Abstract
Without any root contact with the soil, epiphytic bryophytes must experience and explore poor, patchy, and heterogeneous habitats; while, the nitrogen (N) uptake and use strategies of these organisms remain uncharacterized, which obscures their roles in the N cycle. To investigate the N sources, N preferences, and responses to enhanced N deposition in epiphytic bryophytes, we carried out an in situ manipulation experiment via the 15N labelling technique in an Asian cloud forest. Epiphytic bryophytes obtained more N from air deposition than from the bark, but the contribution of N from the bark was non-negligible. Glycine accounted for 28.4% to 44.5% of the total N in bryophyte tissue, which implies that organic N might serve as an important N source. Increased N deposition increased the total N uptake, but did not alter the N preference of the epiphytic bryophytes. This study provides sound evidence that epiphytic bryophytes could take up N from the bark and wet deposition in both organic and inorganic N forms. It is thus important to consider organic N and bark N sources, which were usually neglected, when estimating the role of epiphytic bryophytes in N cycling and the impacts of N deposition on epiphytic bryophytes in cloud forests.
Bryophytes are the earliest land plants1,2,3, they have experienced nearly 450 million years of evolution, and they lack the supracellular transport systems of vascular plants4,5. In total, over 20,000 bryophyte species have been recorded worldwide, making them the second most diverse group of plants6. Bryophytes occur in many ecosystems, from low to high latitudes and altitudes, and generally dominate montane, boreal, and arctic ecosystems where they can strongly influence the carbon and nitrogen (N) cycles7,8,9. Unlike vascular plants, bryophytes lack a cuticle barrier and there is the existence of large cationic exchange properties within the cell walls. They can therefore take up water and nutrients over the entire plant surface9,10. Thus, these organisms often serve as effective traps for environmental nutrients, such as N, which makes them very sensitive to atmospheric N deposition, and in recent years they have been proposed to be good bio-indicators for N pollution9,11,12. Bryophytes can also regulate the ecosystem N dynamic through biological N2 fixation by forming facultative symbioses with diazotrophs, such as Nostoc spp.7,13,14. However, the details of the N preferences and the N sources of bryophytes have been less of a concern, and they remain uncharacterized in natural ecosystems15, which prevents a proper evaluation of their roles in the N cycle and a reasonable prediction of their fates in a changing world.
Most higher plants primarily derive N from the soil16. In contrast, bryophytes lack roots and developed vascular systems, which is thought to limit their access to available nutrients from substrata and affect N transport to the shoots. Previous studies have suggested that bryophytes obtain most of their nutrients from the atmospheric deposition and throughfall17,18 and atmospheric N2 fixation through epiphytic cyanobacteria7,19; however, this is under debate because recent evidence suggests that bryophytes may use a certain amount of N from their substrata15,20,21. For example, Ayres, et al.21 reported that some bryophyte species can obtain N directly from the soil. In addition to terrestrial species, many bryophytes grow on other plants as epiphytes in the canopy habitats in montane and subalpine forests8,22,23. The canopy habitats are usually considered to be harsh, with a variable and sporadic nutrient input, a limited storage capacity for available water and nutrients, and low physical stability, etc.24,25. Accordingly, epiphytic bryophytes must experience and explore poor, patchy, and heterogeneous environments. It remains unknown where and how these organisms obtain nutrients without root contact with the soil.
The N preferences of epiphytic bryophytes remain uncharacterized. The preferences for ammonium (NH4+) or amino acids over nitrate (NO3−) have been observed in vascular plants when different N forms are supplied in equal doses26,27. Some researchers have suggested that bryophytes may not have a preference in N uptake because nutrients can enter moss tissues easily through cation exchange and the proton (H+) pump (e.g., NH4+ and amino acids) and through cotransport (e.g., NO3−) for positively charged ions10,15,28. Other researchers have suggested that the uptake of NH4+ should be higher than that of NO3−15 29. Current knowledge of N cycling in bryophytes and the effects of N deposition on bryophytes largely relates to inorganic N30,31,32. The ability of bryophytes, especially the epiphytic ones, to use organic N as a N source and the ecological significance of this has been largely neglected in past studies, although the preference of amino acids has been reported in several terrestrial bryophytes33,34.
Anthropogenic N deposition has been increasing globally since the 19th century, which has triggered major changes in the dynamics of carbon (C) and N, as well as floral diversity in terrestrial ecosystems35,36,37. Increasing concern has been focused on the effects of enhanced N deposition on bryophytes, which were suggested to be sensitive to N pollution11,18,38. A previous study indicated that species richness and the cover of the epiphytic bryophyte community has significantly decreased because of increased N input. The growth and vitality of the investigated species have declined in locations with high N loads11. However, we still do not know the potential impacts of increased N deposition on the total N uptake and the N preference of epiphytic bryophytes. For example, do bryophytes absorb more N in response to increased N deposition, as has been suggested in some studies39,40? Do bryophytes shift their N preference to increased N deposition when N is much more easily obtained? This information may provide possible explanations for the detrimental effects of the high N loads mentioned above.
Due to their particular biological nature (no cuticle barrier, lacking roots and developed vascular systems, and growing on bark, etc.) and special habitats (poor, patchy, and heterogeneous in N supply), epiphytic bryophytes are likely to have different N uptake and use strategies under natural conditions and under increasing atmospheric N deposition. Epiphytic bryophytes usually dominate the tree trunks and branches in montane forests on moist, undisturbed sites8,41. For example, the subtropical montane cloud forest located in the Ailao Mountain National Nature Reserve of Southwest China, which is generally characterized by persistent and frequent cloud cover at the canopy level, is especially rich in epiphytic bryophytes42. In total, 176 epiphytic bryophyte species have been recorded (accounting for ~30% of the total epiphytes in the study region)41 and the total biomass of the epiphytic bryophytes is 6.7 tons per hectare (accounting for ~63% of the total epiphytes in the study region)41,43. A multifactor in situ manipulation experiment was carried out via the 15N labelling technique in three coexisting and common epiphytic bryophyte species in the subtropical cloud forest. The main objectives were to: 1) Determine whether epiphytic bryophytes can take up N from tree bark or bark surface; 2) Confirm the capacity of epiphytic bryophytes to absorb organic N and quantify its amount in the total N economy of the bryophytes; 3) Address the potential impacts of increased N deposition on the N uptake dynamics of epiphytic bryophytes.
Results and Discussion
Direct uptake of N from bark
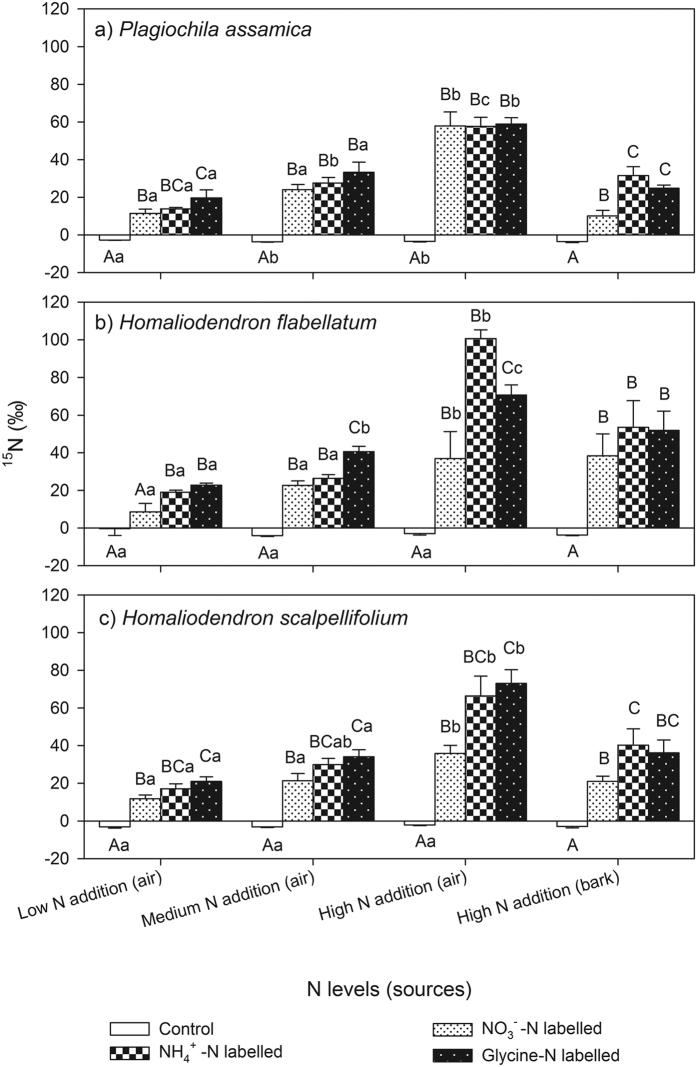
Using a 15N labelling approach, we confirmed that epiphytic bryophytes indeed relied more on N from the air than from the bark of their hosts, but the contribution of the N from the bark should not be neglected (Tables 1 and 2; Fig. 1). Cryptogams, such as bryophytes, which can absorb water directly through their surfaces, have been traditionally suggested to be largely independent of their substrate and were thought to absorb N mostly from precipitations and biological N fixation19,44 as well as the relocation of nutrients from dead moss tissue45. It is surprising that epiphytic bryophytes could use the bark N because bryophytes possess rhizoids rather than roots, which reach only a few cm into the bark surface. However, the recovery of the label in the bryophyte tissue (Fig. S1) does not necessarily reflect active uptake by the bryophyte, since the added N could have reached the bryophyte via passive diffusion along the bryophyte shoots. Endophytic fungi could have retained the added label and get some amino N mineralization and uptake as NH4+. It is also possible that not all bacteria have been killed by the ampicillin once it was injected into the bark. Nevertheless, the 15N enrichment in the bryophyte shoots suggest that the epiphytic bryophytes can acquire N from the bark and translocate it to their shoots via various pathways, e.g. passive diffusion, endophytic fungi, bacteria. Therefore, this still need further investigations in the future.
Table 1. Results from an ANOVA analysis evaluating the effect of species, nitrogen (N) sources, N forms, N levels, and their interactive effects on N uptake rates during treatment periods. Italics indicates p < 0.05.
| DF | MS | F-value | p-value | |
|---|---|---|---|---|
| Species (S) | 2 | 134.85 | 5.676 | 0.006 |
| N sources (Ns) | 1 | 1648.107 | 69.371 | <0.001 |
| N forms (Nf) | 2 | 463.654 | 19.516 | <0.001 |
| S × Ns | 2 | 72.625 | 3.057 | 0.055 |
| S × Nf | 4 | 29.531 | 1.243 | 0.304 |
| Ns × Nf | 2 | 19.09 | 0.804 | 0.453 |
| S × Ns × Nf | 4 | 56.872 | 2.394 | 0.062 |
| Species (S) | 2 | 0.262 | 2.309 | 0.106 |
| N levels (Nl) | 2 | 42.254 | 371.933 | <0.001 |
| N forms (Nf) | 2 | 4.805 | 42.294 | <0.001 |
| S × Nl | 4 | 0.137 | 1.209 | 0.314 |
| S × Nf | 4 | 0.274 | 2.41 | 0.056 |
| Nl × Nf | 4 | 0.633 | 5.574 | 0.001 |
| S × Nl × Nf | 8 | 0.411 | 3.622 | 0.001 |
DF refers to degree of freedom and MS refers to mean square.
Table 2. Percentages of nitrogen (N) absorbed in forms of NO3 −, NH4 +, and glycine by three bryophyte species under air deposition and bark injection at low, medium, and high N addition levels.
| Species | Low N addition (air) | Medium N addition (air) | High N addition (air) | High N addition (bark) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO3− | NH4+ | Glycine | NO3− | NH4+ | Glycine | NO3− | NH4+ | Glycine | NO3− | NH4+ | Glycine | |
| Plagiochila assamica | 25.3 ± 3.3 | 36.5 ± 0.6 | 38.2 ± 3.0 | 26.6 ± 5.9 | 41.3 ± 3.9 | 32.1 ± 2.4 | 23.1 ± 2.2 | 43.7 ± 4.8 | 33.2 ± 3.2 | 21.1 ± 5.0 | 40.3 ± 4.6 | 38.6 ± 6.4 |
| Aa | Ba | Ba | Aa | Ba | ABa | Aa | Ba | ABa | A | B | B | |
| Homaliodendronflabellatum | 21.0 ± 2.7 | 39.7 ± 2.9 | 39.3 ± 5.4 | 27.7 ± 4.6 | 27.7 ± 1.7 | 44.5 ± 3.6 | 21.2 ± 4.3 | 50.4 ± 5.0 | 28.4 ± 4.1 | 27.7 ± 6.9 | 40.0 ± 8.0 | 32.3 ± 5.1 |
| Aa | Bab | Bab | Aa | Ab | Ba | Aa | Ba | Ab | A | A | A | |
| Homaliodendronscalpellifolium | 23.1 ± 3.2 | 40.6 ± 3.1 | 36.3 ± 4.3 | 23.3 ± 3.1 | 39.1 ± 5.5 | 37.5 ± 6.4 | 20.0 ± 3.2 | 31.5 ± 4.4 | 48.5 ± 7.5 | 20.5 ± 2.5 | 41.4 ± 6.0 | 38.1 ± 3.6 |
| Aa | Ba | Ba | Aa | Aa | Aa | Aa | ABa | Ba | A | B | B | |
Means ± SE are presented (n = 4). Capital letters after values indicate significant differences at 0.05 error probability levels among different labelled-N forms under same N levels, while lowercase letters indicate significant differences among different N levels under same labelled-N forms.
Figure 1. Abundance of nitrogen (N) isotope signatures (δ15N) in three epiphytic bryophyte species.
Capital letters after values indicate significant differences at 0.05 error probability levels among different labelled-N forms under same N levels, while lowercase letters indicate significant differences among different N levels under same labelled-N forms.
During the injection procedure, we observed a small amount of leakage of the solution that had been injected into the bark. This was probably due to the limited water-holding capacity of the bark during the injection process. A spot of the leaked solution may flow directly onto the bryophyte shoots, and thus may have been taken up at that point, which obscure the interpretation of our results. Nevertheless, the proportion of 15N recovered in the trunk-dwelling bryophyte P. assamica after 24 h incubation was 3.2% for NO3−, 6.4% for NH4+, and 5.4% for glycine, respectively (Fig. S1). Similar results were found in the other two species, H. flabellatum and H. scalpelifolium (Fig. S1). Although lower than the air deposition counterparts (Fig. S1), the proportion of 15N recovered in the three bryophyte species through bark injection was comparable with previously reported data on the recovery from soil by the Antarctic moss Sanionia uncinata (2% for alanine and 4% for NH4+)46. Thus, the fact that a small amount of liquid leaked out of the bark may not significantly impact the conclusion that epiphytic bryophytes can take up N from bark.
Yet it has been demonstrated that bryophytes can take up N from bark when it is added, but is there actually a significant amount of available N in bark for bryophytes to take up? The average total N concentration in barks of the three dominant host species, i.e. Lithocarpus xylocarpus, L. hancei, and Castanopsis wattii are 7.85 ± 0.61, 8.92 ± 1.33, 8.52 ± 0.49 g kg−1, respectively, which are higher than the average total N concentration in the surface soil (0–20 cm: 6.53 ± 0.83 g kg−1) (Song et al. unpublished data). Mean concentrations of total N, NH4+–N, and NO3−–N in the stemflow in the study region are 2.39 ± 1.11, 1.14 ± 0.50, and 0.42 ± 0.21 mg l−1, respectively, which are significantly higher than that in the precipitation (0.49 ± 0.14, 0.11 ± 0.05, 0.04 ± 0.01 mg l−1)47. The above data indicate that there is actually a significant amount of available N in bark for epiphytic bryophytes to take up, which is comparable to N availability from atmospheric deposition and other substrates in the subtropical cloud forest.
Field investigations have indicated that bryophytes are the dominant epiphytic plants in this ecosystem, as they account for approximately 63% of the total biomass, which is more than any other epiphytic vegetation type (orchids, ferns, and lichens, etc.)41. This indicates that epiphytic bryophytes may be significant competitors for N in the stemflow and throughfall, which could have potential consequences for the plant community structure and nutrient cycling at the ecosystem level47,48. Since all three bryophyte species included in this study have the capacity to absorb the available N from the bark, according to the δ15N signals of the shoots (Fig. 1), this uptake may be common among epiphytic bryophyte species in general. Increasing evidence indicates that both the substratum and the atmosphere are important mineral sources for bryophytes, even in ‘feather mosses’, which have poor soil-moss contact46,49. Recently, Liu, et al.15 estimated that soil N accounted for approximately 40% of the total N in terrestrial bryophytes. If N absorption from bark is common among epiphytic bryophytes, this could partially explain their widespread distribution and importance in many montane and moist ecosystems. If this is the case, the N cycle in the studied forest ecosystem should be modified.
Organic N as important component of N input
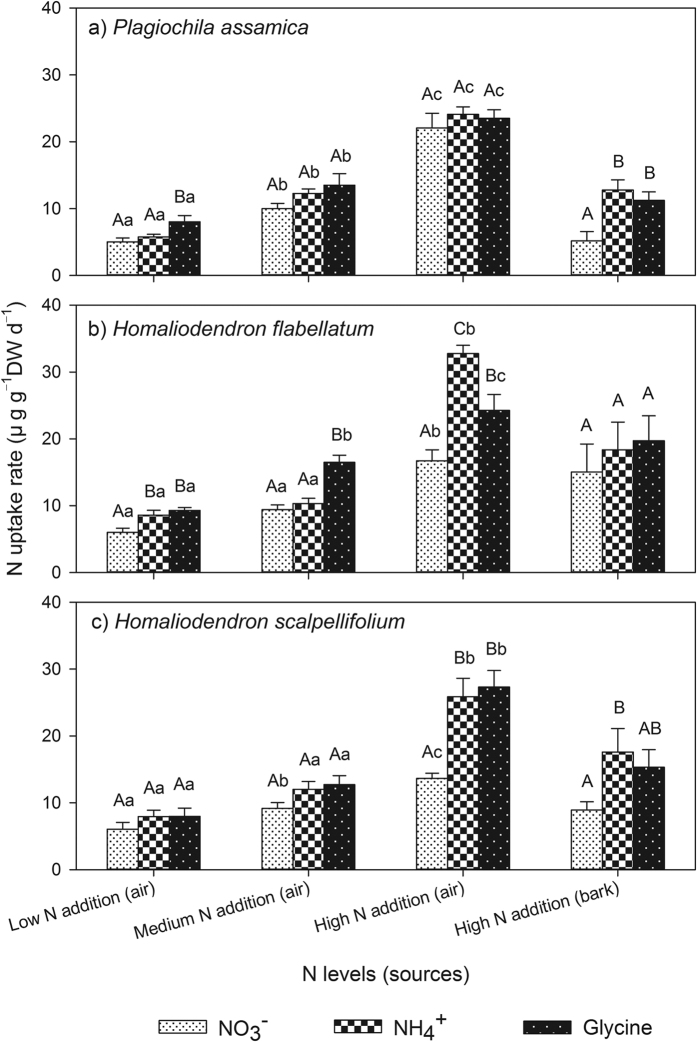
Bryophytes have the ability to absorb organic N besides mineralized, inorganic N (NH4+ and NO3−). In this study, the contribution of organic N (glycine) ranged between 28.4% and 44.5% of the total N uptake, which was comparable with NH4+, but significantly higher than NO3− (Tables 1 and 2; Fig. 2). This was probably due to the greater cation-exchange capacity than the anion-exchange capacity of the cell walls29,50. No significant differences were detected for the acquisition of different N forms between air deposition and bark injection for all three epiphytic bryophyte species (Table 2), which demonstrated that these organisms do not shift their N preference from air to bark N sources. Our study indicated that amino acids, a small but important organic N component, as well as NH4+–N and NO3−–N can serve as important N sources for epiphytic bryophytes. Previous laboratory and field studies have also revealed that amino acids can be absorbed and utilized at substantial rates, which greatly contribute to the total N uptake and effects the N metabolism of bryophytes15,34,51,52; however, this has not been studied in epiphytic bryophyte species. For example, Forsum, et al.33 applied 15N labelled solutions to Hylocomium splendens (Hedw.) and found that this species preferred amino acid N over NO3−, although the assimilation of glycine remained lower than that of NH4+. The mean uptake rates were 1.8 μmol g−1 DW h−1 for NO3−, 3.6 μmol g−1 DW h−1 for NH4+, and 3.4 μmol g−1 DW h−1 for glycine, which indicated that the amino acids could be absorbed by the bryophytes50. The preference for amino acid N or NH4+ over NO3− observed could be partially explained by their differences in assimilation costs. According to Liu, et al.15, and references therein, the assimilation cost of amino acids is expected to be lower than that of NH4+ and much lower than that of NO3−, which is due to the requirement that NH4+ must be attached to a C skeleton before use while NO3− requires additional reduction steps to NH4+. These studies demonstrated that amino acids should be one of the most cost-effective N forms that can be utilized by bryophytes. Considering that amino acids account for only a small proportion of organic N, the bioavailable fraction of organic N is expected to be much larger than that found in amino acids53. Although the organic N input in the studied forest has never been directly measured, the fact that the annual input of total N through precipitation (10.5 kg N ha−1 y−1) was ca. threefold of the sum of the two main inorganic-N forms (NO3−: 0.91 kg N ha−1 y−1, NH4+: 2.69 kg N ha−1 y−1)47, implies that organic N may be an important N form in the studied region. The contribution of organic N to the N economy of epiphytic bryophytes might have been seriously underestimated in the past, although uncertainty exists considering some organic N, e.g. amino acids may have been mineralized and been took up as NH4+.
Figure 2. Comparisons of nitrogen (N) uptake rates by three bryophyte species from NO3−, NH4+, and glycine under air deposition and bark injection at low, medium, and high N addition levels.
Capital letters after values indicate significant differences at 0.05 error probability levels among different labelled-N forms under same N levels, while lowercase letters indicate significant differences among different N levels under same labelled-N forms.
Impact of enhanced N deposition on N uptake
In this study, the shoot 15N concentration and the N uptake rates increased significantly with increasing N concentrations (Table 1; Figs 1 and 2), but the N preferences of the three bryophyte species shifted only slightly in response to the addition of N, except for Homaliodendron flabellatum that preferred glycine under medium N addition, but shifted to NH4+ under high N addition (Table 2). This implies that increased N deposition increases the total amount of N absorbed by the epiphytic bryophytes, but it does not alter the N preference over a short time. Due to N limitations in many ecosystems dominated by bryophytes, a slight increase in N can increase the absorption of N, which enhances the chlorophyll content of the bryophyte cells, thus increasing the photosynthetic capacity54,55. However, excessive N supply is detrimental to these sensitive organisms. For example, it has been demonstrated that the oversupply of N can result in an excess uptake of NH4+ in the cell, which threatens the cell homeostasis and causes toxicity, and thus a subsequent reduction in the growth of the bryophytes55,56. Increased N deposition can alternatively cause increased amino acid accumulation in bryophyte tissues, which may deplete reserves of soluble carbohydrates necessary for growth54. As indicated in this study, high N loads resulted in excessive N uptake, which may induce biochemical disorders in bryophytes57.
In conclusion, this study provides clear evidence that epiphytic bryophytes can uptake N from the bark and can translocate it to their shoots. The ability to translocate the absorbed N to their shoots is of particular importance, since shoots typically have greater N demands for photosynthetic enzymes. This study highlighted that organic N, as opposed to inorganic N, contributed remarkably to the N economy of the epiphytic bryophytes. High N loads may result in excessive N uptake, which may induce biochemical disorders in bryophytes. Thus, it is important to consider organic N and bark N sources when estimating the role of epiphytic bryophytes in N cycling and the impacts of N deposition on epiphytic bryophytes in cloud forests.
Methods
Study site
We conducted this study in the Xujiaba region of Yunnan Province (24° 32′ N, 101° 01′ E), China, in a protected section of a 5,100 ha pristine subtropical cloud forest in the Ailao Mountain National Nature Reserve (23° 35′-24° 44′ N, 100° 54′-101° 01′ E), with an altitude range between 2000 m and 2600 m58. The mean annual temperature is 11.6 °C, with the lowest value in December (6.0 °C) and the highest in July (15.8 °C). The mean annual rainfall is 1859 mm, with 86% of the rain falling during the rainy season (May to October), and a pronounced dry period from December to April42. The forest is primarily co-dominated by Lithocarpus hancei (Benth.) Rehder, Castanopsis rufescens (Hook.f.et Th.) Huang et Y.T. Chang, and Lithocarpus xylocarpus (Kurz) Markgr58. Annual input of total N through precipitation and throughfall were 10.5 and 12.1 kg N ha−1 y−1 47 in the study region, with expectations of increased reactive N deposition with time59.
On account of the persistent, frequent cloud/fog cover, the presence of large, old trees, and long-term effective protection, the forest harbors abundant epiphytes, and is especially rich in epiphytic bryophytes41,42. The most dominant epiphytes growing on tree trunks including Homaliodendron flabellatum (Sm.) Fleisch., Plagiochila arbuscula (Brid. ex Lehm.) Lindenb., H. scalpelifolium (Mitt.) Fleisch., and P. assamica Steph. are bryophyte species41. Epiphytes comprise one of the most diverse and are a conspicuous element of the subtropical cloud forest, and they are also extremely important in carbon, water and nutrient cycling in these ecosystems47,48. For example, epiphytes in the subtropical cloud forest can fix a significant amount of N2; the latest estimated annual N input fixed by epiphytic bryophytes reaches 3.89 kg N ha−1 y−1 60,61.
Experimental design and treatments
Two mosses (H. flabellatum and H. scalpellifolium) belonging to Neckeraceae and one liverworts (P. assamica) belonging to Plagiochilaceae were selected for this manipulation experiment as they were abundant, representative trunk-dwelling species in the study region41,42. In November 2014, four areas (two hectares each) in the cloud forest in Xujiaba were chosen as the experimental plots. In each plot, 48 square quadrats (20 cm × 20 cm) that were dominated by each single species (16 quadrats for each species) were marked on large trunks (Diameter at breast height >20 cm) that were located between 1.0 m–2.0 m above the forest floor. The average total biomass of H. flabellatum, H. scalpellifolium, and P. assamica collected from each experimental quadrat were 1.01, 1.13, and 1.46 g, respectively (Fig. S2).
To study the N preference of the three epiphytic bryophytes, four treatment groups were established. In each treatment, equal proportions of glycine, NH4+, and NO3− (1:1:1) were used. Glycine was adopted because it has been widely used as a model amino acid for studies of organic N uptake by plants62. The first treatment was considered to be the control (Control), and no 15N-labelled N was added. In the other three treatments, only one of the N forms was labelled with 15N, i.e., 15N–glycine (20 atom% 15N) mixed with unlabelled (NH4)2SO4 and KNO3 (Glycine–N labelled); (15NH4)2SO4 (20 atom% 15N) mixed with unlabelled glycine and KNO3 (NH4+–N labelled); and K15NO3 (20 atom% 15N) mixed with unlabelled glycine and (NH4)2SO4 (NO3−–N labelled).
The experiment was divided into two parts. First, we aimed to determine if epiphytic bryophytes could absorb the available N directly from the tree bark. For this, a subset of high N solutions (total N concentration of 12 mM: each N form at 4 mM) with the four treatment groups mentioned above (CK, Glycine-N labelled, NH4+–N labelled, NO3−–N labelled) were injected uniformly into the bark of the trees in the 48 marked quadrats (four N forms × three species × four replicates) with 5 mL syringes. Before the injection process, a steel needle (15 cm in length and 3.5 mm in diameter) was used to squeeze through the bryophyte layer and set up injection holes on barks. The trees were then injected at nine injection points (three rows × three columns) each on the bark of all quadrats to approximately a 5 mm depth using special metal frame sheets (20 cm × 20 cm) to ensure uniformity and consistency. The total amount of N injected to each quadrat corresponded to a dose of 0.21 kg N ha−1.
The second experiment was designed to study the treatment effect of increased N addition on the N preference and uptake. Three N levels, i.e., low (total N concentration 3 mM: each N form at 1 mM), medium (6 mM: each N form at 2 mM), and high (12 mM: each N form at 4 mM), of each treatment were added. In this experiment, 5 ml of the experimental solutions with different N concentrations were sprayed uniformly over 144 experimental quadrats (three N levels × four N forms × three species × four replicates) with small pressure sprayers. The total amounts of N added were 0.05, 0.11, and 0.21 kg N ha−1 (equal to 18.25, 40.15, 76.65 kg N ha−1 y−1) for the low, medium, and high N levels, respectively. The above treatment levels in our simulations of N input were implemented based on background rate (10.5 kg N ha−1 y−1) in the study region47 and a predicted rate measured in a comparable region, i.e. southern China: 30–73 kg N ha−1 y−1 63. Spraying was conducted within special metal frame cubes (20 cm × 20 cm × 20 cm) to avoid the loss of the solutions through air movement. Ampicillin (10 mg L−1) and CaCl2 (100 μM) were added into the solutions to avoid the rapid decomposition of the amino glycine. Bryophyte shoots were harvested one day after the injection or spraying events, following the procedures of Krab, et al.51 and Rousk, et al.46. According to the method used by Warren64, all bryophyte shoots were rinsed in 50 mM KCl to remove any remaining 15N that was still adhering to their surface, and then they were rinsed with ultrapure water, oven-dried (70 °C), and ground for 15N isotope analysis. The total biomasses of the three target bryophyte species are shown in Fig. S2. N contents and 15N/14N ratios were determined using an isotope ratio mass spectrometer (Isoprime 100, Isoprime Ltd., Cheadle, UK) coupled with a vario PYRO cube elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) with a continuous flow mode. The atom% excess 15N (APE) was calculated as the atom% 15N difference between the bryophytes from the 15N treated plots and from the control plots.
Data analysis
According to Xu, et al.65, the uptake of the 15N (mg 15N m−2) by the bryophyte shoots was calculated by multiplying the N content (mg N g−1 d.w.), APE, and biomass (g m−2). The uptake of the available N forms corresponding to the 15N treatment was calculated as in the following:
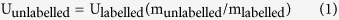 |
where mlabelled is the total mass (g m−2) of the 15N-labelled N injected or sprayed per quadrat and munlabelled is the mass of available N forms measured in solution. Ulabelled is the uptake (g m−2) of 15N from the source mlabelled and Uunlabelled is the uptake of the available N from the source munlabelled.
All data were subjected to normality and homoscedasticity tests before statistical analysis. Two different ANOVA models were adopted: one focused on the N source and the other focused on the N level. To compare N sources, only the high N treatment groups through both air spraying and bark injecting were compared. To compare the treatment effects of the N levels, only the air spraying treatment groups, including low, medium, and high N levels, were considered.
Multiple comparisons of the shoot δ15N, N uptake rates, and contributions of different N forms (percentages of N absorbed in the forms of NO3−, NH4+, and glycine) among the labelled-N forms under the same N addition levels and among different N addition levels under the same labelled-N forms were conducted using LSD’s or Game-Howell’s post hoc tests. All the analyses mentioned above were conducted in SPSS 16.0 (SPSS, Chicago, IL, USA), and all figures were made using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA).
Additional Information
How to cite this article: Song, L. et al. Organic nitrogen uptake is a significant contributor to nitrogen economy of subtropical epiphytic bryophytes. Sci. Rep. 6, 30408; doi: 10.1038/srep30408 (2016).
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31300382, U1133605, 31300459, 31400384, 41471050), the CAS135 Program (No. XTBG-F01), CAS “Light of West China” Program, the Yunnan Natural Science Foundation (2016FB053), and Youth Innovation Promotion Association CAS (No. 2014356). We thank the Ailaoshan Station for Subtropical Forest Ecosystem Studies for granting permission and facilitating this research. We thank the Central Laboratory of Xishuangbanna Tropical Botanical Garden for the assistance in analyzing the isotope abundance. We are also grateful to Prof. Wen-Jie Liu and Prof. Xue-Yan Liu for constructive comments and corrections to the manuscript.
Footnotes
Author Contributions L.S. and H.-Z.L. designed the study; L.S., H.-Z.L., S.Li, X.-M.S., X.C., Y.W., J.-B.H., Q.C., S.Liu and C.-S.W. conducted the study and collected data; L.S., H.-Z.L. and X.-L.X. performed analyses; L.S. wrote the first draft of the manuscript, and L.S., H.-Z.L., X.-L.X., S.Li, X.-M.S. and W.-Y.L. contributed to revisions.
References
- Kenrick P. & Crane P. R. The origin and early evolution of plants on land. Nature 389, 33–39 (1997). [Google Scholar]
- Qiu Y.-L., Cho Y., Cox J. C. & Palmer J. D. The gain of three mitochondrial introns identifies liverworts as the earliest land plants. Nature 394, 671–674 (1998). [DOI] [PubMed] [Google Scholar]
- Gensel P. G. The earliest land plants. Annu. Rev. Ecol. Evol. Syst. 39, 459–477 (2008). [Google Scholar]
- Edwards D., Wellman C. & Axe L. In Bryology for the Twenty-First Century (eds Bates J. W., Ashton N. W. & Duckett J. G.), 15–43 (British Bryological Society (Maney Publishing), 1998). [Google Scholar]
- Proctor M. Mosses and alternative adaptation to life on land. New Phytol. 148, 1–3 (2000). [DOI] [PubMed] [Google Scholar]
- Mishler B. D. The biology of bryophytes—bryophytes aren’t just small tracheophytes. Am. J. Bot. 88, 2129–2131 (2001). [Google Scholar]
- DeLuca T. H., Zackrisson O., Nilsson M.-C. & Sellstedt A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419, 917–920 (2002). [DOI] [PubMed] [Google Scholar]
- Song L. et al. Bole bryophyte diversity and distribution patterns along three altitudinal gradients in Yunnan, China. J. Veg. Sci. 26, 576–587 (2015). [Google Scholar]
- Turetsky M. R. The role of bryophytes in carbon and nitrogen cycling. Bryologist 106, 395–409 (2003). [Google Scholar]
- Glime J. M. Bryophyte ecology, Vol 1. Physiological ecology. Available at: http://www.bryoecol.mtu.edu/. (Assessed: 12th February 2016) (2007).
- Song L., Liu W.-Y., Ma W.-Z. & Qi J.-H. Response of epiphytic bryophytes to simulated N deposition in a subtropical montane cloud forest in southwestern China. Oecologia 170, 847–856 (2012). [DOI] [PubMed] [Google Scholar]
- Harmens H. et al. Nitrogen concentrations in mosses indicate the spatial distribution of atmospheric nitrogen deposition in Europe. Environ. Pollut. 159, 2852–2860 (2011). [DOI] [PubMed] [Google Scholar]
- DeLuca T. H., Zackrisson O., Gundale M. J. & Nilsson M.-C. Ecosystem feedbacks and nitrogen fixation in boreal forests. Science 320, 1181–1181 (2008). [DOI] [PubMed] [Google Scholar]
- Ackermann K., Zackrisson O., Rousk J., Jones D. L. & DeLuca T. H. N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems 15, 986–998 (2012). [Google Scholar]
- Liu X.-Y. et al. Ammonium first: natural mosses prefer atmospheric ammonium but vary utilization of dissolved organic nitrogen depending on habitat and nitrogen deposition. New Phytol. 199, 407–419 (2013). [DOI] [PubMed] [Google Scholar]
- Chapin F. S. I., Matson P. A. & Vitousek P. Principles of Terrestrial Ecosystem Ecology. (Springer Science & Business Media, 2011). [Google Scholar]
- Brown D. H. & Bates J. W. Bryophytes and nutrient cycling. Bot. J. Linn. Soc. 104, 129–147 (1990). [Google Scholar]
- Zechmeister H. G., Dirnb ck T., Hülber K. & Mirtl M. Assessing airborne pollution effects on bryophytes-lessons learned through long-term integrated monitoring in Austria. Environ. Pollut. 147, 696–705 (2007). [DOI] [PubMed] [Google Scholar]
- Lindo Z. & Whiteley J. A. Old trees contribute bio-available nitrogen through canopy bryophytes. Plant Soil 342, 141–148 (2011). [Google Scholar]
- Van Tooren B., Van Dam D. & During H. The relative importance of precipitation and soil as sources of nutrients for Calliergonella cuspidata (Hedw.) Loeske in chalk grassland. Funct. Ecol. 4, 101–107 (1990). [Google Scholar]
- Ayres E., Van der Wal R., Sommerkorn M. & Bardgett R. D. Direct uptake of soil nitrogen by mosses. Biol. Lett. 2, 286–288 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradstein S. & Culmsee H. Bryophyte diversity on tree trunks in montane forests of Central Sulawesi, Indonesia. Trop. Bryol. 31, 95–105 (2010). [Google Scholar]
- Wolf J. H. Diversity patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the northern Andes. Ann. Mo. Bot. Gard. 80, 928–960 (1993). [Google Scholar]
- Cardelús C. L. & Mack M. C. The nutrient status of epiphytes and their host trees along an elevational gradient in Costa Rica. Plant Ecology 207, 25–37 (2010). [Google Scholar]
- Lu H.-Z. et al. Higher clonal integration in the facultative epiphytic fern Selliguea griffithiana growing in the forest canopy compared with the forest understorey. Ann. Bot. 116, 113–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker H. J., Siddiqi M. Y. & Glass A. D. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385, 59–61 (1997). [Google Scholar]
- Wang L. & Macko S. A. Constrained preferences in nitrogen uptake across plant species and environments. Plant, Cell Environ. 34, 525–534 (2011). [DOI] [PubMed] [Google Scholar]
- Raven J., Griffiths H., Smith E. & Vaughn K. In Bryology for the Twenty-First Century (eds Bates J. W., Ashton N. W. & Duckett J. G.), 261–275 (British Bryological Society (Maney Publishing), 1998). [Google Scholar]
- Soares A. & Pearson J. Short-term physiological responses of mosses to atmospheric ammonium and nitrate. Water, Air, Soil Pollut. 93, 225–242 (1997). [Google Scholar]
- Paulissen M. P., Van Der Ven P. J., Dees A. J. & Bobbink R. Differential effects of nitrate and ammonium on three fen bryophyte species in relation to pollutant nitrogen input. New Phytol. 164, 451–458 (2004). [Google Scholar]
- Bragazza L. et al. Nitrogen concentration and δ15N signature of ombrotrophic Sphagnum mosses at different N deposition levels in Europe. Global Change Biol. 11, 106–114 (2005). [Google Scholar]
- Jauhiainen J., Wallén B. & Malmer N. Potential NH4+ and NO3− uptake in seven Sphagnum species. New Phytol. 138, 287–293 (1998). [DOI] [PubMed] [Google Scholar]
- Forsum Å., Dahlman L., NÄSholm T. & Nordin A. Nitrogen utilization by Hylocomium splendens in a boreal forest fertilization experiment. Funct. Ecol. 20, 421–426 (2006). [Google Scholar]
- Kielland K. Role of free amino acids in the nitrogen economy of arctic cryptogams. Ecoscience 4, 75–79 (1997). [Google Scholar]
- Aber J. D., Nadelhoffer K. J., Steudler P. & Melillo J. M. Nitrogen saturation in northern forest ecosystems. Bioscience 39, 378–286 (1989). [Google Scholar]
- Bobbink R. et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30–59 (2010). [DOI] [PubMed] [Google Scholar]
- Townsend A., Braswell B., Holland E. & Penner J. Spatial and temporal patterns in terrestrial carbon storage due to deposition of fossil fuel nitrogen. Ecol. Appl. 6, 806–814 (1996). [Google Scholar]
- Mitchell R. J., Truscot A. M., Leith I. D., Cape J. N. & Van D. A study of the epiphytic communities of Atlantic oak woods along an atmospheric nitrogen deposition gradient. J. Ecol. 93, 482–492 (2005). [Google Scholar]
- Pitcairn C. E. R. et al. Bioindicators of enhanced nitrogen deposition. Environ. Pollut. 126, 353–361 (2003). [DOI] [PubMed] [Google Scholar]
- Solga A. & Frahm J.-P. Nitrogen accumulation by six pleurocarpous moss species and their suitability for monitoring nitrogen deposition. J. Bryol. 28, 46–52 (2006). [Google Scholar]
- Ma W.-Z. The comoposition and biomass of epiphytic materials and their relationships with ecological factors in Xujiaba region from Ailao Mountain, Yunnan PhD thesis thesis, Graduate school of the Chinese Academy of Sciences, (2009).
- Song L. et al. Water relations and gas exchange of fan bryophytes and their adaptations to microhabitats in an Asian subtropical montane cloud forest. J. Plant Res. 128, 573–584 (2015). [DOI] [PubMed] [Google Scholar]
- Xu H.-Q. & Liu W.-Y. Species diversity and distribution of epiphytes in the montane moist evergreen broad-leaved forest in Ailao Mountain, Yunnan. Biodiversity Science 13, 137–147. [In Chinese with English abstract] (2005). [Google Scholar]
- Hietz P., Wanek W., Wania R. & Nadkarni N. M. Nitrogen-15 natural abundance in a montane cloud forest canopy as an indicator of nitrogen cycling and epiphyte nutrition. Oecologia 131, 350–355 (2002). [DOI] [PubMed] [Google Scholar]
- Aldous A. R. Nitrogen translocation in Sphagnum mosses: effects of atmospheric nitrogen deposition. New Phytol. 156, 241–253 (2002). [DOI] [PubMed] [Google Scholar]
- Rousk K., Rousk J., Jones D. L., Zackrisson O. & DeLuca T. H. Feather moss nitrogen acquisition across natural fertility gradients in boreal forests. Soil Biol. Biochem. 61, 86–95 (2013). [Google Scholar]
- Liu W.-Y., Fox J. E. D. & Xu Z.-F. Nutrient fluxes in bulk precipitation, throughfall and stemflow in montane subtropical moist forest on Ailao Mountains in Yunnan, south-west China. J. Trop. Ecol. 18, 527–548 (2002). [Google Scholar]
- Chen L., Liu W.-Y. & Wang G.-S. Estimation of epiphytic biomass and nutrient pools in the subtropical montane cloud forest in the Ailao Mountains, south-western China. Ecol. Res. 25, 315–325 (2010). [Google Scholar]
- Bates J. Mineral nutrient acquisition and retention by bryophytes. J. Bryol. 17, 223–240 (1992). [Google Scholar]
- Wanek W. & Pörtl K. Short-term 15N uptake kinetics and nitrogen nutrition of bryophytes in a lowland rainforest, Costa Rica. Funct. Plant Biol. 35, 51–62 (2008). [DOI] [PubMed] [Google Scholar]
- Krab E. J., Cornelissen J. H., Lang S. I. & van Logtestijn R. S. Amino acid uptake among wide-ranging moss species may contribute to their strong position in higher-latitude ecosystems. Plant Soil 304, 199–208 (2008). [Google Scholar]
- Wiedermann M. M., Gunnarsson U., Ericson L. & Nordin A. Ecophysiological adjustment of two Sphagnum species in response to anthropogenic nitrogen deposition. New Phytol. 181, 208–217 (2009). [DOI] [PubMed] [Google Scholar]
- Neff J. C., Holland E. A., Dentener F. J., McDowell W. H. & Russell K. M. The origin, composition and rates of organic nitrogen deposition: A missing piece of the nitrogen cycle? Biogeochemistry 57, 99–136 (2002). [Google Scholar]
- Baxter R., Emes M. & Lee J. Effects of an experimentally applied increase in ammonium on growth and amino-acid metabolism of Sphagnum cuspidatum Ehrh. ex. Hoffm. from differently polluted areas. New Phytol. 120, 265–274 (1992). [Google Scholar]
- Limpens J. & Berendse F. Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: the role of amino acid nitrogen concentration. Oecologia 135, 339–345 (2003). [DOI] [PubMed] [Google Scholar]
- Pearce I., Woodin S. & Van der Wal R. Physiological and growth responses of the montane bryophyte Racomitrium lanuginosum to atmospheric nitrogen deposition. New Phytol. 160, 145–155 (2003). [DOI] [PubMed] [Google Scholar]
- Nordin A., Strengbom J., Witzell J., Näsholm T. & Ericson L. Nitrogen deposition and the biodiversity of boreal forests: implications for the nitrogen critical load. Ambio 34, 20–24 (2005). [PubMed] [Google Scholar]
- Wu Z.-Y. Research of Forest Ecosystem on Ailao Mountains, Yunnan. (Yunnan Science and Technology Press, 1983). [Google Scholar]
- Liu X.-J. et al. Enhanced nitrogen deposition over China. Nature (2013). [DOI] [PubMed] [Google Scholar]
- Han B. et al. Nitrogen fixation of epiphytic plants enwrapping trees in Ailao Mountain cloud forests, Yunnan, China. Protoplasma 247, 103–110 (2010). [DOI] [PubMed] [Google Scholar]
- Song G.-C. Bilogical nitrogen fixation by canopy epiphytes in montane moist evergreen broad-leave forests and secondary forests in Ailao Mts., Yunnan Master thesis, The University of Chinese Academy of Sciences, (2013).
- Näsholm T., Kielland K. & Ganeteg U. Uptake of organic nitrogen by plants. New Phytol. 182 (2009). [DOI] [PubMed] [Google Scholar]
- Lu X.-K., Mo J.-M., Gilliam F., Zhou G.-Y. & Fang Y.-T. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biol. 16, 2688–2700 (2010). [Google Scholar]
- Warren C. R. Uptake of inorganic and amino acid nitrogen from soil by Eucalyptus regnans and Eucalyptus pauciflora seedlings. Tree Physiol. 29, 401–409 (2009). [DOI] [PubMed] [Google Scholar]
- Xu X.-L. et al. Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 341, 495–504 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.