Abstract
Background
In the past few decades, much has been learned about the pathophysiology of restless legs syndrome (RLS). Investigators have studied neuropathology, imaging, electrophysiology, and genetics of RLS, identifying brain regions and biological systems affected in RLS. This manuscript will review RLS pathophysiology literature, examining the RLS state through consideration of the neuroanatomy, then the biological, organ, and genetic systems.
Methods
Pubmed (1966 to April 2016) was searched for the term “restless legs syndrome” cross-referenced with “pathophysiology,” “pathogenesis,” “pathology,” or “imaging.” English language papers were reviewed. Studies that focused on RLS in relation to another disease were not reviewed.
Results
Although there are no gross structural brain abnormalities in RLS, widespread brain areas are activated, including the pre- and post-central gyri, cingulate cortex, thalamus, and cerebellum. Pathologically, the most consistent finding is striatal iron deficiency in RLS patients. A host of other biological systems are also altered in RLS, including the dopaminergic, oxygen-sensing, opioid, glutamatergic, and serotonergic systems. Polymorphisms in genes including BTBD9 and MEIS1 are associated with RLS.
Discussion
RLS is a neurologic sensorimotor disorder that involves pathology, most notably iron deficiency, in motor and sensory brain areas. Brain areas not subserving movement or sensation such as the cingulate cortex and cerebellum are also involved. Other biological systems including the dopaminergic, oxygen-sensing, opioid, glutamatergic, and serotonergic systems are involved. Further research is needed to determine which of these anatomic locations or biological systems are affected primarily, and which are affected in a secondary response.
Keywords: Restless legs syndrome, pathophysiology, neuroanatomy
Introduction
Restless legs syndrome (RLS) is one of the most common neurologic diseases, affecting up to 10% of adults in industrialized nations.1–3 Clinically significant RLS is estimated to affect 2–3% of adults.4 RLS is a multifaceted neuropsychological disease that consists of abnormalities in sensation, motor activity, arousal, and psychology. More specifically, RLS consists of an inescapable urge to move, often accompanied by sensory discomfort. Other features required for a diagnosis of RLS include worsening of symptoms at night, with inactivity, and development of relief with movement. As a result of these symptoms, sleep is markedly disturbed,5,6 and depression is common.7–9 Still other identifying psychological features of RLS include impulsivity and problems with decision-making.10 This broad description of RLS, although phenomenological, serves to guide a neuroanatomical approach when considering RLS pathophysiology. Neuroanatomic regions likely involved in RLS are those associated with abnormal movement, sensation, arousal, and psychology, and include cortical and spinal areas, multimodal thalamic nuclei, extrapyramidal motor centers, limbic cortices, and integrative cerebellar centers. This anatomical approach is especially pertinent as the biological mechanisms underlying RLS are poorly understood.
Nevertheless, when considering RLS pathophysiology, it is imperative to review biological systems that have been implicated and studied in relation to RLS. These systems occur at the ligand and elemental level, including the dopaminergic and iron systems; at the organ level, including the peripheral arterial and nervous systems; and at the molecular and genetic level, including the oxygen sensing and genetic pathways. This review of RLS pathophysiology will examine the RLS state through consideration of the disease starting with a reflection of the involved neuroanatomy, then attending to the biological, organ, and genetic systems. It will then end with discussion of where pathophysiologic research has been deficient, and propose a direction for future research to aid in uncovering the underlying mechanisms of this common sleep disorder.
Methods
The authors used PubMed (1966 to April 2016) to search for the term “restless legs syndrome” cross-referenced with “pathophysiology,” “pathogenesis,” “pathology,” or “imaging.” English language papers were reviewed. Also reviewed were pertinent papers from the reference list of the above-matched manuscripts. Studies that focused on RLS in relation to another disease such as Parkinson’s disease, multiple sclerosis, or renal failure were not reviewed.
Results
The pathophysiology of RLS will be reviewed in the following sections, first using a neuroanatomical approach, followed by a consideration of the different biological systems involved in RLS patients.
Consideration of neuroanatomy as it relates to RLS
Early magnetic resonance imaging (MRI) studies did not reveal gross structural brain abnormality in RLS.11 Rather, MRI measurement of brain iron demonstrated a deficiency of iron in the substantia nigra, putamen, caudate nucleus, and thalamus.12–14 This brain iron deficiency was confirmed by direct neuropathologic demonstration of low levels of iron, ferritin, and transferrin in the substantia nigra of RLS patients.15,16 Although early studies focused on structures of the basal ganglia, development of functional imaging has allowed an agnostic approach, shifting attention to areas not previously implicated in RLS. During periods when RLS patients were actively experiencing symptoms, functional MRI (fMRI) showed activation of the cerebellum and contralateral thalamus.17 More recent fMRI studies of persons with active RLS symptoms demonstrated activation in these and other areas, including the midbrain, dorsolateral prefrontal cortex, anterior cingulate cortex, left precentral gyrus, posterior central gyrus, pars opercularis, and putamen.18 The numerous regions activated in RLS reflect the complexity of this neuropsychiatric disease. Table 1 lists different brain regions that have been studied in RLS and what these studies have found referable to these areas. The paragraphs below outline these findings.
Table 1. Brain Regions Implicated in Restless Legs Syndrome.
| Brain Region | Abnormality |
|---|---|
| Prefrontal cortex | ↑ activity (fMRI),18 ↓ gray matter density (voxel morphometry MRI)27 |
| Medial frontal | ↑ gray matter density28; ↔ gray matter density (voxel morphometry MRI)29 |
| Precentral gyrus | ↑ activity (fMRI),18 ↓ intra-cortical inhibition (TMS)30; ↑ gray matter (voxel morphometry MRI)31 |
| Postcentral gyrus | ↑ activity (fMRI)18; ↑ gray matter (voxel morphometry MRI)31 |
| Anterior cingulate cortex | ↑ activity (fMRI),18 ↔ gray matter density (Voxel morphometry MRI),32 ↓ white matter volume (voxel morphometry MRI)33 |
| Posterior cingulate cortex | ↑ activity (fMRI)18 |
| Whole brain | ↔ gray matter (voxel morphometry MRI)18,34 |
| Putamen | ↓ iron staining (pathology),15,16,35 ↓ iron (MRI),12 ↓ dopamine-2 binding (PET),36 ↓ dopamine-2 receptor levels (pathology)37 |
| Substantia nigra | ↓ iron (pathology),15,16,35 ↓ iron (MRI),12,13 ↓ iron (phase imaging),22 ↑ tyrosine hydroxylase levels (pathology)37 |
| Caudate | ↓ iron staining (pathology), ↓ iron (MRI)14 |
| Pons, medulla | ↔ Serotonin transporter38 |
| Thalamus | ↑ activity (fMRI),17,18 ↑ gray matter density pulvinar (Voxel morphometry MRI),39 ↔ NAA,40 ↑ glutamate (MRS),40 ↓ β-endorphin positive cells (pathology),41 ↓ iron (MRI)14 |
| Cerebellum | ↑ activity (fMRI),17,18 ↓ gray matter density (voxel morphometry MRI)27 |
Abbreviations: ↑, Increased; ↓, Decreased; ↔, Unchanged; fMRI, Functional Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; NAA, N-acetylaspartate; PET, Positron Emission Tomography; TMS, Transcranial Magnetic Stimulation.
Neuroanatomy of motion and sensation
On the most basic level, RLS consists of sensory discomfort and an urge to move, which drives movement. So it is no surprise that areas involved in sensation and movement, including the post- and pre-central gyri, are activated when RLS symptoms are present. Also activated in RLS are extrapyramidal subcortical structures, including putamen.18,19 Functional imaging in RLS has not shown increased activity in substantia nigra; however, several T2 relaxometry studies have demonstrated reduced iron in substantia nigra of persons with RLS.12,20–22 Striatum has been more extensively studied using single-photon emission computed tomography, which has yielded conflicting results with increased, decreased, and even normal striatal dopamine binding (detailed in the section on dopamine ).23–26
Many studies have focused on the thalamus in RLS,17,19,42 and as a brain region subserving sensation this seems fitting. In several fMRI studies in RLS patients, there is increased activity reported in the thalamus.17–19,42,43 One voxel-based morphometry MRI study demonstrated an increase in gray matter density in the thalamic pulvinar nuclei in RLS compared with controls, suggesting a chronic increase in afferent input, but other similar MRI studies have not replicated this finding.18,29,39 Proton magnetic resonance spectroscopy has demonstrated heightened glutamatergic activity but normal N-acetylaspartate levels in the thalamus of persons with RLS,40,43 suggesting that neurons are intact in the thalamus but hyperactive in RLS. Glutamate is abundant in the thalamus, and is required for communication between the thalamus and the cortex.44,45
When considering the available data regarding motor and sensory areas, there is consistency in that there is widespread activation of neuroanatomical areas, including the somatosensory cortex, thalamus, and parts of the striatum.18,19 There is also consensus from neuropathologic and imaging studies that there is a relative deficiency of iron in extrapyramidal motor regions.15,20–22 There is less consensus on the involvement and/or role of sensory structures, most notably the thalamus, in the pathophysiology of RLS, although there is likely to be increased activation. Lastly, although conventional imaging has not revealed any structural abnormality in motor structures, some but not all voxel-based morphometry studies have revealed increased gray matter density in sensory and motor structures.18,29,31,39 Differences in findings across studies may be related to genetic or population differences (Asian versus Western European), variation in RLS phenotype (early versus late onset), medication exposure status, and differences in imaging analysis techniques.
Neuroanatomy of arousal
In RLS, uncomfortable sensorimotor symptoms alert the sufferer even in the face of sleepiness. When RLS symptoms are present, fMRI has shown activation in the midbrain and pons, and throughout different cortical areas, including the primary motor cortex, somatosensory cortex, and anterior cingulate cortex, the inferior, and superior parietal lobules, and the dorsolateral prefrontal cortex.18,19 Activation of brainstem arousal systems in the dorsal midbrain and pons would result in alertness and excitation of cortical areas. In turn, widespread cortical activation may relate to increased arousal and an inability to sleep.
Bucher and colleagues17 in a fMRI study of persons experiencing sensory RLS symptoms showed bilateral activation of the cerebellum and contralateral activation of the thalamus. In the same study, when sensory RLS symptoms co-occurred with periodic limb movements during sleep (PLMS), there was additional activation in the red nuclei and brainstem close to the reticular formation. PLMS are often associated with cortical arousal, previously thought to occur as a consequence of movement. We now know that arousal can either precede or follow PLMS, suggesting that they occur as a complex rather than one being the consequence of the other.46
Neuroanatomy of emotion
Although the core sensorimotor symptoms of RLS are most conspicuous, there are non-sensorimotor features of RLS that extend its phenomenology, including a tendency toward depression, impulsivity, and PLMS.
Functional imaging in RLS demonstrates activation of brain regions associated with emotion, including the anterior and posterior cingulate gyri and dorsolateral prefrontal cortex.18,19 Voxel-based morphometry MRI shows a decrease in gray matter density in the medial frontal and parietal lobes in persons with RLS,27 whereas a German study showed an increase in gray matter density in the hippocampus and medial frontal lobe.28 However, additional voxel-based morphometry MRI studies showed no difference in the anterior cingulate gray matter density or in the whole brain gray matter compartments.18,32,34 In addition, pathologic study and voxel-based morphometry MRI have demonstrated that there is decreased white matter volume in the corpus callosum and anterior cingulate gyrus in RLS, specifically decreased in myelin integral proteins and myelin basic protein.33 Indeed, there is a well-known association between RLS and depression.47 Lifetime prevalence of depression ranges between 19% and 35% in persons with RLS, representing a greater than twofold increased odds of depression compared with those without RLS.48–50 Another potential clinical correlate of altered limbic and behavioral neuroanatomy is the increased impulsivity shown in persons with RLS, who are more likely than controls without RLS to make risky choices in studies using validated gambling task testing.10
Neuroanatomy of periodic limb movements during sleep
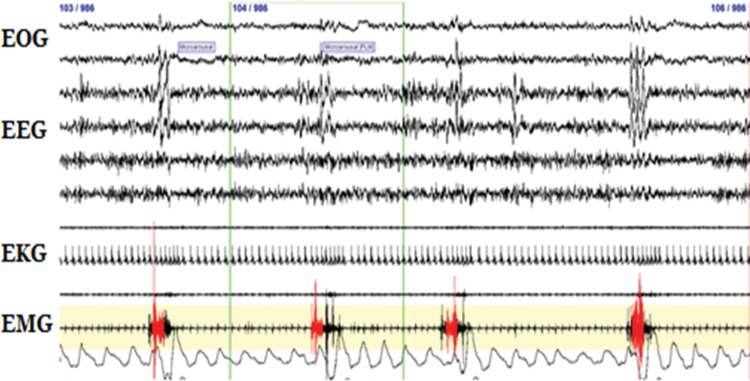
Although not a diagnostic criterion of RLS, PLMS are commonly associated with RLS and may provide insight into its pathophysiology.6 Movements are stereotypic, occur during sleep, and consist of brisk, brief (0.5–5 second) movements of the feet and lower more than upper legs (Figure 1). By definition, PLMS occur in a chain of four or more movements with an inter-movement interval between 5 and 90 seconds. PLMS may number into the hundreds across a night’s sleep. The anatomic generator of these movements is suggested by the pattern of muscular recruitment, which tends to be uniform up or down spinal segments (L3 to S1), suggesting spinal cord hyperexcitability.51 Electrophysiologic study of persons with RLS corroborates spinal cord hyperexcitability, showing a lower threshold and greater spatial spread of the spinal cord flexor response (by medial plantar nerve stimulation).52 That PLMS involve spinal cord hyperexcitability is not surprising, as the movement itself resembles either an extensor plantar response or triple flexion. It is also possible that PLMS arise further upstream in the brainstem. One functional imaging study demonstrated activation in the brainstem near the reticular formation, when both RLS sensorimotor symptoms and PLMS co-occurred.17
Figure 1. Periodic Limb Movements During Sleep Demonstrated By Polysomnography. Periodic limb movements during sleep are displayed as demonstrated by periodically occurring (every 20–30 seconds) activations of anterior tibialis electromyography. Also noticeable in this schematic are activations of electroencephalography, in the form of synchronous cortical firing (K-complexes) and acceleration of heart rate as evidenced by shortening of the R-R interval on electrocardiography.
Other neuroimaging techniques
PLMS are one associated phenomenon that suggest that there is central nervous system hyperexcitability in RLS. Other supporting evidence includes disinhibition of auditory startle in the RLS patients, suggesting activation of reticulospinal pathways from dysfunction in the diencephalon or above.53 Transcranial magnetic stimulation (TMS) studies have consistently shown reduction in intracortical inhibition (mediated by intracortical gamma-aminobutyric acid-A receptors),30 consistent with a hyperexcitable central nervous system. Not every neurophysiologic study of RLS has been compatible with central nervous system disinhibition. Brainstem auditory evoked responses and blink reflex were normal in studies of persons with RLS.11,54,55
Neuroanatomy: bridge to biological pathophysiology of RLS
Clearly, from the above discussion, there are numerous neuroanatomic regions that are involved in RLS or in phenomena associated with RLS. What is not clear is which of these areas is affected primarily by a pathologic process or derangement that causes RLS, and which of the areas becomes involved or activated secondarily as a consequence of either chronic sleep loss or sensorimotor disturbances. Areas of the brain that serve sensation and movement clearly need to be activated/involved when RLS symptoms are present. What isn’t known is what causes the activation of these structures and produces the sensorimotor symptoms of RLS. In addition to brain regions involved in sensation and movement, other areas linked to emotion (frontal lobes, cingulate cortex), arousal (brainstem, cortex), and multimodality (thalamus and cerebellum) have demonstrated activity when RLS symptoms are present. Is this simply because it is impossible not to activate such areas when unpleasant sensations are felt and does activation in these areas simply represent an uninteresting by-product of discomfort? Or is there meaningful pathology that occurs primarily in these areas that drives sensorimotor symptoms of RLS?
Stepping back from neuroanatomy, much investigation has taken place to identify a pathophysiologic substrate of RLS. Biological systems that have been studied include the dopaminergic, opioid, iron, serotonergic, and glutamatergic systems. It is likely that activation and or abnormalities seen in different brain regions result from a primary disturbance of some biological system that in turn drives the symptoms and thus the entity of RLS.
Dopamine function in RLS
It is well known that RLS symptoms can be successfully treated with medicines having dopamine agonist properties. This fact has driven much of the work that has looked at the dopamine system and its relation to RLS. Early on, studies aimed to verify a predominant notion that there was brain dopamine deficiency, but studies have not supported this notion. The first cerebrospinal fluid studies of RLS demonstrated normal dopaminergic and serotonergic metabolites in untreated persons with RLS compared with controls.56 A later study by a separate group showed that persons with RLS have higher levels of 3-ortho-methyldopa, a dopamine metabolite, that correlates with RLS symptom severity.57
Brain imaging studies of the dopaminergic system in RLS have been contradictory. Early positron emission tomography (PET) studies in untreated RLS patients showed lower dopamine (D2) binding potential in the striatum.36,58 Similarly, lower striatal dopamine (D2) binding potential was found in a later PET study of RLS. This study did not find a difference in dopamine receptor affinity or density, and thus the finding of decreased dopamine binding potential was interpreted to suggest an increase in synaptic dopamine.59 Two single photon emission computed tomography studies yielded opposite results, showing both increased and decreased binding potential of dopamine transporter in the striatum of RLS subjects.26,60 Another photon emission computed tomography study found normal dopamine-2 receptor binding in the striatum of RLS patients.23,25 A post-mortem study conducted by Connor et al.37 is consistent with overactivity of the dopamine system in the brain. Brains of patients with RLS demonstrated a decrease in dopamine-2 receptor levels in the putamen and an increase in tyrosine hydroxylase in the substantia nigra. The sum of these metabolic, imaging, and pathologic studies suggests that in RLS there is a relative increase in striatal dopamine, but this supposition needs greater support.
Further insight into the involvement of dopamine comes from considering the phenomenon of augmentation, which is characterized by paradoxical worsening of RLS symptoms in the setting of dopaminergic treatment.61 Increased striatal dopamine stimulation likely produces a post-synaptic downregulation at the receptor level, perhaps enough to maintain an equilibrated dopaminergic state during the day. At night, when circadian influences result in a relative dopamine deficit with low dopamine receptor levels, RLS symptoms may emerge. Treatment with dopaminergic medication at night can temporarily restore balance; however, adding dopamine to a dopamine excess state invariably worsens the situation, and augmentation may result. For this reason, the dopaminergic medications for many RLS practitioners have fallen out of favor, for the alpha-2-delta ligands (gabapentin and pregabalin).
Iron deficiency
Although pharmacological treatment data support involvement of the dopaminergic system in RLS pathophysiology, recent evidence shows that iron depletion is an additional contributing factor. Several studies have shown a relation between low serum ferritin and symptoms of RLS62,63; furthermore, RLS improves with the administration of oral or intravenous iron.64,65 In addition, cerebrospinal fluid (CSF) ferritin levels are 65% lower and CSF transferrin levels are three times higher in RLS patients than age-matched controls.66
Low brain iron has been demonstrated by both imaging and neuropathological studies of the RLS brain. Allen et al.12 evaluated the iron concentration or “iron index” in 10 brain regions with MRI in early and late onset RLS patients and controls. The mean iron index for the substantia nigra was significantly lower for early-onset but not for late-onset RLS patients compared with controls. Neuropathology in RLS has demonstrated a decrease in ferritin and increase in transferrin in neuromelanin containing cells of the substantia nigra in RLS,15 complementing prior CSF findings in RLS patients.66
One inconsistent finding of this autopsy study was a relative decreased staining intensity for transferrin receptors in RLS brains. Normally, an iron-deficient cell should have an increase in transferrin receptors. In addition, there were differences noted in the expression of transporter proteins in neuromelanin cells. The expression of the divalent metal transporter protein 1 was found rarely in neuromelanin cells in RLS brains compared with normal brains. Divalent metal transporter protein 1 helps remove iron from endosomes, making iron available in the intracellular iron pool.67 Another iron transport protein, metal transporter 1, involved in cellular iron efflux, was minimally detected in neuromelanin cells of RLS brains.68 Thus, the lack of appropriate increase in transferrin receptor expression in response to less iron; and a relative decrease in iron transporter protein concentration in RLS suggests that there is a malfunction of central iron acquisition and transport in RLS patients.
Opioid system
The endogenous opioid system may also be involved in RLS pathophysiology,69 again suggested by the often exquisite therapeutic response of RLS to opioid medications.70,71 There is some evidence of endogenous opioid deficiency in RLS. A post-mortem study of RLS patients showed that β-endorphin and met-enkephalin endogenous opioids were significantly decreased in the thalamus of RLS compared with control subjects.41 In an animal model of RLS, µ-opioid receptor knockout mice showed hyperactivity and iron deficiency.72
There are important interactions among the iron, dopamine, and opioid systems, in general, and in how they may relate to RLS. Iron is a necessary cofactor in the formation of L-dopa from L-tyrosine through action of tyrosine hydroxylase. In an in vitro model, Sun et al.73 demonstrated decreased survival of dopaminergic cells exposed to iron deficiency, but that opioid receptor agonism protected dopaminergic cells from cell death.
Glutamatergic system
It is well known that there are sleep–wake and arousal abnormalities associated with RLS, yet it is difficult to attribute these abnormalities to dopamine or iron. Abnormal sensations and heightened arousal are important features of RLS, and both may invoke the thalamus, an area that shows increased activity when RLS symptoms are present. Using this thinking as the impetus, a recent study examined the thalamus in RLS subjects by magnetic resonance spectroscopy, focusing on the glutamatergic system. Glutamatergic activity was increased in the thalamus of RLS patients compared with controls, and intriguingly total activity correlated with time spent awake after sleep onset, but not with PLMS frequency.40 This study suggests that thalamic glutamatergic hyperactivity underlies heightened arousal but not PLMS in RLS. Hyperpolarization of thalamocortical neurons is responsible for sleep spindle generation and decreasing cortical sensory inputs.74 Increased thalamocortical excitation can cause increased wakefulness and decreased non-rapid eye movement stage 2 sleep (seen in RLS). Pertinent to this pathophysiology is the therapeutic response of RLS to α-2-δ ligands, gabapentin, pregabalin, and gabapentin enacarbil, which decrease glutamate release, lessen RLS symptoms, and improve sleep continuity.75–77
Serotonergic system
Medicines that increase serotonin bioavailability (antidepressants) have been associated with increased risk or aggravation of RLS and PLMS.78,79 In a large epidemiological study, Ohayon et al.80 showed that use of serotonin reuptake inhibitor antidepressants was associated with a threefold increased risk of RLS. Antidepressants, serotonin reuptake inhibitors, and venlafaxine have been similarly shown to be associated with increased risk (about fivefold) of having PLMS in a large cohort of patients.81 The pathophysiologic role of serotonin in RLS has been scarcely studied. One study employing magnetic resonance spectroscopy found similar availability of serotonin transporter protein in pons and medulla in RLS and control subjects; but RLS severity did increase with decreased transporter availability. This finding suggests that dysregulation of serotonergic neurotransmission may provoke or exacerbate RLS, implicating yet another neurotransmitter system in RLS pathophysiology.38
Hypoxia
There are clues that hypoxia, both in the periphery and brain, may play a role in RLS. In one study, patients with RLS had significantly lower partial oxygen pressure than controls in the legs but not chest when both groups were subjected to prolonged immobilization.82 Furthermore, increasing severity of RLS correlated with higher chest-to-foot oxygen gradients (ρ = 0.692, p<0.01). Finally, treatment with a dopamine agonist improved peripheral hypoxia toward levels observed in controls. Hypoxia-inducible factor-1α (HIF-1α) is a subunit of the transcription factor HIF-1 that regulates the cellular and developmental response to hypoxia.83 Immunohistochemical analyses of substantia nigra tissue from six RLS and six control patients showed HIF-1α was increased in RLS subjects.
Interestingly, hypoxia may be linked to the dopamine and iron systems. Connor et al.37 showed that tyrosine hydroxylase levels are elevated in post-mortem brains in RLS patients. Tyrosine hydroxylase is the rate-limiting enzyme in dopamine synthesis, and requires iron for its activity. Elevation in tyrosine hydroxylase activity in RLS occurs despite a state of iron deficiency. This could be explained by the presence of hypoxia in RLS, as the tyrosine hydroxylase gene promoter region contains a hypoxia-responsive element that causes increased gene expression of tyrosine hydroxylase in the presence of hypoxia.84
Peripheral and autonomic nervous systems and consideration of PLMS
Although it is fairly well accepted that peripheral neuropathy may be the de novo cause of or exacerbate RLS,85 it is controversial whether or not there is subclinical small fiber neuropathy in patients with primary RLS who have normal neurological examinations and normal conduction studies. Some studies have indicated that patients with primary RLS have sensory abnormalities with quantitative sensory testing, including increased pin prick sensitivity.85–88 However, other examinations of small neuronal fibers in idiopathic RLS, using quantitative sudomotor and skin sympathetic testing, demonstrated normal sweating and intact A-delta and C-fibers, arguing against small fiber neuropathy.88–90 Biopsy has also demonstrated a high concurrence of small fiber neuropathy, especially in late-onset RLS in approximately one-third of patients.91 However, this still leaves two-thirds of RLS patients that cannot be accounted for by subclinical small fiber neuropathy. Measuring vascular response to exercise and sympathomimetic medication, patients with RLS demonstrate a depressed arterial baroreflex response and have greater peripheral vascular resistance, potentially reflecting heightened sympathetic outflow.92
Additionally, increased spinal cord excitability in the form of reduced threshold and increased spatial spread of the flexor reflex has been demonstrated in RLS patients. One study showed multiple late responses in the spinal flexor response with long duration in RLS patients, again suggesting spinal hyperexcitability.93
PLMS occur in up to 90% of persons with RLS.6 Recent studies suggest that the autonomic nervous system is involved in PLMS pathogenesis. Stereotypically, PLMS co-occur with discrete increases in blood pressure measuring about 22 mmHg.94,95 The magnitude of pressure elevations is greatest when there is accompanying arousal from sleep. The co-occurrence of this sympathetic hyperactivity and PLMS suggests activation of the thoracolumbar spinal cord, where sympathetic preganglionic neurons arise. RLS patients have abnormal cardiovascular autonomic function with a tendency toward hypertension, reduced amplitude of both sympathetic and parasympathetic responses during the tilt test, as well as blunted parasympathetic drive to blood pressure changes.96 These findings are in agreement with epidemiological studies that have found a tendency toward hypertension and enhanced cardiovascular risk in RLS.97
Clarification of RLS pathophysiology through genetics
RLS is highly familial with estimates of heritability between 54% and 83% by twin studies.98,99 Positive genetic findings in RLS have come through familial linkage and genome-wide association studies. Genetic linkage studies have found gene regions that are associated with RLS, loci RLS1 through RLS5, but have not implicated specific genes.100,101 On the other hand, genome-wide association studies have identified specific genes that are associated with RLS across different populations and study groups. Genes that are likely associated with the presence of RLS include BTBD9, MEIS1, PTPRD, MAP2K5, SKOR1, and TOX3.102–106 Within these genes, several common single nucleotide polymorphisms have demonstrated significant association with the phenotype of RLS (Table 2). At this point, how and if these different genetic variants result in an RLS phenotype is not known. But animal models, for BTBD9 and MEIS1, which are currently underway, are beginning to disentangle the mechanisms by which these genes may be associated with RLS.107,108 We will further comment on the genetic associations of RLS and BTBD9 and MEIS1. It is important to note, that some of these studies showed association more so for PLMS than for RLS.102,106 There are perhaps competing factors to consider. PLMS occur in the majority of persons with RLS6; however, they also occur commonly in persons without RLS, including those with sleep apnea, narcolepsy, and in normal elderly.109–111 Although, there is non-specificity as to what group of persons has PLMS, it is a phenomenon that is objectively measured rather than subjectively considered, which may decrease false positivity that so often occurs in RLS diagnosis.112
Table 2. Genes Implicated in RLS and PLMS: Cohort, Location, and Function.
| Gene | Chromosome | Function | SNP | Cohort |
|---|---|---|---|---|
| BTBD9 | 6p21.2 | Synaptic plasticity, learning | rs3923809, rs9296249, rs9357271 | Iceland,103 United States,106 Germany,104 Austria,114 Canada,104 Czech Republic,114 Finland,114 Korea115 |
| MEIS1 | 2p14 | Homeobox gene, limb formation, motor neuron connectivity | Rs2300478 | Germany,104 Austria,114 Czech Republic,114 Canada,104 United States106 |
| PTPRD | 9p23-24 | Protein tyrosine phosphatase regulates cell growth, mitosis | rs4626664, rs1975197 | Korea,115 United States116 |
| MAP2K5 | 15q23 | Protein kinase involved in cell proliferation | Rs12593813, rs11635424, rs4489954, rs3784709, rs1026732, rs6494696 | Germany,104 Austria,114 Czech Republic,114 Canada,104 United States106 |
| TOX3 | 16q12.1 | Transcription factor | Rs3104767, rs3104788 | Germany117 |
Abbreviations: PLMS, Periodic Limb Movements during Sleep; RLS, Restless Legs Syndrome; SNP, Single Nucleotide Polymorphism.
BTBD9
BTBD9 is expressed widely in the central nervous system and is involved in synaptic plasticity and learning. Early knockout studies showed that mice and flies lacking BTBD9 expression display an RLS-like phenotype of sleep fragmentation and motor restlessness, which, like RLS, is reversed with the administration of a dopamine agonist.107,108 Also consistent with RLS, flies lacking BTBD9 have a marked reduction in whole brain dopamine compared with wild-type flies. This same group demonstrated in a human embryonic kidney cell line that overexpression of BTBD9 results in increased ferritin.107 These findings are consistent with one or more features of RLS. Much more is likely to be learned about BTBD9, as there is active investigation of this gene and the consequences of its perturbation.
MEIS1
MEIS1 is a homeobox gene, thought to be important in normal limb formation and motor neuron connectivity. Also MEIS1-expressing cells appear to be involved in formation of nigrostriatal projection neurons.117 The presence of the MEIS1 risk allele is associated with a nearly 50% increased odds of having RLS.103 And persons with this MEIS1 risk allele have decreased MEIS1 mRNA and protein in lymphoblastoid cell lines.118 MEIS1 seems to have an association with the iron system and perhaps via this pathway affects RLS. In a Caenorhabditis elegans model, RNA knockdown of MEIS1 increased expression of ferritin.118 A recent study indicated that the allelic variations in this gene that are associated with RLS lead to developmental changes in the telencephalon, suggesting that RLS could be a neurodevelopmental disorder.119 There is much that still needs clarification about the role of MEIS1 in RLS.
Summary and conclusion
Through neuroimaging, neurophysiologic, and neuropathologic analyses many different regions of the central nervous system have been studied in attempts to clarify the pathogenesis of RLS. These areas include those that subserve sensation and movement including the pre- and post-central cortices, thalamus, and striatum, and these may be primarily affected by pathological changes in one or more biological systems. Through sensorimotor discomfort and sleep loss, additional neuroanatomic structures, such as the cingulate and other cortical areas, the cerebellum, and brainstem arousal areas may become involved.
Research to clarify the biological underpinnings of RLS has included studying the role of brain iron and the endogenous opioid, dopamine, glutamate, and serotonin systems, the first two showing deficiency and the last three showing increased function. The paradox that RLS responds to dopaminergic agents despite autopsy evidence that dopamine is hyperfunctional in RLS remains yet to be resolved37; however, this may explain to some extent the phenomenon of augmentation. Findings related to dopamine in RLS have been inconstant. This paired with the likelihood that increased dopamine results secondarily from a problem with iron handling or storage argue against dopamine dysfunction as the primary mechanism in RLS.
On the other hand, the most consistently found biological abnormality in RLS is a deficiency of brain iron in the substantia nigra, red nucleus, putamen, caudate, and thalamus.12–15,22 Ferritin in the blood of RLS patients is commonly low, but iron measures themselves are often normal in RLS. The sum of these findings suggests an impairment of iron transport into the brain, and more specifically into neuromelanin-containing cells of the substantia nigra. Iron, itself, is an essential cofactor for tyrosine hydroxylase in the enzymatic conversion of L-tyrosine to L-dopa, the main precursor to dopamine. Much has been learned about how the iron and dopamine systems interact through the use of iron-deficient animal models. Iron deficiency in the rat is associated with increased presynaptic dopamine and dopamine uptake transporter dysfunction.120,121 These findings suggest that changes in dopamine are secondary to iron deficiency, but it remains unclear why iron is deficient in certain brain regions.
The formation of L-dopa is accomplished by the hydroxylation of L-tyrosine. One oxygen atom is taken from molecular oxygen as it reacts with ferrous iron to form an additional hydroxyl group of L-dopa. Hypoxia, by activating HIF-1α, leads to upregulation of tyrosine hydroxylase and a dopamine transporter.122 Hypoxia in RLS is suggested by increased muscle microvascularization and increased vascular endothelial growth factor in muscle of RLS patients.123,124 Furthermore, increased HIF-1α immunoreactivity has been found in the substantia nigra of RLS patients.83 Although this finding could have been produced by hypoxia itself, HIF-1α can also be activated under conditions of iron deficiency.125 So the question remains when considering the interactions among iron, dopamine, and hypoxia: Are abnormalities in one of these systems driving changes in the others?
Clearly, there are numerous neuroanatomic regions involved in RLS. It is still unclear which areas are affected primarily by a pathologic process that causes RLS, as opposed to affected secondarily as a consequence of either chronic sleep loss or sensorimotor disturbances. It is likely that within these differing neuroanatomic regions, various biological systems are involved in producing the RLS state, including the dopaminergic, opioid, iron, serotonergic, and glutamatergic systems.
Both clinicians and basic researchers will be essential in the effort to determine the underlying causes of RLS. As a common disease, RLS is likely not homogeneous but rather a spectrum that within it may have different subtypes. There needs to be research about the onset of RLS, early versus late, the prominence of certain sensory features such as pain or crawling, the presence or absence of PLMS, and the co-occurrence of RLS with depression or hypertension. Studies of children with RLS will be increasingly important as they will provide clues when and why iron deficiency takes place. The search for a biomarker, either genetic, serologic, or in spinal fluid, should continue fervently. Clinical, animal, genetic, and cellular models should be included.
At the most basic level of this research, an explanation for what causes the peculiar symptoms of RLS that are so central to its clinical identification is still lacking. Yet in consideration of RLS pathophysiology, sensorimotor symptoms and an exploration for what causes the signature, urge to move, are often sidelined. The key to uncovering the RLS mystery may lie in the correct decoding of symptoms that drive movement. And it could be that listening to the patient is what may drive this movement to where it ought to be.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: This study was reviewed by the authors' institutional ethics committee and was considered exempted from further review.
References
- 1.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord. 2011;26:114–120. doi: 10.1002/mds.23430. doi: 10.1002/mds.23430. [DOI] [PubMed] [Google Scholar]
- 3.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–246. doi: 10.1016/j.sleep.2004.03.006. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in Western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–37. doi: 10.1016/j.sleep.2009.03.007. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Borreguero D, Larrosa O, de la Llave Y, Granizo JJ, Allen R. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med. 2004;5:561–565. doi: 10.1016/j.sleep.2004.08.003. doi: 10.1016/j.sleep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–65. doi: 10.1002/mds.870120111. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 7.Celle S, Roche F, Kerleroux J, et al. Prevalence and clinical correlates of restless legs syndrome in an elderly French population: the synapse study. J Gerontol A Biol Sci Med Sci. 2010;65:167–173. doi: 10.1093/gerona/glp161. doi: 10.1093/gerona/glp161. [DOI] [PubMed] [Google Scholar]
- 8.Hornyak M, Kopasz M, Berger M, Riemann D, Voderholzer U. Impact of sleep-related complaints on depressive symptoms in patients with restless legs syndrome. J Clin Psychiatry. 2005;66:1139–1145. doi: 10.4088/jcp.v66n0909. doi: 10.4088/JCP.v66n0909. [DOI] [PubMed] [Google Scholar]
- 9.Kim WH, Kim BS, Kim SK, et al. Restless legs syndrome in older people: a community-based study on its prevalence and association with major depressive disorder in older Korean adults. Int J Geriatr Psychiatry. 2012;27:565–572. doi: 10.1002/gps.2754. doi: 10.1002/gps.2754. [DOI] [PubMed] [Google Scholar]
- 10.Bayard S, Langenier MC, Dauvilliers Y. Decision-making, reward-seeking behaviors and dopamine agonist therapy in restless legs syndrome. Sleep. 2013;36:1501–1507. doi: 10.5665/sleep.3044. doi: 10.5665/sleep.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucher SF, Trenkwalder C, Oertel WH. Reflex studies and MRI in the restless legs syndrome. Acta Neurol Scand. 1996;94:145–150. doi: 10.1111/j.1600-0404.1996.tb07045.x. doi: 10.1111/j.1600-0404.1996.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 12.Allen RP, Barker PB, Wehrl FW, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–265. doi: 10.1212/wnl.56.2.263. doi: 10.1212/WNL.56.2.263. [DOI] [PubMed] [Google Scholar]
- 13.Earley CJ, B Barker P, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–461. doi: 10.1016/j.sleep.2005.11.009. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Godau J, Klose U, Di Santo A, Schweitzer K, Berg D. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord. 2008;23:1184–1187. doi: 10.1002/mds.22070. doi: 10.1002/mds.22070. [DOI] [PubMed] [Google Scholar]
- 15.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. doi: 10.1212/01.WNL.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 16.Connor JR, Ponnuru P, Wang XS, Patton SM, Allen RP, Earley CJ. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134((Pt 4)):959–968. doi: 10.1093/brain/awr012. doi: 10.1093/brain/awr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol. 1997;41:639–645. doi: 10.1002/ana.410410513. doi: 10.1002/ana.410410513. [DOI] [PubMed] [Google Scholar]
- 18.Margariti PN, Astrakas LG, Tsouli SG, Hadjigeorgiou GM, Konitsiotis S, Argyropoulou MI. Investigation of unmedicated early onset restless legs syndrome by voxel-based morphometry, T2 relaxometry, and functional MR imaging during the night-time hours. AJNR Am J Neuroradiol. 2012;33:667–672. doi: 10.3174/ajnr.A2829. doi: 10.3174/ajnr.A2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astrakas LG, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou MI. T2 relaxometry and fMRI of the brain in late-onset restless legs syndrome. Neurology. 2008;71:911–916. doi: 10.1212/01.wnl.0000325914.50764.a2. doi: 10.1212/01.wnl.0000325914.50764.a2. [DOI] [PubMed] [Google Scholar]
- 20.Moon HJ, Chang Y, Lee YS, et al. T2 relaxometry using 3.0-tesla magnetic resonance imaging of the brain in early- and late-onset restless legs syndrome. J Clin Neurol. 2014;10:197–202. doi: 10.3988/jcn.2014.10.3.197. doi: 10.3988/jcn.2014.10.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon HJ, Chang Y, Lee YS, et al. A comparison of MRI tissue relaxometry and ROI methods used to determine regional brain iron concentrations in restless legs syndrome. Med Devices (Auckl) 2015;8:341–350. doi: 10.2147/MDER.S83629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo G, Manners D, Testa C, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Disord. 2013;28:1886–1890. doi: 10.1002/mds.25576. doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 23.Eisensehr I, Wetter TC, Linke R, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57:1307–1309. doi: 10.1212/wnl.57.7.1307. doi: 10.1212/WNL.57.7.1307. [DOI] [PubMed] [Google Scholar]
- 24.Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–170. doi: 10.1007/pl00007859. doi: 10.1007/PL00007859. [DOI] [PubMed] [Google Scholar]
- 25.Tribl GG, Asenbaum S, Happe S, Bonelli RM, Zeitlhofer J, Auff E. Normal striatal D2 receptor binding in idiopathic restless legs syndrome with periodic leg movements in sleep. Nucl Med Commun. 2004;25:55–60. doi: 10.1097/00006231-200401000-00008. doi: 10.1097/00006231-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kim KW, Jhoo JH, Lee SB, et al. Increased striatal dopamine transporter density in moderately severe old restless legs syndrome patients. Eur J Neurol. 2012;19:1213–1218. doi: 10.1111/j.1468-1331.2012.03705.x. doi: 10.1111/j.1468-1331.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Chang HW, Song H, et al. Gray matter alteration in patients with restless legs syndrome: a voxel-based morphometry study. Clin Imaging. 2015;39:20–25. doi: 10.1016/j.clinimag.2014.07.010. doi: 10.1016/j.clinimag.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Hornyak M, Ahrendts JC, Spiegelhalder K, et al. Voxel-based morphometry in unmedicated patients with restless legs syndrome. Sleep Med. 2007;9:22–26. doi: 10.1016/j.sleep.2006.09.010. doi: 10.1016/j.sleep.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Celle S, Roche F, Peyron R, et al. Lack of specific gray matter alterations in restless legs syndrome in elderly subjects. J Neurol. 2010;257:344–348. doi: 10.1007/s00415-009-5320-2. doi: 10.1007/s00415-009-5320-2. [DOI] [PubMed] [Google Scholar]
- 30.Magalhaes SC, Kaelin-Lang A, Sterr A, do Prado GF, Eckeli AL, Conforto AB. Transcranial magnetic stimulation for evaluation of motor cortical excitability in restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2015;16:1265–1273. doi: 10.1016/j.sleep.2015.03.018. doi: 10.1016/j.sleep.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Unrath A, Juengling FD, Schork M, Kassubek J. Cortical grey matter alterations in idiopathic restless legs syndrome: an optimized voxel-based morphometry study. Mov Disord. 2007;22:1751–1756. doi: 10.1002/mds.21608. doi: 10.1002/mds.21608. [DOI] [PubMed] [Google Scholar]
- 32.Pan PL, Dai ZY, Shang HF, et al. Gray matter anomalies in anterior cingulate cortex as a correlate of depressive symptoms in drug-naive idiopathic restless legs syndrome. Neuroscience. 2014;277:1–5. doi: 10.1016/j.neuroscience.2014.06.045. doi: 10.1016/j.neuroscience.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 33.Connor JR, Ponnuru P, Lee BY, et al. Postmortem and imaging based analyses reveal CNS decreased myelination in restless legs syndrome. Sleep Med. 2011;12:614–619. doi: 10.1016/j.sleep.2010.10.009. doi: 10.1016/j.sleep.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comley RA, Cervenka S, Palhagen SE, et al. A comparison of gray matter density in restless legs syndrome patients and matched controls using voxel-based morphometry. J Neuroimaging. 2012;22:28–32. doi: 10.1111/j.1552-6569.2010.00536.x. doi: 10.1111/j.1552-6569.2010.00536.x. [DOI] [PubMed] [Google Scholar]
- 35.Connor JR, Wang XS, Patton SM, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–1567. doi: 10.1212/01.wnl.0000123251.60485.ac. doi: 10.1212/01.WNL.0000123251.60485.AC. [DOI] [PubMed] [Google Scholar]
- 36.Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–937. doi: 10.1212/wnl.52.5.932. doi: 10.1212/WNL.52.5.932. [DOI] [PubMed] [Google Scholar]
- 37.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132((Pt 9)):2403–2412. doi: 10.1093/brain/awp125. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhoo JH, Yoon IY, Kim YK, et al. Availability of brain serotonin transporters in patients with restless legs syndrome. Neurology. 2010;74:513–518. doi: 10.1212/WNL.0b013e3181cef824. doi: 10.1212/WNL.0b013e3181cef824. [DOI] [PubMed] [Google Scholar]
- 39.Etgen T, Draganski B, Ilg C, et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. Neuroimage. 2005;24:1242–1247. doi: 10.1016/j.neuroimage.2004.10.021. doi: 10.1016/j.neuroimage.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–2034. doi: 10.1212/WNL.0b013e318294b3f6. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279:62–65. doi: 10.1016/j.jns.2008.12.022. doi: 10.1016/j.jns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Ku J, Cho YW, Lee YS, et al. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014;15:289–294. doi: 10.1016/j.sleep.2013.09.030. doi: 10.1016/j.sleep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo G, Tonon C, Testa C, et al. Abnormal medial thalamic metabolism in patients with idiopathic restless legs syndrome. Brain. 2012;135((Pt 12)):3712–3720. doi: 10.1093/brain/aws266. doi: 10.1093/brain/aws266. [DOI] [PubMed] [Google Scholar]
- 44.Magnusson KR, Clements JR, Larson AA, Madl JE, Beitz AJ. Localization of glutamate in trigeminothalamic projection neurons: a combined retrograde transport-immunohistochemical study. Somatosens Res. 1987;4:177–190. doi: 10.3109/07367228709144605. doi: 10.3109/07367228709144605. [DOI] [PubMed] [Google Scholar]
- 45.McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferri R, Rundo F, Zucconi M, et al. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. Sleep. 2015;38:19–24. doi: 10.5665/sleep.4740. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin sleep cohort. Sleep Med. 2006;7:545–552. doi: 10.1016/j.sleep.2006.01.004. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–105. doi: 10.1176/jnp.2008.20.1.101. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 50.Rye DB, Trotti LM. Restless legs syndrome and periodic leg movements of sleep. Neurol Clin. 2012;30:1137–1166. doi: 10.1016/j.ncl.2012.08.004. doi: 10.1016/j.ncl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Trenkwalder C, Bucher SF, Oertel WH. Electrophysiological pattern of involuntary limb movements in the restless legs syndrome. Muscle Nerve. 1996;19:155–162. doi: 10.1002/(SICI)1097-4598(199602)19:2<155::AID-MUS5>3.0.CO;2-D. doi: 10.1002/(SICI)1097-4598(199602)19:2<155::AID-MUS5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 52.Aksu M, Demirci S, Bara-Jimenez W. Correlation between putative indicators of primary restless legs syndrome severity. Sleep Med. 2007;8:84–89. doi: 10.1016/j.sleep.2005.12.001. doi: 10.1016/j.sleep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Frauscher B, Loscher W, Hogl B, Poewe W, Kofler M. Auditory startle reaction is disinhibited in idiopathic restless legs syndrome. Sleep. 2007;30((4)):489–493. doi: 10.1093/sleep/30.4.489. [DOI] [PubMed] [Google Scholar]
- 54.Mosko SS, Nudleman KL. Somatosensory and brainstem auditory evoked responses in sleep-related periodic leg movements. Sleep. 1986;9:399–404. doi: 10.1093/sleep/9.3.399. [DOI] [PubMed] [Google Scholar]
- 55.Akyol A, Kiylioglu N, Kadikoylu G, Bolaman AZ, Ozgel N. Iron deficiency anemia and restless legs syndrome: is there an electrophysiological abnormality? Clin Neurol Neurosurg. 2003;106:23–27. doi: 10.1016/j.clineuro.2003.07.004. doi: 10.1016/j.clineuro.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Stiasny-Kolster K, Moller JC, Zschocke J, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Disord. 2004;19:192–196. doi: 10.1002/mds.10631. doi: 10.1002/mds.10631. [DOI] [PubMed] [Google Scholar]
- 57.Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–128. doi: 10.1016/j.sleep.2007.11.012. doi: 10.1016/j.sleep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cervenka S, Palhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129((Pt 8)):2017–2028. doi: 10.1093/brain/awl163. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 59.Earley CJ, Kuwabara H, Wong DF, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–57. doi: 10.5665/sleep.2300. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Earley CJ, Kuwabara H, Wong DF, et al. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep. 2011;34:341–347. doi: 10.1093/sleep/34.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–213. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 62.Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep. 1998;21:371–377. [PubMed] [Google Scholar]
- 63.Kryger MH, Otake K, Foerster J. Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002;3:127–132. doi: 10.1016/s1389-9457(01)00160-5. doi: 10.1016/S1389-9457(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, O’Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: a randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–975. doi: 10.1016/j.sleep.2008.11.003. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–235. doi: 10.1016/j.sleep.2004.03.002. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–1700. doi: 10.1212/wnl.54.8.1698. doi: 10.1212/WNL.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 67.Burdo JR, Menzies SL, Simpson IA, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 68.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 69.Walters AS. Review of receptor agonist and antagonist studies relevant to the opiate system in restless legs syndrome. Sleep Med. 2002;3:301–304. doi: 10.1016/s1389-9457(02)00011-4. doi: 10.1016/S1389-9457(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 70.Walters AS, Wagner ML, Hening WA, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327–332. doi: 10.1093/sleep/16.4.327. [DOI] [PubMed] [Google Scholar]
- 71.Ondo WG. Methadone for refractory restless legs syndrome. Mov Disord. 2005;20:345–348. doi: 10.1002/mds.20359. doi: 10.1002/mds.20359. [DOI] [PubMed] [Google Scholar]
- 72.Deandrade M, Unger E, Zhang L, Yokoi F, Walters A, Li Y. Hyperactivity and alterations in iron homeostasis in mu opioid receptor knockout mice: possible implications for restless legs syndrome/Willis-Ekbom disease. World Association of Sleep Medicine Congress 2013, Valencia, Spain, Oct 1, 2013.
- 73.Sun YM, Hoang T, Neubauer JA, Walters AS. Opioids protect against substantia nigra cell degeneration under conditions of iron deprivation: a mechanism of possible relevance to the Restless Legs Syndrome (RLS) and Parkinson’s disease. J Neurol Sci. 2011;304:93–101. doi: 10.1016/j.jns.2011.02.003. doi: 10.1016/j.jns.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Coulon P, Budde T, Pape HC. The sleep relay – the role of the thalamus in central and decentral sleep regulation. Pflugers Arch. 2012;463:53–71. doi: 10.1007/s00424-011-1014-6. doi: 10.1007/s00424-011-1014-6. [DOI] [PubMed] [Google Scholar]
- 75.Saletu M, Anderer P, Saletu-Zyhlarz GM, et al. Comparative placebo-controlled polysomnographic and psychometric studies on the acute effects of gabapentin versus ropinirole in restless legs syndrome. J Neural Transm (Vienna) 2010;117:463–473. doi: 10.1007/s00702-009-0361-3. doi: 10.1007/s00702-009-0361-3. [DOI] [PubMed] [Google Scholar]
- 76.Rose MA, Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 77.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–1815. doi: 10.1213/01.ane.0000287643.13410.5e. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 78.Page RL, 2nd, Ruscin JM, Bainbridge JL, Brieke AA. Restless legs syndrome induced by escitalopram: case report and review of the literature. Pharmacotherapy. 2008;28:271–280. doi: 10.1592/phco.28.2.271. doi: 10.1592/phco.28.2.271. [DOI] [PubMed] [Google Scholar]
- 79.Rottach KG, Schaner BM, Kirch MH, et al. Restless legs syndrome as side effect of second generation antidepressants. J Psychiatr Res. 2008;43:70–75. doi: 10.1016/j.jpsychires.2008.02.006. doi: 10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. doi: 10.1016/S0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 81.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–514. doi: 10.1016/j.biopsych.2005.04.022. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease) Neurology. 2014;82:1856–1861. doi: 10.1212/WNL.0000000000000454. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 83.Patton SM, Ponnuru P, Snyder AM, Podskalny GD, Connor JR. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol. 2011;18:1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 84.Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem. 2003;85:483–491. doi: 10.1046/j.1471-4159.2003.01696.x. doi: 10.1046/j.1471-4159.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- 85.Bachmann CG, Rolke R, Scheidt U, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133(Pt 3):762–770. doi: 10.1093/brain/awq026. doi: 10.1093/brain/awq026. [DOI] [PubMed] [Google Scholar]
- 86.Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127((Pt 4)):773–782. doi: 10.1093/brain/awh079. doi: 10.1093/brain/awh079. [DOI] [PubMed] [Google Scholar]
- 87.Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251:977–982. doi: 10.1007/s00415-004-0475-3. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- 88.Lim YM, Chang SE, Chung S, Kang BH, Kim KK. Small fiber function in drug naive patients with idiopathic restless legs syndrome. J Clin Neurosci. 2012;19:702–705. doi: 10.1016/j.jocn.2011.07.043. doi: 10.1016/j.jocn.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 89.Tyvaert L, Laureau E, Hurtevent JP, Hurtevent JF, Derambure P, Monaca C. A-delta and C-fibres function in primary restless legs syndrome. Neurophysiol Clin. 2009;39:267–274. doi: 10.1016/j.neucli.2009.06.003. doi: 10.1016/j.neucli.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Isak B, Uluc K, Salcini C, Agan K, Tanridag T, Us O. A neurophysiological approach to the complex organisation of the spine: f-wave duration and the cutaneous silent period in restless legs syndrome. Clin Neurophysiol. 2011;122:383–390. doi: 10.1016/j.clinph.2010.07.005. doi: 10.1016/j.clinph.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Polydefkis M, Allen RP, Hauer P, Earley CJ, Griffin JW, McArthur JC. Subclinical sensory neuropathy in late-onset restless legs syndrome. Neurology. 2000;55:1115–1121. doi: 10.1212/wnl.55.8.1115. doi: 10.1212/WNL.55.8.1115. [DOI] [PubMed] [Google Scholar]
- 92.Bertisch SM, Muresan C, Schoerning L, Winkelman JW, Taylor JA. Impact of restless legs syndrome on cardiovascular autonomic control. Sleep. 2016;39:565–571. doi: 10.5665/sleep.5528. doi: 10.5665/sleep.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–1616. doi: 10.1212/wnl.54.8.1609. doi: 10.1212/WNL.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 94.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 95.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. doi: 10.1016/j.clinph.2007.05.006. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Izzi F, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 2014;15:1392–1397. doi: 10.1016/j.sleep.2014.06.022. doi: 10.1016/j.sleep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 97.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261:1051–1068. doi: 10.1007/s00415-013-7065-1. doi: 10.1007/s00415-013-7065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desai AV, Cherkas LF, Spector TD, Williams AJ. Genetic influences in self-reported symptoms of obstructive sleep apnoea and restless legs: a twin study. Twin Res. 2004;7:589–595. doi: 10.1375/1369052042663841. doi: 10.1375/1369052042663841. [DOI] [PubMed] [Google Scholar]
- 99.Ondo WG, Vuong KD, Wang Q. Restless legs syndrome in monozygotic twins: clinical correlates. Neurology. 2000;55:1404–1406. doi: 10.1212/wnl.55.9.1404. doi: 10.1212/WNL.55.9.1404. [DOI] [PubMed] [Google Scholar]
- 100.Pichler I, Hicks AA, Pramstaller PP. Restless legs syndrome: an update on genetics and future perspectives. Clin Genet. 2008;73:297–305. doi: 10.1111/j.1399-0004.2007.00937.x. doi: 10.1111/j.1399-0004.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 101.Winkelmann J. Genetics of restless legs syndrome. Curr Neurol Neurosci Rep. 2008;8:211–216. doi: 10.1007/s11910-008-0033-y. doi: 10.1007/s11910-008-0033-y. [DOI] [PubMed] [Google Scholar]
- 102.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357((7)):639–647. doi: 10.1056/NEJMoa072743. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 103.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–1006. doi: 10.1038/ng2099. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 104.Schormair B, Kemlink D, Roeske D, et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 2008;40:946–948. doi: 10.1038/ng.190. doi: 10.1038/ng.190. [DOI] [PubMed] [Google Scholar]
- 105.Yang Q, Li L, Chen Q, Foldvary-Schaefer N, Ondo WG, Wang QK. Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med. 2011;12:800–804. doi: 10.1016/j.sleep.2011.06.006. doi: 10.1016/j.sleep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore H, 4th, Winkelmann J, Lin L, Finn L, Peppard P, Mignot E. Periodic leg movements during sleep are associated with polymorphisms in BTBD9, TOX3/BC034767, MEIS1, MAP2K5/SKOR1, and PTPRD. Sleep. 2014;37:1535–1542. doi: 10.5665/sleep.4006. doi: 10.5665/sleep.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freeman A, Pranski E, Miller RD, et al. Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Curr Biol. 2012;22:1142–1148. doi: 10.1016/j.cub.2012.04.027. doi: 10.1016/j.cub.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeAndrade MP, Johnson RL, Jr, Unger EL, et al. Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Hum Mol Genet. 2012;21:3984–3992. doi: 10.1093/hmg/dds221. doi: 10.1093/hmg/dds221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26:717–720. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- 110.Boivin DB, Lorrain D, Montplaisir J. Effects of bromocriptine on periodic limb movements in human narcolepsy. Neurology. 1993;43:2134–2136. doi: 10.1212/wnl.43.10.2134. doi: 10.1212/WNL.43.10.2134. [DOI] [PubMed] [Google Scholar]
- 111.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–1231. doi: 10.1161/CIRCULATIONAHA.111.038968. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”) Sleep Med. 2009;10:976–981. doi: 10.1016/j.sleep.2008.09.015. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kemlink D, Polo O, Frauscher B, et al. Replication of restless legs syndrome loci in three European populations. J Med Genet. 2009;46:315–318. doi: 10.1136/jmg.2008.062992. doi: 10.1136/jmg.2008.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim MK, Cho YW, Shin WC, et al. Association of restless legs syndrome variants in Korean patients with restless legs syndrome. Sleep. 2013;36:1787–1791. doi: 10.5665/sleep.3200. doi: 10.5665/sleep.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Q, Li L, Yang R, et al. Family-based and population-based association studies validate PTPRD as a risk factor for restless legs syndrome. Mov Disord. 2011;26:516–519. doi: 10.1002/mds.23459. doi: 10.1002/mds.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winkelmann J, Czamara D, Schormair B, et al. Genome-wide association study identifies novel restless legs syndrome susceptibility loci on 2p14 and 16q12.1. PLoS Genet. 2011;7:e1002171. doi: 10.1371/journal.pgen.1002171. doi: 10.1371/journal.pgen.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rataj-Baniowska M, Niewiadomska-Cimicka A, Paschaki M, et al. Retinoic acid receptor beta controls development of striatonigral projection neurons through FGF-dependent and Meis1-dependent mechanisms. J Neurosci. 2015;35:14467–14475. doi: 10.1523/JNEUROSCI.1278-15.2015. doi: 10.1523/JNEUROSCI.1278-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Catoire H, Dion PA, Xiong L, et al. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol. 2011;70:170–175. doi: 10.1002/ana.22435. doi: 10.1002/ana.22435. [DOI] [PubMed] [Google Scholar]
- 119.Spieler D, Kaffe M, Knauf F, et al. Restless legs syndrome-associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 2014;24:592–603. doi: 10.1101/gr.166751.113. doi: 10.1101/gr.166751.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–624. doi: 10.1016/0091-3057(94)90323-9. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 121.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 122.Lim J, Kim HI, Bang Y, Seol W, Choi HS, Choi HJ. Hypoxia-inducible factor-1alpha upregulates tyrosine hydroxylase and dopamine transporter by nuclear receptor ERRgamma in SH-SY5Y cells. Neuroreport. 2015;26:380–386. doi: 10.1097/WNR.0000000000000356. doi: 10.1097/WNR.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 123.Wahlin-Larsson B, Ulfberg J, Aulin KP, Kadi F. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve. 2009;40:556–561. doi: 10.1002/mus.21357. doi: 10.1002/mus.21357. [DOI] [PubMed] [Google Scholar]
- 124.Wahlin Larsson B, Kadi F, Ulfberg J, Piehl Aulin K. Skeletal muscle morphology and aerobic capacity in patients with obstructive sleep apnoea syndrome. Respiration. 2008;76:21–27. doi: 10.1159/000126492. doi: 10.1159/000126492. [DOI] [PubMed] [Google Scholar]
- 125.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–2019. doi: 10.1182/blood-2009-05-189985. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]