Abstract
Purpose: The purpose of this study was to develop and evaluate metronidazole loaded floating-mucoadhesive microsphere for sustained drug release at the gastric mucosa.
Methods: Alginate gastroretentive microspheres containing metronidazole were prepared by ionic gelation method using sodium bicarbonate as gas forming agent, guar gum as mucoadhesive polymer, and Eudragit L100 as drug release modifier. Carbopol was used for increasing the bead strength. The microspheres were characterized by scanning electron microscopy and evaluated by means of drug entrapment efficiency, in vitro buoyancy, and swelling studies. In vitro mucoadhesion and drug release studies were carried out in order to evaluate site specific sustained drug release.
Results: All formulations showed 100% buoyancy in vitro for a prolonged period of time. Amount of guar gum influenced the properties of different formulations. The formulation containing drug and guar gum at a ratio of 1:0.5 showed the best results with 76.3% drug entrapment efficiency, 61.21% mucoadhesion, and sustained drug release. Carbopol was found to increase surface smoothness of the microspheres.
Conclusion: Metronidazole mucoadhesive-floating microspheres can be effectively used for sustained drug release to the gastric mucosa in treatment of upper GIT infection.
Keywords: Alginate, Guar gum, Floating microsphere, Metronidazole, Mucoadhesive microsphere, Eudragit
Introduction
Metronidazole is a nitroimidazole antimicrobial which is effective against a variety of anaerobic bacteria.1,2 It is actively used as an adjunct in the treatment of H.pylori infection, responsible for developing gastric ulcers.3 Treatment of upper gastro-intestinal tract (GIT) infection is challenging due to the location of the infection site in stomach mucus lining.4 Treatment of local gastric infection with conventional formulations becomes ineffective due to their short gastric residence time and non-targeted drug release. Gastric emptying, which is highly variable, transfer the conventional formulation quickly to the intestine without significant release of drug to the mucous site. Thus frequent dosing is required.5,6
Gastroretentive drug delivery systems favor prolonged drug release in the stomach.7 Unlike traditional controlled release formulations, they bypass the gastric emptying process which interferes with drug delivery to the upper GIT.8 Gastroretentive drug delivery systems have been developed employing floating technique. Asnaashari et al. developed HPMC based metronidazole floating tablet and showed gastric buoyancy for a prolonged period.9 In general, these floating dosage forms release drugs at multidirections and cannot selectively release drugs on the mucosal surface. Consequently, small amount of drug reaches the target site from multidirectional drug release. Release of drug to the specific site is important for effective treatment.10 It is particularly necessary for eradicating the local infection at the mucous layer where drugs from conventional formulations may not reach.11 Previous studies mainly focused on developing floating formulations. However, site specific metronidazole delivery system was not explored. Mucoadhesive drug delivery systems have recently been explored for sustained release at the mucosa and increasing bioavailability of drugs.12 Indeed, drug delivery systems with floating and mucoadhesive properties may ideally maximize drug release to the specific site, adhering to the mucous layer, in treatment of upper GIT infection.
This study was aimed to develop metronidazole loaded floating-mucoadhesive microsphere for drug release at the mucous layer of upper GIT. Metronidazole microsphere was developed by ionic gelation method using sodium alginate and calcium chloride. Sodium bicarbonate was used to incorporate floating property in the microsphere. Eudragit L100, which dissolves at a pH greater than 6, was used for sustained drug release. Guar gum, a natural viscous polymer having adhesive property, was used for mucoadhesion. Additionally, guar gum was reported to be useful as a gastroprotective agent against peptic ulcer. It reduces gastric acid, and promotes ulcer healing.13 Besides, alginate has in situ gel forming ability, and good mucoadhesive property.14 Mucoadhesive property of guar gum and sodium alginate was compared.
Materials and Methods
Materials
Eudragit L100 and Carbopol 940 were purchased from Evonik Industries and Lubrizol Corporation, respectively. Metronidazole was provided as a gift sample from Beximco Pharmaceuticals Ltd. Sodium alginate and guar gum were supplied by Sigma-Aldrich. Sodium bicarbonate and calcium chloride (crosslinking agent) were obtained from Merck KGaA, Germany, and Wilfrid Smith Ltd., UK, respectively. Deionized water was locally produced in the laboratory, and used for all the experiments. All chemicals used were of analytical grade.
Methods
Preparation of microsphere
Metronidazole floating-mucoadhesive microspheres were prepared by ionic gelation method15 varying the ratio of guar gum and Eudragit (Table 1). Sodium alginate was dissolved in deionized (DI) water (3% w/v). Carbopol (0.06:1 with alginate) was dissolved separately in DI water, and Euragit L100 was mixed in the thick Carbopol solution. In case of formulations containing guar gum, guar gum was added to the Eudragit-Carbopol mixture. Metronidazole (0.6:1 with alginate) was added in the Carbopol-Eudragit matrix and stirred vigorously. Then the gas forming agent sodium bicarbonate (0.2:1 with alginate) was mixed. The prepared slurry was added to sodium alginate solution and mixed continuously. Crosslinking solution was prepared by dissolving calcium chloride in DI water (5% w/v) containing 10% v/v glacial acetic acid. Then the mixture, free from air bubbles, was added drop-wise to the crosslinking solution through a syringe containing 26G needle. The immediately formed beads were collected by filtration and air dried for 8-10 hours. Spherical dried microspheres were stored in air tight vials for further evaluation. All experiments were performed in triplicate, and the data are shown as mean±standard deviations.
Table 1. Polymer ratio (with alginate), particle size, DEE, and swelling index of the developed microspheres.
| Formulations | Eudragit L100 | Guar gum | Avg. Size of Microsphere (µm) | DEE (%) | Swelling Index |
| M1 | 0.15 | - | 725.34± 17.4 | 39.67±3.21 | 0.53±0.01 |
| M2 | 0.3 | - | 739.91± 23.3 | 46.75±4.19 | 1.20±0.02 |
| M3 | 0.15 | 0.15 | 847.44± 29.7 | 65.12±3.87 | 2.18±0.03 |
| M4 | 0.15 | 0.3 | 901.70± 13.3 | 76.30±2.56 | 2.59±0.01 |
Morphology and size
The morphology of the microspheres was studied by electron scanning microscopy (SEM). Microspheres from each formulation were sputter coated with gold under the vacuum. The images were taken at an acceleration voltage (20 kV) by a scanning electron microscope (JEOL JSM – 6490 LA, Japan). Shape and surface texture of the microspheres were analyzed.
Standard sieving method was used to determine the particle size of the microspheres.16 Microspheres from each formulation were spread on the upper sieve of an automatic sieve shaker (AS 200, Germany) and sieved. The amount passed and retained on each sieve was weighed and the average particle size was calculated using the following equation;
Drug entrapment efficiency (DEE)
Microspheres, containing 50 mg of metronidazole, were crushed and immersed into 100mL of simulated gastric fluid (SGF) (0.1N HCl; pH 1.2, without pepsin). The suspension was kept oscillating overnight and filtered through a 0.22 mm filter. The drug concentration was determined by a UV spectrophotometer (Hatch 4000) at the wavelength of 278 nm. DEE was calculated according to the following equation:
In vitro buoyancy
In vitro floating properties of the metronidazole loaded microspheres were evaluated in a USP dissolution apparatus II (paddle type).7 50 individual microspheres from each formulation were immersed into the vessel filled with 500 mL of SGF. The paddles were rotated at 50 RPM and the temperature was maintained at 37±0.5°C. The number of floating microspheres was counted at hourly intervals up to 8 hours. In vitro buoyancy was expressed as a percentage and was calculated from the following equation:
Swelling measurement
Swelling study was conducted using the dissolution test apparatus II. Accurately weighed amount of beads were placed in the vessels containing SGF and allowed to swell. Rotation speed was set at 50 rpm. The microspheres were withdrawn at predetermined time intervals and blotted with filter paper to remove excess amount of water. The changes in weight were measured at different time intervals until maximum weight was gained. The swelling index was then calculated using the following equation:
Swelling index (S):
Where, Wt denotes the initial weight of the microspheres, and Wm denotes the weight at equilibrium.
In vitro mucoadhesion study
Mucoadhesion property was evaluated using rat stomach mucosa.17 The tissue specimen was collected and cut into required size (2cm × 1cm). Microspheres from each formulation were spread onto the rinsed stomach mucosa and kept inside a humidity chamber at 80% relative humidity and 25°C for 20 minutes. Then those were rinsed with SGF placing at an angle of 45°. The number of microspheres adhering to the tissue was calculated and mucoadhession was expressed as the percentage.
In vitro drug release study
Drug release from the floating microspheres was investigated using the USP dissolution apparatus I (basket type). SGF (0.1M HCl; pH 1.2) was used as the dissolution medium and 500mL of it was poured into each dissolution vessel. Microspheres, equivalent to 50 mg of metronidazole, were placed inside the baskets and they were rotated at a speed of 50 rpm, maintained at a temperature of 37±0.5°C. An aliquot of 5mL was withdrawn at hourly intervals up to 8 hours and the volume was replaced with 5mL of fresh medium. The aliquots were diluted and the concentration of metronidazole was determined spectrophotometrically at 278 nm.
Results and Discussion
Metronidazole microsphere, with floating and mucoadhesive properties, was successfully developed by ionic gelation method. The underlying mechanism involves crosslinking of the carboxylate groups of the alginate molecules by divalent calcium ions.18 Sodium bicarbonate was used as the gas forming substance to incorporate floating property, which reacted with glacial acetic acid and formed CO2 [equation 1]. Four different formulations (M1-M4) were developed varying the ratio of Eudragit L100 and guar gum, keeping the amount of alginate, metronidazole, Carbopol, and sodium bicarbonate constant. The amount of sodium bicarbonate was determined by formulating microspheres with lower amounts. The specified amount was found to be optimal for developing floating microsphere.
Equation 1:
NaHCO3 +CH3COOH = CH3COONa +CO2+ H2O ------------- (1)
Production of carbon dioxide by sodium bicarbonate usually impedes the crosslinking of the alginate molecules. As Carbopol is viscous and possesses free carboxylate groups,19 it was used for additional crosslinking by calcium ions to increase bead strength and surface smoothness.
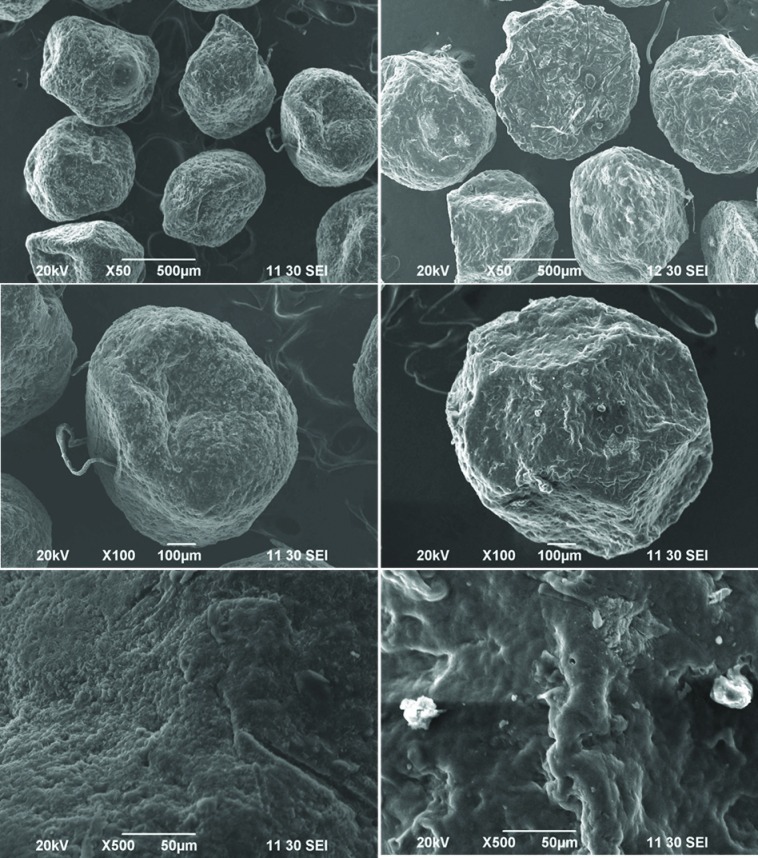
All microspheres were discrete and acceptably spherical. The formulations M1 and M2 were relatively smoother than the microspheres containing guar gum (M3 and M4). Figure 1 shows the shape and surface topography of the microspheres. The SEM images showed that the surface of M2 was smoother than that of M4 (Figure 1). Presence of guar gum on the surface of the microspheres might cause a slightly rough surface as guar gum can interfere with the crosslinking process of alginate by calcium ions.
Figure 1.
Shape and surface texture of the prepared microspheres: left: formulation M2, right: formulation M4.
Table 1 summarizes the formulation variables, size, swelling index, and DEE of the formulations. The sizes of formulation M1 and M2 were smaller than that of M3 and M4. Addition of guar gum increased the viscosity of the slurry resulting in an increase in droplet volume which caused larger particle size. Thus, the size of the microspheres also increased with increasing polymer ratio. Swelling study was performed in order to evaluate solvent uptake by different formulations. It can also be correlated with drug diffusion. Result showed that swelling ability of M3 and M4 was higher than that of other formulations (Table 1). Greater hydrophilicity and high water retaining capacity of guar gum facilitated increased swelling. Moreover, surface guar gum provided rapid hydration. In contrast, swelling of M1 and M2 was low due to lower hydration rate and lack of polymer solubility.
The DEE was affected by both polymer ratio and the type of polymer. Formulations M3 and M4 possessed higher DEE (65.12% and 76.3%, respectively) than that of formulations M1 and M2. Addition of guar gum increased viscosity and prevented diffusion of drug to the crosslinking solution more than Carbopol alone during carbon dioxide formation by sodium bicarbonate. The entrapment efficiency of M1 and M2 was low due to the diffusion of drug into the crosslinking solution as a result of CO2 production. DEE increased with increasing polymer ratio due to increase of viscosity. In vitro floating test revealed that all the formulations had excellent floating ability. 100% floating was observed for all the batches (M1-M4) after 8 hours of study. Eudragit might have aided for prolonged floating because of its low bulk density as previously reported.20
Figure 2 illustrates the in vitro mucoadhesion properties of different formulations. The formulations were compared with the control spheres (prepared with alginate, Eudragit (0.3), and sodium bicarbonate). The formulations M3 and M4 showed higher mucoadhesion than that of formulations M1 and M2. M4 demonstrated the highest mucoadhesion ability (61.21%) among all formulations. According to the mechanism of mucoadhesion, wetting and swelling of the polymer is the initial step employing intimate contact with the mucosa which results in interpenetration and entanglement between the polymer and the mucin chains, promoting hydrogen bond formation.21Rapid hydration of guar gum present on the microsphere surface facilitated expansion of polymer chain and formation of hydrogen bond with the mucous layer. Linear relationship was observed between swelling index and mucoadhesion. In contrast, lack of surface hydration resulted in reduced solvent transfer which might cause low mucoadhesion of formulations M1 and M2. Hence, alginate alone could not sufficiently provide mucoadhesion. Combination of alginate and guar gum can work well to form adhesion bond with the gastric mucosa. This result showed that the developed formulation can provide drug release at the gastric mucosa having both floating and mucoadhesive properties. Previously reported studies focused on floating formulation of metronidazole. However, for treating local infection at the gastric mucosa, site specific drug release would be more beneficial. Moreover, Formulations with only floating property provides multidirectional drug release. Combination of floating and mucoadhesive properties maximizes drug release at the mucus membrane.
Figure 2.
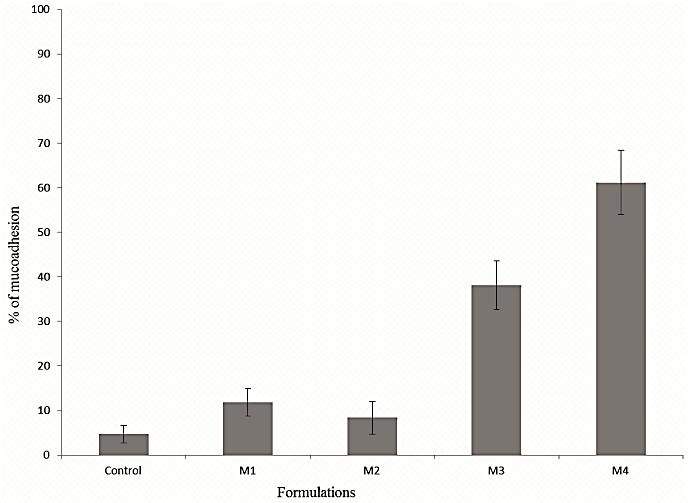
In vitro mucoadhesion property of different formulations. Control: alginate microsphere with Eudragit and sodium bicarbonate only.
Drug release was affected by polymer solubility and surface hydration of the microspheres. Figure 3 shows the drug release profile of different formulations. The release of metronidazole from the formulations M1 and M2 was very low (~ 31% and 29% release, respectively after 8 h). Acid insoluble property of Eudragit L100 and lack of hydration resulted in poor solvent transfer and very slow drug release. A large quantity of drugs might be embedded in Eudragit and could not diffuse out of the microspheres, leading to the low cumulative release of the drug. However, formulations M3 and M4 provided steady and sustained drug release (~ 62% and 73%, respectively, after 8 h). Hydration and swelling of guar gum attributed to the steady drug release. Guar gum is thixotropic in nature which can facilitate solvent transfer into the microspheres. A little faster release in the first hour was possibly associated with the amount of drug present on the particle surface. Mechanism of drug release was determined by fitting the dissolution data in different kinetic models. It revealed that the release of drug followed Higuchi’s model where non-Fickian diffusion was the primary mechanism.
Figure 3.

Drug release profile of the metronidazole loaded floating microspheres in SGF.
Among all the formulations, M4 showed the best results in terms of floating, DEE, mucoadhesion, and drug release. The effect of guar gum was prominent in the formulation. The results substantiate the primary hypothesis for providing specific drug release to the gastric mucosa with prolonged floating and good mucoadhesive property.
Conclusion
In this study, metronidazole loaded floating-mucoadhesive microsphere was developed for drug delivery to the mucous layer in upper GIT. Microspheres were successfully developed with the combination of natural and synthetic polymers. Guar gum was found to be important for DEE, mucoadhesion, and drug release of the microspheres. Formulation M4 showed the best results which contained maximum amount of guar gum among all other formulations. It provided longer floating, good mucoadhesion, and steady drug release implying its potential for the treatment of upper GIT infections.
Acknowledgments
The authors are thankful to the Department of Pharmacy, Stamford University Bangladesh for providing materials and support.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- 1.Cohen-Wolkowiez M, Sampson M, Bloom BT, Arrieta A, Wynn JL, Martz K. et al. Determining population and developmental pharmacokinetics of metronidazole using plasma and dried blood spot samples from premature infants. Pediatr Infect Dis J. 2013;32(9):956–61. doi: 10.1097/INF.0b013e3182947cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de CBC, Berto LA, Venancio PC, Cogo K, Franz-Montan M, Motta RH. et al. Concentrations of metronidazole in human plasma and saliva after tablet or gel administration. J Pharm Pharmacol. 2014;66(1):40–7. doi: 10.1111/jphp.12161. [DOI] [PubMed] [Google Scholar]
- 3.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano M, Cuomo A. Eradication of helicobacter pylori: A clinical update. MedGenMed. 2004;6(1):19. [PMC free article] [PubMed] [Google Scholar]
- 5.Sahasathian T, Praphairaksit N, Muangsin N. Mucoadhesive and floating chitosan-coated alginate beads for the controlled gastric release of amoxicillin. Arch Pharm Res. 2010;33(6):889–99. doi: 10.1007/s12272-010-0612-8. [DOI] [PubMed] [Google Scholar]
- 6.Chun MK, Sah H, Choi HK. Preparation of mucoadhesive microspheres containing antimicrobial agents for eradication of h. Pylori. Int J Pharm. 2005;297(1-2):172–9. doi: 10.1016/j.ijpharm.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Ishak RA, Awad GA, Mortada ND, Nour SA. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-helicobacter pylori therapy. J Control Release. 2007;119(2):207–14. doi: 10.1016/j.jconrel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen YC, Ho HO, Liu DZ, Siow WS, Sheu MT. Swelling/floating capability and drug release characterizations of gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose. PLoS One. 2015;10(1):e0116914. doi: 10.1371/journal.pone.0116914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asnaashari S, Khoei NS, Zarrintan MH, Adibkia K, Javadzadeh Y. Preparation and evaluation of novel metronidazole sustained release and floating matrix tablets. Pharm Dev Technol. 2011;16(4):400–7. doi: 10.3109/10837451003774393. [DOI] [PubMed] [Google Scholar]
- 10.Bakowsky H, Richter T, Kneuer C, Hoekstra D, Rothe U, Bendas G. et al. Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim Biophys Acta. 2008;1778(1):242–9. doi: 10.1016/j.bbamem.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Bytzer P, O'Morain C. Treatment of helicobacter pylori. Helicobacter. 2005;10 Suppl 1:40–6. doi: 10.1111/j.1523-5378.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3(1):89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14(8):581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Kesavan K, Nath G, Pandit JK. Sodium alginate based mucoadhesive system for gatifloxacin and its in vitro antibacterial activity. Sci Pharm. 2010;78(4):941–57. doi: 10.3797/scipharm.1004-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi BY, Park HJ, Hwang SJ, Park JB. Preparation of alginate beads for floating drug delivery system: Effects of co(2) gas-forming agents. Int J Pharm. 2002;239(1-2):81–91. doi: 10.1016/s0378-5173(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 16.Mateovic-Rojnik T, Frlan R, Bogataj M, Bukovec P, Mrhar A. Effect of preparation temperature in solvent evaporation process on eudragit rs microsphere properties. Chem Pharm Bull (Tokyo) 2005;53(1):143–6. doi: 10.1248/cpb.53.143. [DOI] [PubMed] [Google Scholar]
- 17.Rajinikanth PS, Karunagaran LN, Balasubramaniam J, Mishra B. Formulation and evaluation of clarithromycin microspheres for eradication of helicobacter pylori. Chem Pharm Bull (Tokyo) 2008;56(12):1658–64. doi: 10.1248/cpb.56.1658. [DOI] [PubMed] [Google Scholar]
- 18.Amin ML, Jesmeen T, Sutradhar KB, Mannan MA. Development and in vitro evaluation of diclofenac sodium loaded mucoadhesive microsphere with natural gum for sustained delivery. Curr Drug Deliv. 2013;10(6):765–70. doi: 10.2174/15672018113109990054. [DOI] [PubMed] [Google Scholar]
- 19. Neutralizing Carbopol® and Pemulen™ Polymers in Aqueous and Hydroalcoholic Systems. Lubrizol. Technical Data Sheet. https://www.lubrizol.com/Home-Care/Documents/Technical-Data-Sheets/TDS-237-Neutralizing-Carbopol-Pemulen-in-Aqueous-Hydroalcoholic-Systems--HC.pdf. 2015.
- 20.El-Kamel AH, Sokar MS, Al Gamal SS, Naggar VF. Preparation and evaluation of ketoprofen floating oral delivery system. Int J Pharm. 2001;220(1-2):13–21. doi: 10.1016/s0378-5173(01)00574-9. [DOI] [PubMed] [Google Scholar]
- 21.Serra L, Domenech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71(3):519–28. doi: 10.1016/j.ejpb.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]