Abstract
During the past decade, pharmaceutical science has seen rapid growth in interest for nanoscale materials. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are popular research topics recently introduced as nano-scale drug carriers; they have shown numerous merits in drug delivery. Size is the most important index in a nanocarrier affecting its drug delivery efficiency. The influence of preparation conditions and type of lipidic components on the size of SLN and NLC in comparable states seems to be interesting for researchers who investigate these types of carriers. This review highlights the results of SLN and NLC particle size and size distribution comparisons.
Keywords: Solid lipid nanoparticles, SLN, Nanostructured lipid carriers, NLC, Nanoparticle, Drug delivery
Introduction
Improving the pharmacological efficiency of a drug while simultaneously decreasing its toxic side effects are main prerequisites for a perfect drug carrier.1 Colloidal drug carriers are introduced for such an optimal drug delivery.2 However, these investigated delivery systems show their own drawbacks. Frequently reported disadvantages of colloidal carriers such as liposomes, micro and nanoemulsions, nanocapsules, nanosponges, and polymeric nanoparticles include burst drug release in orally-administered drugs due to its rapid degradation by the pH of the stomach or by intestinal enzymes and bile salts, limited physical and chemical stability during storage,3-8 difficulty in large-scale production, residues from organic solvents used in preparation of the carriers, some susceptibilities in polymer toxicity,8-10 and too many more to mention. All of these drawbacks suggest that these colloidal drug delivery systems are not ideal for use as drug carriers.8 Nanolipid carriers have gained much interest in pharmaceutical sciences for several delivery routes, e.g., dermal,11,12 oral,13 parenteral,14 pulmonary,15-18 and ocular.19,20 Solid lipid nanoparticles (SLNs) were introduced in the early 1990s as drug carriers.21 They are prepared from one or blends of two or more solid lipids.22 SLNs merge the superiorities of emulsions, liposomes, and polymeric nanoparticles. The solid matrix can preserve encapsulated drugs against chemical instability and provide controlled drug release patterns (compared to nanoemulsions). SLNs provide stable nanosuspension for a long period of time compared to liposomes. Moreover, SLNs are composed of physiologically well-tolerated and generally recognized as safe (GRAS) excipients, which minimises cytotoxicity and leads to their broad application range (dermal, oral, pulmonary, and intravenous) compared to polymeric nanoparticles.23 The greatest advantage of SLNs is the opportunity for industrial scale fabrication.24-26 To improve the capability of SLNs for drug delivery purposes, second generation lipid nanoparticles (NLC) were developed.27 In NLC, solid lipids are mixed with fluid lipids so that the final particle is solid at room temperature. The differences in SLN and NLC structures are schematically illustrated in Figure 1. Many articles have recently been published regarding the advantages of SLNs and NLCs for drug delivery purposes. Therefore, the comparison of these two carriers in different scopes of drug delivery is interesting and useful for researchers in this field. In a literature review, the authors found no report of such an exciting comparison.
Figure 1.
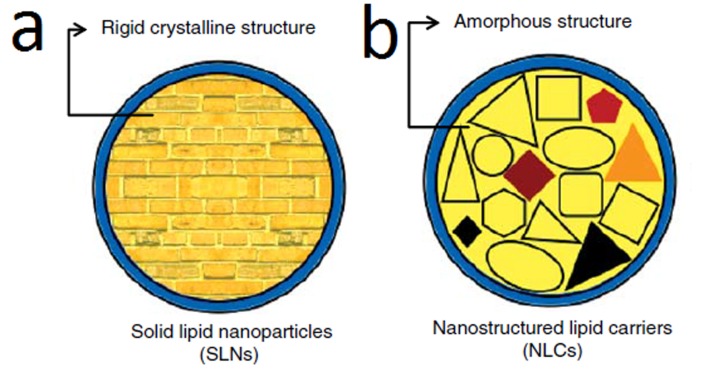
The schematic structure of a) solid lipid nanoparticle b) nanostructured lipid carrier.
Comparison of SLN and NLC particle size and size stability
Particle size and size distribution are two important criteria of nanoparticles as these factors affect drug release rate, bio-distribution, mucoadhesion, cellular uptake of water and buffer exchange to the interior of the nanoparticles, and protein diffusion.28,29 Microspheres with a narrow size distribution are necessary to optimize clinical outcomes.30 The particle size and size distribution are other important indices for evaluating a colloidal dosage form upon storage. The size stability issue is more crucial for nanoparticles than for other drug delivery systems, because nanoparticles have a large specific surface area. The sizes of SLNs were reported as being considerably larger than those of NLCs (297 nm compared to 210 nm) when prepared with the same procedure, surfactant types and concentrations, and total lipid amount.31 Gokce et al. reported that NLCs in all tested oil/lipid ratios (5%, 15%, 30%, and 45%) were smaller than SLNs under identical conditions (the same total lipid concentrations and preparation parameters). The comparison of polydispersity indices for various formulations reported in this study revealed that an increase in the oil/lipid ratio resulted in a decrease in size distribution. Although it seems that there are no statistical differences in polydispersity indices (PDI) between NLC with 5% (PDI 0.280) and 15% (PDI 0.259) oil and the SLN formulation (PDI 0.263), the NLC formulations with 30% (PDI 0.224) and 45% (PDI 0.213) oil showed smaller size distribution than SLN.32 The comparison of 6 SLN and NLC formulations loaded with different amounts of sildenafil citrate and prepared with identical processes revealed that NLC showed a lower particle size than SLN in all formulations. This observation was attributed to the better emulsification of the solid lipid matrix when oil is incorporated in the solid matrix. Size stability results revealed that both SLNs and NLCs kept their particle size during 6 months of storage, demonstrating the good physical stability of the particles.33 Souto et al. prepared clotrimazole-loaded SLN with Dynasan® 116 as the solid lipid and NLC with the same lipid accompanied by 30% Miglyol® 812 as the oil. Their results showed no significant difference in size between NLC and SLN produced with the same total lipid and surfactant composition. The size of both systems remained stable during 90 days of storage.22 Interestingly, the same research group published converse results, reporting that NLC was unstable over 90 days at room temperature and even at 4°C, while drug-loaded SLN was stable under both conditions. This might be attributed to the effect of the solid and liquid lipid types which were different from those in a previous study in which Compritol® 888 ATO and α-tocopherol were administered in preparing NLC instead of Dynasan® 116 and Miglyol® 812, respectively. The difference in surfactant type might be responsible for such a result as well,34 since most topical dosage forms are formulated in the semisolid form. Since lipidic nanoparticles are gaining interest among topical delivery systems,35 few studies are focusing on the stability of SLN and NLC particles after incorporation into hydrogels. Souto et al. prepared hydrogels with different gel-forming agents (xanthan gum, Chitosan, Carbopol, and hydroxyethyl cellulose) in different concentrations containing 5% SLN and NLC dispersion in order to evaluate their stability before and after incorporation into hydrogel formulations. They concluded that the incorporation into hydrogels did not result in particle aggregation after 90 days.36 Bhaskar et al. carried out the same research with Nitrendipene-loaded SLN and NLC dispersion incorporated into hydrogels.25,37 They reported the same conclusion indicating the stability of nanoparticles in the point of size and PDI after 90 days of storage at room temperature. In the case of gel-forming polymers with very polar groups like Chitosan, possible interactions between the negative surface charge of the lipid nanoparticles and the polar groups of this polymer must be considered as well. This interaction caused the prepared particles to be larger than those of other polymers used in the fabrication of SLN-loaded hydrogels. Recently, a comparative research reported the differences between SLN and NLC particles loaded with clotrimazole in various scopes. Changing the surfactant (Chremophore® EL) concentration led to different results regarding the size of NLC and SLN prepared under identical conditions. Lower surfactant concentrations (1-2% w/v) resulted in smaller NLC particles, but contradictorily, NLCs were larger than SLNs at 4% surfactant concentration. There was no significant difference in size between SLNs and NLCs at 3% surfactant concentration. In both cases, particle size increased with increases in clotrimazole concentration. However, the particle size and PDI of SLNs and NLCs at the same drug concentration were not significantly different. It was concluded that NLCs were more stable than SLNs, especially in samples with high drug-loading and those stored at room temperature.38 Saupe et al. compared the size stability of SLN and NLC dispersions. In their study, the only variable was the lipid matrix composition which was changed from lipid matrix without oil (SLN) to 10.0% medium chain triglycerides (Miglyol 812) added to the lipid matrix (NLC). Three main conclusions were revealed in this study: 1-Long term stability was observed for both SLN and NLC (over the investigated period of 12 months); 2-No difference in particle size between SLN (203 nm) and NLC (208 nm) was observed; 3-Particle size and PDI remained unchanged in both SLN and NLC particles in different storage temperatures (4°C, room temperature, and 40°C) over 12 months of storage.39 Two different imidazole antifungal agents (clotrimazole and ketoconazole) were incorporated into SLN and NLC. In this study, Carbopol 934 was used as the gelling agent for the preparation of semi-solid formulations based on SLN and NLC. Unfortunately, different lipids, oils, and surfactants in different amounts were used to prepare SLN and NLC particles with each drug. This makes the results incomparable when judging the role of drug type on the size and size distribution of SLN and NLC particles. SLN was smaller in size than NLC when clotrimazole was loaded into lipid particles prepared from Dynasan 116 (lipid), Miglyol 812 (oil), and Tyloxapol (surfactant). On the other hand, SLN was larger than NLC when Ketoconazole was loaded into lipid particles prepared from Compritol 888 (lipid), Tocopherol (oil), Poloxamer 188 (surfactant) and sodium deoxycholate (co-surfactant). In the same one-year storage period, the steric barrier provided by poloxamer 188 was obviously not sufficient to prevent particle aggregation of both SLN and NLC particles. Contrarily, both SLN and NLC particles prepared from 5% of Tyloxapol remained stable for one year of storage in all studied temperatures (4°C, 25°C, and 40°C). The stable SLN and NLC particles were incorporated in the Carbopol-based hydrogel. The zeta potential values of both SLN and NLC particles were increased significantly after 3 months of incorporation in hydrogels. This result can be attributed to the adsorption of negatively-charged Carbopol molecules onto the surface of the lipid nanoparticles. However, no changes in particle size parameters were observed. This is in agreement with the theory, which says that increased zeta potential provides increased stability by electrostatic repulsion.40 Severino et al. loaded mitotane, an adrenocortical carcinoma treatment agent, into SLN and NLC. Mean particle size and size distribution values obtained for mitotane-loaded SLN (150 nm with a PDI of 0.2) were smaller than those for NLC (250 nm with a PDI of 0.30). Although the total lipid amounts applied for preparing both systems were the same, the type of solid lipid was different. This finding was achieved when the amounts of surfactants (Tween 80 and Span 85) administered for the preparation of NLC were almost two times higher than those used for the fabrication of SLN.41 The two polyhydroxy surfactants, polyglycerol 6-distearate (Plurol® Stearique WL1009), and caprylyl/capryl glucoside (Plantacare® 810) made from renewable resources were investigated for use in the preparation and stabilization of aqueous SLN and NLC dispersions. Both lipid particulate systems were composed of cetyl palmitate as the solid lipid only (SLN) and its mixture with Miglyol 812 (NLC, the mixtures containing increasing amounts of oil from 20% to 60%). It was concluded that the type of surfactant and amount of oil did not influence particle size or polydispersity indices, and all formulations resulted in a size of about 200 nm with a PI below 0.20. All dispersions (SLN and NLC) were physically stable for 3 months at room temperature.42 In another study, SLN and NLC particles were prepared for dermal delivery of fluconazole against cutaneous candidiasis. The identical solid lipid (Compritol 888 ATO), lipid/drug ratio, lipophilic and hydrophilic emulsifiers (egg phosphatidylcholine and pluronic F-68), and manufacturing procedure were used to fabricate SLN and NLC particles. Oleic acid was used as the oil phase of NLC. The ratio of oil to solid phase of NLC particles was not mentioned in the article. The size reported for SLN was 178.9 ± 3.8 nm, while the NLC size was significantly lower at 134.3 ± 5.2 nm. The crystalline lipid core of the SLNs compared to the amorphous core of the NLCs and the OA content in NLCs were introduced as being responsible for the larger size of SLN and lower size of NLC, respectively.43,44 The negative zeta potential values (−25 and −29 mV for SLNs and NLCs, respectively) for both lipidic particles explain the stability of the small size of these systems by maintaining electrostatic repulsion among the particles.45 Topotecan, a cytotoxic drug administered in the treatment of refractory ovarian and small-cell lung cancers, was loaded into NLC particles prepared with oleic acid concentrations ranging from 25% to 50% (w/w); however, no influence on the size and PDI due to liquid lipids was reported, even under higher oil concentrations. An increase in the amounts of topotecan incorporated into the NLC and SLN structures resulted in a decrease in diameter of both NLCs and SLNs, which can be attributed to topotecan’s co-surfactant effect. The reduction in SLN size, however, was not statistically significant.46 Tryptanthrin, an ancient traditional folk medicine, was shown to be useful in treating certain cancers, in particular those that exhibit multidrug resistance. However, since tryptanthrin is insoluble in most biocompatible solvents, delivering it into cancer cells is challenging when using conventional administration techniques, such as oral, intravenous or intramuscular delivery routes. SLN and NLC particles were used to load tryptanthrin to improve its delivery into human breast cancer cells. NLC and SLN were formulated by the same preparation process and with identical compositions (soybean phosphatidylcholine (0.2% w/v) as lipophilic emulsifier, Tween 80 (2.4% w/v) as hydrophilic emulsifier, Precirol and Compritol the solid lipid phase, and squalene as oil phase). The total lipid phase was 12% w/v, and the oil-to-solid lipid in NLC formulations was 50%. Both NLC particles (prepared with 6% Precirol - 6% squalene (198.45 ± 1.53) and 6% Compritol - 6% squalene (269.50 ± 29.71)) were smaller (P < 0.05) than the respective SLN systems (12% precirol (320.39 ± 21.02) and 12% Compritol (539.67 ± 6.62)). The data also revealed that smaller size nanoparticles were achieved when using Precirol than when using Compritol in the composition of both NLC and SLN.47 In one study, the solubility of mefenamic acid, a non-steroidal anti-inflammatory agent with very poor aqueous solubility, was studied in different oil phases; consequently, caprylic acid was selected for the preparation of NLC because of its higher capacity for drug solubility. SLN was prepared using cethyl palmitate as the solid lipid phase, and different formulations of NLC were prepared by ascending amounts of caprylic acid from 10% of the solid lipid phase to 40%. SLN size was 211 nm which linearly decreased until 125 nm when the oil ratio in NLC formulations was increased. However, the size distribution did not change when the formulation composition was varied. Both NLC and SLN showed stable size and size distribution at 4°C for the 21 days of the study. NLC kept its stability at 25°C and exhibited only a few degrees of size instability at 40°C, while SLN showed size instability at both 25°C and 40°C.48 Lidocaine is an efficient rapid onset, intermediate action, and low systemic toxicity local anaesthetic agent. For effective topical delivery, lidocaeine was incorporated into SLN and NLC particles. SLN particles were made with different mixtures of Compritol and Precirol as the solid lipid phase. In agreement with previous reports (41), the SLN formulations mainly composed of Precirol were smaller than those made mostly by Compritol (124 nm versus 247 nm). The incorporation of different ratios of Miglyol 810 as the oil phase from 5% to 40% did not affect the size of lidocaeine-loaded NLC formulations made with Compritol. Although the authors claimed that the PDI of lidocaeine-loaded SLN (0.556 ± 0.09) was higher than the respective NLC formulation (0.463 ± 0.04), the difference does not seem to be statistically significant. The size stability of the SLN formulation was approved after it was studied under different conditions according to ICH guidelines (4 ± 2°C, 25 ± 2°C/60 ± 5% relative humidity (RH) and 40 ± 2°C/75 ± 5% RH for a period of 6 months). The NLC formulation was evaluated for just 1 month in the above-mentioned conditions, and it was found to be stable.49 Domperidone is a dopamine-receptor antagonist widely used in the treatment of motion sickness. Due to its poor water solubility and consequent low oral absorbability, extensive first pass metabolism which leads to poor oral bioavailability (around 15%),50,51 therapeutic administration at low doses (10 mg) or for long-term treatment, and repetitive dosing make this drug an interesting candidate for the development of SLN and NLC. Although the use of different ratios in solid lipids and surfactants provided a difficult comparison, the size range (between 30.4 ± 6.12 nm and 38.1 ± 5.12 nm) indicated that size differences between SLN and NLC are not significant, as the duration of storage increased the NLC formulation was found to be more stable than the SLN one.51 Paclitaxel, an effective natural plant medicine used in treating a broad range of solid tumors, was encapsulated into lipid particles because of its low aqueous solubility and poor permeability across the intestinal barrier. The commercially available paclitaxel parenteral solution (Taxol®) also shows serious side effects in patients, such as allergic reaction, neurotoxicity and nephrotoxicity due to the incorporation of Cremophor EL and dehydrated alcohol as drug dissolvent.52,53 Thus, one study attempted to load paclitaxel in an effective carrier to improve its solubility and reduce its toxicity to normal tissues, as is urgently needed for use in a clinical setting. NLC was prepared using the melted-ultrasonic method to enhance the encapsulation of lipophilic drug in the nanoparticles.52 Glyceryl monostearate, oleic acid, and Tween 20 were used as solid matrix, liquid matrix, and emulsifier, respectively, in the preparation of NLC nanoparticles. Incorporating oil to solid lipid up to 15% increased the size, but the PDI remained unchanged. Increasing oil up to 30% and 50%, however, drastically reduced size. Interestingly, the PDI was also increased. It was found that the size and PDI of NLC dispersion were increased after 4 weeks of storage, but the lyophilized NLC kept its size and PDI characteristics during the same period of storage.53 Puerarin is an efficient traditional Chinese medicine useful in the treatment of cerebrovascular and cardiovascular diseases.54 It does have some drawbacks, however, such as poor solubility, short elimination half-life, and poor oral bioavailability. In addition, various side effects were associated with the injection formulation of puerarin, such as allergic responses, hemolytic anemia, allergic shock, fever, and kidney or liver damage.55 Among the several pharmaceutical methods that have been used to increase the oral absorption of puerarin or decrease the risk of serious adverse effects of its injection, lipid nanoparticles have gained attention because of their biocompatibility and organ-targeting capability, especially their potential for targeted brain drug delivery.56-60 Therefore, puerarin-loaded SLN and NLC particles with blends of glyceryl monostearate (solid lipid phase) and capric/caprylic triglyceride (liquid lipid phase) as the lipid matrices were used. Interestingly, contrary to many other reports, this study reports that SLN exhibited a smaller size than NLC. Moreover, no significant difference in particle size was observed when oil content was increased up to 40% (w/w) with respect to total lipid mass. However, most other investigations have reported changes in size characteristics occurring upon changes in the oil content of NLC (50, 59). A few studies reported that oil content did not influence the size characteristics of NLC formulations.61 Khalil et al. investigated the preparation of meloxicam-loaded lipid nanoparticle-based hydrogels for topical application. They concluded the following results regarding the size of particles: a) the size of SLN particles showed no statistical difference with NLC particles containing 10% oil, b) an increase in the oil ratio in the structure of NLC particles caused a decrease in the size of the particles, c) Compritol and Precirol resulted in the largest and smallest particle sizes, respectively, among the three different solid lipids administered in the formulation of SLN particles.62 Contrary to the above-mentioned results, Gokce et al. concluded the following results for the size properties of resveratrol (a potent antioxidant)-loaded SLN and NLC particles: a) Resveratrol-loaded SLN particles exhibited larger particles than NLC particles, and b) the increase in oil content of the NLC particles decreased the size of the particles.32
Oridonin, a natural tetracycline diterpenoid isolated from the Chinese herb Rabdosia rubescens, was encapsulated into SLN and NLC nanoparticles made of monostearin as the solid lipid and caprylic/capric triglycerides as the liquid lipid and prepared by the emulsion-evaporation and low temperature-solidification technique. The results indicated that increasing liquid oil from 0% to 20% of total lipid resulted in an increase in nanoparticle size from 136 to 217 nm. It was interpreted that SLN nanoparticles could encapsulate liquid oil up to 20 wt%, and the size of the nanoparticles increased because of the swollen core of the nanoparticles loaded with liquid lipid. Nanoparticle size decreased sharply to 121 nm when the content of the liquid lipid was 30 wt%, possibly because the excess oil was excluded during lipid crystallization. The excess oil prevented solid lipid crystallization, generating smaller nanoparticles than even the SLN alone. SLN nanoparticles exhibited the broadest size distribution (the largest PDI).63 The same research group loaded the well-known anti-hepatotoxic polyphenolic substance silybin into lipid nanoparticles to increase its effectiveness which is limited by its poor water solubility and low bioavailability.64 The nanoparticles made of the same lipid and oil compositions were fabricated with the same method described in the above-mentioned study. Surprisingly, the authors of said study reported converse results. They reported that the incorporation of oil in the lipid composition of nanoparticles up to 20 wt% resulted in a decrease in particle size from 397 nm to 206 nm. Increasing oil content up to 30 wt% led to an increase in nanoparticle size from 206 nm to 248 nm. The authors explained that the increase of MCT content reduced the viscosity of the NLC, thereby reducing the surface tension to form smaller particles. As the MCT content reached 30 wt%, the particle size increased due to the swollen core of the particles loaded with liquid lipid. In the previous research, a second reason was given to describe the size enlargement of nanoparticles by adding oil up to 20 wt%.65 The effects of variables on the size of SLN and NLC particles are summarized in Table 1.
Table 1. The effect of variables on the size of SLN and NLC particles .
| Solid Lipid | Liquid Lipid | Preparation Technique | Model drug | SLN, NLC Size comparison | Ref |
| Precirol (Glycerol distearate) | Squalene | Lipid melt-Emulsification with homogenization and ultrasonication | Psoralen | SLN>NLC | 31 |
| Compritol (Glycerol dibehenate/ Behenate) |
Myglyol | Lipid melt-Emulsification with homogenization | Resveratrol | SLN>NLC | 32 |
| Cetylpalmitate | Maisine 35-1[M] | Lipid melt-Emulsification with homogenization | Sildenafil citrate | SLN>NLC | 33 |
| Dynasan116 (Glyceryltripalmitate) |
Miglyol | Lipid melt-Emulsification with homogenization | Clotrimazole | SLN=NLC | 22 |
| Compritol | Α-Tocopherol | Lipid melt-Emulsification with homogenization | Ketoconazole | SLN was stable ,NLC was unstable | 34 |
| Dynasan116 | Miglyol | Lipid melt-Emulsification with homogenization | None | SLN = NLC | 36 |
| Dynasan 116 | Captex 355 (Triglycerides of Caprylic and Capric acid) |
Lipid melt-Emulsification with homogenization | Flurbiprofen | SLN = NLC | 25 |
| Dynasan 114 (Glycerol Trimyristate) |
Captex 355 | Lipid melt-Emulsification with homogenization | Nitrendipine | SLN < NLC | 37 |
| Compritol | Labrafac™ CC (Caprylic/Capric Triglyceride) |
Lipid melt-Emulsification with ultrasonication | Clotrimazole | SLN = NLC | 38 |
| Cetylpalmitate | Miglyol | Lipid melt-Emulsification with homogenization | None | SLN = NLC | 39 |
| Dynasan116, Compritol | Miglyol, Tocopherol | Lipid melt-Emulsification with homogenization | Clotrimazole Ketoconazole |
SLN < NLC SLN > NLC |
40 |
| Cetylpalmitate, PEGylated Stearic acid | Medium chain Triacylglycerols (C8-C10) | Lipid melt-Emulsification with homogenization | Mitotane | SLN < NLC | 41 |
| Cetylpalmitate | Miglyol | Lipid melt-Emulsification with homogenization | None | SLN = NLC | 42 |
| Compritol | Oleic acid | Solvent Emulsion-evaporation | Fluconazole | SLN > NLC | 45 |
| Stearic acid | Oleic acid | Lipid melt-Microemulsion with vigorous stirring | Topotecan | NLC = SLN | 46 |
| Precirol and Compritol | Squalene | Lipid melt-Emulsification with homogenization | Tryptanthrin | NLC>SLN, Precirol SLN and NLC < Compritol ones | 47 |
| Cethylpalmitate | Caprylic acid | Lipid melt- Microemulsion with stirring | Mefenamic acid | SLN > NLC | 48 |
| Compritol and Precirol | Miglyol | Ultrasound dispersion method | Lidocaine | Precirol SLN < Compritol SLN = NLC | 49 |
| Trimyristin | Cetylrecinoleate | Lipid melt- Emulsion with homogenization and ultrasonication | Domperidone | SLN=NLC | 51 |
| Glycerylmonostearate | Oleic acid | Lipid melt-Emulsification with ultrasonication | Paclitaxel | NLC (30% and 50% oil) < SLN < NLC (5%-15% oil) | 53 |
| Glycerylmonostearate | Capric/Caprylic | Lipid melt-Emulsification with ultrasonication | Puerarin | SLN<NLC | 61 |
| Compritol, Precirol, Geleol | Miglyol | Lipid melt-Emulsification with homogenization and ultrasonication | Meloxicam | Compritol SLN > Precirol SLN, SLN=NLC (10% oil) <NLC (20% oil) | 62 |
| Monostearin | Caprylic/Capric triglycerides | Solvent Emulsion-evaporation | Oridonin | NLC (30% oil)> SLN>NLC (10% oil) | 63 |
| Monostearin | Caprylic/Capric triglycerides | Solvent Emulsion-evaporation | Silybin | SLN > NLC | 65 |
Conclusion
A review of the presented data indicated that no certain conclusion can be reached on the comparison of NLC and SLN size characteristics. Several studies reported a smaller NLC size compared with SLN size under identical preparation conditions, while a few reports showed opposite results. Interestingly, one research group reported two contradictory conclusions and interpretations. Therefore, more investigation seems to be necessary for researchers to reach a clear conclusion.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Kreuter J. Evaluation of nanoparticles as drug-delivery systems. 1. Preparation methods. Pharm Acta Helv. 1983;58(7):196–209. [PubMed] [Google Scholar]
- 2.Basu S, Mukherjee B, Chowdhury SR, Paul P, Choudhury R, Kumar A. et al. Colloidal gold-loaded, biodegradable, polymer-based stavudine nanoparticle uptake by macrophages: An in vitro study. Int J Nanomedicine. 2012;7:6049–61. doi: 10.2147/IJN.S38013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamishehkar H, Rahimpour Y, Kouhsoltani M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin Drug Deliv. 2013;10(2):261–72. doi: 10.1517/17425247.2013.746310. [DOI] [PubMed] [Google Scholar]
- 4.Rahimpour Y, Hamishehkar H. Liposomes in cosmeceutics. Expert Opin Drug Deliv. 2012;9(4):443–55. doi: 10.1517/17425247.2012.666968. [DOI] [PubMed] [Google Scholar]
- 5. Gregoriadis G, Florence AT, Patel HM. Liposomes in drug delivery. Harwood Academic Publishers; 1993.
- 6.Choi MJ, Maibach HI. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol Physiol. 2005;18(5):209–19. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- 7.Selvamuthukumar S, Anandam S, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system - review. J Pharm Pharm Sci. 2012;15(1):103–11. doi: 10.18433/J3K308. [DOI] [PubMed] [Google Scholar]
- 8.Selvamuthukumar S, Velmurugan R. Nanostructured lipid carriers: A potential drug carrier for cancer chemotherapy. Lipids Health Dis. 2012;11:159. doi: 10.1186/1476-511x-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A, Hunneyball IM. Evaluation of poly (lactic acid) as a biodegradable drug delivery system for parenteral administration. Int J Pharm. 1986;30(2-3):215–20. doi: 10.1016/0378-5173(86)90081-5. [DOI] [Google Scholar]
- 10.Lherm C, Müller RH, Puisieux F, Couvreur P. Alkylcyanoacrylate drug carriers: II. Cytotoxicity of cyanoacrylate nanoparticles with different alkyl chain length. Int J Pharm. 1992;84(1):13–22. doi: 10.1016/0378-5173(92)90210-S. [DOI] [Google Scholar]
- 11.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1-2):170–84. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hamishehkar H, Shokri J, Fallahi S, Jahangiri A, Ghanbarzadeh S, Kouhsoltani M. Histopathological evaluation of caffeine-loaded solid lipid nanoparticles in efficient treatment of cellulite. Drug Dev Ind Pharm. 2015;41(10):1640–6. doi: 10.3109/03639045.2014.980426. [DOI] [PubMed] [Google Scholar]
- 13.Muchow M, Maincent P, Müller RH. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev Ind Pharm. 2008;34(12):1394–405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 14.Joshi MD, Müller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 2009;71(2):161–72. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Hu L, Jia Y, WenDing WenDing. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Pharmazie. 2010;65(8):585–7. doi: 10.1691/ph.2010.0023. [DOI] [PubMed] [Google Scholar]
- 16.Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144(2):233–41. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emami J, Mohiti H, Hamishehkar H, Varshosaz J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using taguchi and box-behnken design. Res Pharm Sci. 2015;10(1):17–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzati Nazhad Dolatabadi J, Hamishehkar H, Valizadeh H. Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: Solid-state characterization and aerosol dispersion performance. Drug Dev Ind Pharm. 2015;41(9):1431–7. doi: 10.3109/03639045.2014.956111. [DOI] [PubMed] [Google Scholar]
- 19.Luo Q, Zhao J, Zhang X, Pan W. Nanostructured lipid carrier (NLC) coated with chitosan oligosaccharides and its potential use in ocular drug delivery system. Int J Pharm. 2011;403(1-2):185–91. doi: 10.1016/j.ijpharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Araujo J, Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1-2):167–75. doi: 10.1016/j.ijpharm.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 21. Müller RH, Lucks JS. Arzneistoffträger aus festen lipidteilchen–feste lipidnanosphären (SLN). European Patent EP 0605497, 1996.
- 22.Souto EB, Wissing SA, Barbosa CM, Müller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278(1):71–7. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Ezzati Nazhad Dolatabadi J, Hamishehkar H, Eskandani M, Valizadeh H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2014;117:21–8. doi: 10.1016/j.colsurfb.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 24. Müller RH, Lippacher A, Gohla S. Solid lipid nanoparticles (SLN) as a carrier system for the controlled release of drugs. In: Wise D, editor. handbook of pharmaceutical controlled release technology. New York, USA: Marcel Dekker; 2000. P. 377-92.
- 25.Bhaskar K, Anbu J, Ravichandiran V, Venkateswarlu V, Rao YM. Lipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009;8:6. doi: 10.1186/1476-511X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MH, Silva AM. et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv. 2012;2012:750891. doi: 10.1155/2012/750891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müller R, Mäder K, Lippacher A, Jenning V. Solid-liquid (semi-solid) liquid particles and method of producing highly concentrated lipid particle dispersions. German patent application 2000;199(45,203.2).
- 28.Leach WT, Simpson DT, Val TN, Yu Z, Lim KT, Park EJ. et al. Encapsulation of protein nanoparticles into uniform-sized microspheres formed in a spinning oil film. AAPS PharmSciTech. 2005;6(4):E605–E17. doi: 10.1208/pt060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandervoort J, Ludwig A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int J Pharm. 2002;238(1-2):77–92. doi: 10.1016/S0378-5173(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 30.Ma GH, Nagai M, Omi S. Preparation of uniform poly (lactide) microspheres by employing the shirasu porous glass (SPG) emulsification technique. Colloids Surf Physicochem Eng Aspects. 1999;153(1-3):383–94. doi: 10.1016/S0927-7757(98)00460-9. [DOI] [Google Scholar]
- 31.Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur J Pharm Biopharm. 2008;70(2):633–40. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Gokce EH, Korkmaz E, Dellera E, Sandri G, Bonferoni MC, Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int J Nanomedicine. 2012;7:1841–50. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elnaggar YS, El-Massik MA, Abdallah OY. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int J Nanomedicine. 2011;6:3195–205. doi: 10.2147/IJN.S25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souto EB, Müller RH. SLN and NLC for topical delivery of ketoconazole. J Microencapsul. 2005;22(5):501–10. doi: 10.1080/02652040500162436. [DOI] [PubMed] [Google Scholar]
- 35.Lippacher A, Müller R, Mäder K. Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles. Int J Pharm. 2001;214(1-2):9–12. doi: 10.1016/s0378-5173(00)00623-2. [DOI] [PubMed] [Google Scholar]
- 36.Souto EB, Wissing SA, Barbosa CM, Müller RH. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur J Pharm Biopharm. 2004;58(1):83–90. doi: 10.1016/j.ejpb.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Bhaskar K, Krishna Mohan C, Lingam M, Jagan Mohan S, Venkateswarlu V, Madhusudan Rao Y. et al. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug dev Ind Pharm. 2009;35(1):98–113. doi: 10.1080/03639040802192822. [DOI] [PubMed] [Google Scholar]
- 38.Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–51. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Saupe A, Wissing SA, Lenk A, Schmidt C, Müller RH. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) – Structural investigations on two different carrier systems. Biomed Mater Eng. 2005;15(5):393–402. [PubMed] [Google Scholar]
- 40.Souto EB, Müller RH. The use of SLN® and NLC® as topical particulate carriers for imidazole antifungal agents. Pharmazie. 2006;61(5):431–7. [PubMed] [Google Scholar]
- 41.Severino P, Souto EB, Pinho SC, Santana MH. Hydrophilic coating of mitotane-loaded lipid nanoparticles: Preliminary studies for mucosal adhesion. Pharm Dev Technol. 2013;18(3):577–81. doi: 10.3109/10837450.2011.614250. [DOI] [PubMed] [Google Scholar]
- 42.Kovacevic A, Savic S, Vuleta G, Müller RH, Keck CM. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int J Pharm. 2011;406(1-2):163–72. doi: 10.1016/j.ijpharm.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328(2):191–5. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Weyenberg W, Filev P, Van den Plas D, Vandervoort J, De Smet K, Sollie P. et al. Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. Int J Pharm. 2007;337(1-2):291–8. doi: 10.1016/j.ijpharm.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165(4):454–61. doi: 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Souza LG, Silva EJ, Martins AL, Mota MF, Braga RC, Lima EM. et al. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur J Pharm Biopharm. 2011;79(1):189–96. doi: 10.1016/j.ejpb.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Fang YP, Lin YK, Su YH, Fang JY. Tryptanthrin-loaded nanoparticles for delivery into cultured human breast cancer cells, MCF7: The effects of solid lipid/liquid lipid ratios in the inner core. Chem Pharm Bull. 2011;59(2):266–71. doi: 10.1248/cpb.59.266. [DOI] [PubMed] [Google Scholar]
- 48.Khurana S, Bedi P, Jain NK. Development of nanostructured lipid carriers for controlled delivery of mefenamic acid. Int J Biomed Nanosci Nanotechnol. 2012;2(3-4):232–50. doi: 10.1504/IJBNN.2012.051218. [DOI] [Google Scholar]
- 49.Pathak P, Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech. 2009;10(3):985–92. doi: 10.1208/s12249-009-9287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweetman SC. Martindale: the complete drug reference. London: Pharmaceutical Press; 2007. [Google Scholar]
- 51.Madishetti SK, Palem CR, Gannu R, Thatipamula RP, Panakanti PK, Yamsani MR. Development of domperidone bilayered matrix type transdermal patches: Physicochemical, in vitro and ex vivo characterization. DARU. 2010;18(3):221–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Nie SF, Kong J, Li N, Ju CY, Pan Ws. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int J Pharm. 2008;363(1-2):177–82. doi: 10.1016/j.ijpharm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Chen H, Cui S, Xue B, Tian J, Achilefu S. et al. Glucosamine derivative modified nanostructured lipid carriers for targeted tumor delivery. J Mater Chem. 2012;22(12):5770–83. doi: 10.1039/C2JM15830B. [DOI] [Google Scholar]
- 54.Liu B, Zhao J, Liu Y, Zhu X, Zeng J. Physiochemical properties of the inclusion complex of puerarin and glucosyl-β-cyclodextrin. J Agric Food Chem. 2012;60(51):12501–7. doi: 10.1021/jf304447x. [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Tan X, Yun J, Shen S, Zhang S, Tu C. et al. Formulation of poorly water-soluble compound loaded solid lipid nanoparticles in a microchannel system fabricated by mechanical microcutting method: Puerarin as a model drug. Ind Eng Chem Res. 2012;51(35):11373–80. doi: 10.1021/ie300592u. [DOI] [Google Scholar]
- 56.Kumar S, Randhawa JK. Preparation and characterization of paliperidone loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2013;102:562–8. doi: 10.1016/j.colsurfb.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Yu Y, Dai W, Cui J, Wu H, Yuan L. et al. A specific peptide ligand-modified lipid nanoparticle carrier for the inhibition of tumor metastasis growth. Biomaterials. 2013;34(3):756–64. doi: 10.1016/j.biomaterials.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–77. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Bhandari R, Kaur IP. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int J Pharm. 2013;441(1-2):202–12. doi: 10.1016/j.ijpharm.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 60.Kong WH, Park K, Lee MY, Lee H, Sung DK, Hahn SK. Cationic solid lipid nanoparticles derived from apolipoprotein-free ldls for target specific systemic treatment of liver fibrosis. Biomaterials. 2013;34(2):542–51. doi: 10.1016/j.biomaterials.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 61.Hu X, Zhang Y, Yang J, Wan H. Influence of liquid lipid content on the properties of puerarin-loaded lipid nanoparticles. J Chinese Adv Mater Soc. 2014;2(1):9–19. doi: 10.1080/22243682.2013.879369. [DOI] [Google Scholar]
- 62.Khalil RM, Abd-Elbary A, Kassem MA, Ghorab MM, Basha M. Nanostructured lipid carriers (NLCs) versus solid lipid nanoparticles (SLNs) for topical delivery of meloxicam. Pharm Dev Technol. 2014;19(3):304–14. doi: 10.3109/10837450.2013.778872. [DOI] [PubMed] [Google Scholar]
- 63.Dai W, Zhang D, Duan C, Jia L, Wang Y, Feng F. et al. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul. 2010;27(3):234–41. doi: 10.3109/02652040903079526. [DOI] [PubMed] [Google Scholar]
- 64.Pepping J. Milk thistle: Silybum marianum. Am J Health Syst Pharm. 1999;56(12):1195–7. doi: 10.1093/ajhp/56.12.1195. [DOI] [PubMed] [Google Scholar]
- 65.Jia LJ, Zhang DR, Li ZY, Feng FF, Wang YC, Dai WT. et al. Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv. 2010;17(1):11–8. doi: 10.3109/10717540903431586. [DOI] [PubMed] [Google Scholar]


