The most striking manifestation of cutaneous reflexes during locomotor activity is “phase-dependent reflex reversal.” This change of reflex signs (from facilitation to suppression) takes place despite input of the same stimulation to peripheral nerves containing whole axons of foot-sole cutaneous afferents. In the current study, we elucidated that this bidirectional reflex sign arises from selective activation of distinct reflex pathways produced by separate afferent populations innervating specific foot-sole regions depending on walking phase.
Keywords: bipedal walking, cutaneous reflex, reflex reversal, foot sole, humans
Abstract
During walking, cutaneous reflexes in ankle flexor muscle [tibialis anterior (TA)] evoked by tibial nerve (TIB) stimulation are predominantly facilitatory at early swing phase but reverse to suppression at late swing phase. Although the TIB innervates a large portion of the skin of the foot sole, the extent to which specific foot-sole regions contribute to the reflex reversals during walking remains unclear. Therefore, we investigated regional cutaneous contributions from discrete portions of the foot sole on reflex reversal in TA following TIB stimulation during walking. Summation effects on reflex amplitudes, when applying combined stimulation from foot-sole regions with TIB, were examined. Middle latency responses (MLRs; 70–120 ms) after TIB stimulation were strongly facilitated during the late stance to mid-swing phases and reversed to suppression just before heel (HL) strike. Both forefoot-medial (f-M) and forefoot-lateral stimulation in the foot sole induced facilitation during stance-to-swing transition phases, but HL stimulation evoked suppression during the late stance to the end of swing phases. At the stance-to-swing transition, a summation of MLR amplitude occurred only for combined f-M&TIB stimulation. However, the same was not true for the combined HL&TIB stimulation. At the swing-to-stance transition, there was a suppressive reflex summation only for HL&TIB stimulation. In contrast, this summation was not observed for the f-M&TIB stimulation. Our results suggest that reflex reversals evoked by TIB stimulation arise from distinct reflex pathways to TA produced by separate afferent populations innervating specific regions of the foot sole.
NEW & NOTEWORTHY
The most striking manifestation of cutaneous reflexes during locomotor activity is “phase-dependent reflex reversal.” This change of reflex signs (from facilitation to suppression) takes place despite input of the same stimulation to peripheral nerves containing whole axons of foot-sole cutaneous afferents. In the current study, we elucidated that this bidirectional reflex sign arises from selective activation of distinct reflex pathways produced by separate afferent populations innervating specific foot-sole regions depending on walking phase.
during walking, cutaneous input from afferents innervating the foot produce dynamic changes in motoneuron excitability in muscles across the body (Rossignol et al. 2006; Zehr and Duysens 2004). Such modulations contribute to coordinated muscular activation and joint movements that depend on the walking phase, nerve stimulated, and context of the task (Hoogkamer et al. 2015; Rossignol et al. 2006; Zehr and Duysens 2004). Cutaneous reflexes are thought to play crucial roles in stabilizing posture, avoiding obstacles, and supporting progression during bipedal locomotion after disturbances detected from the foot (Rossignol et al. 2006; Zehr et al. 1997, 1998).
One of the most striking manifestations of cutaneous reflexes during locomotor activity is “phase-dependent reflex reversal.” This change of reflex signs (from facilitation to suppression) takes place despite the fact that the stimulation input to peripheral nerves containing axons innervating cutaneous afferents is the same (Duysens et al. 1990; Forssberg et al. 1975; Yang and Stein 1990). For instance, when the distal tibial nerve (TIB) is electrically stimulated during the transition from stance-to-swing phase, facilitatory responses can be observed in the human tibialis anterior (TA) muscle (Duysens et al. 1990; Yang and Stein 1990). Contrastingly, the same stimulation gives rise to a prominent, suppressive response in TA at the late swing phase. From a functional point of view, the facilitatory response in TA helps to generate a rapid dorsiflexion in response to disturbance of the foot, which may serve to keep the foot away from the stimulation and transfer to the swing phase with knee flexion. Furthermore, the suppressive response of TA may be beneficial in generating a quick and firm placing reaction that would ensure stabilization at heel (HL) strike (Zehr et al. 1997). Reflex reversal is not merely confined to TIB-induced reflexes. This phenomenon occurs following stimulation of the superficial peroneal and sural nerves, both of which are thought to play functional roles in persistent walking, such as in the “stumbling corrective reaction” after disturbance of the foot (Duysens et al. 1992; Forssberg 1979; Van Wezel et al. 1997; Zehr et al. 1997).
In previous studies, some neural mechanisms were proposed to explain the reversal of cutaneous reflexes during walking in cats and humans (Conway et al. 1995; De Serres et al. 1995; Duysens et al. 1990; Guertin et al. 1995; Yang and Stein 1990). One plausible explanation is that two discrete groups of motor units (fast or slow types of motoneurons), which predominantly receive facilitatory or inhibitory inputs from spinal interneurons, are differentially activated depending on walking phase (Burke et al. 1970; Yang and Stein 1990). However, De Serres et al. (1995) used motor-unit recordings to demonstrate that a single motoneuron in the TA received both facilitatory and inhibitory inputs from discrete reflex pathways following stimulation of TIB. Thus it is likely that locomotor commands selectively switch reflex pathways at stance-to-swing (facilitatory)- and swing-to-stance (suppressive)-phase transition. However, it is unknown how different skin regions innervated by the TIB contribute to suppressive or facilitatory signs.
Previously, we (Nakajima et al. 2006, 2009; Zehr et al. 2014) and another group (Conway et al. 1995) reported that non-noxious electrical stimulation of discrete foot-sole regions could elicit location-specific suppressive or facilitatory reflexes. For instance, electrical stimulation of the HL region elicited suppression in TA. In contrast, the same stimulation of the forefoot region gave rise to facilitation of TA. Thus topographic organization of pathways mediating cutaneous reflexes within the foot sole led us to hypothesize that specific afferent populations within TIB would partly contribute to generating reflex reversal following whole-nerve stimulation. If so, the investigation of any summation of reflex amplitudes following combined stimulation of the whole nerve and discrete regional afferent populations would clarify the contribution of regional afferents in TIB reflex pathways [cf. Baldissera et al. (1981); LaBella and McCrea (1990); Nakajima et al. (2013, 2014)].
Most recently, we found that cutaneous inputs from discrete regions of the foot sole played a crucial role in enhancing coupling between afferent feedback from the foot-sole and neuromechanical functions during walking (Zehr et al. 2014). Stimulation evoked clear location-specific reflexes in muscles acting at the ankle, and the topographic distribution of responses produced changes in forces under the foot and ankle kinematics. The neural mechanisms accounting for the differences in responses evoked by discrete regional, whole-nerve, and combined stimulation are unclear.
Therefore, here, we investigated possible regional contributions of cutaneous afferents innervating the foot sole on bidirectional reflex signs (reflex reversal) following TIB stimulation during walking. We focused on forefoot-medial (f-M), forefoot-lateral (f-L), and HL regions. To test our hypothesis, we first observed the similarity or dissimilarity of TIB- and foot-sole region-induced reflex patterns during walking. Second, we conducted experiments to answer the question of whether there was a summation effect following combined TIB and medial site of forefoot or HL region stimulation.
MATERIALS AND METHODS
Subjects.
In this study, 15 neurologically intact volunteers (4 women and 11 men, 22–54 yr) participated. Informed, written consent was obtained from all participants before the experiment, under a protocol approved by the Research Ethics Committee of Chiba University, Faculty of Education (Approval Number 35), and in accordance with the Declaration of Helsinki.
Experimental protocol.
Subjects walked on a treadmill (Woodway USA, Waukesha, WI) at a self-reported, comfortable speed of 4 km/h for all experiments. In experiment 1 (Exp. 1), electrical stimuli were delivered to obtain approximately the same number of stimuli for each site (i.e., TIB, f-M, f-L, and HL). Interstimulus interval (ISI) was set at ∼3–5 s to obtain an almost-equal number of stimuli for each site. In experiment 2 (Exp. 2), five different stimuli were delivered randomly within a session: TIB, f-M, HL, f-M&TIB, and HL&TIB. ISI, for the combined stimulation (i.e., f-M&TIB and HL&TIB), was set at 11–21 ms to match the peak of both responses (f-M or HL). ISI was set to avoid the refractory period of the first stimulation on TIB and allow for the possibility of summation of two reflex inputs at the TA motoneurons. ISI was determined by the following: 1) determining the peak of middle latency responses (MLRs) following electrical stimulation of f-M, HL, and TIB stimulation for each subject; 2) calculating the time differences of the peak of MLRs induced by f-M or HL and TIB stimulation; and 3) using the calculated time difference as ISI during stimulation at stance-to-swing- or swing-to-stance-phase transitions.
Before starting these experiments, the subjects were asked to perform three maximum voluntary dorsiflexions for 3–5 s with a 30-s rest interval while in upright stance. The maximum value of the mean amplitude of the full-wave-rectified TA electromyography (EMG) during 1 s was calculated and averaged across the trials. This averaged amplitude was termed EMGmax and was used for normalization.
Foot-sole and TIB stimulation.
Transcutaneous electrical stimulation was delivered to the calcaneous (HL regions on the center of the plantar foot: HL), the head of the first metatarsal (underneath the base of the proximal phalanx of the great toe: f-M), and the distal end of the fifth metatarsal (underneath the proximal phalanx of the fifth toe: f-L) (Nakajima et al. 2006, 2009; Zehr et al. 2014). TIB was electrically stimulated at the medial malleolus (Duysens et al. 1990; Yang and Stein 1990; Zehr et al. 1997). Stimulation was provided by a constant voltage stimulator (SS-100; Nihon Kohden, Tokyo, Japan) [cf. Burke et al. (1991)], controlled with a pulse-regulating system (SEM7201; Nihon Kohden) with trains of 3 × 1.0 ms pulses at 300 Hz. Stimulation to the foot sole and TIB was delivered via flexible, paired surface electrodes with double-sided tape (cathode distal; NS100; Nihon Kohden) that were firmly fixed by elastic surgical tape.
Stimulation intensity.
Before starting each trial, the perceptual threshold (PT) was determined for each stimulation site. PT was defined as the minimum stimulus intensity, where the subject could clearly detect the stimulus beneath the stimulus electrodes. For detecting PT, subliminal electrical stimulation (∼4 V) was gradually increased (∼2 V/step) every 2 s until the subject reported a tactile sensation. Then, the stimulus intensity was increased or decreased by 0.1 or 0.2 V steps until the subject reliably detected the tactile sensation. Approximately 20–30 stimulations were used to detect PT. These procedures are similar to those previously described (Nakajima et al. 2006, 2009; Zehr et al. 2014).
The stimulation intensity for each stimulus site was set to ∼3.0 × PT, chosen to evoke a non-noxious, cutaneous sensation during each trial by activating cutaneous afferents immediately under the electrodes and to provide the same relative activation at all stimulation sites (Zehr et al. 2014).
In Exp. 2, combined electrical stimulation (i.e., f-M&TIB or HL&TIB) was applied during treadmill walking. The f-M and HL areas on the foot sole are innervated by the TIB nerve at ankle level. Whole-nerve stimulation (i.e., TIB) activates afferents innervating f-M and HL foot-sole areas. The combined stimulation of f-M&TIB or HL&TIB thus partly converges on the same interneuronal system [cf. Baldissera et al. (1981)]. Temporal summation could occur within presumed shared reflex pathways. If so, higher-intensity stimulation to TIB and f-M or HL, as used in Exp. 1, could saturate the responsiveness of this putative, shared reflex pathway. To exclude this possibility, therefore, in Exp. 2, the stimulus intensity was reduced to an intensity that could not saturate the pathway (2.4 ± 0.2 × PT). Consequently, this intensity elicited MLR amplitudes with ∼7% of EMGmax (means ± SE: 7.1 ± 0.8%), following each stimulation (i.e., HL, f-M, and TIB) at stance-to-swing-phase transition, and that with ∼13% of EMGmax (means ± SE: 13.0 ± 1.0%) at swing-to-stance-phase transition. This yielded similar reflex sizes following HL, f-M, and TIB stimulation during stance-to-swing- or swing-to-stance-phase transitions [not significant; one-way ANOVA: F(2,18) = 1.09 (stance-to-swing-phase transition), P > 0.05; F(2,18) = 0.45 (swing-to-stance-phase transition), P > 0.05]. Stimulation for stance-to-swing- and swing-to-stance-phase transition was applied at 50.5 ± 1.2% and 88.9 ± 1.1% of gait cycle, respectively. The timing was based on the response from force-sensing resistors (Interlink Electronics, Westlake Village, CA) placed in the insole of the right shoe to detect HL contact and to trigger the electrical stimulation (Zehr et al. 2014).
Electromyography.
Surface EMG signals were simultaneously recorded from TA, medial gastrocnemius, vastus lateralis, and biceps femoris. Pairs of Ag/AgCl disk electrodes (0.8 cm diameter; NE-101; Nihon Kohden) were placed in accordance with the surface EMG for noninvasive assessment of muscles (SENIAM; Enschede, the Netherlands) recommendation (Hermens et al. 2000). The skin was prepared for electrode placement by light abrasion and alcohol cleaning, and skin impedance was reduced to <10 kΩ. The EMG signals were amplified (×1,000) and band-pass filtered (53-1,000 Hz) using a bioamplifier system (1253A; NEC San-ei Instruments, Tokyo, Japan).
All analog data were digitized at 2 kHz using an analog-to-digital converter [CED1401; Cambridge Electronic Design (CED), Cambridge, UK] connected to a computer running a signal acquisition software (Spike2, ver. 6; CED).
Data analysis.
Custom-written software programs were used to separate the step cycle into 16 separate phases, beginning with HL contact and ending with subsequent HL contact at the swing-to-stance-phase transition. With the use of the force-sensing resistor signal, all data for each stimulus condition occurring in the same phase of the step cycle (n = ∼20 responses/phase) were sorted together. Averages from the same phase of walking during unstimulated cycles (“control” EMG, n = ∼50–60/phase) were then subtracted from each of the corresponding 16 averages after stimulation, yielding subtracted traces of reflex EMG (Yang and Stein 1990; Zehr et al. 1997). After this process, these EMG signals were smoothed (5 ms moving average). Cutaneous reflex amplitudes were determined from the maximum facilitatory or suppressive peak responses occurring from 70 to 120 ms after stimulation (MLR) (Nakajima et al. 2008).
Statistics.
For Exp. 1, phasic modulations of cutaneous reflex following electrical stimulation were compared using a two-way ANOVA (16 phases × 4 stimulation modes). In all instances, analyses were conducted on averaged data from each part of the step cycle for each subject. If a significant main effect for “Phase” was detected, then post hoc testing was performed with Dunnett's test. In Exp. 2, one-way ANOVA was used to test differences between group means of MLR obtained by three different stimulus conditions (f-M, TIB, and f-M&TIB or HL, TIB, and HL&TIB) in stance-to-swing- and swing-to-stance-phase transitions. If the result of the one-way ANOVA was significant, then post hoc pair-wise comparisons were computed using Bonferroni's correction. If there is a significant difference in the amount of MLR modulation between the TIB and combined f-M&TIB or HL&TIB, then we refer to this difference as “summation effect.” Statistical significance was set at P < 0.05 in all cases. Group data are shown as means ± SE. All statistical tests were performed using SPSS software, Ver. 11 (SPSS, Chicago, IL). The reported F values and degrees of freedom were those obtained after Greenhouse-Geisser correction when appropriate.
RESULTS
Exp. 1: reflex patterns evoked by TIB and discrete areas of foot-sole stimulation.
The TIB innervates much of the skin of the foot sole, and it is possible that inputs from each discrete site on the foot sole contribute to the whole-nerve TIB reflex. The first experiment was conducted to confirm similarity or differences in TA reflex patterns following TIB stimulation and different foot-sole regions (HL, f-L, and f-M) during walking.
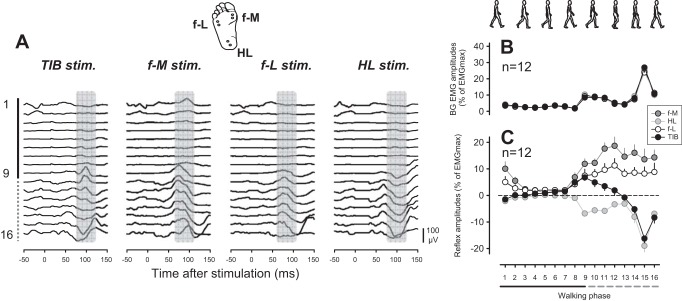
An example from a single subject is shown in Fig. 1A. Each stimulation site evoked unique reflexes, especially at the end of stance and during the whole-swing phase. In particular, MLRs of cutaneous reflexes following TIB stimulation were facilitatory during the late stance to mid-swing phases and reversed to suppression before HL strike (i.e., phase-dependent reflex reversal). Both f-M and f-L stimuli induced facilitation during the late stance to mid-swing phases, but HL stimulation evoked strong suppression during the late stance to the end of swing phases. Phase-dependent reflex reversal of MLR in TA could not be found using regional foot-sole stimulation in this subject.
Fig. 1.
Modulation of cutaneous reflexes in tibialis anterior (TA) muscle following tibial nerve (TIB), forefoot-medial (f-M), forefoot-lateral (f-L), and heel (HL) regional stimulation during walking. A: full-wave-rectified and -averaged electromyography (EMG) recordings from TA during 4 km/h walking on a treadmill. EMG recordings were obtained after subtracting background EMG from nonstimulated walking cycles. The walking cycle was divided into 16 phases and described from top to bottom (e.g., phase 1: initiation of a walking cycle; phase 16: end of walking cycle). From left to right, EMG recordings were obtained following TIB, f-M, f-L, and HL stimulation. Grand means of changes in amplitudes of (B) background (BG) EMG and (C) middle latency response (MLR) in TA muscle following stimulation at 4 different sites across 16 walking phases (n = 12). Vertical (A) and horizontal (C) black, solid lines, stance phase; vertical (A) and horizontal (C) gray, dashed lines, swing phase; numbers next to the vertical line (A), bin numbers in the walking cycle; black, dashed line (C), baseline of reflex amplitude (0 level) after subtraction of EMG traces during unstimulated cycles. EMGmax, maximum value of the mean amplitude of the full-wave-rectified TA EMG.
Figure 1 illustrates the group means for background (BG) EMG and MLR amplitudes (Fig. 1, B and C, respectively) following stimulation at f-M, f-L, and HL foot regions and TIB nerve stimulation during walking obtained from 12 subjects. As shown in Fig. 1B, the mean BG EMG amplitudes were significantly enhanced during swing phases compared with baseline levels at each stimulus site. However, there was no significant effect across stimulus sites. The two-way ANOVA of BG EMG amplitude showed a significant main effect of only walking phase [Phase: F(3.08,33.867) = 36.452, P < 0.0001; Stimulus site: F(3,33) = 1.062, P > 0.05; Task × Stimulus condition: F(4.659,51.244) = 1.161, P > 0.05]. Under these situations, following TIB nerve stimulation, reflexes were significantly enhanced at the stance-to-swing-phase transition (phases 8 and 9) compared with the baseline level (P < 0.05, Dunnett's post hoc test). However, the responses were predominantly suppressive at late swing- and stance-phase transition [phases 14–16 (P < 0.05, Dunnett's post hoc test); Fig. 1C]. The two-way ANOVA of reflex amplitude data showed a significant main effect of walking phase, stimulus site, and their interaction [Phase: F(3.3,36.277) = 9.816, P < 0.0001; Stimulus site: F(1.22,13.445) = 49.551, P < 0.0001; Task × Stimulus condition: F(4.932,54.252) = 19.089, P < 0.0001]. None of the subjects showed phase-dependent reflex reversal of MLR while undergoing regional foot-sole stimulation. In contrast, we elicited this reversal in all subjects following TIB stimulation, where there was a significant difference in reflex amplitude at baseline (i.e., negative or positive directions) across the phases (P < 0.05, Dunnett's post hoc test).
Based on these outcomes, reflex sign and modulation of MLR following TIB stimulation resembled that evoked by f-M stimulation for early swing (phases 8–10; i.e., facilitatory responses) and those following HL stimulation for late swing (phases 13–16; i.e., suppressive responses). In contrast, we found opposite reflex signs between TIB and f-M stimulation at swing-to-stance-phase and those between TIB and HL stimulation at stance-to-swing-phase transition. For instance, large facilitatory responses following f-M and f-L stimulation at swing-to-stance-phase transition were maintained, whereas predominant suppressive responses following TIB stimulation could be elicited at these phases. At the transition from stance-to-swing phase, suppressive responses following HL stimulation were observed when the TIB stimulation gave rise to a facilitatory reflex response.
In Exp. 1, we found both similarities and differences in reflex patterns (e.g., direction of reflex signs) following TIB, f-M, HL, and f-L stimulation, all of which were strongly phase dependent. Reflexes observed with TIB and discrete foot-sole stimulation could be an emergent property of summation selectively revealed by the foot-sole reflex with similar signs. In contrast, it is plausible that afferent populations, which produce opposite signs of foot-sole reflexes, are gated onto TIB-reflex circuits and TA motoneurons. To investigate this possibility in more detail, we conducted the experiment using summation effects of simultaneous stimulation of both TIB and each foot-sole area during walking.
Exp. 2: summation effect on reflexes following combined stimulation of TIB and discrete regions on the foot sole.
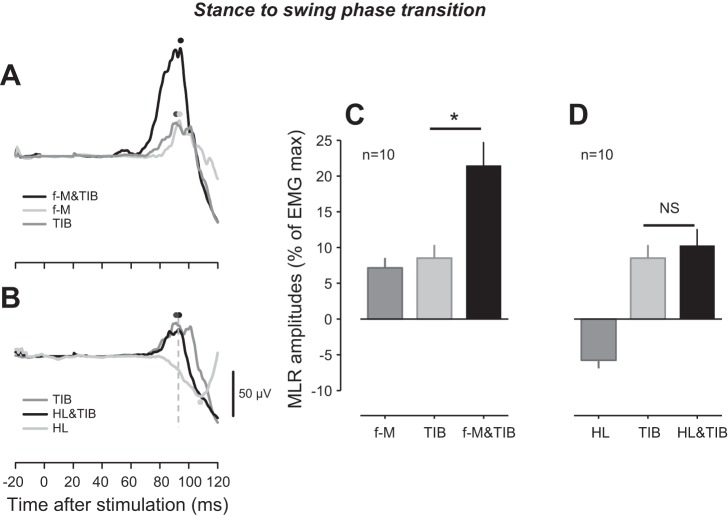
Figure 2, A and B, presents the typical recordings of TA MLR from a single subject following application of all stimuli at the stance-to-swing-phase transition. When TIB was stimulated, a facilitatory MLR can be seen in TA. Furthermore, f-M and HL stimulation evoked a facilitation and suppression response in TA, respectively. When combined f-M&TIB stimulation was given, a summation effect can be seen. In contrast, there was no summation effect following the combined HL&TIB stimulation. Figure 2, C and D, shows grouped data for MLRs evoked by the different stimulation conditions. MLR amplitude for the combined f-M&TIB stimulation condition was significantly larger (21.5 ± 3.2% of EMGmax) than that for separate f-M (7.1 ± 1.3% of EMGmax) or TIB (8.5 ± 1.7% of EMGmax) stimulation across participants (Fig. 2C). One-way ANOVA showed a significant main effect for site [F (2.18) = 16.405, P < 0.0001], and post hoc test showed that there was a significant difference between the means obtained by f-M or TIB stimulation and the combined f-M&TIB stimulation (Fig. 2C; P < 0.001, Bonferroni test). In contrast, no significant change was detected when comparing the MLR amplitude following TIB with that from HL&TIB stimulation (Fig. 2D; P > 0.05, Bonferroni test).
Fig. 2.
Reflex modulation of middle latency response (MLR) following tibial nerve (TIB), forefoot-medial (f-M), forefoot-lateral (f-L), heel (HL), and combined f-M&TIB and HL&TIB stimulation at the stance-to-swing-phase transition. A: superimposed TA EMG recordings following f-M, TIB, and f-M&TIB stimulation obtained from a single subject. B: EMG recordings of TA following HL, TIB, and HL&TIB stimulation, obtained from the same subject showed in A. C: group means of MLR amplitudes following f-M, TIB, and f-M&TIB stimulation. D: group means of MLR following HL, TIB, and HL&TIB stimulation. Note that the EMG traces for TIB stimulation in A and B are the same; furthermore, the group data for TIB in C and D are the same. Black dots, peak latency of MLR following f-M&TIB (A) and HL&TIB (B) stimulation; dark-gray dots, peak latency of MLR following TIB stimulation (A and B); light-gray dots, peak latency of MLR following f-M (A) and HL (B) stimulation; dashed, vertical line (B), latency of peak amplitude following HL&TIB stimulation. *P < 0.01; NS, not significant.
As shown in Fig. 2B, there was a reciprocal influence between the later facilitatory MLR (∼100–110 ms) that followed the f-M&TIB stimulation and the HL-induced suppression. Despite this, the early part of this facilitatory MLR (∼80–95 ms after stimulation) was minimally affected. To confirm this, we performed an additional analysis, in which the amplitude of all reflexes induced by TIB, HL, and HL&TIB stimulation was measured at identical latencies (i.e., Fig. 2B) in all subjects (n = 10), regardless of the sign of the reflexes. This analysis did not reveal significant differences in facilitation amplitudes between HL&TIB (10.25 ± 2.3% of EMGmax) and TIB (8.75 ± 1.9% of EMGmax) stimulation (P > 0.05, Bonferroni test). At this latency, significant suppressions (−3.58 ± 1.1% of EMGmax) were elicited when stimulating HL across all subjects (baseline vs. MLR amplitude using HL stimulation, P < 0.02).
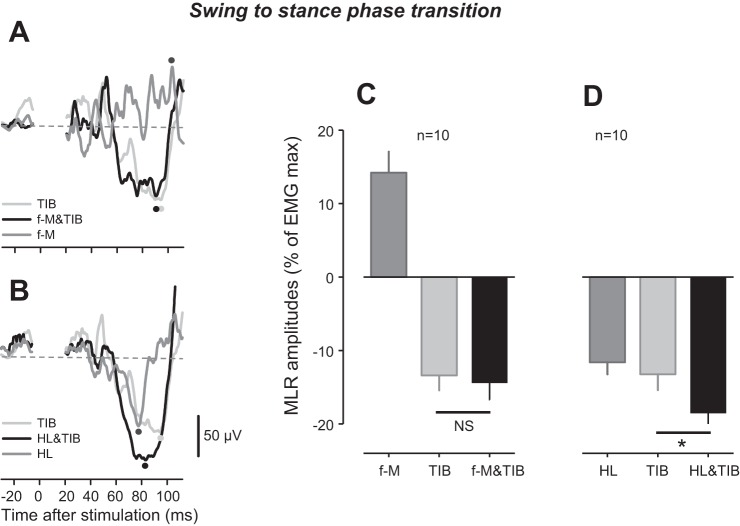
As for swing-to-stance-phase transition, the separate f-M and TIB stimulations evoked facilitation and suppression of MLR amplitudes, respectively (Fig. 3, A and C). Interestingly, almost the same suppressive effect from TIB stimulation was observed following the combined f-M&TIB stimulation (Fig. 3, A and C). Thus the facilitatory effect induced by f-M stimulation did not appear to enhance further the effects of TIB alone. In contrast, a significant increase in suppression was detected following the combined HL&TIB stimulation compared with TIB stimulation alone (P < 0.01, Bonferroni test; Fig. 3, B and D).
Fig. 3.
Reflex modulation of middle latency response (MLR) following tibial nerve (TIB), forefoot-medial (f-M), forefoot-lateral (f-L), heel (HL), and f-M&TIB and HL&TIB stimulation at the swing-to-stance-phase transition. A: superimposed TA EMG recordings following f-M, TIB, and f-M&TIB stimulation obtained from a single subject. B: EMG recordings of TA following HL, TIB, and HL&TIB stimulation, obtained from the same subject as in A. C: group means of MLR amplitudes following f-M, TIB, and f-M&TIB stimulation. D: group means of MLR following HL, TIB, and HL&TIB stimulation. Note that the EMG traces for TIB stimulation in A and B are the same; furthermore, the group data for TIB in C and D are the same. Black dots, peak latency of MLR following f-M&TIB (A) and HL&TIB (B) stimulation; dark-gray dots, peak latency of MLR following f-M (A) and HL (B) stimulation; light-gray dots, peak latency of MLR following TIB stimulation (A and B); dashed, horizontal lines (A and B), baseline of reflex amplitude (0 level) after subtraction of EMG traces during unstimulated cycles. *P < 0.01; NS, not significant.
DISCUSSION
Methodological considerations.
An important consideration is whether discrete stimulation areas on the foot sole were the regions actually innervated by TIB. It is known that the lateral border between the dorsal and plantar surface of the foot is partly innervated by the sural nerve (Agur and Dalley 2009; Neumann 2002). However, we confirmed with all participants that sensations from all three discrete locations (f-M, f-L, and HL) were reported when stimulating TIB. In addition, in some subjects, we stimulated the sural nerve, and participants reported that the sensation was quite different from that following discrete foot-sole stimuli. Thus it is unlikely that our stimulus location included the nerve territory of sural nerve. Furthermore, subjects reported a quite localized sensation underneath the electrodes when stimulating each foot-sole site. Thus we are confident that localized cutaneous afferents in the plantar foot innervated by TIB were simultaneously activated by stimulation through the surface electrodes used in the present study.
After branching from the TIB nerve at the ankle level, f-L and f-M are innervated by the lateral and medial plantar nerves, respectively. However, the skin of the HL is supplied by the TIB nerve above this point (Agur and Dalley 2009). We confirmed that stimulating TIB in the areas of f-M, f-L, and HL generated radiating sensations in all subjects. Thus we are confident that whole-nerve stimulation at the ankle level activated afferents in the branching nerves to HL, f-M, and f-L skin regions.
Afferents branching to the HL have their dorsal roots at S1–S2, whereas the medial plantar nerve has its dorsal roots at L4–L5 (Agur and Dalley 2009). Thus it is plausible that discrete reflex pathways innervating f-M and HL could account for the differing response patterns between them (see Fig. 1A). These are relevant for discussing the convergent effect of simultaneous TIB and discrete foot-area stimulation on reflex responses in relation to the different branches, areas, or structures that they innervate. As such, phase-dependent reflex outputs (i.e., MLRs) that followed the combined f-M&TIB or HL&TIB stimulation could be attributed to the active switching of each reflex pathway by a locomotor pattern-generating system (Duysens et al. 1992).
As an additional consideration, the TIB is a mixed nerve, and the medial and lateral branches innervate different intrinsic foot muscles (Agur and Dalley 2009). Thus it is likely that muscular afferents could play a role in generating phase-dependent reflex reversal when stimulating the TIB. However, it is noteworthy that the general reflex signs of the MLR were substantially similar following TIB and f-M stimulation (the latter innervating the skin underneath the base of the proximal phalanx of the great toe) at the stance-to-swing-phase transition (i.e., facilitatory sign; see Fig. 2A) and that following TIB and HL stimulation (the latter innervating the skin of the HL) at the swing-to-stance-phase transition (i.e., suppressive sign; see Fig. 3B). In addition, purely cutaneous nerve (i.e., sural nerve) stimulation of the foot also elicits this reversal phenomenon, although this is dependent on walking phase (Duysens et al. 1992). Presently, however, we cannot give an explicit explanation for the possible contributions of muscular afferents to reflex reversal on the TA during human walking. Thus further study is needed to elucidate this point.
In Exp. 2, we intended to prevent conscious prediction of stimulation modes in a session (Baken et al. 2006) and to detect reliably any summation effect of reflex amplitudes following combined stimulation. To accomplish this aim, all five stimuli were randomly delivered within a single session for each participant. As a result, the amount of reflex modulations following isolated f-M, HL, and TIB stimulation during both stance-to-swing and swing-to-stance transition phases resembled those obtained in Exp. 1. Thus we consider valid the comparison between the amount of MLR modulation following isolated f-M or HL and the combined f-M&TIB or HL&TIB.
Common and unique effects on reflex signs following TIB and regional sites on the foot sole during walking.
The underlying mechanisms of the reflex reversal of cutaneous reflex amplitudes during walking in cats and humans have been described previously (Conway et al. 1995; Duysens et al. 1980, 1990; Forssberg et al. 1975; Guertin et al. 1995; Nakajima et al. 2008; Van Wezel et al. 1997; Yang and Stein 1990). In humans, one plausible explanation is that two discrete groups of motor units, which receive facilitatory or inhibitory inputs from spinal interneurons, are differentially recruited depending on the walking phase (Burke et al. 1970; Yang and Stein 1990). However, De Serres et al. (1995) argued against this idea and clarified that single motoneurons in TA receive both facilitatory and inhibitory inputs from discrete reflex pathways following stimulation of distal TIB. This suggests that locomotor commands selectively switch access between facilitatory and inhibitory pathways during stance-to-swing- and swing-to-stance-phase transitions, respectively.
Although TIB innervates broad areas of the skin of the foot sole, it is unknown if afferents from discrete regions within the foot-sole innervation territory of TIB give rise to these suppressive or facilitatory effects during human walking. Our prior work and that from others suggest that there are distinct foot-sole regions for eliciting suppressive or facilitatory reflexes (Andersen et al. 2001; Conway et al. 1995; Nakajima et al. 2006, 2009; Sonnenborg et al. 2000; Zehr et al. 2014).
Results from Exp. 1 revealed that the stimulation of discrete regions of the foot sole did not produce phase-dependent reflex reversal in all subjects (see Fig. 1). This suggests that afferent volleys from regional sites on the foot sole do not, in isolation, generate reversal of reflex signs depending on walking phases in TA.
How then is this reflex reversal produced by TIB stimulation? As one possibility, it is likely that suppressive or facilitatory responses elicited by distinct afferent populations within the foot sole converge on the TIB-induced inputs depending on the walking phase. In fact, the signs and modulation patterns of MLR following TIB stimulation resemble those following forefoot stimulation (f-M and f-L) for stance-to-swing (i.e., facilitatory responses) and those following HL stimulation for swing-to-stance (i.e., suppressive responses) phase, respectively. The similarities of reflex patterns and signs infer the possibility of shared pathways elicited by afferent inputs to TIB and foot sole (f-M or HL) (Andersen et al. 2014; Baldissera et al. 1981; LaBella and McCrea 1990; Nakajima et al. 2013, 2014).
In contrast, we found distinct effects on reflex sign induced by stimulating foot-sole regions and TIB during walking. For instance, response signs of MLR following TIB stimulation are opposite to those following HL stimulation for stance-to-swing-phase transition and those following f-M stimulation for swing-to-stance-phase transition, respectively. These findings suggest that the contribution of cutaneous afferents within these regions of the foot sole had relatively small effects on the TIB-induced reflex sign. Therefore, we conducted an experiment to investigate the underlying mechanisms by performing a combined stimulation of discrete foot-sole regions and the TIB.
Evidence for regional contributions of foot-sole afferent populations on the TIB-induced reflex reversal: summation effect of TIB and its innervation area of foot sole during walking.
In Exp. 2, we used summation effects of reflex amplitudes by stimulating TIB and its innervation areas of the foot sole. The summation effects of MLR following simultaneous stimulation were assessed as a kind of temporal summation of the TIB-induced MLR reflex pathway. Thus it is expected that cancellation occurs with simultaneous suppressive and facilitatory MLRs activated by the different regional afferent populations. However, we found that the effects differ depending on foot-sole regions (f-M or HL), and opposite reflex outputs did not cancel out one another. In addition, the relationships were modulated by walking phases (stance-to-swing- or swing-to-stance-phase transitions). TIB-induced, suppressive MLR was less affected by f-M-induced facilitatory MLR at swing-to-stance-phase transition (see Fig. 3, A and C). These findings were consistent with the mechanistic possibilities described above.
To provide a context for our findings, therefore, we propose a schematic representation of the possible neural mechanisms in Fig. 4. This figure is an admittedly oversimplified version of the likely set of connections in the human spinal cord, but it can be a useful approximation for discussing our findings and for framing additional research questions.
Fig. 4.
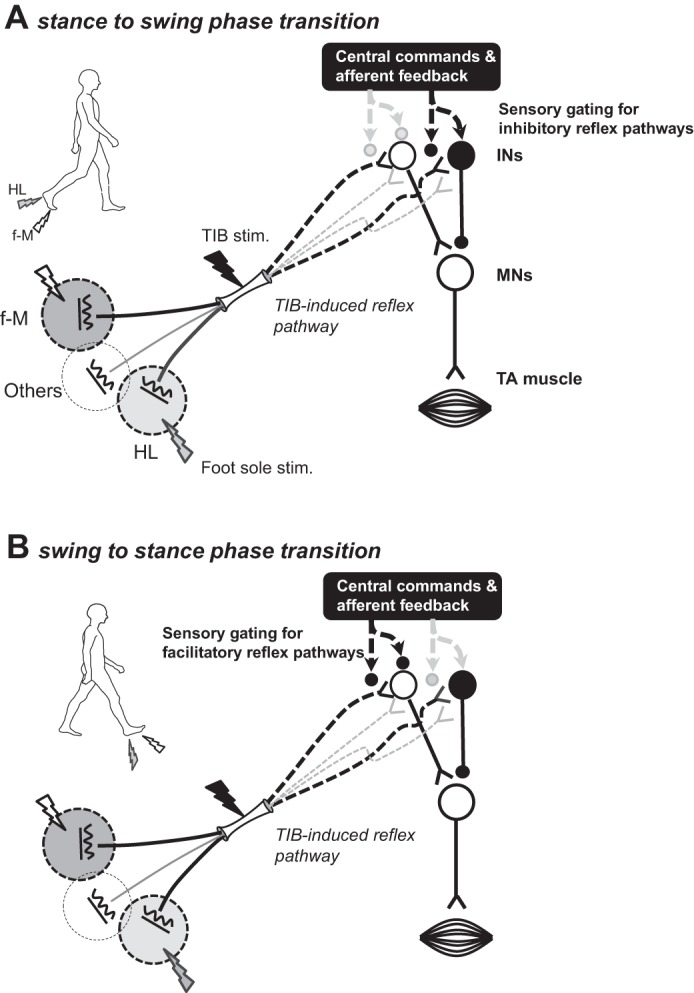
Schematic diagram of a simplified putative neural framework in (A) stance-to-swing- and (B) swing-to-stance-phase transitions. Putative reflex pathways with cutaneous inputs evoked by stimulation of tibial nerve (TIB) and its innervation areas within the foot sole are shown (A and B). These inputs project to interneuronal reflex pathways indicated by the open (excitatory) or filled (inhibitory) circles. The effect of middle latency response (MLR) following forefoot-medial (f-M) and TIB produced temporal summation on TIB-reflex circuits at the stance-to-swing-phase transition (A). Thus the simplest explanation would be that afferent inputs arising from f-M stimulation partly contributed to the facilitation of TIB-induced reflexes (A). In contrast, it is possible that afferent inputs from the heel (HL) were partly gated on TIB-reflex circuitry by locomotor-related commands (downward black arrow in A). The facilitatory effect of TIB input was not modulated when applying inhibitory inputs with HL stimulation. However, during the swing-to-stance transition (B), the facilitatory inputs from f-M onto TIB-induced-reflex circuits were absent, and inhibitory inputs from HL were exhibited. Black lightning bolts, TIB stimulation; gray lightning bolts, HL stimulation; white lightning bolts, f-M stimulation. INs, interneurons; MNs, motoneurons.
Figure 4 shows the simplest putative reflex pathways with cutaneous inputs evoked by stimulation of TIB and its innervation areas of foot sole. These inputs project to interneuronal reflex pathways (excitatory or inhibitory). In our study, the effect of MLR following f-M&TIB stimulation exhibited temporal summation on TIB-reflex circuits at stance-to-swing-phase transition (see Fig. 4A). The simplest explanation would be that afferent inflow arising from f-M stimulation contributed to facilitation of TIB-induced reflexes. In contrast, it is possible that afferent inputs from HL were partly gated from the TIB-induced MLR-reflex circuit by regulation of locomotor-related commands. In fact, the facilitatory effect of TIB reflex was not dramatically modulated when applying suppressive inputs by HL stimulation (i.e., the early part of facilitatory MLR). During swing-to-stance-phase transition (see Fig. 4B), in contrast, the facilitatory inputs from f-M onto the TIB-induced-reflex circuit seem to be omitted, and inhibitory inputs from HL are well accessed.
Although other contributions cannot be denied (e.g., inputs from other areas in the foot sole; see Fig. 4, A and B), we believe that this explanation is the simplest and most reasonable interpretation based on our observations.
Cutaneous reflexes with multiple components have a certain complexity and individual variability [for review, see Nakajima et al. (2015); Zehr and Duysens (2004)]. Although we focused on MLR peaks within 70–120 ms, there are limitations for quantifying the peak facilitation or suppression of the MLR. Thus complementary methods, such as area measurements, could be used in a future study to address this gap in knowledge.
In terms of function, we previously demonstrated that TIB stimulation at the stance-to-swing-phase transition evokes “withdrawal reaction,” with facilitation of TA reflex in concert with knee flexion, and induces a “placing reaction,” with inhibition of TA reflex at the swing-to-stance transition (Zehr and Duysens 2004; Zehr et al. 1997; Zehr and Stein 1999). These reflex actions were suggested to prompt a quick modulation of foot trajectory away from an obstacle and continuance of forward progression (Zehr and Duysens 2004). Thus it is likely that afferent information arising from specific foot-sole regions plays a key role in promoting and shaping these reflex functions, even if whole-nerve stimulation is applied. As for these reflex functions, these are well in line with the data described in our more-recent paper (Zehr et al. 2014), measuring mechanical reflex outputs after stimulation of discrete foot-sole regions and envisioning the skin surface of the foot as a kind of sensory “antenna” that promotes sensory steering. We suggest that reflexes following whole-nerve stimulation (representing foot-sole activation during walking) are weighted with and shaped by feedback from localized regional afferent populations that are themselves differentially modulated. In sum, these provide widespread redundancy and refinement of the finely tuned reflex control of walking. Thus further investigation is needed to elucidate the precise interactions among the components of this phenomenon.
GRANTS
Support for this study was partially provided by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI; No. 26560282; to T. Nakajima). Support for R. A. Mezzarane was provided by the Research Internships Abroad (BEPE) Program (Proc. No. 2012/05304-5) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.N., S.S., H.O., R.A.M., T.S.B., T. Klarner, E.P.Z., and T. Komiyama conception and design of research; T.N., S.S., G.F., H.O., R.A.M., and T. Komiyama performed experiments; T.N., S.S., H.O., T.S.B., T. Klarner, E.P.Z., and T. Komiyama analyzed data; T.N., S.S., G.F., T.S.B., T. Klarner, E.P.Z., and T. Komiyama interpreted results of experiments; T.N., S.S., E.P.Z., and T. Komiyama prepared figures; T.N., R.A.M., E.P.Z., and T. Komiyama drafted manuscript; T.N., S.S., G.F., H.O., R.A.M., T.S.B., T. Klarner, E.P.Z., and T. Komiyama edited and revised manuscript; T.N., S.S., G.F., H.O., R.A.M., T.S.B., T. Klarner, E.P.Z., and T. Komiyama approved final version of manuscript.
REFERENCES
- Agur AM, Dalley AF II. Grant's Atlas of Anatomy. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2009. [Google Scholar]
- Andersen OK, Klimstra M, Thomas E, Loadman PM, Hundza SR, Zehr EP. Human cutaneous reflexes evoked with simultaneous multiple nerve stimulation during rhythmic locomotor-like arm and leg cycling in stroke subjects. In: Replace, Repair, Restore, Relieve—Bridging Clinical and Engineering Solutions in Neurorehabilitation, edited by Jensen W, Anderson OK, Akay M. Switzerland: Springer International, 2014, vol. 7, p. 255–261. [Google Scholar]
- Andersen OK, Sonnenborg FA, Arendt-Nielsen L. Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clin Neurophysiol 112: 641–649, 2001. [DOI] [PubMed] [Google Scholar]
- Baken BC, Nieuwenhuijzen PH, Bastiaanse CM, Dietz V, Duysens J. Cutaneous reflexes evoked during human walking are reduced when self-induced. J Physiol 570: 113–124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Comprehensive Physiology, Supplement 2: Handbook of Physiology, The Nervous System, Motor Control. Bethesda, MD: American Physiological Society, 1981, p. 509–595. [Google Scholar]
- Burke D, Dickson HG, Skuse NF. Task-dependent changes in the responses to low-threshold cutaneous afferent volleys in the human lower limb. J Physiol 432: 445–458, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Jankowska E, ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol 207: 709–732, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Scoott DT, Riddell JS, Hadian MR. The effects of plantar nerve stimulation on the transmission of late flexion reflexes in the decerebrate spinal cat. In: Alpha and Gamma Motor Systems, edited by Taylor A, Gladden MH, Durbaba R. New York: Springer US, 1995, p. 593–595. [Google Scholar]
- De Serres SJ, Yang JF, Patrick SK. Mechanism for reflex reversal during walking in human tibialis anterior muscle revealed by single motor unit recording. J Physiol 488: 249–258, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Loeb GE, Weston BJ. Crossed flexor reflex responses and their reversal in freely walking cats. Brain Res 197: 538–542, 1980. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res 90: 404–414, 1992. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82: 351–358, 1990. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953, 1979. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res 85: 103–107, 1975. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487: 197–209, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Adaptation and after-effects of split-belt walking in cerebellar lesion patients. J Neurophysiol 114: 1693–1704, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella LA, McCrea DA. Evidence for restricted central convergence of cutaneous afferents on an excitatory reflex pathway to medial gastrocnemius motoneurons. J Neurophysiol 64: 403–412, 1990. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Barss T, Klarner T, Komiyama T, Zehr EP. Amplification of interlimb reflexes evoked by stimulating the hand simultaneously with conditioning from the foot during locomotion. BMC Neurosci 14: 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Kamibayashi K, Takahashi M, Komiyama T, Akai M, Nakazawa K. Load-related modulation of cutaneous reflexes in the tibialis anterior muscle during passive walking in humans. Eur J Neurosci 27: 1566–1576, 2008. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Mezzarane RA, Hundza SR, Komiyama T, Zehr EP. Convergence in reflex pathways from multiple cutaneous nerves innervating the foot depends upon the number of rhythmically active limbs during locomotion. PLoS One 9: e104910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Mezzarane RA, Komiyama T, Zehr EP. Reflex control of human locomotion: existence, features, and functions of common interneuronal system induced by multiple sensory inputs in humans. J Sports Med Phys Fitness 4: 197–211, 2015. [Google Scholar]
- Nakajima T, Sakamoto M, Tazoe T, Endoh T, Komiyama T. Location specificity of plantar cutaneous reflexes involving lower limb muscles in humans. Exp Brain Res 175: 514–525, 2006. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Sakamoto M, Tazoe T, Endoh T, Komiyama T. Location-specific modulations of plantar cutaneous reflexes in human (peroneus longus muscle) are dependent on co-activation of ankle muscles. Exp Brain Res 195: 403–412, 2009. [DOI] [PubMed] [Google Scholar]
- Neumann DA. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation. St. Louis, MO: C.V. Mosby, 2002. [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- Sonnenborg FA, Andersen OK, Arendt-Nielsen L. Modular organization of excitatory and inhibitory reflex receptive fields elicited by electrical stimulation of the foot sole in man. Clin Neurophysiol 111: 2160–2169, 2000. [DOI] [PubMed] [Google Scholar]
- Van Wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci 17: 3804–3814, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63: 1109–1117, 1990. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist 10: 347–361, 2004. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77: 3311–3325, 1997. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Nakajima T, Barss T, Klarner T, Miklosovic S, Mezzarane RA, Nurse M, Komiyama T. Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci Med Rehabil 6: 33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB, Komiyama T. Function of sural nerve reflexes during human walking. J Physiol 507: 305–314, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]