Abstract
The search for active catalysts that efficiently oxidize methane under ambient conditions remains a challenging task for both C1 utilization and atmospheric cleansing. Here, we show that when the particle size of zinc oxide is reduced down to the nanoscale, it exhibits high activity for methane oxidation under simulated sunlight illumination, and nano silver decoration further enhances the photo-activity via the surface plasmon resonance. The high quantum yield of 8% at wavelengths <400 nm and over 0.1% at wavelengths ∼470 nm achieved on the silver decorated zinc oxide nanostructures shows great promise for atmospheric methane oxidation. Moreover, the nano-particulate composites can efficiently photo-oxidize other small molecular hydrocarbons such as ethane, propane and ethylene, and in particular, can dehydrogenize methane to generate ethane, ethylene and so on. On the basis of the experimental results, a two-step photocatalytic reaction process is suggested to account for the methane photo-oxidation.
 The search for active catalysts that oxidize methane under ambient conditions is a challenging task. Here, the authors report a nanoscale zinc oxide catalyst that efficiently oxidizes methane under simulated sunlight, with surface plasmon enhanced photo-activity owing to integrated silver nanoparticles.
The search for active catalysts that oxidize methane under ambient conditions is a challenging task. Here, the authors report a nanoscale zinc oxide catalyst that efficiently oxidizes methane under simulated sunlight, with surface plasmon enhanced photo-activity owing to integrated silver nanoparticles.
Methane, as the principal constituent of natural gas, is widely used as a fuel and is an important raw material in industrial chemical processes. In view of its utility for improving the quality of human life the emissions of methane were ignored as a trivial matter for a long time and this has led to a significant increase in the atmospheric methane concentration since the industrial revolution1,2,3. Nowadays, with the increasing concern about environmental pollution and climate change, the negative impact of methane emissions is attracting more attention4,5,6. In comparison with other greenhouse gases, methane is responsible for nearly one-fifth of anthropogenic global warming. Over the course of a century, it has a greenhouse gas effect that is more than twenty times greater than the effect from the equivalent mass of carbon dioxide1,2,7. More seriously, global warming and shale gas exploitation are likely to enhance methane release from a number of sources. Therefore, conversion of atmospheric CH4 into equimolar amounts of CO2 can have a significant impact on reducing global warming.
Given the high C–H bond energy (434 kJ mol−1) and the non-polar nature of the CH4 molecule, thermo-catalysis involving precious metals or transition metal oxides have been extensively studied during the past decades for the conversion of methane8,9,10,11,12,13,14. The high reaction temperature (∼400 °C) and inefficiency in removing trace amounts of methane are drawbacks of this approach. Semiconductor photocatalysis, as a technology utilizing sunlight, has been shown to be promising in both water splitting and environmental remediation15,16,17,18,19. Earlier reports have also shown that by using the approach of photocatalysis, activation and oxidation of methane can take place even at room temperature at atmospheric pressure20,21,22,23. The efficiency of photocatalytic oxidation of methane, however, remains notoriously low even under light irradiation using ultraviolet sources.
In our preliminary studies, we fabricated a range of semiconductors including SrTiO3, KNbO3, CdS, Cu2O, BiVO4, g-C3N4 and Ag3PO4, and so on. that have shown strong capabilities to drive water cleavage under light irradiation, using solid state reaction, hydrothermal, or other modified methods to examine their performance on driving methane photo-oxidation. None of the aforementioned semiconductors, which are known to have strong reduction or oxidation capabilities, exhibit any activity for CH4 photo-oxidation except P25 TiO2 which shows a moderate photo-activity. Heterojunction interface design24, morphology control25 and band edge modulation26 were successively also used to fabricate photoactive materials to address the photo-oxidation of small molecular hydrocarbons. Some small molecular hydrocarbons such as C2H6, C3H8 and C2H4 can be efficiently treated by these techniques, however, effective treatment of methane still remains a great challenge.
In light of the possibility that zinc ions may play an important role in methane activation27, we then turned to zinc containing compounds such as ZnO to examine its activity on photo-oxidizing methane. It should be noted that, although it has been extensively studied, ZnO has never been recognized as an efficient photocatalyst because of its limited light-harvesting ability and serious photo-corrosion problem.
Efficient photocatalysts need to: (1) absorb sunlight across the ultraviolet–visible (UV–vis) region to produce electrons and holes; (2) separate the electrons and holes in space to prevent their recombination; (3) have suitable redox potentials to drive the photo-oxidative reactions. It is challenging to satisfying all the requirements in a single material. In particular, the generation of the active oxygen species O2− and ·OH radicals is crucial step for the photocatalytic oxidation of hydrocarbon species, which means semiconductors with a conduction band minimum higher than the potential of O2/O2− (−0.16 V versus NHE)28 and valence band maximum lower than the ·OH/OH− (+2.59 V versus NHE)24,29 potential are needed for organic pollutant degradation.
Ag decorated ZnO is chosen in this study not only because ZnO is an inexpensive semiconductor with large band gap satisfying the band edge potential requirement, but also because it fulfills the following materials design considerations (Fig. 1): (1) the polar structure renders fast separation and transportation of photo-generated electrons and holes25; (2) rich defective surfaces benefit surface reactions30; (3) nano silver decoration may function as both a co-catalyst and a light-harvesting medium31; (4) applying ZnO in gas phase photo-degradation may halt the photo-corrosion that constantly occurs in aqueous solutions32,33. The experimental results show that a nanoscale ZnO can efficiently oxidize methane under simulated sunlight irradiation and nano silver decoration further improves the activity to a high level even under visible light illumination.
Figure 1. Materials design considerations.
(a) Polar structures favour fast separation and transportation of photo-generated electrons and holes. (b) Rich defective surfaces favour surface reactions. (c) Decorated metallic nanostructures may act as both a co-catalyst and a light-harvesting medium.
Results
Characterization of Ag–ZnO nanocatalysts
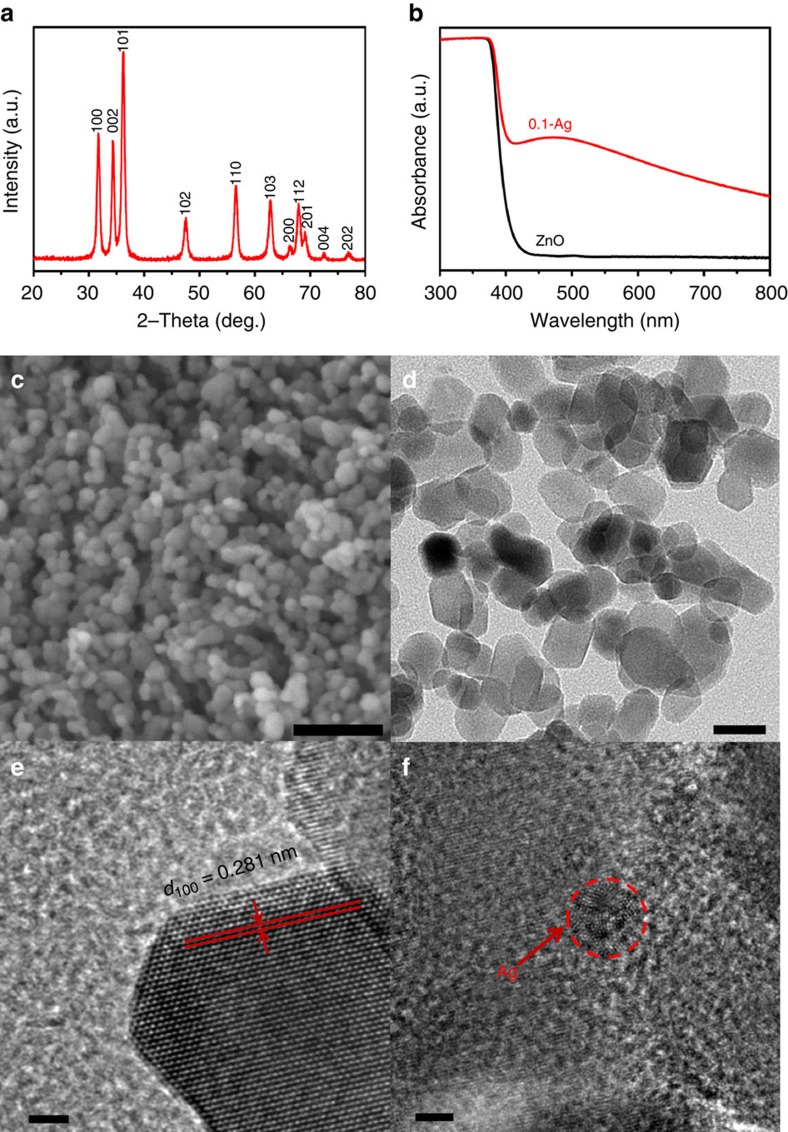
The as-prepared ZnO and Ag–ZnO nanopowders have a Brunauer–Emmett–Teller (BET) surface area of 45.9 and 40.2 m2 g−1, respectively. X-ray diffraction (XRD) analysis identified the hexagonal wurtzite structure type of ZnO (JCPDS file no. 99-0111) for all samples and no diffraction peaks were detected for Ag owing to its low volume fraction (Fig. 2a) and fine particle size (to be shown in Fig. 2e). The UV–vis diffuse reflectance spectra, however, revealed clear distinctions between the bare ZnO and its Ag decorated counterpart. As shown in Fig. 2b, the bare ZnO nanopowder exhibits intense absorption in the ultraviolet region (<400 nm) which is consistent with the wide band gap nature of the ZnO semiconductor. By strong contrast, its silver decorated counterpart exhibits not only the intense ultraviolet absorption expected for the bare ZnO, but also a broad absorption in the visible light region (peaking at ∼470 nm and extending to over 800 nm), owing to the strong surface plasmon resonance of the metallic Ag nanoparticles31.
Figure 2. Physical characterization of the catalysts.
(a) Room temperature XRD patterns of the 0.1 wt% Ag decorated ZnO (0.1-Ag) powders. (b) Ultraviolet–visible diffusive reflectance spectra of the ZnO with and without Ag decoration. (c) SEM image of the 0.1-Ag powders. (d) TEM image of the 0.1-Ag powders. (e,f) HRTEM images of the 0.1-Ag sample. Scale bars, 100 nm (c), 20 nm (d) and 2 nm (e,f).
Morphologies of the samples were characterized by both scanning electron microscopy and transmission electron microscopy (TEM). The ZnO powder shows an irregular morphology with an average particle size of ∼20 nm (Fig. 2c,d). High-resolution TEM observation further confirmed the crystal structure of ZnO where the interplanar lattice spacing of 0.281 nm corresponds well to the (100) plane of hexagonal wurtzite structure type of ZnO (Fig. 2e). Moreover, the high-resolution TEM analysis identified the particle size of silver that decorated on ZnO is only ∼2 nm (Fig. 2f). Elemental mapping was further carried out to examine distribution of the silver nanoparticles and no obvious aggregation was detected.
Photocatalytic properties characterization
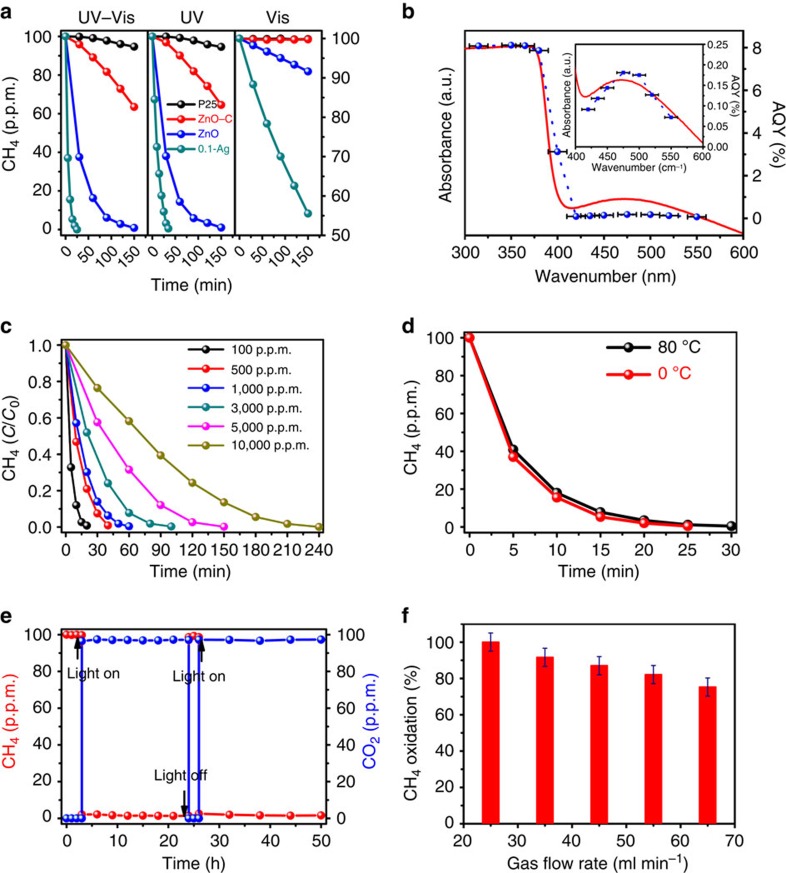
Photocatalytic CH4 oxidation of the as-fabricated samples were examined under simulated sunlight illumination (see Supplementary Fig. 1) with both fixed-bed and flow-bed mode (see Supplementary Fig. 2). Figure 3a shows a typical time evolution of the methane photo-oxidation over the ZnO samples under the fixed-bed mode. For comparison purposes, the performance of commercial ZnO (see Supplementary Fig. 3: 200–300 μm particles size with the BET surface area of ∼3.5 m2 g−1) and P25 (a recognized benchmark photocatalyst with the BET surface area of ∼50 m2 g−1), under the same experimental conditions are also shown. It was found that ZnO possesses an obvious size effect on photocatalytic methane oxidation (see Supplementary Fig. 4), and, the nano-particulate ZnO exhibits exceptional activity for CH4 oxidation either under ultraviolet or UV–vis light illumination. Ag decoration further enhances the photo-oxidation activity. By strong contrast, the commercial ZnO and P25 exhibit only mild and faint activity, respectively, under the same illumination conditions. When illuminated under visible light, neither commercial ZnO nor P25 exhibit any activity for CH4 oxidation, however, the nano-particulate ZnO still shows significant activity and the silver surface plasmon resonance enhancing methane photo-oxidation is undoubtedly corroborated herein.
Figure 3. Photocatalytic oxidation of methane.
(a) Photocatalytic oxidation of methane in a fixed-bed mode with full arc (UV–vis), ultraviolet and visible light illumination, respectively. For comparison purposes, photo-activities of the commercial TiO2 (P25), commercial ZnO (ZnO-C) and as-fabricated ZnO under the same experimental conditions were shown as well. (b) Ultraviolet–visible diffuse reflectance spectrum and AQYs of the 0.1-Ag sample plotted as a function of wavelength of the incident light. AQYs were plotted at the centre wavelengths of the band-pass filters, with error bars showing the deviation of the centre wavelengths (Δλ=±12 nm). (c) Time evolution of the methane photo-oxidation over the 0.1-Ag sample in the fixed-bed mode under full arc illumination with various initial CH4 concentration. (d) Influence of the temperature on the methane photo-oxidation activities over the 0.1-Ag sample under full arc illumination. (e) Methane photo-oxidation activity over the 0.1-Ag sample under full arc illumination and a flow-gas mode with gas flow rate of 25 ml min−1. (f) Influence of the gas flow rate on the rate of methane oxidation under the flow-gas mode with ±5% error bars calculated from the sample introduction uncertainty.
The wavelength dependence of the CH4 oxidation was then further investigated to prove whether or not the reaction really was driven by light. Figure 3b shows the UV–vis diffuse reflectance spectrum of the 0.1 wt% Ag decorated ZnO along with the apparent quantum yield (AQY) of methane oxidation as a function of the incident light wavelength. The AQY decreased with increasing wavelength in the ultraviolet region and the AQY in the visible light region was found to coincide with the characteristic absorption of the silver surface plasmon resonance. This indicates that the methane oxidation reaction is indeed driven by light and that the light-absorption property of the Ag decorated ZnO semiconductor governs the reaction rate. The high quantum yield of 8% at wavelengths <400 nm and over 0.1% at wavelengths ∼470 nm, shows great promise for atmospheric methane oxidation.
In consideration of the knowledge that methane oxidation is an exothermal reaction4,12,17, further experiments such as methane photo-oxidation under various initial hydrocarbon concentrations (Fig. 3c) and under different temperatures (Fig. 3d), were also carried out and the results indicate that temperature fluctuation has little effect on the photo-oxidation process. Careful analysis of the methane photo-oxidation (see Supplementary Fig. 5) revealed that the reactions follow pseudo-first-order kinetics and the apparent reaction rate constant k deduced from the Langmuir–Hinshelwood model34 decrease from 0.24 to 0.02 min−1 when the initial methane concentration increase from 100 to 10,000 p.p.m. These results indicate that by strong contrast to thermal catalysis the approach of photocatalysis is much more promising for the elimination of low concentrations of methane that are difficult to cope with using thermal catalysis.
To examine the mineralization rate and also the carbon balance, the flow mode test was performed as well. Before illumination, CO2 in the reaction system was expelled by flowing carrier gas. After that, the reaction gas consisting of 78.9% N2, 21.1% O2 and 100 p.p.m. methane was flowed through the Ag–ZnO samples and analysed directly by gas chromatography (GC9720 Fuli). During the reaction, a 300 W Xe lamp was used to provide simulated solar light with light density of ∼200 mW cm−2. Figure 3e shows the time dependency of the CH4 photo-oxidation on the Ag decorated ZnO catalysts under simulated sunlight illumination in the flow mode experiment. Before light was turned on, the detected concentration of CH4 was 100 p.p.m. and no CO2 was detected. When the lamp was turned on, the amount of methane decreased rapidly to ∼1.5 p.p.m. Simultaneously, the concentration of CO2 increased promptly to ∼97.3 p.p.m. During the methane photo-oxidation reactions, no CO or other hydrocarbons were detected by gas chromatography. Carbon mass balance of 98.8% is thus obtained based on the ratio of carbon output (1.5 p.p.m. CH4 and 97.3 p.p.m. CO2) to carbon input (100 p.p.m. CH4), which is close to 100% if the experimental uncertainty is considered. When the light was turned off, the concentration of CO2 rapidly decreased to zero, and in the meantime, the amount of methane returned to the constant value. By contrast, the same experiment with thermal catalysis was performed as well. It was found that there is totally no activity of methane oxidation even heating the samples to 250 °C and decreasing the gas flow rate to 10 ml min−1 (see Supplementary Fig. 6). The results again confirm that the methane oxidation occurs through a photo-driven process. Furthermore, the activities of the sample shown in Fig. 3e exhibit no decrease in the 50 h' flow-gas mode experiment, which evidence the high stability of the silver decorated ZnO catalysts.
The influence of gas flow rate on the oxidation of methane was also investigated (Fig. 3f). It was found that increasing the gas flow rate from 25 to 65 ml min−1 caused the ratio of methane oxidation to decrease linearly from almost 100 to ∼76%, which is consistent with the fact that the photocatalytic reaction is a rate-determined process35.
The turnover number (TON) of the CH4 photo-oxidation was obtained by oxidizing a larger amount of CH4 gases over the Ag decorated ZnO catalysts. It has been shown the methane oxidation is a photo-driven process. However, there is no activity if illuminating methane without the presence of the catalyst (see Supplementary Fig. 4c), the fact that the calculated TON for the CH4 photo-oxidization is obviously greater than one (see Supplementary Note 1) indicates that the photo-oxidation reaction is truly driven by a catalytic process.
Photo-oxidation of other hydrocarbons such as ethane, propane, and ethylene were also carried out to further confirm the strong photo-oxidative ability of the silver decorated ZnO catalyst. Similar to methane, these small molecular hydrocarbon gases are difficult to oxidise under mild conditions because of their high bond energy as well as weak molecular polarity22. The highly efficient photo-activity for multiple hydrocarbon gases (see Supplementary Fig. 7) demonstrates that the silver decorated ZnO is a promising candidate for the treatment of atmospheric hydrocarbons under mild conditions.
Stability of a photocatalyst is one of the most important parameters for practical applications. A cycling CH4 photo-oxidation test (see Supplementary Fig. 8) was thus performed for this purpose. After ten cycles, the activity of the silver decorated ZnO semiconductors remains unchanged. After the aforementioned experiments, the Ag–ZnO samples were also carefully examined by XRD, optical absorption and X-ray photoelectron spectroscopy analysis. There are no noticeable distinctions between the freshly prepared and the repeatedly used samples (see Supplementary Fig. 9). These results indicate that the Ag–ZnO catalysts are indeed very stable for hydrocarbon photocatalytic oxidation.
Photocatalytic in situ characterization
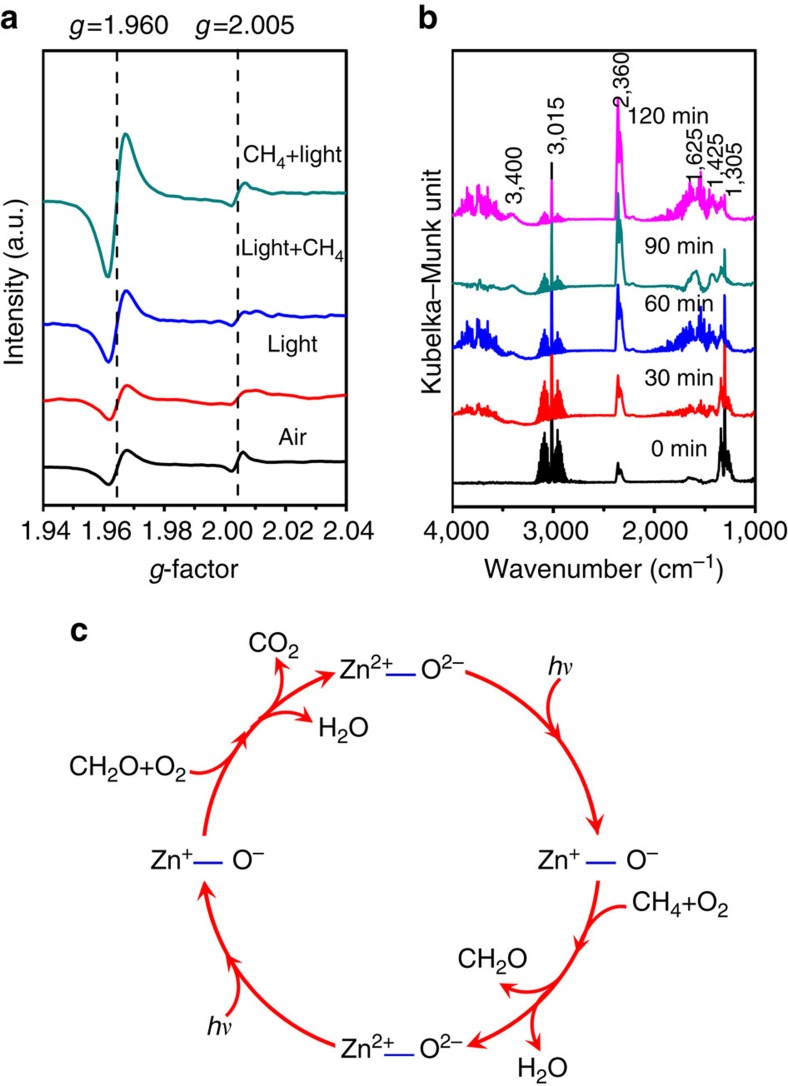
To obtain further insight into the high photo-oxidative activity of the Ag decorated ZnO, in situ electron paramagnetic resonance (EPR) as well as Fourier transform infrared spectroscopy (FT-IR) studies have also been carried out. Figure 4a shows the EPR spectra collected on the Ag–ZnO sample under various atmospheres and illumination conditions. Under the dark and air atmosphere, the sample shows two signals with g=2.005 and g=1.960. The signal of g=2.005 is assigned to single-electron-trapped surface defects such as Vo+ or Os− (refs 36, 37), which is an important feature that is observed only when the particle size of ZnO decreases to the nanoscale. The signal with g=1.960 is attributable to the lattice electron trapping sites (Zn+ or VZn−)36,37 in the defect-rich semiconductor of ZnO. The intensity ratio of the two signals shows less change when illuminating the sample under air atmosphere. However, once methane is injected into the reactor, the signal of Zn+ increases promptly while the signal of the single electron surface defects remains unchanged. For the sample in the atmosphere containing methane and oxygen, continuous illumination caused the signal of Zn+ to keep increasing whereas the signal of surface defects (Vo+ or Os−) increased only slightly (see Supplementary Fig. 10). In view of the fact that single electron defects Zn+ and O− are always generated in pairs when illuminating ZnO, the changes of the EPR signals indicate that the surface defects (Vo+ or Os−) play a vital role in the methane photo-oxidation.
Figure 4. Mechanism of photocatalytic CH4 oxidation.
(a) EPR signals of 0.1-Ag under different environments. From the bottom-up, the traces are for a fresh sample measured in an air atmosphere, measured in an air atmosphere after illumination, measured immediately after injection CH4 to the illuminated system, measured after illumination under CH4 and air atmosphere, respectively. (b) In situ IR spectra of methane photocatalytic oxidation collected at different illumination time intervals. (c) Schematic illustration for the photocatalytic CH4 reaction processes under ambient conditions.
Figure 4b shows in situ diffusive reflectance infrared spectra that was collected during the photocatalytic oxidation of methane. Methane is featured with typical IR vibration modes at ∼1,305 and ∼3,015 cm−1 as well as the multiple IR bands close to 3,015 cm−1 (ref. 38). The IR bands at ∼2,340–2,360 cm−1 are assigned to the characteristic mode of CO2 (ref. 39). With light illumination, the decrease of the intensities of the bands assigned to the v(C-H) vibration of methane is accompanied by gradual increase of the intensities of the IR bands of CO2. Meanwhile, the newly emerged broad peaks at ∼1,625 and ∼3,400 cm−1 keep rising, which correspond to δ (HOH) and ν (HOH) vibrations of chemisorbed H2O40, respectively. Significantly, the newly emerged band at ∼1,425 cm−1 that corresponds to the δ (CHO) mode of chemisorbed aldehyde41, shows less increase with the proceeding of light illumination. During the experiment, no other intermediate species was detected. These results revealed that the methane photo-oxidation, in all likelihood, proceeds via a two-step process (Fig. 4c): first, CH4 reacts with O2 and produces H2O and HCHO (CH4+O2→HCHO+H2O), and then the intermediate product HCHO further reacts with O2 and produces H2O and CO2 (HCHO+O2→CO2+H2O).
Discussion
As we known, the primary step of methane activation on oxide materials frequently involves reaction with surface O− radical ions42,43,44:
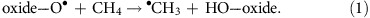 |
When ZnO was illuminated under simulated solar light, surface electron (Zn+) and hole (O−) centres will generate via the reaction45:
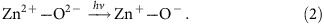 |
Earlier research has demonstrated that the Zn+ cations can attract three hydrogen atoms of methane and the fourth hydrogen is on the opposite side27, whereas the O− anion has a strongly attractive force for the hydrogen atoms of methane and can abstract the fourth hydrogen from methane36. Therefore the surface-adsorbed CH4 would be activated which will initiate the following reactions:
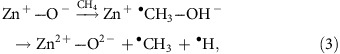 |
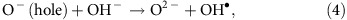 |
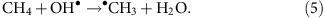 |
Since oxygen was present in the reactor, the surface electron (Zn+) would either get recombined with hole (O−) to form Zn2+ and O2− or react with surface-adsorbed oxygen molecule to form Zn2+ and adsorbed superoxide anion radicals:
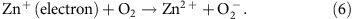 |
The generation of superoxide anion radicals will initiate further oxidation of the methyl radicals:
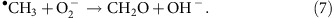 |
Since the superoxide anion radicals react very easily with the surface OH− to form their conjugated acid46,47, the following route to generate formaldehyde cannot be ruled out:
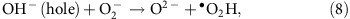 |
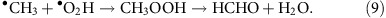 |
We know the oxidation of formaldehyde has been extensively investigated. With the involvement of active oxygen species O2−, ·OH and O−, the intermediate product formaldehyde can conveniently be oxidized to CO2 and H2O in a similar manner48,49.
The aforementioned analysis distinguishes photocatalytic methane oxidation from the thermocatalytic approach, where the latter requires the thermal activation of oxygen to drive the methane oxidation. This process is temperature dependent. Since CH4 oxidation is an exothermic reaction, a higher concentration of methane releases more heat, which is beneficial for the activation of oxygen. Therefore, the thermocatalytic approach is more efficient for the treatment of methane if it is in high concentration. Whereas for the photocatalytic methane oxidation, the lattice oxygen activated by photo-generated hole is the main active species for abstracting the hydrogen of CH4. This process is not determined by the reaction temperature but closely related to the light energy and intensity. Therefore, the photocatalytic oxidation is less sensitive to temperature fluctuations. Instead, once the illumination condition is fixed, the reaction rate will depend on the concentration of methane, and proceed more quickly for lower concentrations.
The function of nano silver decoration lies at least in: (I) as electron sink reducing the recombination of electrons and holes in the surface of ZnO (see the significantly reduced photoluminescence spectra intensity in Supplementary Fig. 11); (2) as a photo-sensitizer extending the utilization of the visible light.
On the basis of the above understanding, one could predict that if no oxygen is involved in the methane photo-oxidation, ethane will be produced owing to the oxidative dehydrogenation of methane, and, if ethane further abstracts hydrogen the generation of ethylene and other hydrocarbons will occur. We then further performed the flow mode methane conversion experiments under oxygen-free conditions and a methane conversion of 0.35% and a selectivity of 89.47% for ethane were obtained (see Supplementary Fig. 12).
Methods
Sample preparation
The nano-particulate ZnO powders were prepared by a method of precipitation: 0.005 mol Zn(NO3)2 and 0.005 mol oxalic acid were dissolved, respectively, in 100 ml distilled water at room temperature. Then, the oxalic acid solution was added into the Zn(NO3)2 solution drop by drop to get zinc oxalate precipitates. After that, the precipitates were filtered and calcined at 350 °C in air atmosphere for 6 h. The Ag–ZnO composite photocatalysts were prepared as follows: First, 1.00 g ZnO powers were dispersed into 100 ml aqueous solution that containing various amount of AgNO3 in a quartz reactor under vigorous stirring. Then, the suspension was evaporated at 80 °C until dryness. After that, the precipitates were treated at 350 °C in air atmosphere for 2 h. For simplicity, the resultant Ag–ZnO composites with 0.1 wt% Ag (compared with ZnO) deposition were denoted as 0.1-Ag.
Physical characterization
The structure and crystallinity of the samples were investigated by XRD (Rigaku Miniflex II) using Cu Kα (λ=0.15418, nm) radiation (30 kV, 15 mA). A scan rate of 5o min−1 was applied to record the powder XRD patterns in the 2θ range of 20–80o. The diffuse reflectance UV-visible spectra of the samples were recorded on a PerkinElmer Lambda 900 UV/VIS/NIR spectrometer that was equipped with an integrating sphere covered with BaSO4 as the reference. The BET-specific surface areas of the samples were measured by a TriStar II 3020-BET/BJH Surface Area analyzer. Images of TEM and high-resolution TEM as well as electron diffraction patterns were obtained using a JEM 2010 EX instrument at an accelerating voltage of 200 kV. The X-ray photoelectron spectroscopy measurements were performed on a Phi Quantum 2000 spectrophotometer with Al Kα radiation (1,486.6 eV). The binding energies were calibrated using that of C 1 s (284.8 eV). Photoluminescence spectra of the photocatalysts were collected on a Varian Cary Eclipse spectrometer with an excitation wavelength of 325 nm. In situ FT-IR studies were performed on a spectrometer Nexus FT-IR (Thermo Nicolet) by using a diffuse reflectance attachment equipped with a reaction chamber. The 128 single-beam spectra had been co-added at a resolution of 4 cm−1 and the spectra were presented as Kubelka–Munk function referred to adequate background spectra. The background and samples spectra were taken (the average of accumulated 32 scans) over the frequency range 4,000–600 cm−1. The EPR spectra were obtained on a Brucker A300 spectrometer. The details of the instrumental parameters are as follows: scanning frequency: 9.85 GHz, central field: 3350 G, scanning width: 1,260 G, scanning power: 20 mW, and scanning temperature: 25 °C.
Photocatalytic experiments
The photocatalytic oxidation of hydrocarbons were carried out in a homemade fixed-bed pyrex reactor of 450 ml capacity (see Supplementary Fig. 2a) and a homemade flow-bed pyrex reactor of 0.6 ml (30 × 20 × 1 mm3) capacity (see Supplementary Fig. 2b), respectively. All of the experiments were performed at atmospheric pressure and room temperature unless otherwise stated. In a typical fixed-bed reaction: First, 0.5 g photocatalysts were dispersed uniformly on the bottom of reactor. Then, the reactor was flushed with 78.9% N2 and 21.1% O2 mix gas repeatedly to remove water and CO2 that adsorbed on the catalyst and the inwall of reactor. Subsequently, different amounts of hydrocarbons were injected into the reactor by a micro-syringe. Before the illumination, the reactor was kept in the dark for 2 h to ensure the establishment of an adsorption-desorption equilibrium between the photocatalyst and reactants. Then, the reactor was illuminated by a 300 W Xe lamp from the upper part with light intensity of ∼200 mW cm−2. At a certain time interval, 4 ml gas was sampled from the reactor and analysed by a gas chromatograph (GC9720 Fuli) equipped with a HP-Plot/U capillary column, a molecular sieve 13 × column, a flame ionization detector and a thermal conductivity detector. A typical flow-bed reaction proceeded as follows: first, 0.5 g photocatalysts were fully filled in the flow-bed pyrex reactor; second, the mixed gas consisting of 78.9% N2, 21.1% O2 and 100 p.p.m. hydrocarbons was flowed through the samples and analysed directly by the gas chromatograph (GC9720 Fuli). The reactor was illuminated using 300 W Xe lamp from both the top and bottom surfaces during the photoreactions. The oxygen-free conversion of methane was carried out using the same procedure and the only difference was the reaction gas which consisted of 95% N2 and 5% CH4 that free of oxygen.
The AQY measurements were performed with the fixed-bed mode and monochromatic light illumination for 2 h under different wavelength was used during the experiment. On the basis of the reaction CH4+2O2→CO2+2H2O and the assumption that all electrons are excited by light, the AQYs are calculated by the following formula:
AQYs (%)=100 × (the number of reacted electrons or holes)/(the number of incident photons)=100 × (the number of reacted CH4 molecules × 8)/(the number of incident photons).
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Additional information
How to cite this article: Chen, X. et al. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 7:12273 doi: 10.1038/ncomms12273 (2016).
Supplementary Material
Supplementary Figures 1-12 and Supplementary Note 1.
Acknowledgments
This work was financially supported by the National Key Project on Basic Research (Grant No. 2013CB933203), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000), the Natural Science Foundation of China (Grant No. 21373224, 21577143 and 51502289), the Natural Science Foundation of Fujian Province (Grant No. 2014H0054 and 2015J05044) and the One Hundred Talents Program of the Chinese Academy of Sciences.
Footnotes
Author contributions X.C. prepared the samples and carried out the experiments; Y.L. assisted the photocatalytic tests; X.P. directed the IR analysis; D.C. contributed the manuscript revision; X.H. and Z.Y. co-supervised the project; X.C. and Z.Y. wrote the paper and all authors discussed the results and commented on the manuscript.
References
- Forster P. et al. in Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change eds Solomon S.et al. Cambridge Univ. Press (2007). [Google Scholar]
- Kirschke S. et al. Three decades of global methane sources and sinks. Nat. Geosci. 6, 813–823 (2013). [Google Scholar]
- Brandt A. R. et al. Methane leaks from North American natural gas systems. Science 343, 733–735 (2014). [DOI] [PubMed] [Google Scholar]
- Farrauto R. J. Low-temperature oxidation of methane. Science 337, 659–660 (2012). [DOI] [PubMed] [Google Scholar]
- Alvarez R. A., Pacala S. W., Winebrake J. J., Chameides W. L. & Hamburg S. P. Greater focus needed on methane leakage from natural gas infrastructure. Proc. Natl Acad. Sci. USA 109, 6435–6440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale J., Shindell D., Schneidemesser E. V., Chabay I. & Lawrence M. Air pollution: clean up our skies. Nature 515, 335–337 (2014). [DOI] [PubMed] [Google Scholar]
- Lashof D. A. & Ahuja D. R. Relative contributions of greenhouse gas emissions to global warming. Nature 344, 529–531 (1990). [Google Scholar]
- Cullis C. F. & Willatt B. M. Oxidation of methane over supported precious metal catalysts. J. Catal. 83, 267–285 (1983). [Google Scholar]
- Gélin P. & Primet M. Complete oxidation of methane at low temperature over noble metal based catalysts: a review. Appl. Catal. B 39, 1–37 (2002). [Google Scholar]
- Choudhary T. V., Banerjee S. & Choudhary V. R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A 234, 1–23 (2002). [Google Scholar]
- Zarur A. J. & Ying J. Y. Reverse microemulsion synthesis of nanostructured complex oxides for catalytic combustion. Nature 403, 65–67 (2000). [DOI] [PubMed] [Google Scholar]
- Cargnello M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. In situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nat. Commun. 6, 7181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F. F. et al. Understanding complete oxidation of methane on spinel oxides at a molecular level. Nat. Commun. 6, 7798 (2015). [DOI] [PubMed] [Google Scholar]
- Fujishima A. & Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). [DOI] [PubMed] [Google Scholar]
- Zou Z., Ye J., Sayama K. & Arakawa H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 414, 625–627 (2001). [DOI] [PubMed] [Google Scholar]
- Kudo A. & Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009). [DOI] [PubMed] [Google Scholar]
- Yi Z. et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 9, 559–564 (2010). [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R., Martin S. T., Choi W. & Bahnemann D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). [Google Scholar]
- Graetzel M., Thampi K. R. & Kiwi J. Methane oxidation at room temperature and atmospheric pressure activated by light via polytungstate dispersed on titania. J. Phys. Chem. 93, 4128–4132 (1989). [Google Scholar]
- Lien C. F., Chen M. T., Lin Y. F. & Lin J. L. Photooxidation of methane over TiO2. J. Chin. Chem. Soc. 51, 37–42 (2004). [Google Scholar]
- Yuliati L. & Yoshida H. Photocatalytic conversion of methane. Chem. Soc. Rev. 37, 1592–1602 (2008). [DOI] [PubMed] [Google Scholar]
- In S. I. et al. Photocatalytic methane decomposition over vertically aligned transparent TiO2 nanotube arrays. Chem. Commun. 47, 2613–2615 (2011). [DOI] [PubMed] [Google Scholar]
- Chen X., Huang X. & Yi Z. Enhanced ethylene photodegradation performance of g-C3N4–Ag3PO4 composites with direct Z-scheme configuration. Chem. Eur. J. 20, 17590–17596 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang L., Zhang Y., Qiu Y. & Yi Z. Improved photocatalytic activity by utilizing the internal electric field of polar semiconductors: a case study of self-assembled NaNbO3 oriented nanostructures. RSC Adv. 4, 3165–3170 (2014). [Google Scholar]
- Long P., Zhang Y., Chen X. & Yi Z. Fabrication of Y1-xBi1-xVO4 solid solutions for efficient C2H4 photodegradation. J. Mater. Chem. A 3, 4163–4169 (2015). [Google Scholar]
- Li L. et al. Efficient sunlight-driven dehydrogenative coupling of methane to ethane over a zn+-modified zeolite. Angew. Chem. 123, 8449–8453 (2011). [DOI] [PubMed] [Google Scholar]
- Wood P. M. The potential diagram for oxygen at pH 7. Biochem. J. 253, 287–289 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol W. H. & Liebman J. F. The oxidizing nature of the hydroxyl radical. a comparison with the ferryl ion (FeO2+). J. Phys. Chem. 88, 99–101 (1984). [Google Scholar]
- McCluskey M. D. & Jokela S. J. Defects in ZnO. J. Appl. Phys. 106, 071101–071112 (2009). [Google Scholar]
- Linic S., Christopher P. & Ingram D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 10, 911–921 (2010). [DOI] [PubMed] [Google Scholar]
- Spathis P. & Poulios I. The corrosion and photocorrosion of zinc and zinc oxide coatings. Corros. Sci. 37, 673–680 (1995). [Google Scholar]
- Han J., Qiu W. & Gao W. Potential dissolution and photodissolution of ZnO thin films. J. Hazard. Mater. 178, 115–122 (2010). [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R., Martin S. T., Choi W. & Bahnemann D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). [Google Scholar]
- Finger M., Haeger A. & Hesse D. Kinetics and mechanisms of photocatalyzed total oxidation reaction of hydrocarbon species with titanium dioxide in the gas phase. Chem. Eng. Technol. 28, 783–789 (2005). [Google Scholar]
- Volodin A. M. & Cherkashin A. E. ESR spectrum of methyl radicals on ZnO surface. React. Kinet. Catal. Lett. 18, 243–246 (1981). [Google Scholar]
- Kaftelen H. et al. EPR and photoluminescence spectroscopy studies on the defect structure of ZnO nanocrystals. Phys. Rev. B 86, 014113–0141139 (2012). [Google Scholar]
- Deschenaux C., Affolter A., Magni D., Hollenstein C. & Fayet P. Investigations of CH4, C2H2 and C2H4 dusty RF plasmas by means of FTIR absorption spectroscopy and mass spectrometry. J. Phys. D Appl. Phys. 32, 1876–1886 (1999). [Google Scholar]
- Ramis G., Busca G. & Lorenzelli V. Low-temperature CO2 adsorption on metal oxides spectroscopic characterization of some weakly adsorbed species. Mater. Chem. Phys. 29, 425–435 (1991). [Google Scholar]
- Panayotov D. A. & Yates J. T. Depletion of conduction band electrons in TiO2 by water chemisorption–IR spectroscopic studies of the independence of Ti–OH frequencies on electron concentration. Chem. Phys. Lett. 410, 11–17 (2005). [Google Scholar]
- Hauchecorne B. et al. Photocatalytic degradation of ethylene: an FTIR in situ study under atmospheric conditions. Appl. Catal. B 105, 111–116 (2011). [Google Scholar]
- Ito T. & Lunsford J. H. Synthesis of ethylene and ethane by partial oxidation of methane over lithium-doped magnesium oxide. Nature 314, 721–722 (1985). [Google Scholar]
- Driscoll D. J., Martir W., Wang J. X. & Lunsford J. H. Formation of gas-phase methyl radicals over magnesium oxide. J. Am. Chem. Soc. 107, 58–63 (1985). [Google Scholar]
- Ito T., Wang J., Lin C. H. & Lunsford J. H. Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst. J. Am. Chem. Soc. 107, 5062–5068 (1985). [Google Scholar]
- Anpo M. & Kubokawa Y. Photoluminescence of zinc oxide powder as a probe of electron-hole surface processes. J. Phys. Chem. 88, 5556–5560 (1984). [Google Scholar]
- Thampi K. R., Kiwi J. & Grätzel M. Room temperature photo-activation of methane on TiO2 supported molybdena. Catal. Lett. 1, 109–116 (1988). [Google Scholar]
- Maldotti A., Molinari A. & Amadelli R. Photocatalysis with organized systems for the oxofunctionalization of hydrocarbons by O2. Chem. Rev. 102, 3811–3836 (2002). [DOI] [PubMed] [Google Scholar]
- Noguchi T. & Fujishima A. Photocatalytic degradation of gaseous formaldehyde using TiO2 film. Environ. Sci. Technol. 32, 3831–3833 (1998). [Google Scholar]
- Sun S. et al. Photocatalytic oxidation of gaseous formaldehyde on TiO2: an in situ DRIFTS study. Catal. Lett. 137, 239–246 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-12 and Supplementary Note 1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.