventrolateral part |
| 147, 148 | Lateral posterior thalamic nucleus, laterorostral part |
| 149, 150 | Lateral posterior thalamic nucleus, mediorostral part |
| 151, 152 | Mediodorsal thalamic nucleus, central part |
| 153, 154 | Mediodorsal thalamic nucleus, lateral part |
| 155, 156 | Mediodorsal thalamic nucleus, medial part |
| 157, 158 | Paracentral thalamic nucleus |
| 159, 160 | Parafascicular thalamic nucleus |
| 161, 162 | Posterior thalamic nuclear group |
| 163, 164 | Posterior thalamic nuclear group, triangular part |
| 165, 166 | Paratenial thalamic nucleus |
| 167, 168 | Reticular thalamic nucleus |
| 169, 170 | Ventral anterior thalamic nucleus |
| 171, 172 | Ventrolateral thalamic nucleus |
| 173, 174 | Ventromedial thalamic nucleus |
| 175, 176 | Ventral posterolateral thalamic nucleus |
| 177, 178 | Ventral posteromedial thalamic nucleus |
| 179, 180 | Medial geniculate nucleus, dorsal part |
| 181, 182 | Medial geniculate nucleus, ventral part |
| 183, 184 | Dorsal lateral geniculate nucleus |
| 185, 186 | Zona incerta |
| 187, 188 | Zona incerta, dorsal part |
| 189, 190 | Zona incerta, ventral part |
| 191, 192 | Subincertal nucleus |
| 193, 194 | Anterior hypothalamic area, central part |
| 195, 196 | Dorsal hypothalamic area |
| 197, 198 | Dorsomedial hypothalamic nucleus, dorsal part |
| 199, 200 | Lateral hypothalamic area |
| 201, 202 | Posterior hypothalamic area |
| 203, 204 | Lateral preoptic area |
| 205, 206 | Medial preoptic area |
| 207, 208 | Magnocellular preoptic nucleus |
| 209, 210 | Tuber cinereum area |
| 211, 212 | Field ca3 of hippocampus, ventral part |
| 213, 214 | Hippocampus fronto-dorsal |
| 215, 216 | Hippocampus posterior, dorsal part |
| 217, 218 | Hippocampus posterior, ventral part |
| 219, 220 | Perirhinal cortex |
| 221, 222 | Subiculum dorsal part |
| 223, 224 | Ectorhinal cortex |
| 225, 226 | Primary auditory cortex |
| 227, 228 | Secondary auditory cortex, dorsal area |
| 229, 230 | Secondary auditory cortex, ventral area |
| 231, 232 | Temporal association cortex |
| 233, 234 | Primary visual cortex, binocular area |
| 235, 236 | Primary visual cortex, monocular area |
| 237, 238 | Secondary visual cortex, lateral area |
| 239, 240 | Secondary visual cortex, mediolateral area |
| 241, 242 | Secondary visual cortex, mediomedial area |
| 243, 244 | Superior colliculus, deep gray layer |
| 245, 246 | Superior colliculus, intermediate gray layer |
| 247, 248 | Superior colliculus, intermediate white layer |
| 249, 250 | Superior colliculus, optic nerve layer |
| 251, 252 | Superior colliculus, superficial gray layer |
| 253, 254 | Anterior pretectal nucleus |
| 255, 256 | Interstitial nucleus of medial longitudinal fasciculus |
| 257, 258 | Deep mesencephalic |
| 259, 260 | Prerubral field |
| 261, 262 | Periaqueductal grey |
| 263, 264 | Ventral tegmental area |
ROI, region of interest.
RESULTS
Motion artifact evaluation.
The primary goal of this study was to develop an innovative procedure for fMRI in awake rodents, with optimized motion and stress level. First, we wanted to compare head motion in awake rodents to the minimum that can be expected under anesthesia; thus we used a high concentration of isoflurane at 3%. Head motion during fMRI experiments was estimated with standard post hoc image registration. The motion was detected by measurement of the relative RMS relative displacement. The motion time course of each rodent is shown in Fig. 2.
Fig. 2.
Head motion and stress evaluation. A: time course of head motion during fMRI, calculated as root-mean-square (RMS) displacement, shown for awake (top) and anesthetized (bottom) conditions (n = 10). Each rat's head movement is represented in one color. Timings of air-puff stimuli in the periodic-stimulus fMRI scans are shown by black traces. B: average head motion in awake and anesthetized conditions. C: stress level, measured by plasma corticosterone, was evaluated on an additional set of rats at baseline, immediately after the acclimation sessions and in resting-state fMRI scans. Values are means ± SE; n = 5 ∼ 6.
For resting and periodic-stimulus scans, average head motion was low (the maximum of average head motion: awake = 74 μm, anesthetized = 47 μm). Although the rat was awake, random-stimulus scans exhibited greater and more frequent movement; the maximum of average head motion was less than 100 μm. There were borderline significant increases in average motion during the awake condition for resting (paired t-test, P = 0.03; mean of average motion in awake = 27 μm, anesthetized = 19 μm) and periodic-stimulus (P = 0.06; mean of average motion in awake = 29 μm, anesthetized = 22 μm) scans, and a highly significant increase of motion for the random-stimulus scans (P < 0.001; mean of average motion in awake = 43 μm, anesthetized = 17 μm) (Fig. 2B).
The effects of large head motions on fMRI signal can be mitigated by post hoc motion scrubbing and regression (Power et al. 2012; Satterthwaite et al. 2013). The spurious change in signal intensity caused by motion is quantified using DVARS, the temporal derivative of RMS variance. Motion scrubbing essentially involves removing parts of the fMRI signal during single time points of excessive motion and is acceptable for functional connectivity analyses as long as 125 temporal data points remain (Satterthwaite et al. 2013). Our scans exhibited no more than 27 time points with excessive motion (Table 2). Thus all functional connectivity assessments were reliable, with at least 210 time points (7 min) of data remaining after motion scrubbing. The effects of motion can also be minimized by regressing motion time courses (as in Fig. 2A) out of the fMRI signal. However, there is risk in reducing detection of a signal of interest (i.e., fMRI signals related to a stimulus) if motion is temporally correlated to it. We found that the timing of stimuli did not correlate highly with head motion in any condition (r < 0.6), suggesting subsequent analyses of stimulus-based fMRI signal is not contaminated with stimulus-induced movement (data are not shown). Overall, our awake-scanning procedure kept motion within a range that did not reduce the quality of the fMRI signal.
Table 2.
The number of time points in an fMRI dataset corrupted by large motion
| Number of Time Points with Excessive Motion |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | fMRI Scan | Rat 1 | Rat 2 | Rat 3 | Rat 4 | Rat 5 | Rat 6 | Rat 7 | Rat 8 | Rat 9 | Rat 10 |
| Awake | Resting-state | 12 | 6 | 3 | 18 | 0 | 0 | 0 | 12 | 8 | 3 |
| Periodic-stimulus | 11 | 3 | 2 | 3 | 9 | 1 | 4 | 8 | 4 | 7 | |
| Random-stimulus | 21 | 12 | 9 | 10 | 4 | 1 | 16 | 15 | 27 | 15 | |
| Anesthetized | Resting-state | 0 | 0 | 0 | 26 | 11 | 0 | 0 | 0 | 3 | 2 |
| Periodic-stimulus | 0 | 1 | 0 | 17 | 7 | 1 | 0 | 0 | 0 | 1 | |
| Random-stimulus | 0 | 2 | 0 | 7 | 14 | 2 | 2 | 0 | 2 | 0 | |
Outlier time points were those that fell outside 1.5 times the interquartile range (0.25–0.75 quartile).
Stress level evaluation.
In addition to motion, stress can also have negative effects on fMRI data quality, as well as the general health of the animal. To test the effects of our scanning procedure on stress levels, we measured plasma corticosterone at baseline before acclimation, immediately after 30 min of acclimation (for the 1st, 4th, and 8th training session), and immediately after a 30-min resting-state scan. We observed a slight but not significant elevation in plasma corticosterone in acclimation session 1 compared with baseline (repeated-measures ANOVA, post hoc Fisher least significant difference comparison, P = 0.14). Corticosterone levels persisted during acclimation session 4 and showed a trend of decreasing during acclimation session 8. During the actual fMRI scan, plasma corticosterone increased significantly over baseline (P < 0.01) and compared with all acclimation sessions (P < 0.05) (Fig. 2C). In general, we found that the process of acclimating rodents to the experimental environment did not increase stress, and thus rodents were not at risk of persistent stress-induced ailments from our procedure. Scanning procedure, however, did increase stress levels, although the rats were able to display minimal movement during the scan after all acclimation sessions.
Respiration rate evaluation.
To compare stress-related physiological parameters with other fMRI studies, we analyzed the mean and standard deviation of breaths per minute of each animal during the resting scan. Our awake rats group had respiration rate of 73.9 ± 6.1 breaths/min (Fig. 3), which was lower than those recently published, all of which exhibit at least ∼80 breaths/min (King et al. 2005; Reed et al. 2013), and in some cases much higher than ∼100 breaths/min (Ferenczi et al. 2016; Harris et al. 2015; Upadhyay et al. 2011).
Fig. 3.
Respiration rate evaluation. Breathing characteristics of awake rats and rats under anesthesia in the present study are compared with a previous study using 1–2% isoflurane. A: mean of beats per minute (bpm). B: standard deviation of bpm.
fMRI studies of anesthetized rats with isoflurane typically use 1 ∼ 2% isoflurane, delivered via a bite bar in nose-cone apparatus. To evaluate the depth of anesthesia of the present study (3% isoflurane with a loose nose-cone apparatus), we analyzed the respiration rate of a group of 20 anesthetized rats from an earlier resting-state study in which all rats received 1–2% isofluorane using a conventional nose-cone approach (Chang et al. 2014). Both anesthetized group had significantly lower mean respiration rate than awake group (73.9 ± 6.1 breaths/min) (t-test, P < 0.05). An unpaired t-test indicated that mean respiration rate for the present group of anesthetized animals (56.7 ± 4.6 breaths/min) and the group exposed to 1–2% isofluorane (55.4 ± 1.4 breaths/min) was not significantly different (P = 0.74). Furthermore, for both anesthesia groups, variance of breathing was significantly lower (t-test, P < 0.05) than in the awake animals. An unpaired t-test comparing the variance of breathing rate (standard deviation of breaths/min across the length of the scan) showed that average variance for the present group (3.1 ± 0.7 breaths/min) was significantly higher (P = 0.01) than that for the 1–2% isofluorane group (1.6 ± 0.1 breaths/min). This is more similar to the breathing rate variance when rats were awake in the present study (5.2 ± 0.7 breaths/min), indicating that the effective level of anesthesia here may have been, in fact, lighter than 1–2%.
Brain activity for stimulation-evoked sensations.
Many studies have reported the difference between fMRI data in awake and anesthetized animals (Masamoto et al. 2009; Pawela et al. 2009; Peeters et al. 2001; Pisauro et al. 2013). While dosage and different anesthetics such as medetomidine and α-chloralose may yield different brain responses, testing these effects were beside the primary goal of the study, which was to demonstrate the quality of data that can be obtained using our awake-scanning method compared with that under anesthesia.
To evaluate awake and anesthetized brain response to common block-design stimulus, we delivered periodic, unilateral air-puffs to the right paw and submitted the fMRI data to a standard GLM analysis. Awake-scanning resulted in significantly increased (P < 0.05, FWE corrected) activation in bilateral primary sensory cortex hindlimb region, bilateral thalamus, and cingulate cortex (Fig. 4A, Tables 3 and 4). Significant decrease in fMRI signal was observed in the bilateral secondary sensory cortex, hypothalamus, and ipsilateral insular cortex. While activation of these areas was stronger in the hemisphere contralateral to the stimulus, bilateral activation is commonly seen in unilateral stimulus paradigms in humans (Baliki et al. 2009). No significant change in fMRI signal was found while rodents were anesthetized. However, using a more liberal statistical criterion [P < 0.01, uncorrected, cluster-size threshold (k) = 26 voxels], subthreshold activation of the contralateral sensory cortex hindlimb region could be seen in the anesthetized condition (Fig. 4B, Table 5). The time courses of activation (from peak voxels in each condition) in the contralateral somatosensory cortex hindlimb region, contralateral thalamus, and cingulate cortex clearly indicate that fMRI signal strength in awake rodents increased during each epoch of air-puffs and gradually returned to baseline after the end of stimulation. Unexpectedly, time to peak activation in the thalamus proceeded that in the cortex. This is opposite of the hierarchy of the somatosensory pathway, but could be caused by interactions from descending corticothalamic input, or slow fMRI hemodynamic response in this region of the thalamus. The stimulus-induced signal change in anesthetized rodents was not as obvious as in awake scans (Fig. 4C). Because awake scanning resulted in stimulus-related activation maps that more accurately represent supraspinal pathways of the somatosensory system, our awake scanning procedure is likely to provide a more realistic account of sensory processing than scanning rodents under anesthesia.
Fig. 4.
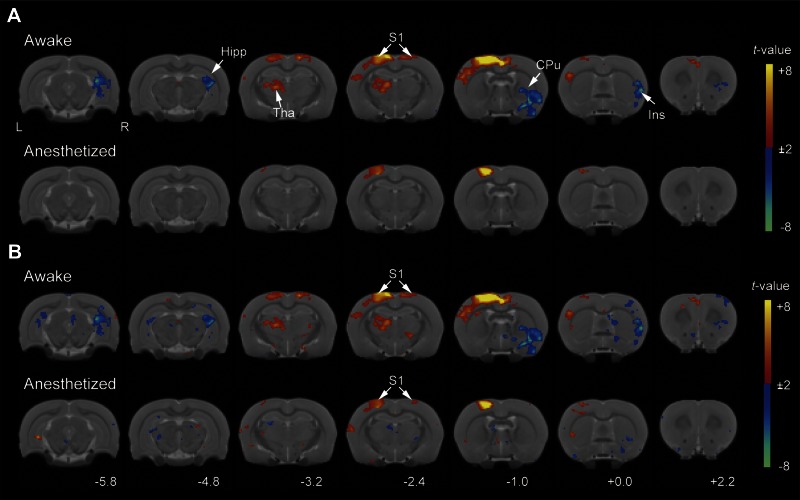
Brain activations to periodic air-puff applied to the right paw, in awake and anesthetized conditions. The group-averaged maps are displayed as statistical t-value maps overlaid on corresponding T2-weighted anatomical images. Color bars represent range of t-values. A, top: while rats were awake, significant activation was seen in many brain regions involved in somatosensation, including bilateral S1HL, Tha, and Cg1 (P < 0.05, FWE corrected, n = 10). Bottom: no significant change in brain response was observed in the anesthetized condition (P < 0.05, FWE corrected). B: the statistical t-value maps with less stringent thresholds for multiple comparisons (uncorrected P < 0.01, k = 26 voxels) are displayed. C: percent signal change as a function of time in contralateral S1HL, contralateral Tha, and Cg1 in awake (red) and anesthetized (blue) condition. Values are means ± SE. Stimulus period is labeled in gray. Error bars (SE of the mean) indicate across animal response variability. S1HL, primary somatosensory cortex hindlimb region; S2, secondary somatosensory cortex; Tha, thalamus; Hypo, hypothalamus; Cg1, cingulate cortex area 1; Ins, insular cortex; R, right; L, left side of the brain.
Table 3.
Brain activity for periodic-stimulus evoked sensations in awake rats
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Activation | ||||||
| 3,442 | L | Primary somatosensory cortex, hindlimb region | 7.6 | −2.3 | −0.8 | −1.6 |
| R/L | Cingulate cortex area 1 | 6.0 | 0.4 | −2.5 | 2.2 | |
| 4.3 | 0.8 | −2.3 | 3.2 | |||
| 4.1 | −0.2 | −2.3 | 0.4 | |||
| 1,107 | R | Secondary motor cortex | 4.1 | 1.4 | −1.0 | −3.6 |
| R | Primary somatosensory cortex, hindlimb region | 3.8 | 2.3 | −1.6 | −0.6 | |
| R | Laterodorsal thalamic nucleus, dorsomedial part | 3.9 | 1.6 | −4.7 | −2.6 | |
| R | Lateral posterior thalamic nucleus, mediorostral part | 3.7 | 1.6 | −4.3 | −3.6 | |
| L | Laterodorsal thalamic nucleus, ventrolateral part | 3.6 | −1.7 | −4.7 | −3.2 | |
| L | Anterodorsal thalamic nucleus | 3.6 | −1.0 | −4.5 | −1.8 | |
| L | Lateral habenular nucleus, lateral part | 3.4 | −0.8 | −4.3 | −3.6 | |
| L | Central medial thalamic nucleus | 3.4 | −0.4 | −5.8 | −2.4 | |
| Deactivaton | ||||||
| 1,149 | L | Secondary somatosensory cortex | −3.4 | −5.8 | −5.0 | −1.0 |
| L | Dysgranular insular cortex | −3.5 | −5.4 | −5.8 | 0.8 | |
| −3.4 | −4.7 | −5.6 | 1.6 | |||
| 548 | L | Primary somatosensory cortex, jaw region | −3.5 | −4.9 | −4.9 | 2.6 |
| L | Primary somatosensory cortex, barrel field | −3.6 | −5.0 | −3.3 | −1.0 | |
| L | Agranular insular cortex, posterior part | −3.8 | −5.6 | −7.2 | −0.4 | |
| R | Dysgranular insular cortex | −3.0 | 5.8 | −6.2 | −0.2 | |
| R | Primary somatosensory cortex upper, lip region | −4.0 | 5.2 | −4.9 | 0.4 | |
| −3.1 | 4.9 | −1.9 | −0.2 | |||
| 356 | R | Agranular insular cortex, dorsal part | −3.1 | 5.0 | −5.4 | 2.0 |
| R | Primary somatosensory cortex, dysgranular region | −3.4 | 5.0 | −2.9 | 0.2 | |
| R | Secondary somatosensory cortex | −3.4 | 6.2 | −5.0 | −1.2 | |
| R/L | Nucleus of the horizontal limb of the diagonal band | −3.4 | −0.2 | −8.0 | 0.4 | |
| −3.3 | 1.4 | −8.1 | 0.4 | |||
| R | Lateral septal nucleus, ventral part | −3.4 | 0.8 | −6.4 | −0.4 | |
| R | Paraventricular hypothalamic nucleus, anterior parvicellular part | −3.6 | 0.4 | −7.0 | −1.6 | |
| R | Medial preoptic nucleus, medial part | −3.8 | 0.2 | −8.0 | −1.2 | |
List of activated and deactivated brain regions in response to air-puff stimulation (P < 0.05, FWE corrected) is shown. Volumes are expressed in no. of voxels. Coordinates (mm) are in standard space. R, right of the brain; L, left of the brain.
Table 4.
Brain activity for periodic-stimulus evoked sensations in awake rats with a more liberal statistical criterion
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Activation | ||||||
| 3,629 | L | Primary somatosensory cortex, hindlimb region | 7.8 | −2.3 | −0.8 | −1.6 |
| R/L | Cingulate cortex area 1 | 6.1 | 0.4 | −2.5 | 2.2 | |
| R/L | Cingulate cortex area 2 | 6.1 | 0.0 | −2.3 | −0.2 | |
| R/L | Cingulate cortex area 2 | 4.9 | −0.4 | −2.5 | −1.4 | |
| R/L | Retrosplenial agranular cortex | 5.2 | 1.0 | −1.4 | −3.6 | |
| L | Primary motor cortex | 5.2 | −2.1 | −1.2 | −0.2 | |
| 1,130 | R | Laterodorsal thalamic nucleus, dorsomedial part | 5.2 | 1.7 | −4.3 | −2.6 |
| L | Centrolateral thalamic nucleus | 4.2 | −1.0 | −4.5 | −3.8 | |
| L | Anteromedial thalamic nucleus | 3.9 | −0.6 | −5.6 | −2.2 | |
| 3.6 | −1.0 | −4.7 | −1.8 | |||
| 202 | R | Posterior thalamic nuclear group | 3.2 | 2.5 | −4.5 | −3.8 |
| R/L | Retrosplenial granular B cortex | 4.1 | 0.0 | −1.7 | −6.4 | |
| R/L | Retrosplenial granular A cortex | 3.6 | −0.4 | −2.7 | −6.0 | |
| R/L | Retrosplenial agranular cortex | 3.0 | 1.0 | −1.0 | −6.2 | |
| 172 | R | Ectorhinal cortex | 4.2 | 5.6 | −6.0 | −3.4 |
| R | Secondary auditory cortex, ventral area | 2.8 | 6.4 | −5.4 | −4.4 | |
| 161 | L | Secondary auditory cortex, dorsal area | 3.9 | −5.8 | −2.5 | −5.0 |
| L | Primary somatosensory cortex, barrel field | 3.1 | −5.6 | −2.3 | −4.0 | |
| 129 | L | Primary auditory cortex | 3.3 | −5.8 | −5.4 | −3.8 |
| L | Secondary somatosensory cortex | 3.1 | −6.0 | −5.2 | −2.8 | |
| 126 | R | Primary auditory cortex | 3.6 | 6.6 | −4.1 | −5.6 |
| 118 | L | Medial geniculate nucleus, dorsal part | 3.8 | −3.1 | −5.0 | −5.6 |
| L | Medial geniculate nucleus, ventral part | 2.5 | −3.7 | −6.0 | −5.2 | |
| 84 | L | Ectorhinal cortex | 3.9 | −6.8 | −6.2 | −5.4 |
| 36 | L | Caudate putamen | 3.2 | −1.9 | −4.9 | 0.4 |
| 31 | L | Primary auditory cortex | 3.1 | −7.0 | −3.9 | −5.6 |
| Deactivation | ||||||
| 1,167 | L | Primary somatosensory cortex, barrel field | −5.1 | −5.2 | −3.5 | −0.8 |
| L | Secondary somatosensory cortex | −4.7 | −5.8 | −5.4 | −0.6 | |
| L | Dysgranular insular cortex | −4.0 | −5.2 | −5.8 | 1.0 | |
| L | Agranular insular cortex, ventral part | −3.9 | −4.7 | −5.8 | 2.0 | |
| L | Agranular insular cortex, posterior part | −3.8 | −5.6 | −7.2 | −0.4 | |
| L | Piriform cortex | −3.3 | −2.1 | −6.8 | 3.0 | |
| 584 | R | Primary somatosensory cortex, jaw region oral surface | −4.5 | 4.9 | −2.9 | 0.6 |
| R | Primary somatosensory cortex, upper lip region | −4.0 | 5.2 | −4.9 | 0.4 | |
| −3.8 | 6.0 | −4.5 | −0.2 | |||
| 391 | R | Secondary somatosensory cortex | −3.4 | 6.2 | −5.0 | −1.2 |
| R | Agranular insular cortex, dorsal part | −3.1 | 5.0 | −5.4 | 2.0 | |
| R | Primary somatosensory cortex, upper lip region | −3.1 | 4.9 | −1.9 | −0.2 | |
| R | Medial preoptic nucleus | −4.9 | 0.4 | −7.8 | −1.4 | |
| R/L | Nucleus of the horizontal limb of the diagonal band | −3.6 | 1.4 | −8.3 | 0.0 | |
| −3.4 | −0.2 | −8.0 | 0.4 | |||
| 266 | R | Lateral septal nucleus, ventral part | −3.4 | 0.8 | −6.4 | −0.4 |
| L | Bed nucleus of the stria terminalis medial division posterolateral part | −2.8 | −1.0 | −7.4 | −1.2 | |
| L | Primary visual cortex, monocular area | −5.2 | −2.9 | −0.8 | −6.0 | |
| L | Parietal association cortex | −3.2 | −4.5 | −1.4 | −4.8 | |
| −2.7 | −3.1 | −0.4 | −4.8 | |||
| 208 | L | Primary somatosensory cortex, jaw region | −4.3 | −4.1 | −3.9 | 1.6 |
| −3.8 | −3.9 | −2.3 | 2.0 | |||
| −3.0 | −3.3 | −3.1 | 1.2 | |||
| 157 | L | Lateral globus pallidus | −3.9 | −3.3 | −6.8 | −1.6 |
| −2.6 | −2.3 | −7.0 | −0.8 | |||
| 104 | L | Hippocampus posterior, dorsal part | −3.0 | −2.7 | −3.3 | −4.2 |
| 88 | L | Bed nucleus of the stria terminalis lateral division | −3.3 | −1.2 | −6.8 | −0.2 |
| L | Interstitial nucleus of the posterior limb of the anterior commissure | −2.9 | −2.1 | −7.6 | 0.2 | |
| 70 | R | Primary somatosensory cortex, barrel field | −3.4 | 4.5 | −1.2 | −0.8 |
| 62 | R | Primary somatosensory cortex, barrel field | −3.5 | 4.5 | −2.5 | −3.4 |
| 54 | L | Primary somatosensory cortex, jaw region | −3.4 | −4.5 | −1.2 | 1.2 |
| −2.7 | −4.5 | −2.1 | 0.4 | |||
| −2.5 | −4.1 | −3.1 | 0.4 | |||
| 52 | R | Hippocampus posterior, dorsal part | −3.3 | 2.3 | −2.9 | −4.4 |
| 50 | R | Amygdala intermediate tissue | −3.3 | 2.7 | −8.7 | −2.2 |
| 44 | R | Ventral endopiriform nucleus | −3.1 | 4.9 | −8.3 | −2.0 |
| 34 | L | Medial amygdaloid nucleus, anterior dorsal | −3.3 | −2.5 | −8.7 | −2.8 |
| 31 | L | Primary motor cortex | −3.5 | −1.9 | −1.2 | 1.8 |
| 29 | R | Parietal association cortex | −3.8 | 3.9 | −0.6 | −4.8 |
| 28 | R | Primary somatosensory cortex, barrel field | −3.2 | 6.4 | −3.7 | −2.6 |
| 26 | R | Caudate putamen | −3.0 | 4.1 | −5.0 | −1.0 |
List of activated and deactivated brain regions in response to air-puff stimulation using voxel-wise threshold of P < 0.01, uncorrected for multiple comparison and in more than 20 contiguous voxels (k) is shown. Volumes are expressed in no. of voxels. Coordinates (mm) are in standard space.
Table 5.
Subthreshold brain activity for periodic-stimulus evoked sensations in anesthetized rats
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Activation | ||||||
| 166 | L | Primary somatosensory cortex, barrel field | 3.9 | −5.0 | −2.9 | −2.0 |
| Primary somatosensory cortex, barrel field | 3.3 | −5.2 | −3.9 | −1.2 | ||
| 143 | L | Primary somatosensory cortex, hindlimb region | 4.2 | −1.7 | −0.6 | −1.2 |
| L | Primary motor cortex | 3.5 | −0.8 | −0.6 | −0.8 | |
| 101 | L | Secondary visual cortex, mediolateral area | 4.1 | −2.9 | −0.6 | −5.8 |
| 78 | L | Secondary motor cortex | 3.5 | −0.8 | −0.8 | 1.6 |
| R/L | Cingulate cortex area 1 | 3.1 | −0.4 | −1.9 | 1.6 | |
| 58 | R/L | Prelimbic cortex | 3.1 | 1.0 | −2.9 | 1.8 |
| R | Secondary motor cortex | 2.7 | 1.2 | −1.9 | 1.4 | |
| 56 | R | Primary somatosensory cortex, trunk region | 3.3 | 3.1 | −1.2 | −2.6 |
| 54 | R | Ventral posterolateral thalamic nucleus | 3.1 | 2.7 | −6.2 | −2.8 |
| R | Ventrolateral thalamic nucleus | 2.6 | 1.6 | −6.0 | −2.8 | |
| 51 | R | Lateral entorhinal cortex | 3.0 | 6.4 | −6.8 | −5.4 |
| 39 | R/L | Prelimbic cortex | 3.4 | 0.8 | −3.7 | 2.8 |
| 34 | L | Dorsal lateral geniculate nucleus | 3.4 | −3.3 | −4.9 | −5.0 |
| 28 | L | Lateral septal nucleus, intermediate part | 3.6 | −0.2 | −5.8 | 1.4 |
| Deactivaton | ||||||
| 52 | L | Caudate putamen | −3.4 | −3.1 | −6.2 | 0.6 |
| 35 | L | Lateral posterior thalamic nucleus, mediorostral part | −3.5 | −1.6 | −3.5 | −4.2 |
| 30 | L | Amygdala intermediate tissue | −3.1 | −4.1 | −8.0 | −1.4 |
List of activated and deactivated brain regions in response to air-puff stimulation when rats were under anesthesia (uncorrected P < 0.01, and k = 26 voxels) is shown. Volumes are expressed in no. of voxels.
Resting-state functional connectivity.
Besides its application to brain mapping, fMRI is also commonly used for studying functional connectivity of the brain. Functional connectivity refers to the synchrony of spatially separated brain areas by assessing the temporal correlation between their fMRI signals (Gusnard and Raichle 2001). Given that the somatosensory cortex displayed the most prominent response to periodic stimulation, we investigated how spontaneous activity from this brain region synchronized with other parts of the brain during the resting-state scan. To do this, we extracted the average fMRI signal time series from a 0.5-mm-radius sphere centered on the peak activity voxel from the GLM analysis and measured its temporal correlation to all other voxels in the brain. We found significant correlations with the secondary somatosensory cortex, insular cortex, and contralateral somatosensory cortex (P < 0.05, FWE corrected) when rodents were awake (Fig. 5A and Table 6 and 7). Under anesthesia, their somatosensory cortexes mainly displayed local connections (Fig. 5A, Table 8); however, connections between bilateral sensory cortexes were present at lower statistical thresholds (P < 0.01, uncorrected, and k = 26 voxels) (Fig. 5B, Table 9).
Fig. 5.
Resting-state functional connectivity of the somatosensory cortex, in awake and anesthetized conditions. The seed for the functional connectivity analysis was derived from the peak activation of the left somatosensory cortex in respond to periodic air-puff stimulation (Fig. 3). The group-averaged maps are t-value maps overlaid on corresponding T2-weighted anatomical images. Color bars represent the range of t-values. A: group-averaged resting-state functional connectivity maps in awake and anesthetized conditions (P < 0.05, FWE corrected, n = 10). B: the statistical t-value maps with less statistical stringent thresholds for multiple comparisons (uncorrected P < 0.01, k = 26 voxels) are shown. For abbreviations, refer to Fig. 4 legend.
Table 6.
Somatosensory cortex resting-state functional connectivity in awake condition
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 2,334 | L | Primary somatosensory cortex, hindlimb region | 20.6 | −2.1 | −0.8 | −1.0 |
| 10.8 | −2.1 | −0.2 | −2.4 | |||
| L | Primary somatosensory cortex, dysgranular region | 7.1 | −3.9 | −1.9 | −0.6 | |
| L | Forelimb region | 17.0 | −2.9 | −0.6 | −1.8 | |
| L | Primary motor cortex | 7.4 | −1.0 | −0.4 | −1.8 | |
| 6.4 | −1.4 | −0.4 | −3.2 | |||
| 1,314 | L | Secondary somatosensory cortex | 7.2 | −6.0 | −5.6 | −2.0 |
| L | Dysgranular insular cortex | 6.6 | −6.0 | −5.6 | −3.0 | |
| L | Caudate putamen | 6.5 | −3.1 | −7.0 | 0.8 | |
| 6.1 | −2.5 | −5.8 | 1.2 | |||
| L | Primary somatosensory cortex, barrel field | 6.1 | −5.0 | −1.9 | −1.4 | |
| 5.7 | −6.4 | −3.7 | −1.4 | |||
| 280 | R | Primary somatosensory cortex, hindlimb region | 9.0 | 2.3 | −1.0 | −2.4 |
| R | Primary somatosensory cortex, forelimb region | 6.8 | 3.3 | −0.4 | −1.8 | |
| R | Primary somatosensory cortex, trunk region | 3.7 | 1.7 | −0.2 | −3.8 | |
| Negative correlation | ||||||
| 407 | R | Primary auditory cortex | −6.9 | 5.8 | −3.9 | −4.6 |
| −6.7 | 6.2 | −4.9 | −4.0 | |||
| R | Temporal association cortex | −5.0 | 6.6 | −5.8 | −4.0 | |
| R | Perirhinal cortex | −3.8 | 6.2 | −7.0 | −3.4 | |
| R | Primary somatosensory cortex, barrel field | −3.7 | 5.4 | −3.1 | −4.0 | |
| 399 | R | Zona incerta, dorsal part | −7.1 | 2.5 | −6.8 | −5.6 |
| R | Anterior pretectal nucleus | −6.1 | 1.6 | −5.0 | −4.8 | |
| R | Intergeniculate leaf | −5.7 | 3.7 | −5.4 | −5.0 | |
| R | Hippocampus posterior, ventral part | −2.9 | 4.5 | −6.0 | −5.8 | |
List of regions functionally connected with somatosensory cortex (P < 0.05, FWE corrected) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 7.
Somatosensory cortex resting-state functional connectivity in awake condition with a more liberal statistical criterion
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 2,208 | L | Primary somatosensory cortex hindlimb region | 20.6 | −2.1 | −0.8 | −1.0 |
| 10.8 | −2.1 | −0.2 | −2.4 | |||
| L | Primary somatosensory cortex, forelimb region | 17.0 | −2.9 | −0.6 | −1.8 | |
| L | Primary somatosensory cortex, dysgranular region | 7.1 | −3.9 | −1.9 | −0.6 | |
| L | Primary motor cortex | 7.4 | −1.0 | −0.4 | −1.8 | |
| 6.4 | −1.4 | −0.4 | −3.2 | |||
| 1,237 | L | Secondary somatosensory cortex | 7.2 | −6.0 | −5.6 | −2.0 |
| L | Dysgranular insular cortex | 6.6 | −6.0 | −5.6 | −3.0 | |
| L | Caudate putamen | 6.5 | −3.1 | −7.0 | 0.8 | |
| 6.1 | −2.5 | −5.8 | 1.2 | |||
| L | Primary somatosensory cortex, barrel field | 6.1 | −5.0 | −1.9 | −1.4 | |
| 5.7 | −6.4 | −3.7 | −1.4 | |||
| 268 | R | Primary somatosensory cortex, hindlimb region | 9.0 | 2.3 | −1.0 | −2.4 |
| R | Primary somatosensory cortex, forelimb region | 6.8 | 3.3 | −0.4 | −1.8 | |
| R | Primary somatosensory cortex, trunk region | 3.7 | 1.7 | −0.2 | −3.8 | |
| 169 | L | Primary somatosensory cortex, forelimb region | 4.6 | −3.3 | −2.9 | −0.2 |
| 3.8 | −2.9 | −2.9 | −1.2 | |||
| 106 | L | Primary somatosensory cortex, jaw region | 6.0 | −4.3 | −3.3 | 1.4 |
| L | Caudate putamen | 4.0 | −3.3 | −4.9 | 1.6 | |
| 74 | R/L | Cingulate cortex area 1 | 5.8 | −0.2 | −1.7 | 2.8 |
| 4.2 | 0.4 | −2.5 | 2.2 | |||
| 69 | L | Primary motor cortex | 4.3 | −1.9 | −2.1 | 0.8 |
| 4.1 | −2.7 | −1.4 | 1.4 | |||
| 69 | L | Reticular thalamic nucleus | 4.9 | −1.7 | −5.0 | −1.4 |
| L | Triangular septal nucleus | 3.8 | −0.2 | −4.9 | −1.0 | |
| 52 | R | Anterior perifornical nucleus | 5.4 | 0.4 | −6.4 | −1.0 |
| R | Striohypothalamic nucleus | 3.8 | 0.6 | −7.6 | −1.2 | |
| R | Substantia innominata | 3.2 | 1.0 | −6.6 | −2.0 | |
| 27 | L | Caudate putamen | 3.8 | −3.3 | −5.4 | −1.0 |
| 27 | L | Medial preoptic area | 5.0 | −1.0 | −7.6 | −1.0 |
| 26 | L | Secondary motor cortex | 3.4 | −0.8 | −1.0 | 1.2 |
| R | Secondary motor cortex | 3.2 | 0.4 | −1.4 | 1.2 | |
| 26 | R | Primary somatosensory cortex, hindlimb region | 4.3 | 2.5 | −1.6 | −0.6 |
| 26 | L | Magnocellular preoptic nucleus | 4.6 | −2.3 | −8.1 | −0.8 |
| Negative correlation | ||||||
| 391 | R | Substantia nigra, compact part, dorsal tier | −7.1 | 2.5 | −6.8 | −5.6 |
| R | Anterior pretectal nucleus | −6.1 | 1.6 | −5.0 | −4.8 | |
| R | Intergeniculate leaf | −5.7 | 3.7 | −5.4 | −5.0 | |
| R | Hippocampus posterior, ventral part | −2.9 | 4.5 | −6.0 | −5.8 | |
| 386 | R | Primary auditory cortex | −6.9 | 5.8 | −3.9 | −4.6 |
| −6.7 | 6.2 | −4.9 | −4.0 | |||
| R | Temporal association cortex | −5.0 | 6.6 | −5.8 | −4.0 | |
| R | Perirhinal cortex | −3.8 | 6.2 | −7.0 | −3.4 | |
| R | Primary somatosensory cortex, barrel field | −3.7 | 5.4 | −3.1 | −4.0 | |
| 135 | L | Primary somatosensory cortex, jaw region | −6.0 | −4.7 | −1.6 | 1.4 |
| −4.2 | −5.2 | −2.7 | 1.4 | |||
| 131 | R | Anterior amygdaloid area, ventral part | −7.2 | 3.3 | −9.3 | −0.8 |
| R | Piriform layer, internal | −4.8 | 5.0 | −8.0 | −0.4 | |
| R | Basomedial amygdaloid nucleus, anterior part | −4.4 | 4.1 | −8.3 | −1.4 | |
| 118 | R | Hippocampus posterior, dorsal part | −6.8 | 4.5 | −5.2 | −6.0 |
| R | Hippocampus posterior, ventral part | −4.9 | 5.6 | −5.6 | −5.8 | |
| 112 | R | primary motor cortex | −8.9 | 4.1 | −1.6 | 2.8 |
| −4.7 | 3.7 | −1.0 | 1.8 | |||
| −4.3 | 2.9 | −0.6 | 1.2 | |||
| 100 | L | Dorsal lateral geniculate nucleus | −5.6 | −3.7 | −5.0 | −5.0 |
| 87 | R | Primary somatosensory cortex, jaw region | −4.3 | 4.9 | −1.6 | 0.8 |
| −3.9 | 4.9 | −2.5 | 1.6 | |||
| −3.5 | 4.5 | −2.5 | 0.6 | |||
| 68 | R | Primary visual cortex, binocular area | −5.7 | 5.2 | −1.4 | −5.8 |
| −3.9 | 4.7 | −1.2 | −6.6 | |||
| 60 | R/L | Retrosplenial agranular cortex | −6.0 | 1.4 | −1.0 | −6.6 |
| R | Secondary visual cortex, mediomedial area | −3.2 | 2.1 | −0.4 | −5.6 | |
| 59 | R | Secondary visual cortex, lateral area | −5.2 | 4.1 | −1.2 | −5.0 |
| 55 | R | Hippocampus posterior, dorsal part | −4.6 | 3.5 | −3.7 | −4.8 |
| R | Hippocampus fronto-dorsal | −3.5 | 2.9 | −3.3 | −3.8 | |
| 43 | R | Hippocampus posterior, dorsal part | −3.0 | 4.9 | −4.1 | −5.4 |
| 39 | L | Hippocampus fronto-dorsal | −5.7 | −3.7 | −2.9 | −3.8 |
| 38 | R | Hippocampus fronto-dorsal | −6.7 | 2.7 | −2.1 | −4.2 |
| 35 | R | Dorsal endopiriform nucleus | −9.2 | 3.5 | −6.8 | 1.4 |
| 29 | R | Lateral amygdaloid nucleus, dorsolateral part | −3.5 | 5.2 | −6.8 | −4.2 |
| 28 | R | Piriform layer region, external | −4.2 | 5.6 | −8.9 | −2.6 |
List of regions functionally connected with somatosensory cortex (uncorrected P < 0.01, and k = 26 voxels) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 8.
Somatosensory cortex resting-state functional connectivity in anesthetized condition
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 781 | L | Primary somatosensory cortex, hindlimb region | 12.7 | −2.3 | −0.6 | −1.2 |
| L | Primary somatosensory cortex, forelimb region | 4.6 | −2.9 | −1.6 | −2.4 | |
| L | Primary motor cortex | 4.7 | −1.6 | −1.9 | −0.2 | |
List of regions functionally connected with somatosensory cortex (P < 0.05, FWE corrected) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 9.
Somatosensory cortex resting-state functional connectivity in anesthetized condition with a more liberal statistical criterion
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 763 | L | Primary somatosensory cortex, hindlimb region | 12.7 | −2.3 | −0.6 | −1.2 |
| L | Primary somatosensory cortex, forelimb region | 4.6 | −2.9 | −1.6 | −2.4 | |
| L | Primary motor cortex | 4.7 | −1.6 | −1.9 | −0.2 | |
| 48 | R | Primary somatosensory cortex, trunk region | 4.6 | 2.7 | −1.0 | −3.2 |
| 36 | R/L | Cingulate cortex area 1 | 4.8 | −0.2 | −1.4 | −1.4 |
| 3.6 | 0.0 | −1.4 | −0.4 | |||
| 32 | L | Piriform cortex | 5.3 | −2.5 | −7.2 | 2.6 |
| 26 | L | Primary motor cortex | 4.3 | −4.7 | −3.3 | 3.2 |
| Negative correlation | ||||||
| 112 | R | Primary motor cortex | −5.9 | 3.5 | −0.8 | 2.0 |
| R | Primary somatosensory cortex, jaw region | −5.4 | 4.9 | −1.6 | 1.6 | |
| 76 | R | Claustrum | −6.1 | 5.2 | −6.8 | −0.4 |
| 75 | R | Caudate putamen | −5.6 | 3.1 | −6.0 | 0.6 |
| −3.9 | 2.3 | −5.2 | 0.2 | |||
| 59 | R/L | Dorsal peduncular cortex | −3.9 | 0.0 | −5.4 | 2.2 |
| R/L | Infralimbic cortex | −3.9 | −0.8 | −5.0 | 3.0 | |
| R/L | Prelimbic cortex | −3.8 | −0.6 | −4.1 | 2.2 | |
| 49 | R | Zona incerta ventral part | −4.4 | 3.1 | −6.4 | −4.8 |
| 46 | L | Caudate putamen | −5.0 | −4.5 | −5.0 | −1.6 |
| 46 | R | Primary somatosensory cortex | −4.0 | 5.6 | −2.9 | −4.6 |
| 42 | R | Accumbens nucleus, core | −5.5 | 2.1 | −7.2 | 1.4 |
| 34 | L | Caudate putamen | −4.1 | −2.7 | −7.0 | 0.8 |
| 30 | R | Hypothalamus dorsal intermediate tissue | −3.5 | 1.2 | −7.6 | −2.0 |
| R | Medial preoptic area | −3.5 | 1.2 | −7.8 | −1.0 | |
| 27 | L | Ectorhinal cortex | −4.2 | −5.8 | −6.6 | −3.0 |
List of regions functionally connected with somatosensory cortex is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis (uncorrected P < 0.01, and k = 26 voxels). Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Stimulus-related functional connectivity.
We further evaluated functional connectivity while rodents were exposed to a stimulus of randomly timed air-puffs delivered to the right hindpaw. Stimulus timing was designed to simulate the dynamics of sensation that is more common to patients with chronic pain and thus were not time-locked to the TR of the scanner. For this reason, we chose to assess data quality with functional connectivity, as opposed to an event-related analysis, which is a common approach to evaluating the brain networks associated with pain. In awake scans, we found significant (P < 0.05, FWE corrected) positive correlations in somatosensory cortex with the thalamus, contralateral somatosensory cortex, and negative correlations to caudate putamen and hippocampus (Fig. 6A, Table 10 and 11). Under anesthesia, somatosensory cortex again displayed mostly local connections (Fig. 6A, Table 12), with bilateral sensory cortex connections only present at a lower statistical threshold (P < 0.01, uncorrected, and k = 26 voxels) (Fig. 6B, Table 13). In general, we found awake scanning, during both resting and stimulus delivery, resulted in functional connectivity maps of the somatosensory cortex that better reflect the well-known pathways of supraspinal somatosensory information processing.
Fig. 6.
Stimulus-related functional connectivity of somatosensory cortex, for a random continuous sequence of air-puff stimuli. The seed for this functional connectivity analysis was the same as in Fig. 4. The maps display statistical t-values overlaid on corresponding T2-weighted anatomical images. Color bars represent range of t-values. A: group-averaged, stimulus-related functional connectivity maps in awake and anesthetized conditions (P < 0.05, FWE corrected, n = 10). B: the statistical t-value maps for a more liberal threshold for multiple comparisons (uncorrected P < 0.01, k = 26 voxels). Hipp, hippocampus; CPu, caudate putamen. See also abbreviations in Fig. 4.
Table 10.
Somatosensory cortex stimulus-related functional connectivity in awake condition
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 3,943 | L | Primary somatosensory cortex, hindlimb region | 16.2 | −1.9 | −0.8 | −0.8 |
| L | Primary somatosensory cortex, forelimb region | 13.3 | −2.9 | −0.6 | −1.6 | |
| L | Primary motor cortex | 11.4 | −0.8 | −0.6 | −2.4 | |
| L | Secondary motor cortex | 11.3 | −0.4 | −1.0 | −1.2 | |
| L | Primary motor cortex | 7.7 | −1.9 | −1.4 | 1.4 | |
| R | Primary somatosensory cortex, trunk region | 6.8 | 1.7 | −0.6 | −2.8 | |
| 863 | L | Mediodorsal thalamic nucleus, central part | 7.0 | −0.6 | −5.0 | −3.4 |
| L | Anterodorsal thalamic nucleus | 6.4 | −1.0 | −4.7 | −2.0 | |
| L | Posterior thalamic nuclear group | 5.5 | −1.7 | −4.9 | −3.2 | |
| L | Ventral posteromedial thalamic nucleus | 4.4 | −2.1 | −5.6 | −2.6 | |
| L | Medial habenular nucleus | 4.1 | 0.0 | −4.3 | −4.6 | |
| Negative correlation | ||||||
| 1,657 | R | Caudate putamen | −9.8 | 4.3 | −7.2 | −1.2 |
| R | Secondary somatosensory cortex | −8.4 | 5.6 | −5.4 | 0.0 | |
| R | Agranular insular cortex posterior part | −7.1 | 5.6 | −7.4 | −1.8 | |
| R | Piriform layer | −6.5 | 4.9 | −8.7 | −1.0 | |
| R | Caudate putamen | −6.3 | 4.5 | −5.6 | −1.8 | |
| R | Primary somatosensory cortex, upper lip region | −5.9 | 5.0 | −4.3 | 0.0 | |
| 905 | R | Hippocampus posterior, dorsal part | −14.6 | 4.1 | −3.7 | −5.6 |
| −8.3 | 4.9 | −4.3 | −5.2 | |||
| R | Hippocampus posterior, ventral part | −4.8 | 4.7 | −6.2 | −5.8 | |
| 532 | R | Dorsal endopiriform nucleus | −8.4 | 3.5 | −7.0 | 1.4 |
| R | Caudate putamen | −4.7 | 2.7 | −5.8 | 1.2 | |
| R | Lateral orbital cortex | −4.3 | 2.9 | −4.9 | 2.4 | |
| R | primary somatosensory cortex, jaw region | −4.2 | 4.1 | −4.3 | 2.4 | |
List of regions functionally connected with somatosensory cortex (P < 0.05, FWE corrected) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 11.
Somatosensory cortex stimulus-related functional connectivity in awake condition with a more liberal statistical criterion
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 3,636 | L | Primary somatosensory cortex, hindlimb region | 16.2 | −1.9 | −0.8 | −0.8 |
| L | Primary somatosensory cortex, forelimb region | 13.3 | −2.9 | −0.6 | −1.6 | |
| R | Primary somatosensory cortex, trunk region | 6.8 | 1.7 | −0.6 | −2.8 | |
| L | Primary motor cortex | 11.4 | −0.8 | −0.6 | −2.4 | |
| 7.7 | −1.9 | −1.4 | 1.4 | |||
| L | Secondary motor cortex | 11.3 | −0.4 | −1.0 | −1.2 | |
| 821 | L | Mediodorsal thalamic nucleus, central part | 7.0 | −0.6 | −5.0 | −3.4 |
| L | Anterodorsal thalamic nucleus | 6.4 | −1.0 | −4.7 | −2.0 | |
| L | Posterior thalamic nuclear group | 5.5 | −1.7 | −4.9 | −3.2 | |
| L | Ventral posteromedial thalamic nucleus | 4.4 | −2.1 | −5.6 | −2.6 | |
| L | Medial habenular nucleus | 4.1 | 0.0 | −4.3 | −4.6 | |
| 174 | L | Primary somatosensory cortex, barrel field | 5.1 | −5.2 | −3.9 | −1.8 |
| L | Secondary auditory cortex, dorsal area | 4.2 | −6.6 | −3.3 | −3.4 | |
| 88 | R | Reticular thalamic nucleus | 4.9 | 3.1 | −6.4 | −3.4 |
| R | Ventromedial thalamic nucleus | 4.6 | 2.1 | −6.4 | −2.6 | |
| 53 | L | Primary motor cortex | 5.2 | −2.3 | −2.3 | 2.4 |
| 50 | L | Caudate putamen | 4.2 | −3.7 | −6.2 | 0.6 |
| 42 | L | Primary somatosensory cortex, barrel field | 4.4 | −3.5 | −3.1 | −1.0 |
| 32 | L | Secondary somatosensory cortex | 4.6 | −4.7 | −5.2 | −0.2 |
| 27 | R | Primary auditory cortex | 5.1 | 7.0 | −3.9 | −5.0 |
| Negative correlation | ||||||
| 1,593 | R | Caudate putamen | −9.8 | 4.3 | −7.2 | −1.2 |
| R | Secondary somatosensory cortex | −8.4 | 5.6 | −5.4 | 0.0 | |
| R | Agranular insular cortex, posterior part | −7.1 | 5.6 | −7.4 | −1.8 | |
| R | Piriform layer | −6.5 | 4.9 | −8.7 | −1.0 | |
| R | Caudate putamen | −6.3 | 4.5 | −5.6 | −1.8 | |
| R | Primary somatosensory cortex, upper lip region | −5.9 | 5.0 | −4.3 | 0.0 | |
| 850 | R | Hippocampus posterior, dorsal part | −14.6 | 4.1 | −3.7 | −5.6 |
| −8.3 | 4.9 | −4.3 | −5.2 | |||
| R | Hippocampus posterior, ventral part | −4.8 | 4.7 | −6.2 | −5.8 | |
| R | Medial geniculate nucleus, ventral part | −3.6 | 3.7 | −5.2 | −6.0 | |
| 514 | R | Dorsal endopiriform nucleus | −8.4 | 3.5 | −7.0 | 1.4 |
| R | Caudate putamen | −4.7 | 2.7 | −5.8 | 1.2 | |
| R | Lateral orbital cortex | −4.3 | 2.9 | −4.9 | 2.4 | |
| R | Primary somatosensory cortex, jaw region | −4.2 | 4.1 | −4.3 | 2.4 | |
| 159 | L | Hippocampus posterior, dorsal part | −5.4 | −4.3 | −4.3 | −5.0 |
| −3.7 | −4.5 | −5.0 | −5.8 | |||
| −3.5 | −3.5 | −3.5 | −5.8 | |||
| 142 | R | Primary motor cortex | −4.2 | 4.3 | −1.4 | 2.4 |
| −4.2 | 3.1 | −0.8 | 2.0 | |||
| −4.1 | 2.5 | −0.4 | 3.2 | |||
| R | Primary somatosensory cortex, jaw region | −3.8 | 3.9 | −2.7 | 1.6 | |
| 113 | R | Caudate putamen | −4.8 | 1.7 | −4.5 | −0.2 |
| 108 | R | Ventral pallidum | −5.3 | 2.1 | −7.2 | −0.4 |
| 95 | L | Primary motor cortex | −4.3 | −4.3 | −2.9 | 3.6 |
| L | Agranular insular cortex | −3.8 | −3.9 | −4.5 | 3.2 | |
| 90 | R | Secondary visual cortex, lateral area | −5.5 | 4.9 | −2.3 | −5.6 |
| R | Parietal association cortex | −3.9 | 4.5 | −2.1 | −4.6 | |
| R | Primary somatosensory cortex, barrel field | −3.9 | 5.6 | −1.7 | −4.2 | |
| 82 | R | Superior colliculus | −4.8 | 0.4 | −4.1 | −5.6 |
| R/L | Retrosplenial granular a cortex | −4.0 | 1.0 | −3.1 | −6.2 | |
| 53 | R | Hippocampus posterior, dorsal part | −8.0 | 1.9 | −2.1 | −4.2 |
| 52 | R | Lateral septal nucleus, intermediate part | −6.2 | 1.0 | −5.6 | −0.6 |
| 42 | R | Primary motor cortex | −6.2 | 2.5 | −3.1 | 3.0 |
| 40 | R | Primary somatosensory cortex, forelimb region | −3.9 | 4.1 | −1.6 | 0.0 |
| 37 | L | Accumbens nucleus, shell | −4.4 | −0.6 | −6.4 | 2.0 |
| 31 | L | Piriform layer region, internal | −4.0 | −4.1 | −7.4 | 0.6 |
List of regions functionally connected with somatosensory cortex (uncorrected P < 0.01, and k = 26 voxels) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 12.
Somatosensory cortex stimulus-related functional connectivity in anesthetized condition
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 963 | L | Primary somatosensory cortex, hindlimb region | 24.3 | −2.1 | −0.8 | −1.2 |
| L | Primary somatosensory cortex, forelimb region | 5.3 | −3.3 | −0.8 | 0.2 | |
| L | Primary somatosensory cortex, dysgranular region | 4.0 | −3.7 | −1.0 | −2.8 | |
| L | Primary motor cortex | 3.1 | −0.8 | −0.6 | −2.0 | |
List of regions functionally connected with somatosensory cortex (P < 0.05, FWE corrected) is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis. Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Table 13.
Somatosensory cortex stimulus-related functional connectivity in anesthetized condition with a more liberal statistical criterion
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Volume | L/R | Anatomical Structure | t-Value | x | y | z |
| Positive correlation | ||||||
| 949 | L | Primary somatosensory cortex, hindlimb region | 24.3 | −2.1 | −0.8 | −1.2 |
| L | Primary somatosensory cortex, forelimb region | 5.3 | −3.3 | −0.8 | 0.2 | |
| L | Primary somatosensory cortex, dysgranular region | 4.0 | −3.7 | −1.0 | −2.8 | |
| L | Primary motor cortex | 3.1 | −0.8 | −0.6 | −2.0 | |
| 124 | L | Secondary auditory cortex, ventral area | 5.2 | −7.0 | −5.2 | −4.2 |
| L | Secondary somatosensory cortex | 4.7 | −7.0 | −4.9 | −3.0 | |
| L | Secondary somatosensory cortex | 3.1 | −6.4 | −4.9 | −1.4 | |
| L | Primary somatosensory cortex, barrel field | 3.5 | −6.0 | −3.9 | −3.2 | |
| 45 | R | Primary somatosensory cortex, hindlimb region | 4.6 | 2.7 | −0.4 | −1.8 |
| 39 | L | Primary somatosensory cortex, forelimb region | 4.7 | −3.5 | −2.5 | −0.4 |
| L | Primary somatosensory cortex, dysgranular region | 3.7 | −4.5 | −1.7 | −0.2 | |
| 37 | L | Primary somatosensory cortex, barrel field | 4.6 | −4.9 | −3.7 | −1.6 |
| 36 | L | Hippocampus posterior, ventral part | 6.3 | −4.5 | −5.8 | −5.6 |
| 33 | L | Primary somatosensory cortex, barrel field | 4.0 | −3.3 | −1.6 | −3.8 |
| L | Parietal association cortex | 3.0 | −2.9 | −0.8 | −4.4 | |
| 27 | L | Caudate putamen | 4.4 | −4.5 | −5.4 | 0.0 |
| 27 | L | Hippocampus posterior, ventral part | 4.3 | −5.2 | −6.2 | −4.2 |
| 26 | L | Olfactory tubercle, polymorph layer | 3.8 | −2.3 | −8.5 | 0.8 |
| Negative correlation | ||||||
| 147 | R | Caudate putamen | −7.5 | 3.7 | −6.0 | 0.4 |
| R | Piriform layer region, internal | −4.6 | 3.7 | −6.6 | 1.8 | |
| R | Dysgranular insular cortex | −4.2 | 4.5 | −5.6 | 1.6 | |
| 117 | L | Anterodorsal thalamic nucleus | −8.2 | −1.6 | −4.5 | −2.2 |
| L | Septofimbrial nucleus | −6.8 | −1.2 | −4.3 | −1.2 | |
| R | Paratenial thalamic nucleus | −4.0 | 0.6 | −5.0 | −2.0 | |
| 82 | L | Hippocampus fronto-dorsal | −6.5 | −2.5 | −3.5 | −3.2 |
| −4.0 | −1.7 | −3.3 | −4.0 | |||
| 71 | R | Primary motor cortex | −4.9 | 3.9 | −2.3 | 1.4 |
| 59 | R | Magnocellular preoptic nucleus | −4.8 | 2.9 | −8.5 | −0.6 |
| R | Caudate putamen | −4.1 | 2.9 | −7.4 | −0.2 | |
| 57 | L | Substantia innominata | −5.3 | −0.2 | −8.5 | −1.8 |
| 55 | L | Zona incerta, dorsal part | −6.4 | −2.9 | −6.0 | −4.6 |
| 47 | L | Granular insular cortex | −5.8 | −5.0 | −5.2 | 1.4 |
| 44 | L | Piriform layer region, internal | −4.1 | −2.9 | −7.4 | 2.0 |
| 32 | R | Granular insular cortex | −4.8 | 5.4 | −5.6 | 0.8 |
| 30 | L | Lateral septal nucleus, intermediate part | −5.5 | −0.4 | −6.4 | 0.6 |
| 27 | R | Caudate putamen | −6.8 | 5.0 | −6.0 | −0.6 |
List of regions functionally connected with somatosensory cortex is shown. Left somatosensory cortex hindlimb region was used for seed-based functional connectivity analysis (uncorrected P < 0.01, and k = 26 voxels). Coordinates (mm) are in standard space. Volumes are expressed in no. of voxels.
Whole-brain functional connectivity.
Recent experiments in monkeys have shown that resting-state functional connectivity across the whole brain becomes weaker under anesthesia (Barttfeld et al. 2015). However, it is unknown how anesthesia influences global connectivity while an animal is receiving a stimulus. To measure the overall strength of connectivity in our rodents' brains, we calculated the pairwise temporal correlations of fMRI signals between 264 regions covering the entire brain (Table 1) and measured the FWHM of their distributions. If correlations are close to zero (i.e., if the correlation distribution is centered narrowly on zero and has a low FWHM), functional connections are weak. Here, stimulus-related scans are with random stimulus conditions.
In all conditions, the peaks of correlation distributions were centered at zero, but awake-scanning resulted in a qualitatively wider distribution for both resting-state and stimulus-related scans (Fig. 7A), suggesting greater absolute connection strengths. Additionally, FWHM was borderline significantly greater in awake than anesthetized rats during the resting-state (paired t-test, P = 0.05), and significantly (P < 0.01) greater in stimulus-related scans (Fig. 7B). These stronger connections appeared to be widespread across the brain, as functional connectivity matrices exhibited pervasive shifts in connection strength from anesthetized- to awake-state scans (Fig. 7C). Overall, we found anesthetized scanning resulted in whole brain reduction in connectivity strength, and this reduction occurred regardless of whether the rodent received stimulation or not.
Fig. 7.
Whole brain resting state and stimulus-related functional network properties, in awake and anesthesia conditions. The brain was segmented into 264 anatomical regions of interest (ROI) as nodes, based on a standard rat atlas. A: average distribution of functional connectivity strength for all node pairs, in awake (red) and anesthetized condition (blue) (n = 10). B: the full width at half maximum (FWHM) of functional connectivity strength for each rat. The color code corresponds to those for motion evaluation. C: matrix representation of group-averaged brain functional correlation networks (r). The color is proportional to the strength of connections.
DISCUSSION
Imaging the brains of conscious animals is key to establishing a useful link to conscious brain function in humans. There are two main concerns of imaging awake animals: 1) motion and its effect on image quality; and 2) stress and its effect on animal behavior and ultimately on the brain activity measured. As such, most previous fMRI studies involving awake animals have used some form of initial anesthesia to secure animals and then scan them upon waking, with the accepted tradeoff of the residual effects of anesthesia on the brain and the stress in the animal caused by waking locked and uncomfortable in an unfamiliar environment. In the present study, we demonstrate that with a new approach relying on training rats to enter the setup independently, to habituate them over the course of 8–10 days of acclimation, rats can be conscious, remain still and comfortable during fMRI experiments under minimal stress, avoiding the use of anesthesia entirely, and thus more closely matching human scanning conditions. Using this procedure, we showed that fMRI in the awake condition permitted better evaluation of functional networks and brain responses to external stimuli, compared with the same animals under anesthesia. We believe the guidelines presented here will make fMRI in awake, small animals more accessible and improve the value of imaging data in translational neuroscience.
Motion artifact is a considerable problem in fMRI studies. Any minor head movement can lead to distortion of the image and create a change in signal intensity that can be mistaken for changes in brain activity (Birn et al. 1999). Therefore, properly controlled head motion is essential. One way movement can be controlled in conscious animals is to introduce a low-dose anesthesia to place them in the magnet, then carry out scanning 15 or 30 min after rodents have regained consciousness (Ferris et al. 2006; Liang et al. 2012; Upadhyay et al. 2011). Although this waiting period is helpful to reduce the lingering effects of anesthesia, little research exists on what happens to the brain when anesthetics are wearing off (Hudson et al. 2014). Patients who undergo general anesthesia can experience impaired cognitive ability (e.g., forgetfulness, or inability to concentrate, etc.) up to 3 days after they return to consciousness (Tzabar et al. 1996). These long-lasting effects of anesthesia can perturb brain function and potentially lead to erroneous interpretation of experimental findings. A major improvement in our method is the complete elimination of anesthesia. We found, in properly trained rats, that motion was kept within an acceptable range during awake scanning. For both resting-state and stimulus-based scans, awake rodents moved comparably to those that were anesthetized and met the standard subject inclusion criteria for motion scrubbing (Power et al. 2012). The mean of average movements during resting scan was no more than 50 μm, which is one-half of the motion that has been typically observed in previous awake rat fMRI studies (∼100 μm) (Upadhyay et al. 2011). Even during random-stimulus scans, the rat showing maximum motion had average movements less than 100 μm. Additionally, movements did not correlate with the timing of stimuli, suggesting head motion did not interfere with the ability to detect the stimulus-evoked signal changes. Awake scanning of rodents can therefore be done practically, under minimal movement, and without the use of any anesthesia.
Acclimating rodents for awake scanning can also introduce experimental problems: repeated immobilization of the rodent may impose chronic stress and could lead to psychological and neurological disorder, such as depression. Many awake rat fMRI studies have shown repeated acclimation reduces stress-like behaviors and neurochemical signs at day 4 or day 5 compared with day 1 (King et al. 2005; Reed et al. 2013), suggesting that these rats do not develop any psychological and neurological disorder from their acclimation approach. But it remains to know how much these stress-like behaviors and neurochemical signs are during scanning and relative to the baseline prior to the acclimation. Our study is the first to report stress levels not only during acclimation, but also during scanning. The training approach in the present study takes advantage of graded exposure techniques to keep the difficulty of each acclimation session sufficiently low. Moreover, our design provides an enclosure that meets a rodent's need for a secure, sheltered environment and a comfortable, natural posture. It is important to note that the aims of animal welfare and science are the same: the more comfortable the rodent is, the more stable a performance it has. With the current acclimation procedure, significant increases of plasma corticosterone levels were not observed. In fact, corticosterone levels lessened across sessions, which is consistent with previous studies showing reduced stress with repeated acclimation (King et al. 2005; Upadhyay et al. 2011). We did, however, find that stress levels were higher during scanning than acclimation sessions. Although we tried to match scanning conditions as closely as possible by acclimating in a mock scanner, it is likely that the unfamiliar environment and acoustic noise contributed to this effect. Our method could be improved by tailoring acclimation to the fMRI environment to aid in the habituation process. Although the rats showed increased stress hormone levels during the scan, our awake-scanning method exhibits a lower average breathing rate than those recently published (Ferenczi et al. 2016; Harris et al. 2015; King et al. 2005; Reed et al. 2013; Upadhyay et al. 2011), suggesting that our method may be the least stress-inducing awake-animal scanning procedure to date. Overall, we demonstrate that, using our practical training procedure, awake scanning can be carried out without introducing repeated, high stress to rodents during acclimation. Our method therefore introduces minimal risk to rodents in developing chronic stress ailments.
The ultimate goal of our method was to obtain meaningful, quality fMRI signal from the rodent. We found that, when rats were awake (compared with when they were anesthetized), fMRI signal was more consistent with activity patterns and network behavior typically seen in awake humans. Unilateral air-puff stimulation resulted in significant activation of bilateral sensory cortex, bilateral thalamus, and cingulate cortex. These brain regions are well-characterized as part of the somatosensory pathway (Hayes and Northoff 2012), but they were mostly silent when rats were anesthetized. This is consistent with previous fMRI reports indicating higher fMRI brain activity in awake compared with anesthetized condition (Peeters et al. 2001). In addition, the lack of thalamic activation under anesthesia is consistent with previous studies using anesthetized rodents (Lowe et al. 2007; Silva et al. 1999; Weber et al. 2006), suggesting anesthesia reduces the detectability of neural signals normally picked up with fMRI. Similarly, anesthesia can modulate communication between different parts of the brain (Martuzzi et al. 2010; Peltier et al. 2005), which may pose further problems in evaluating brain functional networks. Our awake-scans revealed stronger connectivity across the entire brain, and an anatomically specific somatosensory network with greater interhemispheric synchrony, which is consistent with seminal connectivity studies in humans (Biswal et al. 1995). The somatosensory network of the same rodents under anesthesia lacked long-range synchrony and exhibited mostly local connections. Long-range connectivity is characteristic of conscious brain function (Barttfeld et al. 2015), and these results further demonstrate the need for awake-animal scanning in translational research involving conscious humans.
Note that, to have the best comparison within the same animals, we induce anesthesia when rats were in the scanner. To avoid discomfort to rats, we did not muzzle them in an anesthesia nose cone with their incisors on the bite bar as in a conventional setup. Although the dose of anesthetics used in the present study may seem to be higher than those used in published papers (Liang et al. 2012; Liu et al. 2013), our analysis of respiration rate between this and a previous study (Chang et al. 2014) shows that giving our rats 3% isofluorane through a loose nose cone is no more severe than a conventional 1–2% dose through a more secure nose cone. Mean respiration was no different from the 1–2% group, and surprisingly variance in respiration was more similar to that during awake scans, suggesting that, while our rats were anesthetized, the effective dose may have been even lighter than 1–2%. Therefore, we believe the use of 3% isoflurane does not minimize our ability to compare fMRI results under different conditions.
In conclusion, the present study demonstrates an innovative and practical procedure of fMRI in awake rat that minimizes movement and stress in the animal, and eliminates the need for anesthesia all together. We also confirmed that awake scanning results in brain functional networks and responses to stimuli that are more consistent with fMRI studies in humans. This is an important step forward in translational research, allowing for more realistic evaluation of awake-brain processing, and providing a more meaningful, preclinical platform to translate the outcome between human and animal research.
GRANTS
This work was supported by National Institutes of Health Grants NS-057704 and DE-022746.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.-C.C. and A.V.A. conception and design of research; P.-C.C., Q.B., and M.V.C. performed experiments; P.-C.C. analyzed data; P.-C.C., D.P., A.B., and A.V.A. interpreted results of experiments; P.-C.C. prepared figures; P.-C.C. and D.P. drafted manuscript; P.-C.C., D.P., A.B., and A.V.A. edited and revised manuscript; P.-C.C., D.P., Q.B., A.B., and A.V.A. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Manuel Amaro at Rehabilitation Institute of Chicago for assistance in designing the equipment for awake rodent fMRI, and also to Elizabeth Hunt for editing the manuscript.
REFERENCES
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25: 169–169, 2002. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26: 12165–12173, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101: 875–887, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310–319, 2002. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A 112: 887–892, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Chang PC, Bishop J, Borsook D. CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw. Neuroimage 54: 1355–1366, 2011. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. [DOI] [PubMed] [Google Scholar]
- Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 155: 1128–1139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, Kopell N, Buckner RL, Graybiel AM, Moore CI, Boyden ES. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol 105: 1393–1405, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351: aac9698, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Febo M, Luo F, Schmidt K, Brevard M, Harder JA, Kulkarni P, Messenger T, King JA. Functional magnetic resonance imaging in conscious animals: a new tool in behavioural neuroscience research. J Neuroendocrinol 18: 307–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694, 2001. [DOI] [PubMed] [Google Scholar]
- Hansen TD, Warner DS, Todd MM, Vust LJ, Trawick DC. Distribution of cerebral blood flow during halothane versus isoflurane anesthesia in rats. Anesthesiology 69: 332–337, 1988. [DOI] [PubMed] [Google Scholar]
- Harris AP, Lennen RJ, Marshall I, Jansen MA, Pernet CR, Brydges NM, Duguid IC, Holmes MC. Imaging learned fear circuitry in awake mice using fMRI. Eur J Neurosci 42: 2125–2134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci 13: 60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AE, Calderon DP, Pfaff DW, Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci U S A 111: 9283–9288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods 148: 154–160, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20: 937–945, 1998. [DOI] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Intrinsic organization of the anesthetized brain. J Neurosci 32: 10183–10191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Watson GD, Alloway KD, Lee G, Neuberger T, Zhang N. Mapping the functional network of medial prefrontal cortex by combining optogenetics and fMRI in awake rats. Neuroimage 117: 114–123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr 26: 363–377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage 35: 719–728, 2007. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32: 33–48, 2006. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage 49: 823–834, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci 30: 242–250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li RP, Jones SR, Cho YR, Matloub HS, Hyde JS. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage 46: 1137–1147, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging 19: 821–826, 2001. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport 16: 285–288, 2005. [DOI] [PubMed] [Google Scholar]
- Pisauro MA, Dhruv NT, Carandini M, Benucci A. Fast hemodynamic responses in the visual cortex of the awake mouse. J Neurosci 33: 18343–18351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59: 2142–2154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MD, Pira AS, Febo M. Behavioral effects of acclimatization to restraint protocol used for awake animal imaging. J Neurosci Methods 217: 63–66, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor P, Nyary I, Reivich M, Kovach AG. Comparative effects of chloralose anesthesia and Sernylan analgesia on cerebral blood flow, CO2 responsiveness, and brain metabolism in the baboon. Stroke 8: 432–436, 1977. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64: 240–256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel F, Schroeter A, Rudin M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: implications on analysis of mouse fMRI data. Neuroimage 116: 40–49, 2015. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Danckaert A, Reese T, Gozzi A, Paxinos G, Watson C, Merlo-Pich EV, Bifone A. A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. Neuroimage 32: 538–550, 2006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab 19: 871–879, 1999. [DOI] [PubMed] [Google Scholar]
- Tzabar Y, Asbury AJ, Millar K. Cognitive failures after general anaesthesia for day-case surgery. Br J Anaesth 76: 194–197, 1996. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Chandran P, Miller L, Lee Y, Marek GJ, Sakoglu U, Chin CL, Luo F, Fox GB, Day M. Default-mode-like network activation in awake rodents. PloS One 6: e27839, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303: 1162–1167, 2004. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage 29: 1303–1310, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.