Abstract
Purpose: Telomere is a nucleoprotein complex at the end of eukaryotic chromosomes and its length is regulated by telomerase. The number of DNA repeat sequence (TTAGGG)n is reduced with each cell division in differentiated cells. The aim of this study was to evaluate the effect of SCF (Stem Cell Factor), Flt3 (Fms- Like tyrosine kinase-3), Interleukin-2, 7 and 15 on telomere length and hTERT gene expression in mononuclear and umbilical cord blood stem cells (CD34+ cells) during development to lymphoid cells.
Methods: The mononuclear cells were isolated from umbilical cord blood by Ficoll-Paque density gradient. Then cells were cultured for 21 days in the presence of different cytokines. Telomere length and hTERT gene expression were evaluated in freshly isolated cells, 7, 14 and 21 days of culture by real-time PCR. The same condition had been done for CD34+ cells but telomere length and hTERT gene expression were measured at initial and day 21 of the experiment.
Results: Highest hTERT gene expression and maximum telomere length were measured at day14 of MNCs in the presence of IL-7 and IL-15. Also, there was a significant correlation between telomere length and telomerase gene expression in MNCs at 14 days in a combination of IL-7 and IL-15 (r = 0.998, p =0.04). In contrast, IL-2 showed no distinct effect on telomere length and hTERT gene expression in cells.
Conclusion: Taken together, IL-7 and IL-15 increased telomere length and hTERT gene expression at 14 day of the experiment. In conclusion, it seems likely that cells maintain naïve phenotype due to prolonged exposure of IL-7 and IL-15.
Keywords: Telomere, Telomerase, Interleukin, Mononuclear cells, CD34+ cells
Introduction
Telomeres are nucleoprotein structures at the end of eukaryotic chromosomes. As it is proven, DNA hexamer (TTAGGG)n repeat have an important role in chromosomes stability.1,2 In differentiated cells, the number of DNA hexamer repeats decreases with each cell division.3-5
Telomerase is encoded by the telomerase reverse transcriptase (TERT) gene and it associated with a telomerase RNA component (TERC) as RNA template. This enzyme adds telomeric repeats to the chromosome ends and prevents telomere shortening in germ line cells and the majority of tumor cells.6,7 Therefore, telomere length and expression level of telomerase positively correlate with the immortality of cancer cells, germ-line cells and embryonic stem cells.8-11
In many previous studies has been demonstrated that telomere lengths change in lymphocytes with activation and differentiation of T and B cells. In parallel telomerase is expressed in normal lymphocytes, and this expression is regulated in the time of development and activation. These findings indicate that the maintenance of telomere length may be important to regulate T- and B-cell responses.12,13 There is a strong correlation between telomere length and replicative capacity in all normal cells. This correlation extends in T cells to differences in telomere length and replicative capacity in subsets such as naive and memory CD4+ cells. Furthermore, telomerase expression is highly regulated during development and activation of both T and B cells.14
IL-2, IL-7 and IL-15 are critical cytokines, regulating in hematopoiesis and proliferation, self-renewal, differentiation and senescence of HSCs (Hematopoietic stem cells).15,16 Interleukin- 2 (IL-2) supports hematopoiesis, T cell proliferation (both CD4+ and CD8+) and cellular metabolism.17-20 As well as the expansion of activated T lymphocytes (T cells) and differentiation of B cells.21 In addition, previous studies revealed that in T-cells recently exposed to IL-2 apoptosis is more likely induced upon antigen-dependent T-cell receptor stimulation.22 IL-2 along with Interleukin-15 (IL-15) are growth factors for natural killer cells.23 It is shown that IL- 7 influences the expansion of B cells, promotes T cell differentiation and has an important role in natural killer cell development.24 Additionally, Interleukin-15 (IL-15) promotes expansion, proliferation and homeostasis of T cells.25
We have previously shown that B and T cell expansion increased in cord blood mononuclear cells using IL-2 and IL-7.26 In another study, we illustrated that IL-2 and IL-15 are important for the expansion of NKP46 positive cells (NK cells) in cord blood cells in vitro.27
Cord blood is a major source of CD34 positive cells used HSC-transplantation in leukemia treatment or cancer immunotherapy. So far, it remains unclear if cytokines can interfere with telomerase dynamic in umbilical cord blood cells.
The aim of this study was to evaluate the influence of IL-2, IL-7 and IL-15 on the telomere length and hTERT gene expression on mononuclear and CD34+ umbilical cord blood stem cells during development to the lymphoid cell.
Materials and Methods
Mononuclear cord blood isolation and CD34+cells enrichment
Umbilical cord blood sample were obtained from full-term normal deliveries in Tabriz Alzahra hospital, Iran. The collected cord blood was diluted 1:2 with phosphate buffered saline (PBS) plus 10% fetal bovine serum (FBS). Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (GE Healthcare, Piscatta, NJ, USA) gradient centrifugation at 400×g for 25 min at 4º C. Subsequently MNCs were collected and washed twice in PBS plus 5% FBS. The isolated MNCs were co-incubated with 100 µl of CD34+ micro beads (Miltenyi Biotec, Germany Cat no: 130100453) for 30 minutes. Thereafter resuspended cells were passed through one LS MACS column (Miltenyi Biotec, Germany). Thereafter enriched CD34+ cells were retrieved by flushing the column. For purity assessment of CD34+ cells, flow cytometry was performed by FACSCalibur (BD Bioscience) and the output data were processed with Flow software version X.0.7.
Culture condition
MNCs and isolated CD34+ cells were seeded in 96-well flat bottom cell culture plates at the density of 5×105 cells per well. For culturing RPMI 1640 supplemented with 20% (v/v) FBS and 1% (v/v) penicillin/streptomycin was used. Different combinations of cytokines (Pepro Tech, London, UK) were added to the culture medium in final concentrations of 80 ng/ ml and cell cultures were maintained for 21 days. SCF and Flt3 were same in all groups. Cells were harvested and analyzed at day 7, 14 and 21. All cytokines used in this study including SCF, FLT3, IL-2, IL-7 and IL-15 were purchased from Peprotech.
Flow cytometry
Monoclonal antibodies were CD3 (UCHT1; R&D) for T cells, CD20 (PE; clone 2H7; BD Biosciences) for B cells and Anti-NKp46-PE (BD,biosience) for NK cells. Flow cytometry was performed at 14 day of culture period time. Shortly, the cells were incubated with these antibodies in each group (for 20 min at 4ºC). We used of Propidium iodide (1.0 mg/mL; Invitrogen) to eliminate of dead cells. The flow cytometry analysis of the cell suspensions was performed on a BD FACSCalibur analyser (BD Biosciences). Between 10000 and 30000 events were collected and analysed by the flow cytometry software Perttu Terho (version: 2.5.1., Cyflogic, Finland).
RNA and DNA extraction
Total RNA was extracted from harvested MNCs and CD34+ cells at different time points using Thermo Scientific Gene JET RNA Purification Kit (K0731) according to the manufacturer's instruction. Extracted RNA was dissolved in nuclease-free water. For integrity assessment of extracted RNA we use of %1.5 agarose gel electrophoresis and for purity assessment we use of spectrophotometry. DNA was extracted using the pure link genomic DNA mini kit (Invitrogen, USA) according to the manufacturer's instruction. Briefly, the cells were collected and digested with Proteinase K (Invitrogen, USA) at 55 °C. Any residual RNA was removed by incubation with RNase A (Invitrogen, USA) for 2min. After digestion, 200 µl ethanol (96~100%) was added, mixed thoroughly by pulse vortexing for 30 sec, and transferred carefully to a DNA binding pure link spin column. Finally, impurities were removed by washing buffer. The genomic DNA was then eluted in the low salt elution buffer. For integrity assessment we use of %1 agarose gel electrophoresis and purity assessment was analysed by spectrophotometry.
c-DNA synthesis and Real-time PCR
Reverse transcription was carried out using the RevertAidTM first strand cDNA synthesis kit (K1622; Fermentas, Germany). Two microgram RNA was used for the first-strand cDNA synthesis in a total volume of 20 µL according to the manufacturer's guidelines. Briefly, the reaction tubes were put at 65°C for 10 min, 42°C for 60 min preceded with 70°C for 10 min and followed at 4°C for 5 min. For every reaction set, one RNA sample was prepared without RevertAidTM M-MuLV reverse transcriptase (RT reaction) to provide a negative control in the subsequent PCRs.
All PCRs were performed using the Corbett Rotor-Gene™ 6000 HRM (Corbett Research, Australia) in a total volume of 20 µL containing Power SYBR Green master mix (2x) (TaKaRa Ex Taq HS, Japan), Primer (0.4 μM), cDNA (20 ng/μl) plus nuclease free water. The mRNA-specific primers were designed using Oligo 7 software (v. 7.52, Molecular Biology Insights, Inc, USA). The sequences are listed in Table 1 and 2. Both β-actin and hTERT amplification were done in triplicates for each sample. β-actin was selected as an endogenous housekeeping gene. Forty-five thermal cycles were performed in the following order: 2 min at 94ºC, 40 cycles, 94ºC for 15 sec and 63ºC for 1 min. PCR data were analyzed using Rotor-Gene 6000 Software (version: 1.7) to determine CT values. Delta CT values were calculated in relation to β-actin CT values by the 2-RΔΔCT method, in which ΔCt represents the difference between the CT value of target genes and the CT value of β-actin.
Table 1. Primers for quantitative Real-time RT-PCR .
| No. | Gene | Primer pair sequence (5'-3') | Product length (bp) |
| NM_001193376.1 | TERT | CCGCCTGAGCTGTACTTTGT CAGGTGAGCCACGAACTGT | 234 |
| NM_001101.3 | β-actin | AAACTGGAACGGTGAAGGTG TATAGAGAAGTGGGGTGGCT | 174 |
Table 2. Oligomers used for aTL assay .
| Oligomer name | Oligomer sequence (5'-3') | Amplicon size (bp) |
| Telomere standard | (TTAGGG)14 | 84 |
| 36B4 standard | 5'CAGCAAGTGGGAAGGTGTAATCCGTCTCCACAGACAAGGCCAGGACTCGTTTGTACCCGTTGATGATAGAATGGG-3' | 75 |
| Telo | Fwd:CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT Rev:GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
>76 |
| 36B4 | Fwd:CAGCAAGTGGGAAGGTGTAATCC Rev:CCCATTCTATCATCAACGGGTACAA |
75 |
Standard curves and associated calculations for aTL
A standard curve was obtained from dilution series of known quantities of a synthesized 84 mer oligonucleotide (84 bp in length) containing only TTAGGG repeated 14 times. The number of repeats in each standard is calculated as previously described by O’Callaghan.2 For generating a standard curve the serial dilutions of TEL STD A (10-1[1.18 ×108] through to 10-6 [1.18 × 103] dilution) is performed. Plasmid DNA (pet 28a) was added to each standard to maintain a constant 20 ng of total DNA per reaction tube (Table 3).
Table 3. Amounts of calculation for aTL .
| Oligomer | Molecular weight (MW) | Weight of telomere standard and 36B4 (g) | Number copies of 36B4 | Number molecules of oligomer in TEL STD A | Amount of telomere sequence in TEL STD A (kbp) | SCG STD A |
| (TTAGGG)14 | 26667.2. | 2.6667 × 104/6.02 × 1023= 0.44 × 10-19 | - | 60 × 10-12/0.44 × 1019=1.36×109 | 1.36 × 109× 84= 1.18 × 108 |
- |
| synthesized 36B4 oligomer standard | 23268.1 | 2.32681 × 104/6.02 × 1023= 0.38 × 10-19 | 200 × 10-12/0.44 × 10-19= 5.26×109 | - | - | 2.63 × 109 |
For the single copy gene (SCG) standard curve, we routinely used 36B4, which encodes the acidic ribosomal phosphoprotein P0. Although telomeric DNA sequence is consistent in mammals, the SCG will be different, thus, an SCG standard curve and amplicon must be generated for each target species. SCG amplification is crucial for the accuracy and reliability of the results generated in the aTL assay.
For generating a standard curve the serial dilutions of SCG STD a (10-1through to 10-6 dilution) is performed (Figure 1). As same as telomere standard, plasmid DNA (pet 28a) is added to each standard to maintain a constant 20 ng of total DNA per reaction tube.
Figure 1.
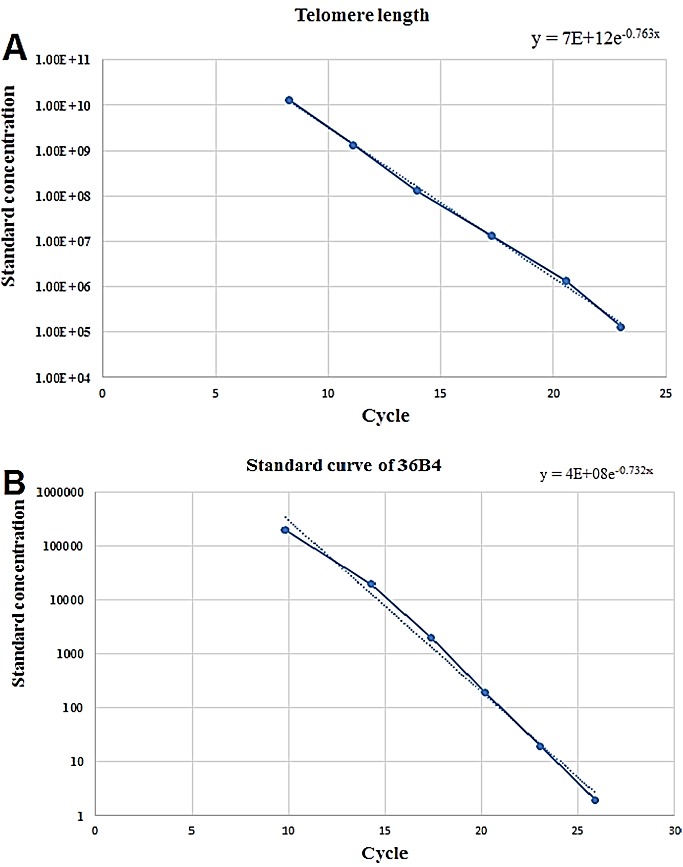
Standard curve used to calculate absolute telomere length.(A) Graph shows standard curve for calculating length of telomere sequence per reaction tube. X-axis represents number of cycle and Y-axis show the standards concentration.(B) Graph shows standard curve for calculating genome copies using 36B4 copy number. X-axis represents number of cycle and Y-axis show standard concentrations per each reaction.
Statistical analysis
In this study data were studied by one-way ANOVA followed by the Tukey test. For graph we used of Prism software (GraphPad Software, Inc., San Diego, CA version; 6). Values were measured statistically significant at P < 0.05.
Results
The IL-2, IL-7 and IL-15 can stimulate T, B and NK cells expansion in cord blood mononuclear cells
5×105 cells were cultured in presence of different combination of cytokines including IL-2, IL-7 and IL-15 for 14 days. Harvested cells evaluated at 14 day by flow cytometry using CD 20 for B cells, CD3 for T cells and NKP46 for NK cells.
As shown in Figure 2 the IL-2 are involved in expansion of T (94%), B (96%) and NK cells(38%). However IL-7 increased T cell expansion (92%) as well as B cells (97%), but not significantly increased NK cells. Our data were shown that IL-15 can increase the expansion of NK cells (28%), but it was not important for B cells. The Figure 2 shows that IL-2 and IL-15 can stimulate expansion of mononuclear cells toward T and NK cells and IL-7 was important for B cells.
Figure 2.
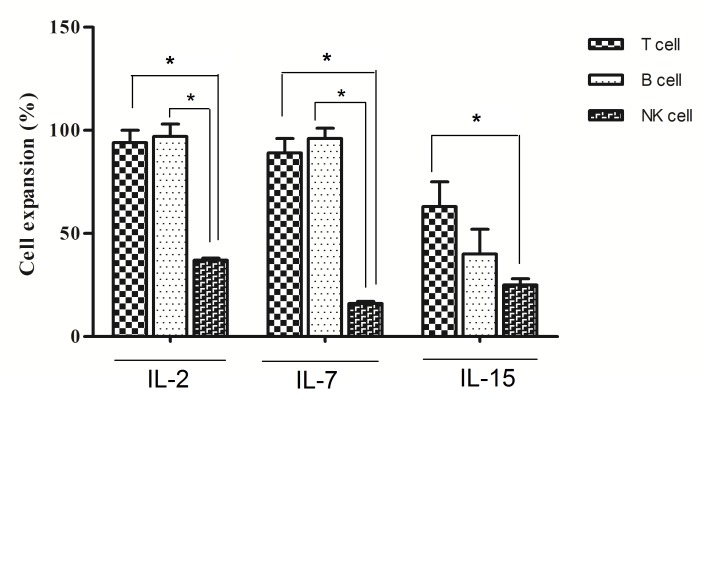
Cord blood mononuclear cell derived T, B and NK cells in presence of IL-2, IL-7 and IL-15 at day 14 in vitro. Harvested cells evaluated by flow cytometry and measured the percentage of B, T and NK cells. In all groups SCF and FLt3 included. All data showed mean ± SD. Differences between groups are significant at *p <0.05.
The effect of Interleukin-7 on telomere length and hTERT gene expression in cord blood mononuclear cells
To investigate the effects of IL-7 on both telomere length and hTERT gene expression, quantitative real-time PCR was carried out in MNCs cultured with IL-7 for 21 days. We found that IL-7 could actively alter the dynamic of telomere length over a period of 21 days: The minimal telomere length was found in non-cultured cells (0.86 kb). The absolute telomere length was elongated in day14 (56.82 kb) in compared to day7 while it shortened (9.09 kb) in day 21 days in compared to day14 (Figure 3A). A simultaneous evaluation of mean fold of hTERT in day 7, 14 and 21 was 2.36, 3.94 and 0.97, respectively (Figure 3B). In parallel highest hTERT expression was seen at day 14 (3.94) and the significant difference was seen in days 14 and 21 (P<0.01).
Figure 3.
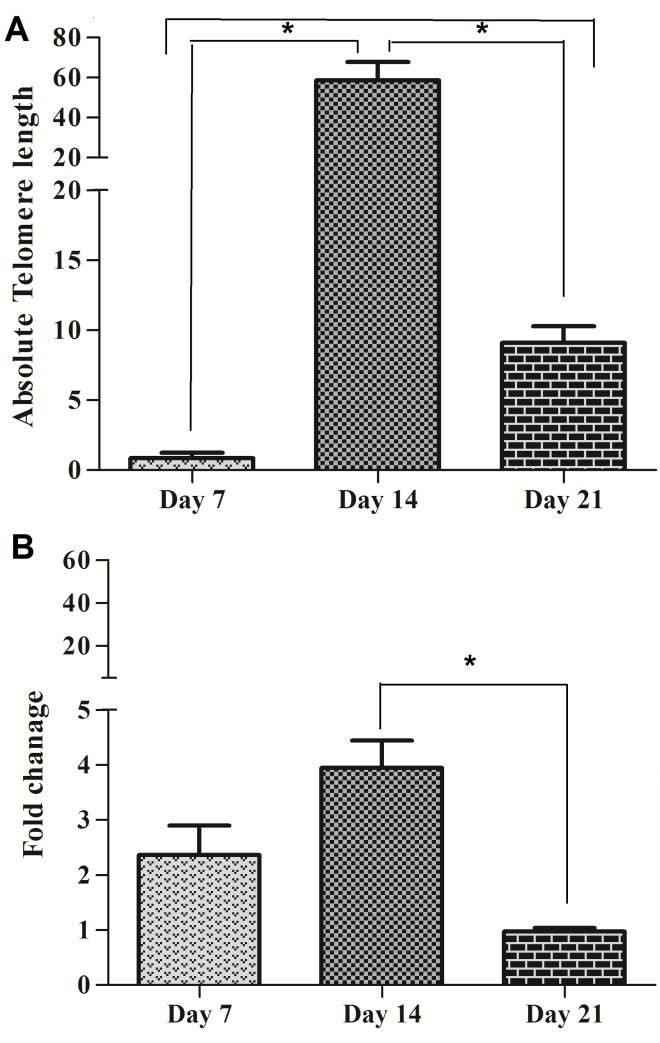
comparison of telomere length and hTERT gene expression folds in MNCs after treatment with 80 ng/ml of IL-7.( A) Telomere length (Kbp) in 7, 14 and 21 days of MNCs. (B) hTERT gene expression fold in MNCs in 7, 14 and 21 days. Differences between groups are significant at *p <0.05, **p <0.01, and ***p<0.001.
Association between telomere length and hTERT gene expression in cord blood mononuclear cells is an outcome of CD34+ stem cells expression using Interleukin-7 (IL-7)
We cultured 4 × 105 CD34+ enriched cord blood cells for 21 days with IL-7. Telomere length and hTERT gene expression were measured innon-cultured and late time point of our experiment (day 21). Similar to MNCs, telomere length and hTERT gene expression were measured. The mean telomere length of CD34+ cells was recorded 0.12 and 0.129 kb at day 0 and 21 respectively. No significant differences were seen in telomere length at the beginning and day 21 (P>0.05) (Figure 4A). Accordingly, the mean hTERT gene expression fold was 0.14 in day 0 and reached 9.79 at day 21 while no significant differences were observed in gene expression levels of hTERT between day 0 and 21 (P= 0.06) (Figure 4B).
Figure 4.
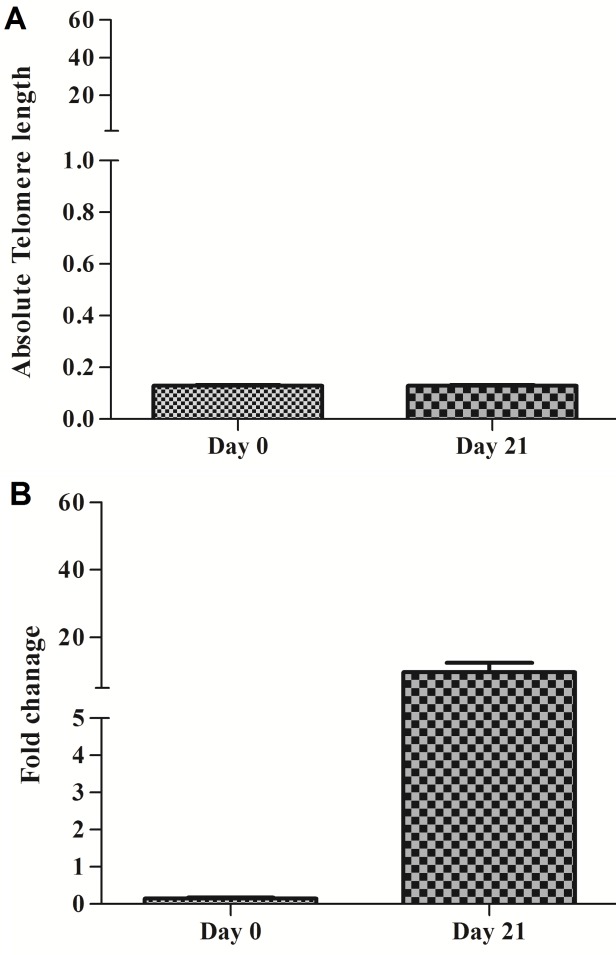
Relationship between of telomere length and hTERT gene expression folds in CD34+ cells in using 80 ng/µl of IL- 7. (A) Telomere length (Kbp) of CD34+cells in 0 and 21 days. (B) hTERT gene expression fold of CD34+ in 0 and 21 days. Differences between groups are significant at *p <0.05, **p <0.01, and ***p<0.001.
Association between Immune cell key cytokines with Telomere length and hTERT gene expression in cord blood MNCs
To evaluate telomere length and hTERT gene expression, quantitative real-time PCR was performed at day 7, 14 and 21 for harvested cells.
Average telomere length of MNCs after 14 days of co-culture with IL- 2, IL- 7 and IL- 15 was 7.42 kb, 58.62 kb and 22.52 kb. Interestingly, the highest length of telomere was observed after co-culture with IL- 7 (Figure 5A). Also, the mean hTERT gene expressions measured in cultured cells with IL-2, IL-7 and IL-15 and were in order 1.35, 17.34, 3.94 and 48.54. The significant differences were indicated for cultured with IL- 2 (P< 0.01), IL-7 (P<0.05) and IL-15 (P<0.001) (Figure 5B). The highest hTERT gene expression fold was seen in cultured with IL-15.
Figure 5.
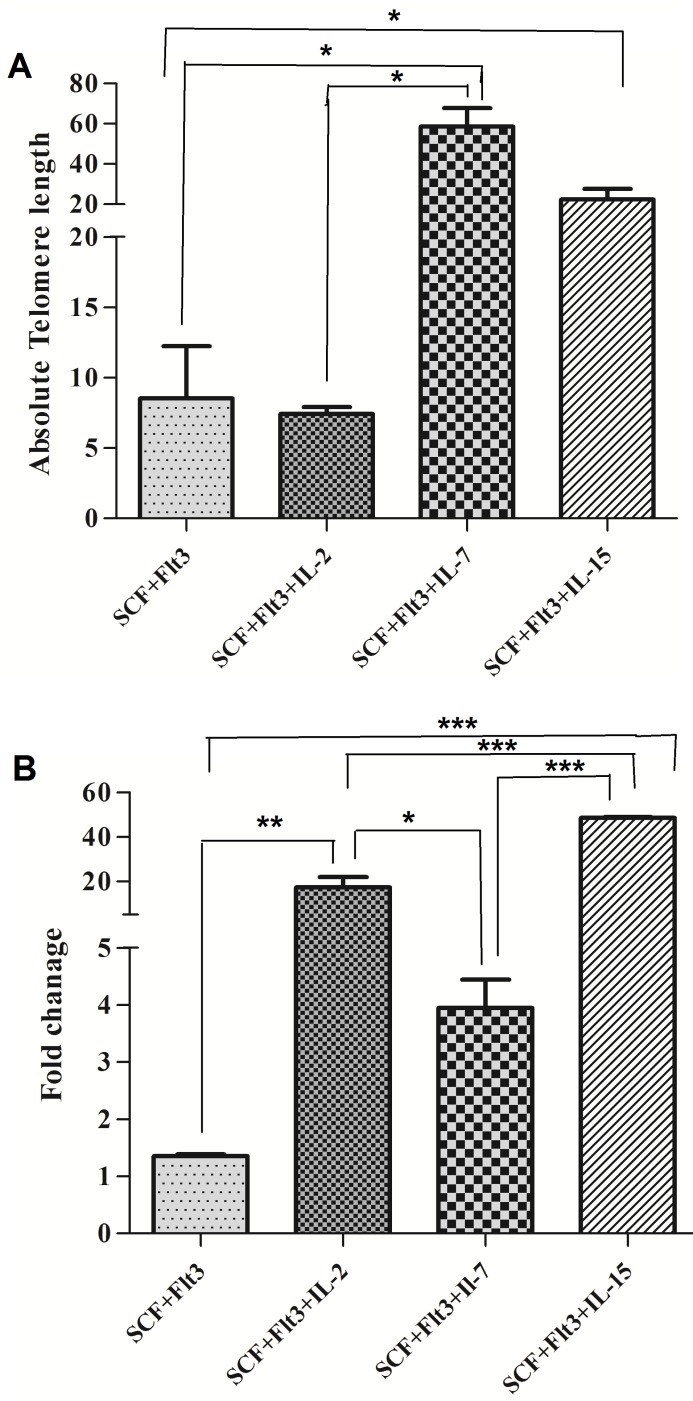
Comparisons of telomere length and hTERT gene expression fold in different groups at 14th day of MNCs (using 80ng/µl of each cytokines). (A) telomere length (Kbp) in SCF+FLT3, SCF+FLT3+IL-2, SCF+FLT3+IL-7 and SCF+FLT3+IL-15 groups at 14th day of MNCs.(B) hTERT gene expression fold in SCF+FLT3, SCF+FLT3+IL-2, SCF+FLT3+IL-7 and SCF+FLT3+IL-15 groups at 14th day of MNCs. Differences between groups are significant at *p <0.05, **p <0.01, and ***p<0.001.
Correlation between hTERT gene expression and cellular turnover
Telomere length and hTERT gene expression were evaluated by real-time PCR in distinct time points for cultured cells with the combination of IL-2, IL-7 and IL-15.
Telomere length at day 7, 14 and 21 accounted for 17.2 kb, 14.4 kb and 14.4 kb, respectively and there were no significant differences between groups (P>0.05) (Figure 6A). Mean hTERT gene expression was 3.26, 33.11 and 1.44 in days 7, 14 and 21 of culture respectively. Significant differences were seen at day 14 and 21 (P<0.001) (Figure 6B). Also there was correlation between telomere length and hTERT gene expression at 14 day (r = 0.998, p =0.04).
Figure 6.
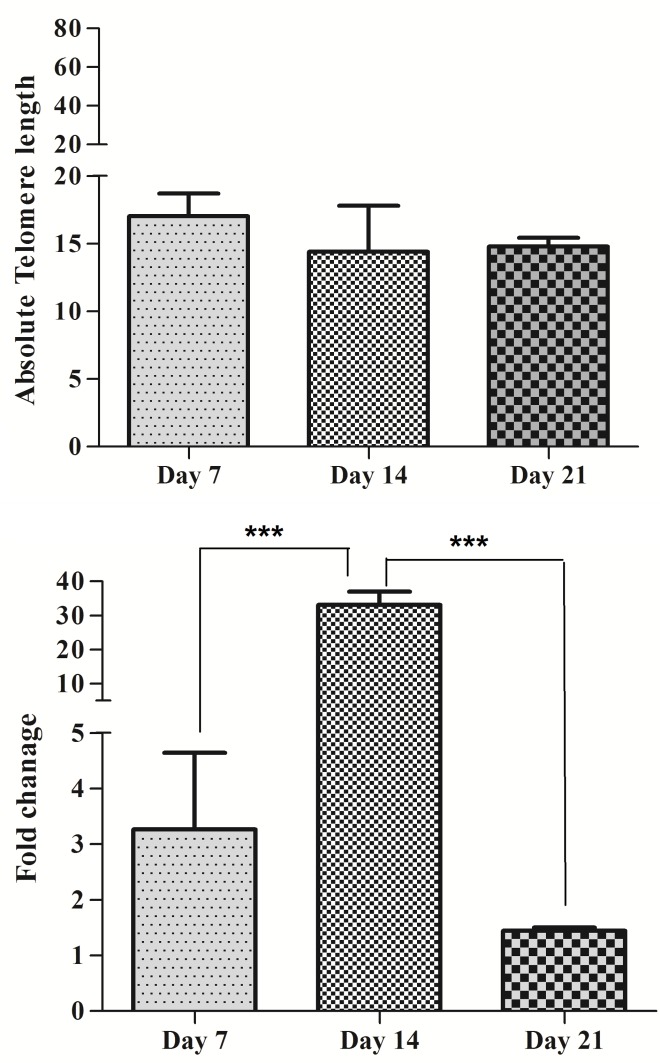
association between of telomere length and hTERT gene expression Fold in SCF+FLT3+IL-2+IL-7+IL-15 group of MNCs (using 80ng/µl of each cytokines). (A) Telomere length (Kbp) in 7, 14 and 21 days of MNCs. (B) hTERT gene expression fold in 7, 14 and 21 days of MNCs. Differences between groups are significant at *p <0.05, **p <0.01, and ***p<0.001.
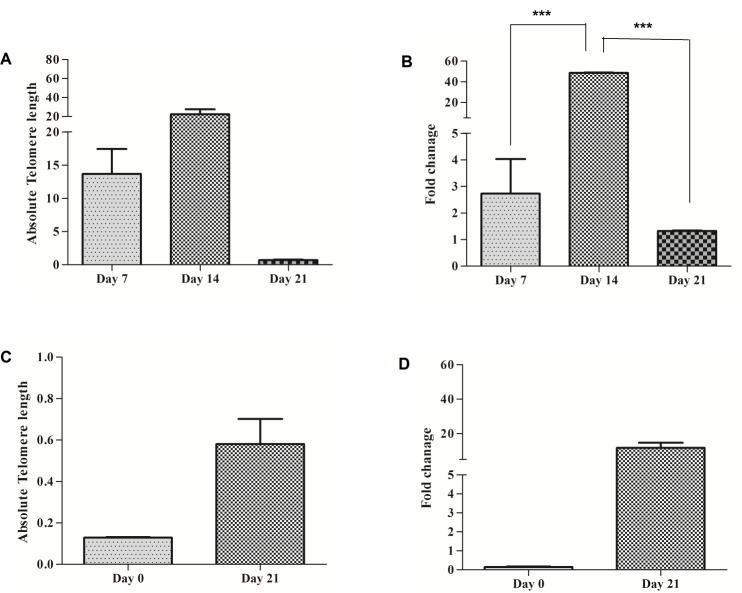
Both Telomere length and hTERT gene expression in MNCs and CD34 positive cells are high using Interleukin-15 (IL-15)
Both MNC and CD34+ cells (n= 4 ×105) wereco_cultured with IL-15 and telomere length and hTERT gene expression were measured at different time points.
Telomere length was measured in MNCs by real-time PCR. Absolute telomere length was 13.68 kb at day 7, 22.52 and 0.7 kb at day 14 and 21, respectively. The highest telomere length was seen at day 14. There was no significant difference between groups (P>0.05) (Figure 7A). hTERT gene expression fold at day 7, 14 and 21 day was 2.73, 48.54 and 1.34, respectively. Results showed that there are significant differences in hTERT gene expression between day 14 and 21(P<0.001). The highest hTERT gene expression fold was observed at day 14 (Figure 7B).
Figure 7.
Comparison of telomere length and hTERT gene expression folds in MNCs and CD34+ in using 80 ng/ µl of IL-15. (A) Telomere length (Kbp) in MNCs in different days of culture period time. (B) hTERT gene expression fold of MNCs in different days of culture period time. (C) Telomere length (Kbp) of CD34+cells in 0 and 21 days. (D) hTERT gene expression fold of CD34+ in 0 and 21 days. Differences between groups are significant at *p <0.05, **p <0.01, and ***p<0.001.
Final telomere length of CD34+ accounted for 0.12 kb in non-cultured cells and 0.58 kb in cells cultured for 21 days. There was no significant difference between telomere length at noncultured cells and cells cultured for 21 days (P>0.05) (Figure 7C). hTERT gene Expression fold was 0.14 at noncultured and 0.11at day 21. There was no significant difference between hTERT expression in non-cultured and at day 21 in CD34+ cells (P>0.05) (Figure 7D).
Discussion
Natural killer cell, T and B lymphocyte development are controlled by immune cell cytokines (IL-2, IL-7 and IL-15) which known as common cytokine gamma chain. Previous study demonstrated that there is a relationship between B cell development and IL-2 and IL-7.28 Our results showed that IL-2 and IL-7 affect the expansion of T and B cells more than NK cells at 14 day of culture. NK cell development is dependent to two member of common cytokine gamma chain on the other hand IL-2 and IL-15.29 Similar to previous study, NK cells expansion percent was higher in IL-2 and Il-15 group more than IL-7 group.
In the present study, absolute telomere length and hTERT gene expression were evaluated in cord blood-derived MNCs and CD34+ cells after treatment with SCF, FLT3, IL- 2, IL- 7 and IL- 15.We found that, telomere length and hTERT expression were higher after 14 days of exposure to IL-7 and IL-15. Blackburn et al reported reduced telomerase function in proliferating cells and the population of heterogeneously sized telomeres with time shortens and the cell population undergoes telomere shortening and in experiments that telomerase enzyme was disrupted the length of telomere-shortened in cells.30,31 In line with this statement, disturbed hTERT function resulted in telomere shortening and cellular senescence.30,32 It was previously shown several times that the up-regulation of hTERT expression has a key role in the maintenance of telomere length and cell proliferation capacity.33 It is commonly believed that IL-7 is required for homeostatic proliferation of naïve T cells while IL-15 stimulates maturation of memory T cells.34 Previously, Diana et al unveiled that a long-term culture of cells in the presence of both IL-7 and IL-15 cytokines resulted in the delayed expansion of naïve CD8+ T cells that cellular expansion rate peaked only after 14 days.34 In line with our current results, previous investigations of human fibroblasts revealed that telomere shortening correlated with replicative senesces in vitro.35 Here, we demonstrate that IL-7 or IL-15 treated cells could retain their naïve phenotype and telomere length through up-regulation of hTERT expression.36 The telomere length of IL-7 treated MNCs reached its maximum level at day 14 of the experiment which may be due to the late expansion of T cell in response to IL-7. Yu Li et al described the potency of IL-15 to induce telomerase expression during activation of the Jak3 and PI3K/ AKT signaling pathways in T lymphocytes. Particularly in memory CD8+T cells.37 On the other hand, IL-15-induced telomerase dynamic is associated with the relative stable telomere length in long-term cultured memory phenotype CD8+T cells.37 The induction of hTERT via cytokines is distinctly mediated by PI3K/Akt, or the NF-kB pathways. Among the possible mechanism suggested for NF-kB pathway, protein kinase C (PKC) has been shown to have a critical role in up-regulation of TERT expression.38
In CD34+ cells, telomere length and hTERT gene expression analysis showed that telomere length was higher at the 21th day, thereby indicating that prolonged exposure to IL-7 its effects. The CD34+ cord blood cells proliferated faster in response to cytokine stimulation and total cell proliferation was increased sevenfold compared to their counterpart bone marrow.39 Study of colony-forming dynamic by sorted cord blood and bone marrow CD34+ cells revealed that, although the number of CD34+ cells in cord blood is less than bone marrow, cord blood CD34+cells have a significantly higher number of colony formation.36 This is possible that telomere length and hTERT gene expression in MNCs is an outcome of CD34+ cells.
In this experiment cells were treated with different cytokines including; SCF, FLT3, IL- 2, IL- 7 and IL- 15. In consequence telomere length and hTERT gene expression were altered. We showed that telomere length in combination with cytokines was constant at different time point but hTERT expression at 14th day was highest. These results can be explained by roles of cytokines in cell grows regulation of subtypes of immune cells and may be in this situation hTERT gene expression level have no effect on telomere length and telomere length controlled by alternative lenghething telomere mechanism. Another theory may be is when cytokines used with together telomere length not affected by hTERT expression level and remodeling in chromatin structure cause to this condition. In the other manner, in this condition telomere length not regulated by hTERT level.40,41
Conclusion
In conclusion, this study showed that telomere length and hTERT gene expression are affected by IL-7 ad IL-15. The longest telomere length and highest hTERT gene expression were observed at 14th day of culture when cells treated with Il-7 and Il-15. But IL-2 has no distinct effect on telomere length and hTERT gene expression.
Acknowledgments
The authors thank the Stem Cell Research Center, Tabriz University of Medical Sciences. This work was supported by a grant (5/104/618) from the Tabriz University of Medical Sciences. We further thank Mrs. Montazer saheb for her great help in this study.
Ethical Issues
Not applicable.
Conflict of Interest
All author declared no conflict of interest.
References
- 1.Lu W, Zhang Y, Liu D, Songyang Z, Wan M. Telomeres-structure, function, and regulation. Exp Cell Res. 2013;319(2):133–41. doi: 10.1016/j.yexcr.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arenas-Aranda DJ, Hernández-Caballero E, Salamanca-Gómez F. Cellular Senescence and Its Relation with Telomere. INTECH Open Access Publisher; 2012.
- 4.Oeseburg H, de Boer RA, van Gilst WH, van der Harst P. Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459(2):259–68. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1561):76–84. doi: 10.1098/rstb.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D. et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123(6):1121–31. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3(10):640–9. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 9.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584(17):3826–30. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96(7):1020–4. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21(6):349–53. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38(10):854–9. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M. et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1-2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dioni L, Hoxha M, Nordio F, Bonzini M, Tarantini L, Albetti B. et al. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ Health Perspect. 2011;119(5):622–7. doi: 10.1289/ehp.1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell. 2012;10(6):690–7. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol. 2008;15(4):307–11. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172(8):4661–5. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 18.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28(3):109–23. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK. et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11(1):R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6(3):683–92. doi: 10.1016/S1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 21.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–77. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q, Chen X, Du Y, Guo J, Su Y. Cyclic AMP-Responsive Element Modulator alpha Polymorphisms Are Potential Genetic Risks for Systemic Lupus Erythematosus. J Immunol Res. 2015;2015:906086. doi: 10.1155/2015/906086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki S, Iwamoto M, Saito Y, Fuchimoto D, Sembon S, Suzuki M. et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell. 2012;10(6):753–8. doi: 10.1016/j.stem.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Janot-Sardet C, Assouline B, Cheynier R, Morre M, Beq S. A validated assay to measure soluble IL-7 receptor shows minimal impact of IL-7 treatment. J Immunol Methods. 2010;353(1-2):115–23. doi: 10.1016/j.jim.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Anthony SM, Schluns KS. Emerging roles for IL-15 in the activation and function of T-cells during immune stimulation. Rese Rep Biol. 2015;6:25–37. doi: 10.2147/RRB.S57685. [DOI] [Google Scholar]
- 26.Aliyari Z, Alami F, Mostafavi T, Taiefi Nasrabadi H, Soleimanirad J, Nozad Charoudeh H. The roles of IL-2, IL-7, and IL15 ligands in B cells development from cord blood mononuclear cells. Iran J Ped Hematol Oncol. 2015;5(3):155–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Aliyari Z, Alemi F, Brazvan B, Tayefi Nasrabadi H, Nozad Charoudeh H. CD26+ Cord Blood Mononuclear Cells Significantly Produce B, T, and NK Cells. Iran J Immunol. 2015;12(1):16–26. [PubMed] [Google Scholar]
- 28.Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW. et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182(7):4255–66. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol. 2009;9(7):480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Qian D, Ma HH, Jin R, Yang PX, Cai MY. et al. Anthracyclines disrupt telomere maintenance by telomerase through inducing PinX1 ubiquitination and degradation. Oncogene. 2012;31(1):1–12. doi: 10.1038/onc.2011.214. [DOI] [PubMed] [Google Scholar]
- 32.Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res. 2008;18(7):725–32. doi: 10.1038/cr.2008.74. [DOI] [PubMed] [Google Scholar]
- 33.Schuller CE, Jankowski K, Mackenzie KL. Telomere length of cord blood-derived CD34(+) progenitors predicts erythroid proliferative potential. Leukemia. 2007;21(5):983–91. doi: 10.1038/sj.leu.2404631. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DL, Berard M, Soares MV, Oldham J, Cook JE, Akbar AN. et al. Prolonged exposure of naive CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology. 2006;119(2):243–53. doi: 10.1111/j.1365-2567.2006.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens UM, Brass V, Sedlacek L, Pantic M, Exner C, Guo Y. et al. Telomere maintenance in human B lymphocytes. Br J Haematol. 2002;119(3):810–8. doi: 10.1046/j.1365-2141.2002.03910.x. [DOI] [PubMed] [Google Scholar]
- 36.Cao JN, Gollapudi S, Sharman EH, Jia Z, Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell. 2010;9(1):19–31. doi: 10.1111/j.1474-9726.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174(7):4019–24. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 38.Barsov EV. Telomerase and primary T cells: biology and immortalization for adoptive immunotherapy. Immunotherapy. 2011;3(3):407–21. doi: 10.2217/imt.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan S, Jutzy JMS, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson SP, Hoare SF, Glasspool RM, Keith WN. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005;65(17):7585–90. doi: 10.1158/0008-5472.CAN-05-1715. [DOI] [PubMed] [Google Scholar]
- 41.Londoño-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64(7):2324–7. doi: 10.1158/0008-5472.CAN-03-4035. [DOI] [PubMed] [Google Scholar]