Abstract
Cocaine addiction is a debilitating neuropsychiatric disorder characterized by uncontrolled cocaine intake, which is thought to be driven, at least in part, by cocaine-induced deficits in dopamine system function. A decreased ability of cocaine to elevate dopamine levels has been repeatedly observed as a consequence of cocaine use in humans, and preclinical work has highlighted tolerance to cocaine's effects as a primary determinant in the development of aberrant cocaine taking behaviors. Here we determined that cocaine self-administration in rats produced tolerance to the dopamine transporter-inhibiting effects of cocaine in the nucleus accumbens core, which was normalized following a 14 or 60 d abstinence period; however, although these rats appeared to be similar to controls, a single self-administered infusion of cocaine at the end of abstinence, even after 60 d, fully reinstated tolerance to cocaine's effects. A single cocaine infusion in a naive rat had no effect on cocaine potency, demonstrating that cocaine self-administration leaves the dopamine transporter in a “primed” state, which allows for cocaine-induced plasticity to be reinstated by a subthreshold cocaine exposure. Further, reinstatement of cocaine tolerance was accompanied by decreased cocaine-induced locomotion and escalated cocaine intake despite extended abstinence from cocaine. These data demonstrate that cocaine leaves a long-lasting imprint on the dopamine system that is activated by re-exposure to cocaine. Further, these results provide a potential mechanism for severe cocaine binge episodes, which occur even after sustained abstinence from cocaine, and suggest that treatments aimed at transporter sites may be efficacious in promoting binge termination following relapse.
SIGNIFICANCE STATEMENT Tolerance is a DSM-V criterion for substance abuse disorders. Abusers consistently show reduced subjective effects of cocaine concomitant with reduced effects of cocaine at its main site of action, the dopamine transporter (DAT). Preclinical literature has shown that reduced cocaine potency at the DAT increases cocaine taking, highlighting the key role of tolerance in addiction. Addiction is characterized by cycles of abstinence, often for many months, followed by relapse, making it important to determine possible interactions between abstinence and subsequent drug re-exposure. Using a rodent model of cocaine abuse, we found long-lasting, possibly permanent, cocaine-induced alterations to the DAT, whereby cocaine tolerance is reinstated by minimal drug exposure, even after recovery of DAT function over prolonged abstinence periods.
Keywords: abstinence, addiction, psychostimulant, relapse, tolerance, voltammetry
Introduction
Cocaine addiction is a debilitating neurological disorder that results in severe negative health and economic outcomes (O'Connor et al., 2014). In the United States alone, >700,000 people received treatment for cocaine use in 2014 (Substance Abuse and Mental Health Services Administration, 2015). The disorder is characterized by repeated attempts at abstinence, which often end in relapse to cocaine use. Although relapse can occur following short periods of abstinence, which has been suggested to be driven by the effects of withdrawal or negative reinforcement, relapse can occur even following years of successful abstinence (Gawin, 1991). Further, once relapse occurs, addicts often continue using the drug for extended periods of time before attempting abstinence again (Grella et al., 2003); thus, elucidating the neurochemical consequences of cocaine abuse, specifically the interaction between abstinence and cocaine re-exposure, is critical to understanding the neurobiology of addiction.
Years of clinical and preclinical research have implicated cocaine-induced alterations to dopamine system function as a primary factor in the development of excessive cocaine taking behaviors (Dackis and O'Brien, 2001; Hyman et al., 2006; Koob, 2013; Willuhn et al., 2014). In particular, decreases in the ability of cocaine to elevate dopamine levels and produce euphoric effects have been observed in human cocaine addicts (Volkow et al., 1997, 2014). This tolerance to cocaine results in increased drug consumption to overcome decreased subjective effects (Dackis and O'Brien, 2001). These effects have been recapitulated following cocaine exposure in preclinical studies where escalation of intake over time results in a blunted ability of cocaine to increase extracellular dopamine levels (Hurd et al., 1989, 1990; Weiss et al., 1992; Mateo et al., 2005; Ferris et al., 2011). We have shown that this reduction in cocaine effects on the dopamine system occurs directly at the dopamine transporter (DAT), where cocaine is less effective at inhibiting dopamine uptake from the synapse (Ferris et al., 2011; Calipari et al., 2013a; Siciliano et al., 2015a,b). Whereas previous work has shown that abstinence can result in apparently normalized dopamine system function (Satel et al., 1991; Kalivas and Duffy, 1993; Pierce et al., 1995; Beveridge et al., 2009; Ferris et al., 2011), here we show that, although many of the markers of dopamine system function appear normal, the system is in a state that is effectively “primed” to return to the tolerant state following a single exposure to the drug.
Using ex vivo fast scan cyclic voltammetry (FCSV) in the nucleus accumbens (NAc) core of cocaine self-administering rats, we assessed the ability of cocaine to inhibit the DAT following self-administration and abstinence. We found that, consistent with previous investigations (Ferris et al., 2011), cocaine self-administration resulted in a reduction in the ability of cocaine to inhibit the DAT. Further, we found that prolonged abstinence from cocaine (14 or 60 d) resulted in restoration of apparently normal DAT function; however, when rats self-administered a single cocaine injection, DAT dysfunction was fully reinstated, suggesting that after extended abstinence, the DAT remains in a labile state such that cocaine-induced deficits are reestablished by subthreshold cocaine exposures. These results have broad implications for understanding of long-lasting impact of cocaine on dopamine system function, and provide a putative mechanism for high-intake cocaine binges, which can occur in cocaine addicts even after years of abstinence (Washton and Stone-Washton, 1990; Wallace, 1992; McKay et al., 1995).
Materials and Methods
Animals.
Male Sprague-Dawley rats (350–400 g; Harlan Laboratories), were maintained on a 12 h reverse light/dark cycle (3:00 A.M. lights off; 3:00 P.M. lights on) with food and water ad libitum. All rats were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
Self-administration.
Rats were anesthetized and implanted with chronic indwelling jugular catheters as previously described (Siciliano et al., 2015a). Rats were singly housed, and each 6 h session took place in the home cage during the active/dark cycle (09:00–15:00 h). Without any prior operant training, rats were given access on a fixed-ratio one schedule to a cocaine-paired lever, which, upon responding, initiated an intravenous injection of cocaine (1.5 mg/kg, infused over ∼4 s, depending on animal weight). Concurrent with the start of each infusion, the lever was retracted and a stimulus light was illuminated for a 20 s timeout period. Sessions lasted 6 h or until 40 injections were earned. Acquisition (Day 1) was counted when the animal reached 35 or more responses with a stable and consistent inter-injection interval. Following acquisition of cocaine maintained responding, the rats were given access to 40 injections per day (1.5 mg/kg/infusion) for a period of 5 consecutive days before beginning abstinence or voltammetry experiments. Abstinence periods lasted for 14 or 60 d, during which catheter patency was maintained by hourly infusions of heparinized saline. During abstinence, rats were housed in their home cage, but levers were not extended. For 60 d experiments, at the end of the abstinence period, catheter patency was determined by infusion of sodium methohexital (0.5–1 mg); rats were considered patent if consciousness was lost within 1–2 s following infusion. Note that both saline and cocaine re-exposure rats were exposed to methohexital. Following abstinence, rats were given access to cocaine (1.5 mg/kg) or saline with a maximum of 1 injection. Cocaine doses for the initial self-administration as well as for the self-administration re-exposure were selected because 1.5 mg/kg/infusion has been shown to be the most reinforcing dose, at the peak of the progressive ratio dose–response curve (Richardson and Roberts, 1996). Further, we have demonstrated previously that this dose results in high cocaine intake, and escalation in rate of cocaine intake over days, concomitant with disruption of cocaine effects the DAT (Ferris et al., 2011; Siciliano et al., 2015a). Rats were excluded from the experiment based on two criteria: (1) failure to meet acquisition criteria within 14 cocaine self-administration sessions, and (2) loss of catheter patency. Approximately 15% of rats were excluded for failure to meet criteria, and five rats were excluded based on loss of patency.
Voltammetry experiments were performed the morning after (∼18 h) following completion of the final self-administration session. Control rats were either naive or surgery control rats, age-matched and housed under the same reversed light/dark light cycle for the same length of time as experimental rats. Consistent with several previous studies from our laboratory, we found that surgery alone did not alter cocaine effects, thus surgery and naive controls were collapsed into one group (Calipari et al., 2013a,b, 2015).
Ex vivo voltammetry.
Rats were euthanized for FSCV experiments the morning following the final self-administration session (∼18 h), when no drug was present. FSCV was used to characterize the ability of cocaine to inhibit dopamine uptake in the NAc core. A vibrating tissue slicer was used to prepare 400-μm-thick coronal brain sections containing the NAc core, as previously described (Siciliano et al., 2014a). The tissue was immersed in oxygenated artificial CSF (aCSF) containing the following (in mm): 126 NaCl (126), 2.5 KCl (2.5), 1.2 NaH2PO4, 2.4 CaCl2, 1.2 MgCl2, 25 NaHCO3, 11 glucose, 0.4 l-ascorbic acid, and pH was adjusted to 7.4. Once sliced, the tissue was transferred to the testing chambers containing bath aCSF (32°C), which flowed at 1 ml/min. A carbon fiber microelectrode (100–200 μm length, 7 μm diameter) and bipolar stimulating electrode were placed into the core of the NAc, which was selected because of its role in the reinforcing and rewarding actions of cocaine. Dopamine release was evoked by a single electrical pulse (350 μA, 4 ms, monophasic) applied to the tissue every 3 min. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). Once the extracellular dopamine response was stable, cocaine (0.3–30 μm) was applied cumulatively to the brain slice.
Ki values.
As described previously (Siciliano et al., 2014b), inhibition constants (Ki) were calculated by the equation: [(Km)/(S)], where Km is equal to the Km of dopamine for the DAT, or 1.6 μm, and S is equal to the slope of the linear concentration–response regression for cocaine. The Ki, reported in micromoles and is a measure of the cocaine concentration that is necessary to decrease the rate of dopamine–DAT interactions to 50% of their uninhibited rate.
Locomotor assessment.
All locomotor testing occurred during the dark/active cycle (9:00 A.M.). Rats were first transferred to the locomotor testing room (lights off) and allowed to habituate within their home cages for 1 h. Rats were then placed in activity monitors (Med Associates) and their horizontal activity was monitored. The activity chambers were acrylic boxes measuring 43 × 43 × 30 cm and contained two infrared beam arrays. Horizontal activity was measured by beam breaks, which were recorded by a computer. Control rats were age-matched and received identical surgery and housing conditions.
Self-administration re-exposure.
To test the effects of a history of cocaine self-administration and abstinence on subsequent self-administration behavior, a separate group of rats was allowed to self-administer cocaine for 5 d post-acquisition, identical to the parameters described above. Control rats went through identical procedures but were allowed to self-administer saline for 5 d. Both groups then went through 14 d of abstinence, followed by cocaine self-administration under identical parameters. For both groups, during abstinence catheter patency was maintained by hourly infusions of heparinized saline. For rats with a history of cocaine self-administration, post-abstinence data presented were from the first 5 d of self-administration following abstinence. For rats that had only had prior access to saline, post-abstinence data presented were from the day acquisition criteria were met and the 4 d following. For all control rats, acquisition occurred 1–8 d following abstinence.
Data analysis.
For all analysis of FSCV data Demon Voltammetry and Analysis software was used (Yorgason et al., 2011). To evaluate dopamine kinetics and drug potency, evoked levels of dopamine were modeled using Michaelis–Menten kinetics. Recording electrodes were calibrated by recording responses (in electrical current; nA) to a known concentration of dopamine (3 μm) using a flow-injection system. This was used to convert electrical current to dopamine concentration. For cocaine concentration-response curves, apparent Km, a measure of affinity for the DAT, was used to determine changes in ability of the cocaine to inhibit dopamine uptake. As apparent Km increases, uptake inhibition increases, and changes in drug potency are seen as shifts in the curve.
Statistics.
Graph Pad Prism (v6) was used to statistically analyze datasets and create graphs. Concentration response curves for cocaine and locomotor data were subjected to a mixed-model two-way repeated-measures ANOVA with either concentration or time as the within-subjects factor, and experimental group as the between-subjects factor. Ki values were compared using a one-way ANOVA. Differences between groups for both one- and two-way ANOVAs were tested using a Bonferroni post hoc test. Escalation data from the self-administration re-exposure experiment were fit with linear regression lines, and the slope of the line was compared to zero to determine whether escalation was occurring over time. Area under the curve for the locomotor experiment and rate of intake before and after abstinence for the self-administration re-exposure experiment were compared using two-tailed unpaired and paired samples Student's t tests, respectively. All p values of < 0.05 were considered to be statistically significant.
Results
Cocaine self-administration-induced decreases in cocaine potency are reinstated by a single cocaine injection following 2 weeks of abstinence
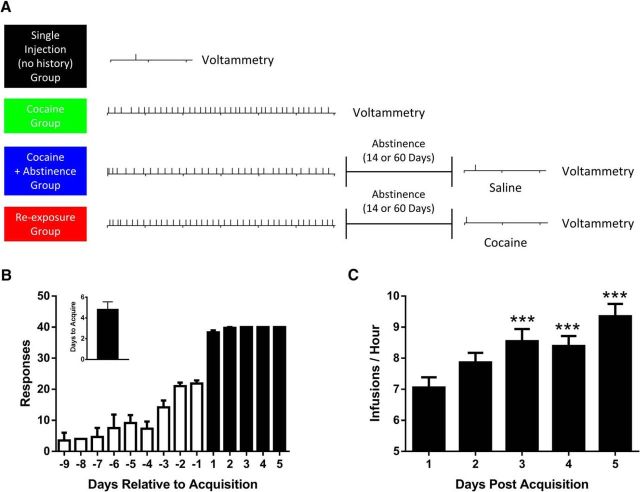
Rats were allowed to self-administer either a single cocaine injection, or for 5 d with a maximum of 40 injections per day (Fig. 1A). Following completion of self-administration, rats were subjected to a 14 or 60 d forced abstinence period. At the end of the abstinence period, rats were allowed to self-administer a single cocaine or saline infusion. As illustrated in Figure 1A, this design yielded four groups: (1) rats that self-administered a single cocaine infusion with no prior history [single injection (no history); n = 4 for 14 d experiments, n = 4 for 60 d experiments], (2) rats that went through 5 d of cocaine self-administration (Cocaine; n = 5 for 14 experiments, n = 6 for 60 d experiments), (3) rats that went through 5 d of cocaine self-administration followed by 14 or 60 d of abstinence with a single self-administered saline injection at the end of the abstinence period (cocaine + 14/60 days off; n = 5 for 14 d experiments, n = 6 for 60 d experiments), and (4) rats that went through cocaine self-administration and abstinence following by a single self-administered cocaine infusion (re-exposure at Day 14/60; n = 8 for 14 d experiments and n = 6 for 60 d experiments).
Figure 1.
Timeline of cocaine self-administration and voltammetric recordings. A, Our experimental design yielded four groups: (1) rats that self-administered a single cocaine infusion with no prior cocaine experience [single injection (no history), black], (2) rats that performed 5 d of cocaine self-administration [cocaine, green], (3) rats that performed cocaine self-administration followed by a 14 or 60 d abstinence and were subsequently allowed to self-administer a single injection of saline [cocaine + abstinence, blue], and (4) rats that performed cocaine self-administration followed by a 14 or 60 d abstinence and were subsequently re-exposed to a single self-administered injection of cocaine (re-exposure, red). B, Number of reinforcers earned relative to day of acquisition. Lack of error post-acquisition reflects the session limit of 40 injections being reached by all rats. Inset, Average number of self-administration sessions before acquisition criteria were met. C, Over the course of cocaine self-administration rats escalate in rate of cocaine intake over days. ***p < 0.001 versus day 1. Error bars indicate ± SEM.
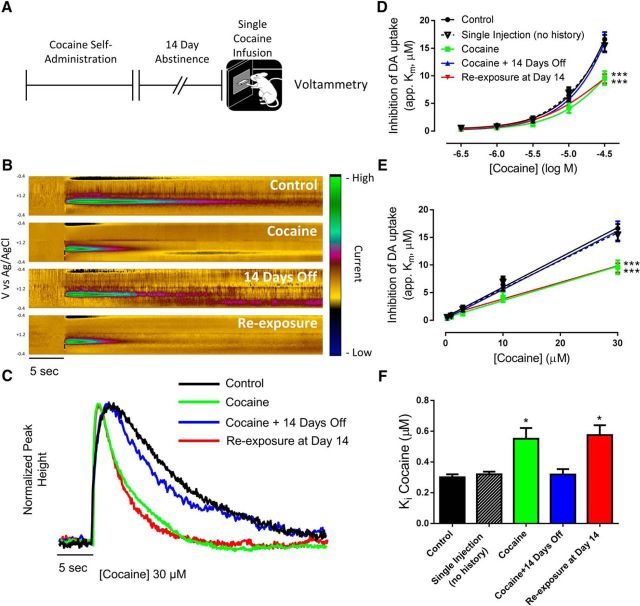
Number of reinforcers earned pre- and post-acquisition are plotted in Figure 1B. We found that rats who were allowed to self-administer for 5 d increased in their rate of cocaine intake, reaching the 40 injection maximum faster on each subsequent self-administration session (Fig. 1C; one-way ANOVA, F(4,44) = 14.91, p < 0.0001). Eighteen hours after completion of the final cocaine self-administration session, rats were euthanized for ex vivo voltammetry recordings in the core of the NAc (Fig. 2A). Cumulative concentrations of cocaine were then bath applied to brain slices to assess the ability of cocaine to inhibit the DAT (Fig. 2B,C). We found that, consistent with previous work (Siciliano et al., 2015b), there was a marked decrease in the ability of cocaine to inhibit the DAT following cocaine self-administration (Fig. 2D). Further, tolerance to cocaine effects returned to control levels following a 14 d abstinence period. When rats were allowed to self-administer a single cocaine infusion following cocaine self-administration and abstinence, tolerance to cocaine effects were fully reinstated [Fig. 2D; two-way ANOVA (concentration × group); concentration: F(4,84) = 389.1, p < 0.0001; group: F(4,21) = 5.257, p = 0.0043; interaction: F(16,84) = 6.915, p < 0.0001]. In addition, linear concentration response curves were plotted to determine Ki values for cocaine across groups (Fig. 2E). We found that Ki values were increased (ie, decreased potency) only in the cocaine and re-exposure groups (Fig. 2F; one-way ANOVA: F(4,21) = 6.064, p = 0.0021). Rats that self-administered a single cocaine injection, without any prior cocaine exposure, showed no change in cocaine potency compared with controls (Fig. 2F), demonstrating that cocaine self-administration leaves the DAT in a labile state whereby tolerance to cocaine effects is rapidly reinstated by minimal cocaine re-exposure following abstinence.
Figure 2.
Cocaine self-administration-induced cocaine tolerance in the NAc core is reinstated by a single cocaine injection. A, Experimental timeline. B, Dopamine (DA), as indicated by current (z-axis) occurring at its oxidation (+0.6 V) and reduction (−0.2 V) peaks (y-axis) across time (x-axis) is represented as pseudo-color plots following 30 μm cocaine. Representative color plots and traces (C) show that the ability of cocaine to slow dopamine uptake is blunted in rats with a history of cocaine self-administration, and after cocaine re-exposure. Logarithmic (D) and linear (E) concentration–response curves for cocaine show a downward shift in cocaine effects in the cocaine and re-exposure groups. F, Ki values for cocaine are increased in the cocaine and re-exposure groups, indicating decreased cocaine potency at the DAT. *p < 0.05, ***p < 0.001 versus control. Control, n = 4; single injection, n = 4; cocaine, n = 5; cocaine + 14 days off, n = 5; re-exposure at Day 14, n = 8. Error bars indicate ± SEM.
Cocaine self-administration-induced deficits are reinstated by a single cocaine injection following prolonged abstinence
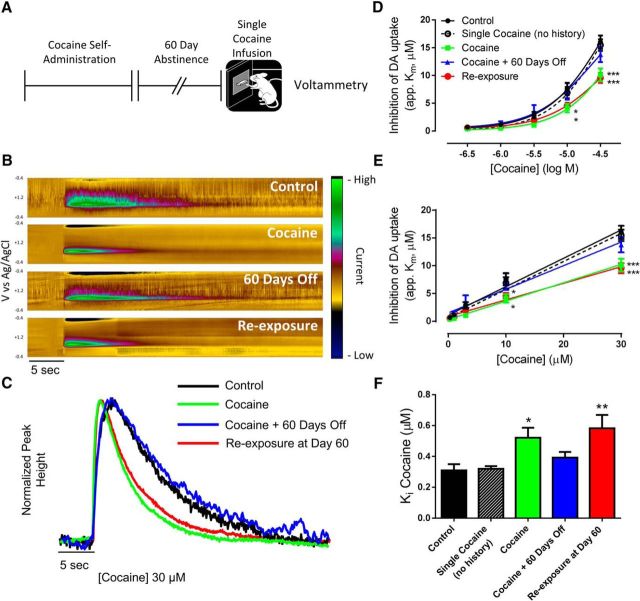
Having demonstrated that cocaine self-administration produces a lasting effect on the DAT that can be activated by a subthreshold cocaine exposure, we next determined whether this neurochemical phenotype persisted over a prolonged abstinence period. In this experiment, rats were given a 60 d abstinence period (approximately equivalent to 4 years in humans) (Quinn, 2005) after cocaine self-administration before subsequent re-exposure to a single infusion of cocaine and voltammetric assessment of cocaine effects on dopamine signaling (Fig. 3A). Importantly, this time-point is much longer than the time course of DAT protein turnover, which has a half-life of 3–6 d (Fleckenstein et al., 1996; Kimmel et al., 2003) and thus rats are expressing DATs that have not previously interacted with cocaine. Follow bath application of cocaine (Fig. 3B,C), we found that tolerance was reinstated by a single self-administered cocaine infusion following a 60 d abstinence period [Fig. 3D; two-way ANOVA (concentration × group); concentration: F(4,96) = 386.5, p < 0.0001; group: F(4,24) = 3.762, p = 0.0164; interaction: F(16,96) = 4.752, p < 0.0001]. Linear concentration response curves were plotted to determine Ki values for cocaine across groups (Fig. 3E). We found that Ki values were increased only in the cocaine and re-exposure groups (Fig. 3F; one-way ANOVA: F(4,24) = 4.555, p = 0.007). Thus, cocaine self-administration leaves a long-lasting change in the dopamine system such that re-exposure to subthreshold doses of cocaine reinstates DAT deficits.
Figure 3.
Cocaine self-administration leaves the DAT in a labile state following prolonged abstinence. A, Experimental timeline showing binge cocaine self-administration and subsequent cocaine re-exposure following a 60 d abstinence period. B, Dopamine (DA), as indicated by current (z-axis) occurring at its oxidation (+0.6 V) and reduction (−0.2 V) peaks (y-axis) across time (x-axis) is represented as pseudo-color plots and traces (C) following 30 μm cocaine. Logarithmic (D) and linear (E) cocaine concentration–response curves indicate tolerance to the uptake inhibiting effects of cocaine following cocaine self-administration, and this tolerance is reinstated by a single cocaine injection following a 60 d abstinence period. F, Ki values for cocaine are increased (ie, decreased cocaine potency) in the cocaine and re-exposure groups. *p < 0.05, **p < 0.01, ***p < 0.001 versus control. Control, n = 7; single injection, n = 4; cocaine, n = 6; cocaine + 60 days off, n = 6; re-exposure at Day 60, n = 6. Error bars indicate ± SEM.
Reinstatement of DAT tolerance is concomitant with decreased cocaine-induced locomotor activity and increased rate of cocaine intake after abstinence
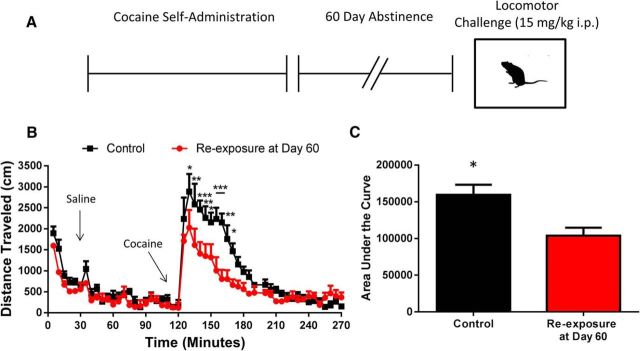
To determine whether decreased cocaine potency at the DAT translated to decreased cocaine effects in vivo, we gave rats a challenge injection of cocaine (15 mg/kg, i.p.) following cocaine self-administration and 60 d of abstinence (re-exposure, n = 6; or age-matched surgery controls: control, n = 5) to determine how cocaine-induced locomotor activity was altered (Fig. 4A). Indeed, we found that following cocaine self-administration and 60 d of abstinence, rats exhibited tolerance to the locomotor stimulating effects of cocaine [Fig. 4B; two-way ANOVA (time × group); time: F(53,477) = 29.81, p < 0.0001); group: F(1,9) = 10.09), p = 0.0112; interaction: F(53,477) = 2.981, p < 0.0001]. Further, we found that the area under the curve following the cocaine injection was reduced in the re-exposure group as compared with controls (Fig. 4C; two-tailed unpaired t test, t(9) = 3.237, p = 0.0102).
Figure 4.
Cocaine re-exposure produces tolerance to the locomotor-activating effects of cocaine. A, Experimental timeline. Rats performed cocaine self-administration followed by a 60 d abstinence period before subsequent re-exposure to a cocaine challenge (15 mg/kg, i.p.). B, Rats with a history of cocaine self-administration show tolerance to the locomotor activating effects of cocaine, with no change in spontaneous locomotor activity. C, Area under the locomotor activity curve following the cocaine challenge is decreased in rats with a history of cocaine self-administration. *p < 0.05, **p < 0.01, ***p < 0.001 versus re-exposure group. Control, n = 5; re-exposure at Day 60, n = 6. Error bars indicate ± SEM.
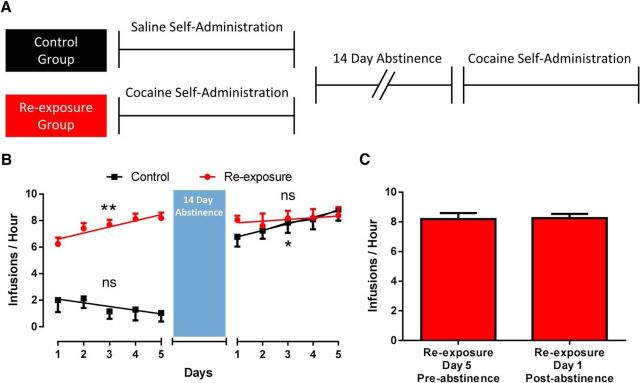
We have shown that cocaine self-administration results in DATs to be in a primed state whereby both neurochemical and behavioral tolerance to cocaine is rapidly reinstated by subthreshold cocaine exposure. Thus, we hypothesized that rapid re-induction of tolerance may underlie high intake cocaine binges, which can occur even after extended abstinence periods in human cocaine addicts. To test this hypothesis, we allowed rats to self-administer cocaine for 5 d of 40 injections followed by a 14 d abstinence period and re-exposure to cocaine for an additional 5 d (re-exposure, n = 7; Fig. 5A). Control rats self-administered saline for 5 d before going through a 14 d abstinence period followed by 5 d of cocaine self-administration (control, n = 5). To determine whether escalation in rate of cocaine intake occurred, we fit each self-administration period with a linear regression line (Fig. 5B). For the re-exposure group, we found that the slope of the line differed from zero in the initial 5 d of cocaine self-administration (slope = 0.46 ± 0.13; F(1,33) = 12.56, p = 0.001), indicating escalation of intake over this period. However, the slope of the regression did not differ from zero during the 5 d of post-abstinence self-administration (slope = 0.13 ± 0.20), indicating that the animals did not further escalate following abstinence. For control rats, the slope of the linear regression did not differ from zero during the 5 d of saline self-administration before abstinence (slope = −0.28 ± 0.22), but was different from zero during the 5 d of cocaine self-administration after the abstinence period (slope = 0.49 ± 0.22; F(1,23) = 5.03, p = 0.03), indicating that the condition of abstinence alone (ie, time spent in box, hourly saline infusions for catheter patency) did not alter escalation in rats without a history of cocaine self-administration. Indeed, the slope of the regression line was not significantly different between the initial escalation (days 1–5 pre-abstinence, slope = 0.46 ± 0.13) in the re-exposure group and the first cocaine exposure (days 1–5 post-abstinence, slope = 0.49 ± 0.22) in the control group (p = 0.89). Giving additional support that animals with prior escalated cocaine self-administration are quickly reinstated to the pre-abstinence state, in the re-exposure group, we found that the rate of cocaine intake did not differ between the fifth day of initial cocaine self-administration and the first day of re-exposure following abstinence (Fig. 5C). Together, these results demonstrate that in rats with a history of escalated cocaine self-administration, their rates of cocaine intake remain escalated despite an abstinence period.
Figure 5.
Escalated rate of cocaine intake remains following abstinence. A, Experimental timeline. Rats performed 5 d of cocaine or saline self-administration before a 14 d abstinence period. Both groups were then allowed to self-administer cocaine for 5 d post-abstinence. B, Control rats did not escalate in rate of intake during the saline self-administration period, but did escalate during 5 d of cocaine self-administration after abstinence. Conversely, re-exposure rats escalated during the initial 5 d of self-administration, but did not continue to escalate following the abstinence period. C, Rate of cocaine intake did not differ between the final day of pre-abstinence self-administration and the first day of post-abstinence self-administration in the re-exposure group. *p < 0.05, **p < 0.01 for the slope of the regression line versus zero. Control, n = 5; re-exposure, n = 7. Error bars indicate ± SEM.
Discussion
Here we show that cocaine use leads to long lasting plasticity of the DAT whereby alterations to the DAT are reinstated by a single cocaine exposure, even after long-term abstinence from cocaine. Indeed, we found that cocaine self-administration produced marked tolerance in the ability of cocaine to inhibit the DAT, and although DAT function returned to control levels over a 14 or 60 d abstinence period, the system remained primed, and tolerance was reinstated by a single self-administered cocaine injection. Further, reinstatement of tolerance was concomitant with decreased cocaine-induced locomotor activity, and rapid reinstatement of escalated cocaine intake, even in the first self-administration session post-abstinence. This priming effect, which may be permanent, may contribute to the severity of relapse episodes in cocaine addicts.
Behavioral implications of cocaine tolerance
Clinical studies have identified tolerance to the dopamine-elevating and euphoric effects of cocaine to be a ubiquitous phenotype of human cocaine addicts, and preclinical studies have demonstrated that cocaine tolerance is a primary determinant of aberrant cocaine taking behaviors (Volkow et al., 1997, 2014; Dackis and O'Brien, 2001; Ferris et al., 2011; Siciliano et al., 2015b). Despite the clear involvement of dopamine in the acute effects of cocaine and on-going self-administration behavior (Phillips et al., 2003; Willuhn et al., 2014), most preclinical studies show that cocaine-induced dopamine system alterations dissipate relatively rapidly during abstinence. Thus, it has been suggested that alterations to dopamine signaling are relevant for the acute effects of withdrawal, but are not a major factor in relapse events occurring after long-term abstinence (Kalivas and Volkow, 2005). We show that despite apparent recovery of normal function, cocaine self-administration leaves the DAT in a reactive state even after a prolonged abstinence. These results may help to reconcile findings that show that dopamine system alterations are typically not present in late withdrawal periods (Satel et al., 1991; Kalivas and Duffy, 1993; Pierce et al., 1995; Beveridge et al., 2009; Ferris et al., 2011) though manipulations of the dopamine system alter drug seeking and taking behaviors during these periods (De Vries et al., 1999; Bossert et al., 2007). We show here that in rats with a history of cocaine self-administration, escalated cocaine intake is present even on the first day of re-exposure following an abstinence period, and animals do not continue to escalate over subsequent days. These behavioral data match the rapid reinstatement of cocaine tolerance at the DAT where tolerance is restored by a single cocaine injection, but is not exacerbated beyond the level of tolerance induced by the initial 5 d of cocaine self-administration.
Methodological considerations and alternative interpretations
Although quickened reinstatement of escalated cocaine intake following abstinence has been previously reported (Ahmed and Koob, 1998; Guillem et al., 2014), these investigations have found that pre-abstinence levels of cocaine intake are not reach until ∼5 d post-abstinence as opposed to the first day post-abstinence reported in the current study. This discrepancy may be a result of several methodological disparities. These differences include dose of cocaine available (0.25 mg/infusion in previous studies, 1.5 mg/kg/infusion in the current study), length of abstinence (30–35 d in previous studies, 14 d in the current study), context of abstinence (home cage in previous studies, self-administration was in the home cage in the current study), number of infusions per day (unlimited for 6 h in previous studies, capped at 40 injections per day in current study), and number of days of self-administration prior to abstinence (16–22 in previous studies, 5 in current study). We hypothesize that the most important factor in the development and reinstatement of cocaine tolerance and escalation of intake is the dose of cocaine available. We have shown previously show that 14 d of long-access self-administration reinforced by 0.75 mg/kg/infusion, similar to Guillem et al. (2014) and Ahmed and Koob (1998), results in decreased cocaine potency at the DAT (Calipari et al., 2013a). However, the magnitude of tolerance appears to be less robust than is seen with higher cocaine doses (1.5 mg/kg/infusion), even after a single day of cocaine self-administration (cf. Ferris et al., 2011). This apparent difference in the magnitude of cocaine tolerance is reflected in the behavioral data as well, where escalation is not apparent until ∼5 d of low-dose long-access self-administration (Ahmed and Koob, 1998), but is seen by day 2 or 3 using high-dose self-administration (current study; Ferris et al., 2012). Thus, our working hypothesis is that due to the dose-dependency of the development of cocaine tolerance, low-dose self-administration (Guillem et al., 2014) results in slower escalation and slower reinstatement of escalated intake after abstinence, whereas under high-dose conditions these processes occur more quickly.
It is important to note that although reinstatement of cocaine tolerance at the DAT was concomitant with reduced cocaine-induced locomotor activity and heighted rates of cocaine intake, reduced cocaine potency may not be the only contributing factor to changes in the behavioral actions of cocaine. For example, changes in cocaine action may also reflect, in part, state-dependent learning of the relationship between the operant response and cocaine infusion. These factors are unlikely to contribute to changes in cocaine-induced locomotion because locomotor experiments were performed in a novel environment and measured the response to an experimenter delivered cocaine injection. Thus, rats had no prior experience with either the context or mode of delivery of cocaine. Although factors involving learning may be at play in the self-administration re-exposure experiment, it is likely that decreased cocaine potency is the determining factor in escalated cocaine intake. Several studies have shown that escalation is dissociable from simple task acquisition (Ahmed and Koob, 1998, 1999), and we have shown that the development of cocaine tolerance at the DAT tracks increases in rate of cocaine intake over days (Ferris et al., 2011). Thus, we hypothesize that decreased cocaine-induced locomotor activity and augmented rate of cocaine intake are a direct consequence of alterations in pharmacodynamic actions of cocaine.
Considerations of molecular mechanisms
The fact that tolerance was reinstated by a subthreshold cocaine exposure, even following 60 d of abstinence, suggests that reinstatement of tolerance to cocaine is not a result of simple cocaine-DAT interactions, because 60 d is well past the turnover rate of individual DAT molecules (Fleckenstein et al., 1996; Kimmel et al., 2003) and therefore the transporters present at the plasma membrane have not previously interacted with cocaine. Thus, there appear to be epigenetic or other long-lasting changes in intracellular signaling cascades or regulatory proteins, which control DAT structure or function that remain irreversibly altered by cocaine self-administration. Further, these signaling mechanisms are not activated by cocaine application to the slice, as we do not see tolerance in rats that went through cocaine self-administration and 60 d of abstinence, but were not re-exposed to cocaine in vivo. There are several possible explanations as to why cocaine application to brain slices does not reinstate tolerance. For example, the mechanisms underlying reinstatement of tolerance may be triggered by activity in the dopamine cell bodies in the ventral midbrain, which are not present in the acute slice preparation, or expression of tolerance may require cocaine to bind to and then be completely removed from the vicinity of the DAT (ie, metabolized) before these mechanisms can be engaged. Because ∼18 h elapse between the single cocaine re-exposure and procuring brain slices, all cocaine would be eliminated from the system in animals exposed in vivo. Further studies will be required to identify the exact molecular mechanisms of tolerance reinstatement.
Clinical implications and concluding remarks
Regardless of mechanism, these finding have broad implications for understanding the persistent impact of cocaine even after long abstinence periods. There are currently no Food and Drug Administration-approved pharmacotherapeutic treatments for cocaine addiction, and addicts attempting to abstain from using cocaine are highly prone to relapse. Longitudinal studies of cocaine addicts seeking treatment have revealed a pattern of repeated bouts of abstinence and relapse despite attempts at sustained abstinence (Washton and Stone-Washton, 1990; Wallace, 1992). Although the determinants of when a relapse episode will occur are well studied, less is known about the factors that determine the severity of the relapse episode (McKay et al., 1995). These episodes typically last for extensive periods of time before abstinence can be re-established (Grella et al., 2003). Due to the reoccurring nature of the binge, abstinence, and relapse cycle, successful treatment often requires many interventions and treatment utilizations over time (Hser et al., 1997; Leshner, 1997). Identifying the mechanisms underlying these extended binges, which occur even following years of abstinence, may allow for pharmacotherapeutics aimed at limiting relapse severity, and decreasing latency to re-enter treatment (Grella et al., 2003). Here we show that DATs remain in an irrevocable primed state following cocaine self-administration such that deficits are rapidly reinstated by extremely limited cocaine re-exposure. These results provide a putative mechanism for binge drug-taking relapse episodes in cocaine addicts, and outline a neurobiological precedent for permanent cocaine-induced plasticity of the dopamine system that may drive addiction long after cessation of cocaine use.
Footnotes
This work was supported by NIH Grants R01 DA021325, R01 DA030161 (S.R.J.), F31 DA037710 (C.A.S.), and T32 AA007565 (C.A.S., S.C.F.). We thank Steven Albertson for his assistance in performing behavioral experiments.
The authors declare no competing financial interests.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013a;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJ, Jones SR, Porrino LJ. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci. 2013b;38:3749–3757. doi: 10.1111/ejn.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano CA, Zimmer BA, Jones SR. Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology. 2015;40:728–735. doi: 10.1038/npp.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/S0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology. 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69:201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology. 2012;37:1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Pögün S, Carroll FI, Kuhar MJ. Recovery of dopamine transporter binding and function after intrastriatal administration of the irreversible inhibitor RTI-76 [3 beta-(3p-chlorophenyl) tropan-2 beta-carboxylic acid p-isothiocyanatophenylethyl ester hydrochloride] J Pharmacol Exp Ther. 1996;279:200–206. [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Hsieh SC. Predictors of drug treatment re-entry following relapse to cocaine use in DATOS. J Subst Abuse Treat. 2003;25:145–154. doi: 10.1016/S0740-5472(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–39. doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Grella C, Longshore D, Prendergast ML. Drug treatment careers: a conceptual framework and existing research findings. J Subst Abuse Treat. 1997;14:543–558. doi: 10.1016/S0740-5472(97)00016-0. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob G, Ungerstedt U. The influence of cocaine self-administration on in vivo dopamine and acetylcholine neurotransmission in rat caudate-putamen. Neurosci Lett. 1990;109:227–233. doi: 10.1016/0304-3940(90)90568-T. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine: II. Dopamine perikarya. J Neurosci. 1993;13:276–284. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ. Withdrawal from repeated cocaine alters dopamine transporter protein turnover in the rat striatum. J Pharmacol Exp Ther. 2003;304:15–21. doi: 10.1124/jpet.102.038018. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Alterman AI, Cacciola JS, Kaplan MR. An examination of the cocaine relapse process. Drug Alcohol Depend. 1995;38:35–43. doi: 10.1016/0376-8716(95)01098-J. [DOI] [PubMed] [Google Scholar]
- O'Connor PG, Sokol RJ, D'Onofrio G. Addiction medicine: the birth of a new discipline. JAMA Intern Med. 2014;174:1717–1718. doi: 10.1001/jamainternmed.2014.4211. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Duffy P, Kalivas PW. Sensitization to cocaine and dopamine autoreceptor subsensitivity in the nucleus accumbens. Synapse. 1995;20:33–36. doi: 10.1002/syn.890200106. [DOI] [PubMed] [Google Scholar]
- Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci. 2014a;34:5575–5582. doi: 10.1523/JNEUROSCI.4050-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Jones SR. Amphetamine potency varies with dopamine uptake rate across striatal subregions. J Neurochem. 2014b;131:348–355. doi: 10.1111/jnc.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Ferris MJ, Jones SR. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur J Neurosci. 2015a;42:2091–2096. doi: 10.1111/ejn.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci. 2015b;6:27–36. doi: 10.1021/cn5002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville MD: SAMHSA, Office of Applied Studies; 2015. Receipt of services for behavioral health problems: results from the 2014 national survey on drug use and health. Available at http://www.samhsa.gov/ [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, Fowler JS, Wong C, Yin P, Du C. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry. 2014;19:1037–1043. doi: 10.1038/mp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC. Treating crack cocaine dependence: the critical role of relapse prevention. J Psychoactive Drugs. 1992;24:213–222. doi: 10.1080/02791072.1992.10471641. [DOI] [PubMed] [Google Scholar]
- Washton AM, Stone-Washton N. Abstinence and relapse in outpatient cocaine addicts. J Psychoactive Drugs. 1990;22:135–147. doi: 10.1080/02791072.1990.10472539. [DOI] [PubMed] [Google Scholar]
- Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF. Neurochemical correlates of cocaine and ethanol self-administration. Ann N Y Acad Sci. 1992;654:220–241. doi: 10.1111/j.1749-6632.1992.tb25970.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]