Abstract
Background
Patients with cytopenia are increasingly undergoing molecular genetic tests of periperal blood or bone marrow for diagnostic purposes. These tests can detect genetic mutations that do not have any morphological correlate in hematologic neoplasia such as myelodysplastic syndrome (MDS). A new entity was recently defined to lessen the risk of incorrect diagnoses of MDS. This new entity is a potential precursor of myeloid diseases, analogously to monoclonal gammopathy of undetermined significance as a potential precursor of multiple myeloma.
Method
This review is based on pertinent articles retrieved by a selective search in PubMed employing the terms “clonal hematopoiesis,” “acute myeloid leukemia,” and “myelodysplastic syndrome.”
Results
Clonal hematopoiesis of indeterminate potential (CHIP) is a new entity in which somatic mutations are found in cells of the blood or bone marrow, but no other criteria for hematologic neoplasia are met. Its prevalence rises with age and is roughly 10% among persons aged 70 to 80. It is estimated that, in Germany, about 2.75 million people are affected. The most common mutation is on the DNMT3A gene, followed by TET2 and ASXL1. The rate of transformation to a hematological neoplasia is 0.5–1% per year, and thus about 13 times higher than the incidence of such neoplasias in general. If CHIP is discovered incidentally in a patient with a normal blood count, a complete blood count with differential should be repeated three months later and then at annual intervals.
Conclusion
CHIP must be included in the differential diagnosis of peripheral blood cytopenia. This new entity can help us understand the clinical significance of clonal hematopoiesis.
Human bone marrow produces several billions of cells every day. These cells are produced by hematopoietic stem cells, the total number of which is estimated at 11 000 to 22 000 per person (1). While the progeny of hematopoietic stem cells (known as progenitor or precursor cells) divide rapidly and so take on the main burden of hematopoiesis, stem cells only rarely divide in the course of their lifetime, thus contributing to stability of the genome.
For many decades now, genetic mutations have been used as markers of clonal development to demonstrate that all the mutated cells have their origin in one mutated “mother cell.” In normal individuals, it is assumed that hematopoietic stem cells contribute equally to hematopoiesis. By contrast, leukemia is an extreme case of clonal hematopoiesis, in which one blood stem cell takes over almost the whole of the (abnormal) hematopoiesis and drives it into severe disorders of maturation (blasts) and to suppression of normal hematopoiesis (resulting in anemia, neutropenia, and/or thrombocytopenia).
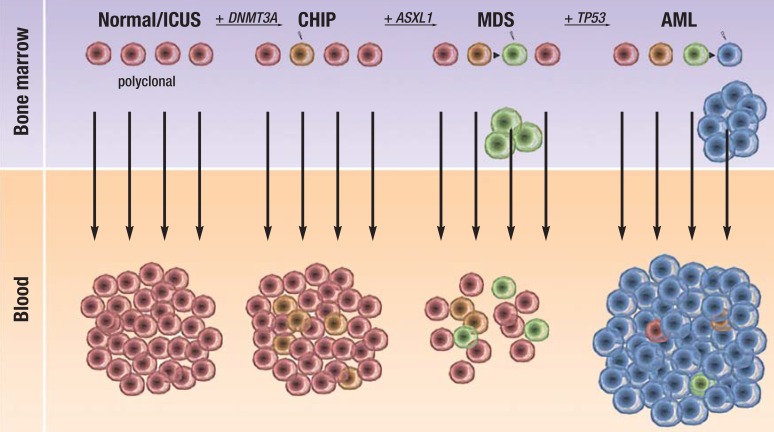
Over the past 5 years, the genetic mutations that can trigger acute myeloid leukemia (AML) have been exhaustively studied by exome/genome sequencing (2). Among the most frequently mutated genes are DNMT3A, TET2, and ASXL1, all of which play an important role in epigenetic regulation (DNMT3A for DNA methylation, TET2 for DNA hydroxymethylation, and ASXL1 for histone methylation and histone ubiquitination) (3). DNMT3A mutations are found in 18% of AML patients (4). In up to 40% of these leukemia patients an interesting phenomenon is observed: although the leukemia cells disappear from the bone marrow after intensive chemotherapy, and most of the leukemia-specific mutations are no longer shown to be present, a mutation in the DNMT3A gene that was present at diagnosis does persist, even in patients in complete remission (5, 6). This suggests that a DNMT3A mutation, but no other leukemia-specific mutations, can be shown to be already present in a hematopoietic stem cell. This stem cell is termed a “premalignant stem cell” and contributes more to hematopoiesis than do other stem cells (that is, it has expanded clonally), without inducing leukemia (Figure 1). Three recently published studies have now investigated whether clonally expanded hematopoietic stem cells or premalignant stem cells can be shown to be present even in normal individuals (7– 9). Based on these studies, a new disease entity was defined that is associated with an increased risk of hematologic neoplasms and increased mortality. The name given to this entity is “clonal hematopoiesis of indeterminate potential” (CHIP) (10). We carried out a selective literature search of PubMed using the terms “clonal hematopoiesis,” “acute myeloid leukemia,” and “myelodysplastic syndrome.” In this article, we present this new disease entity and discuss its clinical significance. We expect the phenomenon of clonal selection on the basis of somatic mutations to be applicable to other tissues in which tumor incidence is observed to increase with age.
Figure 1.
Stepwise development from polyclonal hematopoiesis (normal, no evidence of somatic mutations) to clonal hematopoiesis of indeterminate potential (CHIP), myelodysplastic syndromes (MDS), and acute myeloid leukemia (AML). ICUS, idiopathic cytopenia of indeterminate significance. The mutated genes (DNMT3A, ASXL1, TP53) are shown as examples; other genes may be affected, and in a different order.
CHIP clonal hematopoiesis of indeterminate potential
Disorders of hematopoiesis occur more frequently with age and are associated with increased morbidity, hospitalization, and mortality. Thus, they represent a medical problem of the aging population in particular: for example, while the prevalence of anemia is about 10% in patients over the age of 60, in patients aged over 85 it goes up to 20%. In a third of cases, no cause is found (11).
CHIP is predominantly defined by evidence of somatic mutations, i.e., those acquired after birth. Patients undergoing molecular genetic investigation for cytopenia (anemia, leukopenia, thrombocytopenia) are the most likely to be given this diagnosis. Important differential diagnoses in patients with cytopenia in peripheral blood include clonal hematopoietic diseases such as myelodysplastic syndrome (MDS). The myelodysplastic syndromes, which are malignant diseases, are defined by:
Dysplastic cells or ring sideroblasts or increase of myeloblasts up to 19% in bone marrow or peripheral blood
Cytopenia in peripheral blood
Absence of reactive causes of the cytopenia (12).
The increasing use of gene sequencing in the diagnosis of MDS has meant that somatic mutations are increasingly being identified while the other criteria for MDS remain unfulfilled. This is the reason for the introduction of CHIP, a benign disease entity with a low risk of transformation into myeloid or lymphoid neoplasms. Similar entities are already known in the form of monoclonal gammopathy of undetermined significance (MGUS) for multiple myeloma and monoclonal B-lymphocytosis (MBL) for chronic lymphocytic leukemia—diagnoses that do not cause morbidity by themselves, but like CHIP are associated with a 1% to 2% rate of transformation into malignant disease. CHIP is defined by:
Demonstration of clonal hematopoiesis (somatic mutation)
Absence of hematopoietic dysplasia in bone marrow
Absence of blast increase in bone marrow (Box).
Box. Definition of CHIP (modified from Steensma et al. 10).
A No evidence of morphologic criteria for any hematologic neoplasm; especially, no dysplasia or blast increase (DD: MDS and AML)
B PNH, MGUS, and MBL ruled out
C Evidence of a somatic mutation that is associated with a hematologic neoplasm and has an allele frequency of at least 2% (= evidence of clonality)
D Cytopenia in peripheral blood may be present but is not part of the definition of CHIP (DD: ICUS and MDS).
E Annual risk of progression to hematologic neoplasm 0.5% to 1%
AML, acute myeloid leukemia; CHIP, clonal hematopoiesis of indeterminate potential; DD, differential diagnosis; ICUS, idiopathic cytopenia of indeterminate significance; MBL, monoclonal B-lymphocytosis; MDS, myelodysplastic syndrome; MGUS, monoclonal gammopathy of undetermined significance; PNH, paroxysmal nocturnal hemoglobinuria
Cytopenia in peripheral blood may be present but is not obligatory. The following must be ruled out:
Paroxysmal nocturnal hemoglobinuria (PNH)
MGUS
MBL.
Clonal hematopoiesis must be demonstrated by a somatic mutation with a variant allele frequency of at least 2% (10). This very low allele frequency is only just above the detection limit of the new high-throughput sequencing techniques and can only be reliably demonstrated by deep sequencing (13), so that for allele frequencies between 2% and 10% we recommend a reading depth of at least 500 copies and repeating the sequencing for confirmation. The main feature that distinguishes CHIP from low-risk MDS is the absence of hematopoietic dysplasia, while it can be distinguished from high-risk MDS by a normal blast count in the bone marrow. Figure 1 illustrates the distinctions between normal hematopoiesis, CHIP, MDS, and AML. The existing definition of idiopathic cytopenia of unknown significance (ICUS) remains; this entity differs from CHIP in the absence of clonality, i.e., a lack of somatic aberrations (14, 15). In everyday clinical practice, patients with cytopenia and evidence of somatic mutations but insufficient criteria for a diagnosis of MDS should be given a diagnosis of CHIP. On the other hand, for the foreseeable future it will continue to be unusual for a patient without cytopenia to be diagnosed with the new entity CHIP on the basis of incidentally discovered somatic mutations in hematopoietic cells.
Clinical significance of CHIP
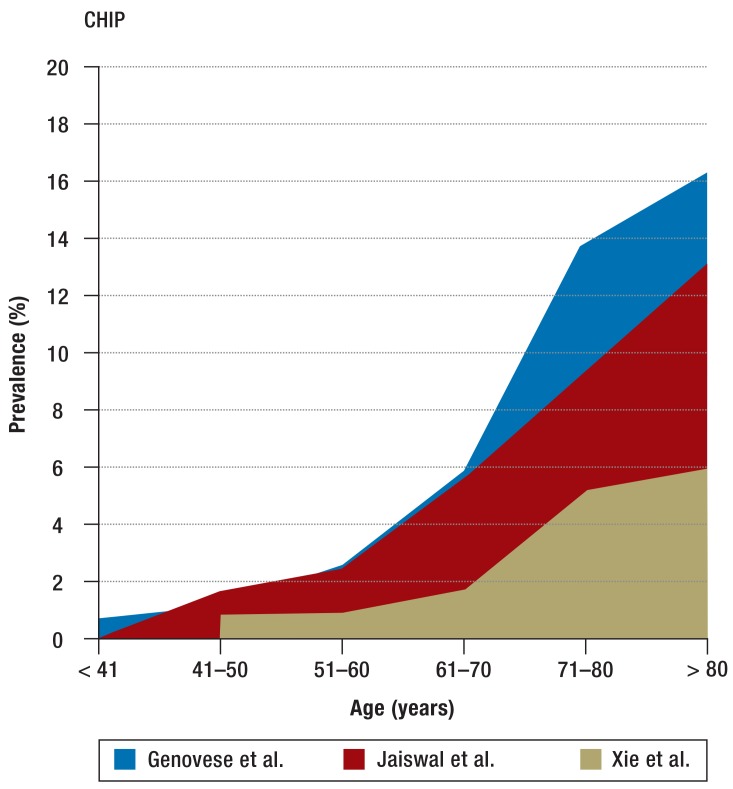
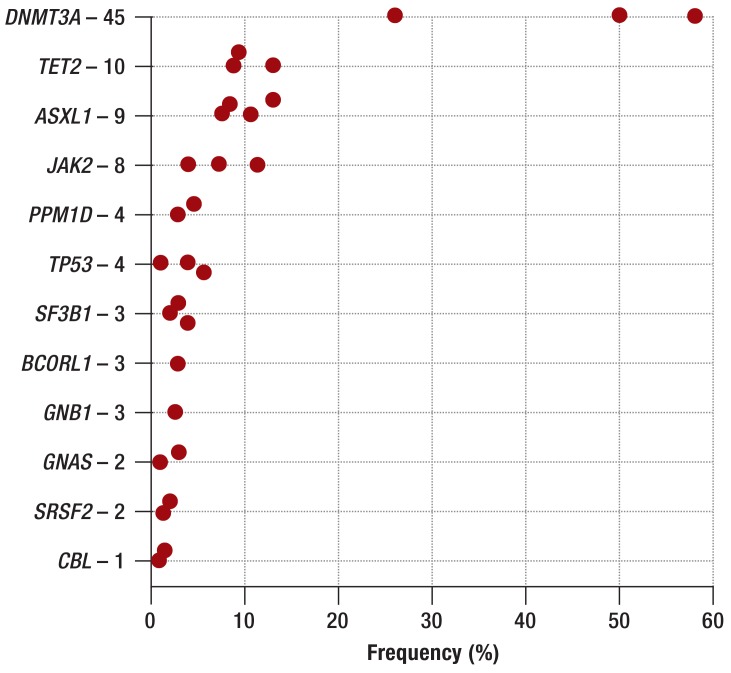
Three large studies have investigated the clinical significance of clonal hematopoiesis in humans without a known hematopoietic disease. Most of the 32 290 patients studied were enrolled in cohort studies of diabetes mellitus (7) and psychiatric illness (8); 2728 patients had solid tumors (9). In all patients, exome/genome sequencing was carried out on cells from peripheral blood. Clonal hematopoiesis was found in fewer than 1% of patients under the age of 40. Among persons aged between 71 and 80, by contrast, clonal hematopoiesis was shown in 9.5% to 13.9%, while in those aged over 80 the percentage rose to 16.4% (Figure 2). Related to the age structure in Germany, this would mean that about 2.75 million persons are affected (Federal Statistical Office, Statistisches Bundesamt; as of 31 December 2010). Men are affected 1.3 times as often as women (7). Anemia, thrombocytopenia, and leukopenia are not observed more often in patients with than in patients without CHIP. However, persons with CHIP more often had bi- or tricytopenia in peripheral blood (7). The most frequently mutated genes are those that are also the most frequently mutated in patients with AML and MDS: DNMT3A, TET2, and ASXL1 (2, 13, 16) (Figure 3). Mutations in the DNA-methyltransferase DNMT3A were the most frequent (26% to 58%) (7, 9). The risk of developing a malignant hematologic disease was 11 to 13 times higher for patients with somatic mutations; however, the absolute risk of developing a malignant hematologic disease was low, with an annual rate of 0.5% to 1% (7, 8). Of the patients with CHIP who showed a malignant hematologic disease, about 60% had a my eloid neoplasm (AML, MDS, chronic myelomonocytic leukemia [CMML], myeloproliferative neoplasm [MPN]) and about 40% a lymphoid neoplasm (chronic lymphocytic leukemia [CLL], multiple myeloma, B-cell lymphoma) (8).
Figure 2.
Age-related prevalence of CHIP (7–9)
CHIP, clonal hematopoiesis of indeterminate potential
Figure 3.
Frequency of mutated genes in CHIP. The numbers following the gene names give the mutation frequency in percent (7– 9). CHIP, clonal hematopoiesis of indeterminate potential
Interestingly, overall mortality was increased for patients with CHIP (hazard ratio [HR] 1.4; p = 0.02). Whereas in one study this finding was due to increased cancer mortality (HR 2.0; p = 0.02) (8), a second study showed an increased risk of fatal cardiovascular events (coronary heart disease and stroke, HR 2.6; p = 0.003) (7). Patients with a higher allele frequency of the somatic mutations were at higher risk both for developing a hematologic neoplasm and for overall mortality. This indicates a causal relationship between clonal hematopoiesis and these outcome parameters. The findings also suggest that CHIP, in addition to its hematologic significance, could even be an indicator of the general aging process.
Etiology and pathogenesis of CHIP
CHIP shows a clear association with age. There are two phenomena that could be causing this. First, as life advances, each hematopoietic stem cell undergoes more and more replication cycles. At each replication, mutations occur, and some of these may be irreparable. The age-dependent increasing frequency of mutations was impressively shown recently by DNA sequencing of individual hematopoietic stem cells from healthy people (17). While 0 to 1 mutation per stem cell was shown in newborns, 4 to 7 mutations were shown in 40- to 50-year-olds, and 8 to 12 mutations in 70- to 80-year-olds. However, clonal expansion of the stem cell will only occur if these mutations hit genes that are important for hematopoiesis. On the other hand, stem cell exhaustion, e.g., due to telomere loss and apoptosis, can reduce diversity and competition between the various stem cells, so that in old age, stem cells can win out with only a small growth advantage over other stem cells. This is the assumption underlying a large, recently published study on aplastic anemia (18). In aplastic anemia, there is a stepwise loss of hematopoietic stem cells and severe cytopenia. Unsurprisingly, in a third of patients somatic mutations with low allele frequency were found largely in the same genes as are also found in healthy people with clonal hematopoiesis (DNMT3A, ASXL1, TP53, TET2, BCOR, and BCORL1) (18).
Genotoxic stressors such as chemicals or radiation can increase the mutation rate in cells. In the study by Genovese et al., an association was found between smoking and clonal hematopoiesis (HR 2.2; p <0.001) (8), such as is also known for MDS (19, 20). This relationship could also explain the increased cardiovascular risk found in the study by Jaiswal et al. (7).
Clinical management of CHIP
The recommendations provided here are based on expert opinions, since to date there are no clinical studies of patients with CHIP. CHIP was defined as a provisional entity to make it possible to avoid a false diagnosis of MDS and the negative consequences of unnecessary treatment. In addition, the definition will help to ensure that in future these patients are observed more closely and their clinical course is better documented, and thus knowledge about this provisional entity will accumulate. At the present time, screening for CHIP should not be carried out in healthy people with normal blood values, as (1) the risk of hematologic neoplasm is low and (2) currently no intervention is available.
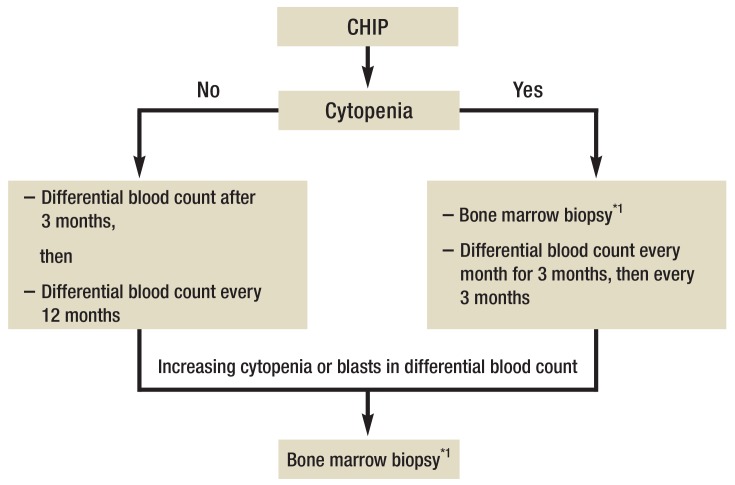
When CHIP is an incidental finding in patients with normal blood counts, we recommend a differential blood count 3 months later, and after that every 12 months, in order to identify any development/progression of cytopenia or the appearance of blasts in the blood.
In patients with CHIP and peripheral cytopenia (Hb <10 g/dL, thrombocytes <100 000/µL and/or neutrophils <1000/µL), we recommend, in addition to initial bone marrow biopsy, a differential blood count at 1, 2, and 3 months, and then every 3 months, to identify worsening of cytopenia and/or appearance of blasts in the differential blood count. Upon aggravating cytopenia or appearance of blasts in the differential blood count, repeat bone marrow biopsy is indicated for further diagnostic investigations including cytomorphology, histology, cytogenetic and molecular genetic studies (Figure 4).
Figure 4.
Algorithm for clinical management of CHIP
Cytopenia defined as Hb <10 g/dL, platelets <100 000/µL, neutrophils <1000/µL.
CHIP, clonal hematopoiesis of indeterminate potential
*1To acquire samples for cytologic, histologic, cytogenetic, and molecular genetic studies
Conclusion
Recent studies suggest that mutations in the DNMT3A, TET2, ASXL1, and other genes induce clonal hematopoiesis. Clonal hematopoiesis is an age-dependent phenomenon and is associated with increased risk for hematologic neoplasms. The rate of transformation is 0.5% to 1% per year, similar to the rate for the benign conditions MGUS and monoclonal B-lymphocytosis. The definition of CHIP enables identification of patients with somatic mutations who do not fulfill the criteria for MDS, allowing them to be further investigated in future in a targeted manner. Screening for CHIP is not indicated in healthy persons. However, elucidating the causes of CHIP and evaluating possible ways of preventing it from transforming into a hematologic neoplasm will be interesting areas of for future research. In treatment for MDS and AML, also, it will be important to answer the question of whether CHIP-associated mutations are effective targets for therapy, or whether treatment should rather be aimed at the genetic changes that have caused the progression from CHIP to AML. The concept of clonal expansion of stem cells in healthy persons ought also to be applied to other tissues in patients that show increasing tumor incidence with age—such as colorectal carcinoma, breast cancer, or prostate cancer—to help further elucidate the pathogenesis of these diseases.
Key Messages.
Clonal hematopoiesis of indeterminate potential (CHIP) is a newly defined entity.
Clonal hematopoiesis is defined by evidence of a genetic mutation that is associated with a hematologic neoplasm.
Dysplasias and leukemic blasts must be ruled out.
Clonal hematopoiesis increases with age (10% in the 8th decade of life).
The annual risk of transformation into a hematologic neoplasm is 0.5% to 1%.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Ganser has received consultancy fees from Boehringer-Ingelheim, Celgene, Takeda, and Tolero.
PD Dr. Thol has received lecture fees from Celgene. She has received fees from Celgene and Boehringer-Ingelheim for carrying out clinical studies.
Professor Heuser has received lecture fees from Celgene. He has received funding into a third-party account from Novartis, Karyopharm, Suneris, Tetralogic, BerGen Bio, and Daiichi Sankyo. He has received support from Illumina in the form of equipment for a research project he initiated.
References
- 1.Abkowitz JL, Catlin SN, McCallie MT, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- 2.TCGA. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121:3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thol F, Damm F, Lüdeking A, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29:2889–2896. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- 5.Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debarri H, Lebon D, Roumier C, et al. IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the acute leukemia french association. Oncotarget. 2015 doi: 10.18632/oncotarget.5645. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steensma R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 12.Thol F, Heuser M, Ganser A. Myelodysplastic syndromes. Internist (Berl) 2015;56:364–373. doi: 10.1007/s00108-014-3598-3. [DOI] [PubMed] [Google Scholar]
- 13.Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valent P, Horny HP. Minimal diagnostic criteria for myelodysplastic syndromes and separation from ICUS and IDUS: update and open questions. Eur J Clin Invest. 2009;39:548–553. doi: 10.1111/j.1365-2362.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 15.Valent P, Bain BJ, Bennett JM, et al. Idiopathic cytopenia of undetermined significance (ICUS) and idiopathic dysplasia of uncertain significance (IDUS), and their distinction from low risk MDS. Leuk Res. 2012;36:1–5. doi: 10.1016/j.leukres.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35–47. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjork J, Albin M, Mauritzson N, Stromberg U, Johansson B, Hagmar L. Smoking and myelodysplastic syndromes. Epidemiology. 2000;11:285–291. doi: 10.1097/00001648-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia. 2005;19:1912–1918. doi: 10.1038/sj.leu.2403945. [DOI] [PubMed] [Google Scholar]