Abstract
Purpose: Exposure to diazinon can trigger acute and chronic toxicity and significantly induces DNA damage and proapoptotic effects in different human cells. Due to the significance of probiotic bacteria antitoxin effect, this study aimed to investigate the effect of Lactobacillus casei on diazinon (DZN) cytotoxicity in human umbilical vein endothelial cells (HUVEC) in vitro.
Methods: The cytotoxicity assessments were performed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test, DAPI (4',6-diamidino-2-phenylindole) staining and flow cytometric methodologies.
Results: Cytotoxic assessments through flow cytometry/ DAPI staining demonstrated that apoptosis is the main cytotoxic mechanism of diazinon in HUVEC cells and L. casei could decrease the diazinon cytotoxic effects on toxicants.
Conclusion: the screen of total bacterial secreted metabolites can be considered as a wealthy source to find the new active compounds to introduce as reducing agricultural remained pesticide cytotoxicity effects on the human food chain.
Keywords: Apoptosis, Cytotoxicity, Diazinon, Lactobacillus casei, Probiotic
Introduction
Pesticides are widely used to increase the agricultural production through the control of the harmful insects’ populations. Among these pesticides, the organophosphorus pesticides accounted for 50 percent of all insecticide applications.1,2 Diazinon, (O, O -diethyl O -2-isopropyl-6-methylpyrimidin-4-yl phosphorothioate), a broad-spectrum organophosphorus pesticide, is used on a wide range of crops such as rice, fruits, wine grapes, sugarcane, corn, and potatoes.3 The main concern about the utilization of pesticides such as diazinon (DZN) is their residual amount in agricultural products, soil and water.4,5 Pesticides are stored in various tissues of plants that enter the human food chain through the consumption of the edible segments. According to the commission of the European communities report, 45 percent of fruits and vegetables contain maximum residue levels of pesticides (MRLs).6 Monitoring of pesticide residue in Brazilian fruits has indicated that 14.3% of samples exceeded European Union MRLs.7
DZN inhibits the acetylcholinesterase enzyme activity that hydrolyzes the neurotransmitter acetylcholine in cholinergic synapses and neuromuscular junctions.3,8 Exposure to DZN leads to acute symptoms such as anorexia, diarrhea, generalized weakness, muscle tremors, abnormal posturing and behavior, depression and even death as well as chronic effects including genotoxicity, immunotoxicity, effects on the reproductive system, damage to brain and intestinal cells.3,9-11 The organophosphorus pesticides significantly induce DNA damage and pro-apoptotic effects in many different healthy cells.12,13 Environmental protection agency (EPA) classifies DZN as “not likely a human carcinogen”, but experimental studies have confirmed its carcinogenicity such as leukemia, non-Hodgkin’s lymphoma besides lung, brain and prostate cancers.14
Probiotic bacteria are living microorganisms and when are administered in adequate amounts, confer health benefits on the host.15 Probiotics may have positive effects on toxic substances and performance or on toxicity of transferred drugs or toxins into the body.16 Numerous studies have previously shown the probiotics beneficial effects on human health through their antimicrobial effects, antitoxin effects, the improvement of intestinal barrier function, the modulation of immune responses, the impacts on apoptosis and cell proliferation, and anti-oxidant function.17,18 Several studies have assayed the viability, the colonization ability, and the binding capacity of probiotics to toxic substances.19 Lactobacillus casei strain DN114001 can bind to heterocyclic aromatic amines in vitro and can decrease the concentration and the genotoxicity of these amines.19 Various strains of bifidobacteria hand in hand with Lactobacillus reuteri strain NRRL14171 and Lactobacillus casei strain Shirota were able to bind to aflatoxin B1 that can be attributed to the presence of these bacteria in the gastrointestinal tract which may prevent the absorption of aflatoxins.20,21 Lactobacillus kefir strains (CIDCA 83115, 8321, 8345 and 8348) were able to bind to Clostridium difficile toxins by surface layer (s-layer) proteins.22 By considering the aforementioned issues, this study aimed to evaluate the effects of lactobacillus casei secretion metabolites on the toxicity of agricultural organophosphorus pesticide (diazinon) through investigating the metabolites effects on diazinon treated/untreated human normal cell line, HUVEC in vitro.
Materials and Methods
Bacteria isolation
Lactobacillus casei was isolated from the traditional yogurt samples collected from East Azarbayjan, the northwest province in Iran. 5 g of each sample was suspended in 2% w/v sodium citrate solution and homogenized using the Stomacher 400 Circulator (Seward Laboratory Systems Inc, USA) for 2 min. Afterwards, 1 ml of the samples was added to 24 ml of de Man Rogosa and Sharpe (MRS, Merck, Germany) broth medium and incubated at 37 °C for 24 h. After the incubation time, the bacteria were isolated by spreading them on a de Man Rogosa and Sharpe (MRS, Merck, Germany) agar plate similar to the previous condition. Many single colonies were randomly selected and again incubated in 5 ml MRS broth for 24 h. The individual colonies were subjected to morphological evaluation. Gram positive and catalase negative bacilli colonies were stored at -80°C in MRS supplemented with glycerol 25% (v/v).
Molecular identification by 16S rDNA
Total genomic DNA was extracted from the cultures inoculated with a single colony using the previously described procedure by Drisko.23 For this purpose, 1.5 ml of the bacterial culture, a single sub-cultured colony in MRS broth for 24 h at 37°C, was centrifuged at 10000×g for 5 min and the cell pellet was used to isolate the DNA. All the extracted genomic DNAs of the samples, resuspended in 50 µl distilled water, were then checked and visualized via 0.8% agarose gel electrophoresis. Subsequently, the gel monitoring apparatus (Biometra, Gottingen, Germany) and spectrophotometric method were used to evaluate the quality and quantity of the extracted DNA, respectively. The PCR amplification was conducted in a thermal cycler PTC 200 (MJC research, Waltham, USA) by using a pair of LAB-specific universal primers (LABF 5´-AGATTTTGATCMTGGCTCAG-3´ and LABR 5´-TACCTTGTTAGGACTTCACC-3´). PCR amplification was performed using the following temperature profile: an initial denaturation at 94°C for 4 min, followed by 32 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 1 min, and a final extension step at 72°C for 5 min.24 The PCR products were determined by electrophoresis in a 1% (w/v) agarose gel and were visualized through ethidium bromide staining. The PCR products were sequenced at Sinaclone Corporation, Tehran, Iran. The sequences were then analyzed using the BLAST program of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST).
Acid and bile salt tolerance
The isolated cells were harvested from the cultures incubated overnight followed by centrifugation for 10 min at 6000×g and 4°C . The cell pellets were then resuspended in PBS (80 mM Na2HPO4, 1.5 M NaCl, 20 mM KH2PO4, 30 mM KCl, pH 3.0) and were incubated at 37°C for 3 h in MRS broth. The viable cells after low pH treatment were subjected to PBS (80 mM Na2HPO4, 1.5 M NaCl, 20 mM KH2PO4, 30 mM KCl, pH 7.2) containing 0.3% (w/v) of bile salt (Sigma Chemical Co., St. Louis, Mo., USA) then were incubated at 37°C for 4 h in MRS broth. Proper dilutions based on 1 h time intervals were performed, and the dilutions were centrifuged for 10 min at 6000×g and 4°C then the cell plates (acid and bile salt resistance bacteria) were incubated at 37°C in anaerobic conditions for 24 h. The survival of the bacterial cells was evaluated using log phase cultures (8 log10CFU ml-1) by plating them on MRS agar after 0, 1, 2, and 3 h of incubation in acidic (pH 3.0)/bile supplemented MRS broth at 37°C via the standard pour plate technique.
The survival rate (for both acid/bile resistance) was calculated using the following equation: survival rate (%) = (log cfu N1/log cfu N0) × 100, where N1 corresponds to the total clones treated with extra bile salts or acids and N0 corresponds to the total clones before they were incubated under harsh conditions.
Antimicrobial activity
Antibacterial activity assessments were conducted against clinically important human pathogens, including native isolate of E. coli (026), Candida albicans (PTCC 5027), Escherichia coli (057) (PTCC 1276), Salmonella enterica subsp. typhimurium (ATCC 14028), Klebsiella pneumoniae (PTCC 1053), Shigella flexneri (PTCC 1234), Pseudomonas aeruginosa (PTCC 1181), Serratia marcesens (PTCC 1187), Staphylococcus aureus (ATCC 25923), Bacillus cereus subsp. kenyae (PTCC 1539), Listeria monocytogenes (PTCC 1163), Enterococcus faecalis (PTCC 1394), Staphylococcus saprophyticus subsp. saprophyticus (PTCC 1440), and Streptococcus mutans (PTCC 1683). The overnight cultured isolated strains in MRS broth medium at 37 °C were filtered through 0.2 µm filter, and then 50 µl of each filtrate was added to 7 mm diameter wells on the indicator growth medium agar, which were previously incubated overnight by indicator pathogens at 37 °C. The pH of the isolated fresh supernatants was adjusted to the pH of the each indicator pathogens growth media (Table 1). After the overnight incubation of plates at 37 °C, the clear zones around of each well were measured and considered as positive antibacterial activity. Based on the diameter of the inhibition zone, anti-pathogen activities were divided to strong (diameter ≥ 20 mm), moderate (20 mm ≤ diameter ≥ 10 mm), and weak (diameter ≤ 10 mm).25
Table 1. Indicator microorganisms and their growth condition .
| Indicator microorganism | Growth condition |
| Candida albicans (PTCC 5027) | Yeast mold agar, pH 6.2 ± 0.2 |
| E. coli (026) | Nutrient agar, pH 7.0 |
| Escherichia coli (057) | Nutrient agar, pH 7.0 |
| Salmonella enterica subsp. typhimurium (ATCC 14028) | Nutrient agar, pH 7.0 |
| Shigella flexneri (PTCC 1234) | Nutrient agar, pH 7.0 |
| Serratia marcesens (PTCC 1187) | Nutrient agar, pH 7.07.0 |
| Staphylococcus aureus (ATCC 25923) | Micrococcus medium, FDA, pH 7.2 |
| Staphylococcus saprophyticus subsp. saprophyticus (PTCC 1440) | Nutrient agar, pH 7.0 |
| Streptococcus mutans (PTCC 1683) | Blood agar, Difco 0045 |
| Klebsiella pneumoniae (PTCC 1053) | Nutrient agar, pH 7.0 |
| Pseudomonas aeruginosa (PTCC 1181) | Nutrient agar, pH 7.0 |
| Bacillus cereus subsp. kenyae (PTCC 1539) | Nutrient agar, pH 7.0 |
| Listeria monocytogenes (PTCC 1163) | Blood agar, Difco 0045 |
| Enterococcus faecalis (PTCC 1394) | Blood agar, Difco 0045 |
Antibiotic susceptibility
To determine the antibiotic susceptibility, the disc diffusion method against some clinically important antibiotics such as chloramphenicol (30 µg), vancomycin (30 µg), tetracycline (30 µg), erythromycin (15 µg), Ampicillin (10 µg), gentamycin (10 µg), clindamycin (2µg), sulfamethoxazol (25µg) and penicillin (10,000 units) was used. The isolated bacteria were completely incubated on the Mueller-Hinton agar plate and then the antibiotic disks (Padtan Teb Co., Tehran, Iran) were manually placed on plates by using the sterile forceps and after 24 h incubation at 37 °C, the clear zones were measured according to disks producer’s guidelines. Based on the areola size, the isolates were classified to sensitive, intermediate and resistant groups through the analysis of data.
Cell culture
The Human umbilical vein endothelial cells (HUVEC) were obtained from Pasteur Institute, National Cell Bank of Iran. The cells were cultured into 25 cm2 T-flask with cell density (1×106 cell/ml)in RPMI 1640 (GIBCO, Uxbridge, UK) medium containing 10% fetal bovine serum (HyClone, Logan, UT, USA) inactivated at 45 °C for 1 h, 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma, St. Louis, MO, USA) were incubated at 37˚C in a humidified atmosphere with 5% CO2. The media of t-flasks were renovated each 48 h intervals. For the cell passage, all cells were detached using 0.025% trypsin-0.02% EDTA (Sigma, St. Louis, MO, USA).
Cell treatment
Lactobacillus casei was cultured overnight in de Man Rogosa (MRS) broth medium (Merck, Darmstadt, Germany). To prepare cell free supernatant, the culture was centrifuged at 4000 rpm for 5 min and the supernatant’s pH was adjusted to 7.2 before sterilizing through filtering using 0.2 µm membrane filter (Millipore, Eschborn, Germany). The filtered supernatant was used for the treatment of HUVEC cell line (treated with median-lethal concentration of DZN (IC50: 70 µg/ml) or the untreated control). Diazinon was purchased from Merck (Merck, Darmstadt, Germany) and stored at 25 °C and kept in dark condition. Dissolved DZN in dimethyl sulfoxide (DMSO), in which DMSO was <1% (safe concentration for human cells at in vitro condition), with final concentration 100 µg/ml was sterilized using a 0.2 µm filter (Millipore, Eschborn, Germany). Ten concentrations of supernatant, 10-100 µg/ml with 10 intervals, were prepared to determine of DZN IC50. HUVEC cells (with seeding density 1.2×104 cells/well) were directly treated by the formerly-prepared concentrations of DZN with six repetitions for each concentration in 96-well microplates and were incubated for 24 hours at 37 °C and 5% CO2. After incubation time the treated/untreated HUVEC cell line, cultured in microplate, was used to MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma, St. Louis, MO, USA) assay.
50 µl of MTT solution (2 mg/ml in phosphate buffer saline) was added to each well after replacing the previous media with 150 µl of fresh culture medium, then these cells were incubated for 4 h similar to the culture condition. After that, the medium containing MTT was completely removed from each well; subsequently, 200 µl DMSO plus 25 µl of Sorenson’s glycine buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5) were added into each well and the microplates were incubated for 25 min at room temperature and finally the absorbance was measured by employing a microplate reader (Biotek, ELx 800, USA) at 570 nm.
DAPI staining
All treated/untreated HUVEC cells’ culture medium was removed from the 6 well plates and the attached cells were washed twice by PBS (pH=7.2). The washed cells were fixed by 500 µl of 1% paraformaldehyde for 5 min. To increase the permeability of fixed cells, paraformaldehyde was replaced with 500 µl of 0.1% Triton X-100 and incubated for 10 min at room temperature. The permeabilized cells were subjected to 100 µl of 4', 6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) solution (250 ng/ml for each well) and then were incubated for 10 min at room temperature. The morphological changes were analyzed using a fluorescent microscope (Olympus BX64, Olympus, Japan) equipped with a U-MWU2 fluorescence filter (excitation filter BP 330e385, dichromatic mirror DM 400, and emission filter LP 420).
Flow cytometric analysis
To remove RPMI medium, the detached all treated/untreated control HUVEC cells were centrifuged at 335 ×g for 10 min and the cell plates were washed with PBS (pH=7.2) then were centrifuged again similar to previous condition. During the next phase, the cells were re-suspended in 100 µl binding buffer (1×106 cells/ ml) of Annexin V-FITC kit (eBioscience, San Diego, CA). The binding buffer containing cells were mixed with 5 µl Annexin V-FITC, next 10 µl propidium iodide solution was added to the cell suspension and were kept in a 5 ml culture tube for 15 min at room temperature in dark conditions. Binding buffer (400 µl) was again added to each culture tube and assessments were conducted using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). The analysis on 100,000 cells was accomplished at a rate of 1000 cells/s. Quadrant setting was conducted using the untreated cell line as the negative control. Data analysis was performed using CellQuest Pro software (BD Biosciences, San Jose, CA, USA). Flow cytometry assessments were conducted thrice with three repetitions for each time.
Statistical Analysis
The statistical analysis was performed by SPSS software version 18.0 (SPSSInc, Chicago, IL, USA). The normal distribution of data was tested by Kolmogorov-Smirnov test. ANOVA and Tukey's post hoc test were used for analyzing data and multiple mean comparisons, respectively. Statistical significance was considered a value of P≤ 0.05 and quantitative data were reported as mean ± SD. All experiments were repeated three times with six replicates for each experiment.
Results
Isolation and identification
A total of 22 grown hemispherical white or achromatic colonies was separately propagated for further assessments. The presence of lactic acid bacteria strains in the isolated samples were confirmed by amplifying the 16S rDNA gene using gene-specific primers. Sequences of 16S rDNA gene 1500 bp fragments were blasted with the deposited sequences in GenBank. Isolates with 99% to 100% homology were identified by considering the threshold values of taxonomical studies (97%).26 These 22 colonies belonged to Lactobacillus casei strain YSH, Lactobacillus paracasei strain YJ, Lactobacillus rhamnosus strain YI, Lactobacillus fermentum strain YAL, Lactobacillus plantarum strain YSH1, and Lactobacillus delbrueckii strain YJI.
Resistance to acid and bile salt
The survival rates of the six isolated LAB strains after incubating for 3 h at pH 3 and in 0.3% bile salt (oxgall; Sigma Chemical Co., St. Louis, Mo., USA) are shown in Table 2. Based on the results, all six selected strains retained their viability at the mentioned harshness condition where the tolerance to acidic/high bile salt conditions was strain specific. The survival rates ranging from 73% to 85% were observed in Lactobacillus strains at acidic condition, whereas the survival rates, ranging from 92% to 98%, were observed in bile salt condition. The strains with the most efficient tolerance to acidic conditions were L. plantarum strain YSH1, L. rhamnosus strain YI, L. delbrueckii strain YJI, and L. casei strain YSH with survival rates of 85%, 82%, 81%, and 78%, respectively. Meanwhile, the six isolates showed high survival rates with >90% under high bile conditions. The strains with the highest tolerance to 0.3% oxgall were L. casei strain YSH, L. fermentum strain YAL, L. rhamnosus strain YI with the survival rates of 98%, 98%, and 96%, respectively.
Table 2. The survival rates of isolated LAB after 3 h incubation at pH 3 and 0.3% bile salts .
| Bacteria |
pH 3
Final counts (log cfu/ml) after incubation at: |
0.3% bile salt
Final counts (log cfu/ml) after incubation at: |
||||||||
| 0 h | 1h | 2h | 3 h | SR(%) | 0h | 1h | 2h | 3 h | SR(%) | |
| L. paracasei strain YJ | 8.873 | 8.754 | 7.124 | 6.743 | 76 | 8.768 | 8.754 | 8.124 | 8.066 | 92 |
| L. plantarum strain YSH1 | 8.401 | 8.112 | 7.325 | 7.14 | 85 | 8.783 | 8.712 | 8.325 | 8.256 | 94 |
| L. delbrueckii strain YJI | 8.974 | 8.589 | 7.412 | 7.268 | 81 | 8.875 | 8.789 | 8.512 | 8.431 | 95 |
| L. fermentum strain YAL | 8.900 | 8.613 | 6.704 | 6.497 | 73 | 8.687 | 8.613 | 8.604 | 8.513 | 98 |
| L. casei strain YSH | 8.683 | 8.454 | 7.012 | 6.772 | 78 | 8.434 | 8.354 | 8.312 | 8.265 | 98 |
| L. rhamnosus strain YI | 8.291 | 8.004 | 6.918 | 6.798 | 82 | 8.394 | 8.304 | 8.118 | 8.058 | 96 |
SR: Survival Rate
Antimicrobial activity
Table 3 shows the 6 isolated strains, including L. casei strain YSH, L. paracasei strain YJ, L. rhamnosus strain YI, L. fermentum strain YAL, L. plantarum strain YSH1, and L. delbrueckii strain YJI displayed significant anti-pathogenic activities against indicator microorganisms.
Table 3. The inhibitory effect of isolated strains against pathogenic microorganisms .
| Pathogens | Diameter of inhibition zone (mm) | |||||
| YJ | YSH | YI | YAL | YSH1 | YJI | |
| P. aeruginosa | 13.3±1.2 | 12.3±0.3 | 14.0±1.0 | 15.3±0.7 | 16.7±0.3 | 16.0±0.6 |
| C. albicans | 0.0±0.0 | 11.0±0.0 | 12.3±0.3 | 0.0±0.0 | 15.0±0.0 | 0.0±0.0 |
| S. marcesens | 13.7±0.3 | 18.3±0.0 | 14.7±0.3 | 13.0±0.6 | 14.0±1.0 | 13.0±0.0 |
| E. faecalis | 0.0±0.0 | 11.0±0.0 | 12.0±0.0 | 0.0±0.0 | 15.3±1.2 | 0.0±0.0 |
| S. saprophyticus | 0.0±0.0 | 12.3±0.7 | 0.0±0.0 | 14.0±0.6 | 0.0±0.0 | 0.0±0.0 |
| S. mutans | 12.0±0.0 | 18.3±0.6 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 13.3±1.2 |
| E. coli (0157) | 14.7±1.2 | 11.0±0.0 | 12.0±1.0 | 0.0±0.0 | 15.3±0.3 | 15.0±0.0 |
| S. typhimurium | 0.0±0.0 | 13.3±1.2 | 12.3±1.2 | 0.0±0.0 | 13.3±0.3 | 0.0±0.0 |
| S. aureus | 0.0±0.0 | 12.7±0.3 | 15.7±0.3 | 0.0±0.0 | 15.0±0.0 | 15.7±1.2 |
| E. coli (026) | 0.0±0.0 | 13.3±0.7 | 13.3±0.6 | 0.0±0.0 | 15.7±0.3 | 0.0±0.0 |
| B. cereus | 0.0±0.0 | 11.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| L. monocytogenes | 0.0±0.0 | 14.7±0.9 | 16.7±0.7 | 0.0±0.0 | 17.0±0.0 | 0.0±0.0 |
| K. pneumoniae | 14.3±0.7 | 13.0±0.6 | 12.3±0.7 | 12.7±1.2 | 13.3±1.2 | 13.0±1.0 |
| S. flexneri | 14.0±0.0 | 13.0±0.0 | 13.0±0.0 | 12.0±0.0 | 14.0±0.6 | 13.3±0.7 |
Notes: values are mean ± standard error
S (strong r ≥20 mm), M (moderate r<20 mm and >10 mm), and W (weak ≤10 mm)
Lactobacillus casei strain YSH, Lactobacillus paracasei strain YJ, Lactobacillus rhamnosus strain YI, Lactobacillus fermentum strain YAL, Lactobacillus plantarum strain YSH1, and Lactobacillus delbrueckii strain YJI
Lactobacillus species, particularly L. casei strain YSH, showed the most efficient antagonistic activity and inhibited the growth of 13 indicator pathogens among the isolated bacteria. Meanwhile, L. rhamnosus strain YI, and L. paracasei strain YJ exhibited an overall good antagonistic activity and inhibited the growth of indicator pathogens.
Antibiotic susceptibility
The antibiotic susceptibility of the isolated bacteria against the high consumption antibiotics was evaluated using the measurements of inhibition zone diameter. The antibiotic susceptibility results of the six isolated LAB against clinically important antibiotics are presented in Table 4. Based on our findings, all six isolated bacteria were sensitive or semi-sensitive to tetracycline and clindamycin. Lactobacillus strains generally displayed the highest susceptibility to the majority of antibiotics. L. casei strain YSH displayed the best results and was sensitive or semi-sensitive to all antibiotics.
Table 4. Antibiotic susceptibility of isolated LAB against the high consumption antibiotics by disc diffusion assay .
| Isolated Strains | Diameter of inhibition zone (mm) | ||||||||
| C | TE | ER | AM | GE | CC | SLX | P | V | |
| L. casei strain YSH | 30S | 30S | 23I | 32S | 15S | 26S | 22S | 23S | 22S |
| L. plantarum strain YSH1 | 20S | 20S | 20I | 40S | 11S | 18S | 25S | 40S | 0R |
| L. delbrueckii strain YJI | 23S | 25S | 25S | 28S | 25S | 27S | 0R | 23S | 0R |
| L. fermentum strain YAL | 28S | 18S | 26S | 26S | 18S | 27S | 0R | 15I | 24S |
| L. paracasei strain YJ | 20S | 20S | 0R | 24S | 13S | 30S | 15I | 24S | 30S |
| L. rhamnosus strain YI | 22S | 30S | 20I | 25S | 12S | 20S | 22S | 24S | 15S |
chloramphenicol; TE: tetracycline; ER: erythromycin; AM: Ampicillin; GE: gentamycin; CC: clindamycin; SLX: sulfamethoxazol; P: penicillin; V: vancomycin
Erythromycin results based on R ≤13 mm; I: 13–23 mm; S≥23 mm.
Gentamycin results based on R ≤6 mm; I: 7–9 mm; S≥10 mm.
Vancomycin results based on R ≤12 mm; I: 12–13 mm; S≥13 mm.
I: intermediate (zone diameter, 12.5–17.4mm); R: resistant (zone diameter, ≤12.4mm); S: susceptible (zone diameter, ≥17.5).
The effect of L. casei on diazinon toxicity
The effect of different concentrations of DZN on cell proliferation was measured by MTT assay. DZN at concentration of 70 µg/ml reduced the viability of HUVEC cell lines by 50.97% in a dose-response manner, so, this concentration was selected as IC50 of diazinon in this study and used in other treatments. Among the other strains, L. casei cell free supernatant (50 µg/ml) significantly increased the cell viability compared with control (p≤0.01) while DZN IC50 increased cell death at a concentration of 70 µg/ml (p≤ 0.01).The treatment of HUVEC cell line with IC50 of diazinon and L. casei secreted metabolites showed a significant decrease in the cytotoxicity of DZN on HUVEC cell lines (Figure 1).
Figure 1.
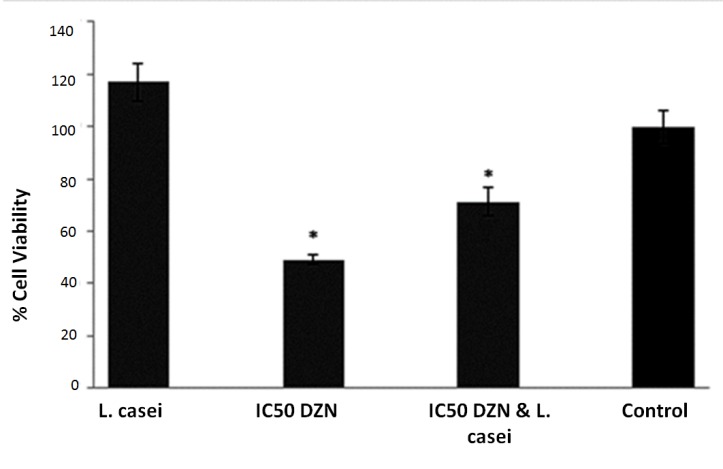
The effect of L. casei on diazinon toxicity. Error bares represent standard deviations. *P≤0.05
Apoptosis assessment
To analyze the apoptosis incidence in DZN treated cells and prove the effect of L. casei on the viability of HUVEC cells, the latter was exposed to 50 µg/ml of filtered supernatant of late stationary phase growth of L. casei after 24 h incubation to visualize the apoptosis using Dapi staining and observation by fluorescent microscopy (Olympus BX64, Olympus, Japan).
The intact viable cells displayed a plenary health nucleus (Figure 2B), whereas the apoptotic cells were characterized by shrinking cells with condensed (early apoptosis) or fragmented (late apoptosis) nuclei (Figure 2A). The DZN treated HUVEC cells illustrated the very distinctive signs of apoptosis, including the formation of micronuclei, cell shrinkage, membrane blebbing, nucleus fragmentation, and apoptotic bodies (Figure 2A). None of these signals were observed in untreated HUVEC cells (Figure 2C). The L. casei secretion metabolites could decrease the apoptosis-related signals in DZN-treated HUVEC cells (Figure 2D).
Figure 2.
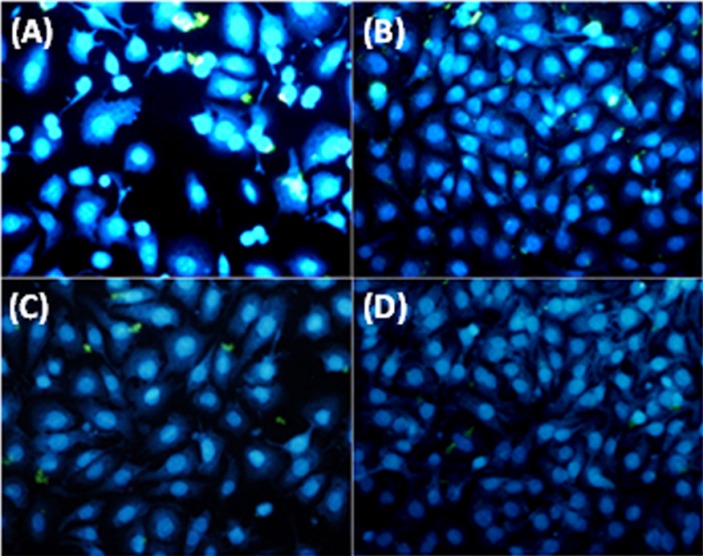
Nuclear morphology analysis using DAPI of HUVEC cell line, A) HUVEC cells treated with IC50 dose of diazinon, B) Untreated HUVEC cells, C) HUVEC cells treated with L. casei and D) HUVEC cells treated with L. casei and IC50 dose of diazinon
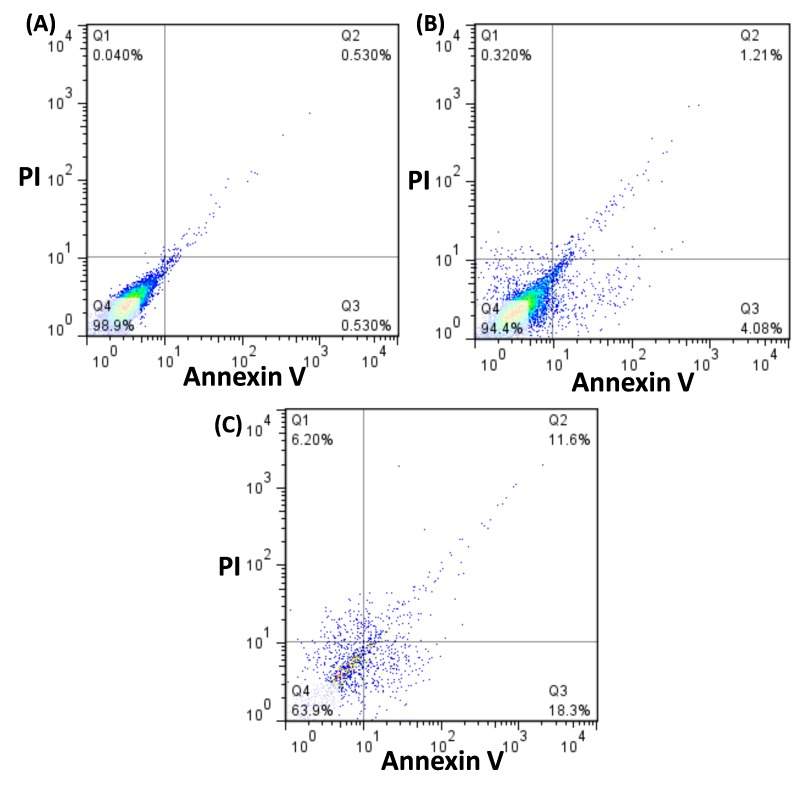
Annexin V-FITC/PI flow cytometry analysis was performed to confirm the metabolite secreted from selected L. casei strain effects on apoptosis of HUVEC cells. These cells were treated with 50 µg/ml L. casei supernatant for 24 h. The dual parameter fluorescent dot blots revealed the viable cell population in the lower left quadrant (annexin V-/PI-), the cells at the early apoptosis are in the lower right quadrant (annexin V+/PI-), and the cells at the late apoptosis are in the upper right quadrant (annexin V+/PI+). Figures 3A to 3C show that 0.53 percent of the cells was annexin V+/PI-in untreated cells after 24 h of post seeding. Our findings indicated that the cytotoxicity of DNZ on HUVEC cells occurred during apoptosis. A total of 18.3% and 11.6% of the treated cells was induced at early and late stages of apoptosis after 24 of incubation. These results revealed that significant differences existed in apoptotic induction by DNZ. The cells stained by PI alone (V-/PI+) underwent necrosis. As shown in Figure 3, the DZN-treated cells demonstrated an increase in the necrotic population (6.2%) shown in the upper left quadrant whereas the toxin treated cells exposed to L. casei supernatant showed a slight increase in necrotic cells (0.32%) compared to the untreated cells (0.04%). These findings proved that L. casei secretion metabolites could decrease the DZN cytotoxicity effects on human normal cells (HUVEC) at in vitro.
Figure 3.
The flow cytometry assessment on HUVEC cell line, A) Untreated HUVEC cells, B) HUVEC cells treated with L. casei and IC50 dose of diazinon. C) HUVEC cells treated with IC50 dose of diazinon, Lower left column: Annexin V/PI (viable cells), lower right column: Annexin V+/PI (early apoptotic cells), upper right column: Annexin V+/P+ (late apoptotic cells) and upper left column Annexin V/P+ (necrotic cells).
Discussion
The results of the present study indicated the treatment of human poisoned cells with L. casei showed the protective effects against DZN induced cytotoxicity at in vitro. Human and animal studies have shown the toxicity of DZN in different tissues such as hematological disorders,27 cardiotoxicicty,27 hepatotoxicity,28 nephrotoxicity,29 neurotoxicity,30 and both female and male reproductive toxicity.29 The exposure of the NTera2/D1 (NT2) cell line to diazinon at concentrations ranging between 10-4 and 10-5 M enhanced cell death with a number of special features of apoptosis including membrane and mitochondrial potential changes.31 Slotkin and Seidler indicated that the target genes of the organophosphates including chlorpyrifos and diazinon are the cell cycle and apoptosis-regulating genes in the developing brain and in neuronotypic cells in vitro.32
In our study, the co-treatment of the HUVEC cells with L. casei supernatant and diazinon decreased cell death and apoptosis induced by diazinon. The previous studies have suggested that some probiotics may regulate apoptosis.33 The activation of the antiapoptotic Akt/protein kinase B and inhibition of the activation of proapoptotic p38/mitogen-activated protein kinase by probiotic bacteria were suggested to prevent apoptosis in the colon epithelial cells.34,35 Also, probiotics possess the antitoxin effects through binding to the toxins ;thus, can protect cells against both membrane and DNA-damaging toxins.36
Although the main mechanism of DZN toxic effects on target and non-target organisms is the inhibition of acetylcholinesterase,37 the researchers have shown that it is not in charge of all of toxic effects and several studies suggest that DZN induces oxidative stress and produces free radicals in biological systems which is the main mechanism of chronic organophosphates (Ops) toxicity.38 The chronic Ops elevate the level of reactive oxygen species which is a major apoptotic stimulant in different organs.31 Indeed, apoptosis is a common outcome for the exposures to toxicant that evoke oxidative stress.2,5,28,39 Diazinon increase lipid peroxidation and decrease antioxidant biomarkers including reduced glutathione, glutathione peroxidase, superoxide dismutase, catalase and total antioxidant capacity in male wistar albino rats.29 The injection of DZN at high doses has increased the level of malondialdehyde, superoxide dismutase and glutathione S-transferase activities and has decreased glutathione (GSH) level, lactate dehydrogenase, and cholinesterase activities in the brain, heart, and spleen of female Wistar and Norway rats.38 Sub-acute exposure to DZN has induced oxidative stress-mediated apoptosis in rat liver through the activation of caspases-9 and -3, and increasing Bax/Bcl-2 expression ratio.28
Probiotics with antioxidant effects, Lactobacillus acidophilus, could decrease malondialdehyde and could increase the levels of antioxidants, glutathione reductase, superoxide dismutase, and glutathione peroxidase in Sprague–Dawley rats.40 Therefore, it seems that anti-apoptotic effects of L. casei in HUVEC cells treated with DZN probably due to a decrease in oxidative stress and anti-oxidant effects.
Conclusion
The results of the present study reveal that diazinon has cytotoxic effects on normal cells and apoptosis is the main cytotoxic mechanism of diazinon; however, L. casei secretions is able to decrease its cytotoxic effects. Although the exact mechanism of anti-toxic effects of L. casei is not clear but the reduction of DZN cytotoxicity on human normal cells is because of its anti-apoptotic mechanisms, antitoxin effects and/or via decreasing the oxidative stress.
Ethical Issues
No ethical issues to be promulgated.
Conflict of Interest
The authors declare that there are no conflicts of interests.
References
- 1.Mosaddegh MH, Emami F, Asghari G. Evaluation of residual diazinon and chlorpiryfos in children herbal medicines by headspace-spme and GC-FID. Iran J Pharm Res. 2014;13(2):541–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Slotkin TA, Seidler FJ. Does mechanism matter? Unrelated neurotoxicants converge on cell cycle and apoptosis during neurodifferentiation. Neurotoxicol Teratol. 2012;34(4):395–402. doi: 10.1016/j.ntt.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal V, Deng X, Tuli A, Goh KS. Diazinon-chemistry and environmental fate: A california perspective. Rev Environ Contam Toxicol. 2013;223:107–40. doi: 10.1007/978-1-4614-5577-6_5. [DOI] [PubMed] [Google Scholar]
- 4.Boobis AR, Ossendorp BC, Banasiak U, Hamey PY, Sebestyen I, Moretto A. Cumulative risk assessment of pesticide residues in food. Toxicol Lett. 2008;180(2):137–50. doi: 10.1016/j.toxlet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. 2013;203(3):547–55. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Ciscato CH, Bertoni Gebara A, Henrique Monteiro S. Pesticide residue monitoring of brazilian fruit for export 2006-2007. Food Addit Contam Part B Surveill. 2009;2(2):140–5. doi: 10.1080/19440040903330326. [DOI] [PubMed] [Google Scholar]
- 8.Pomeroy-Black M, Ehrich M. Organophosphorus compound effects on neurotrophin receptors and intracellular signaling. Toxicol In Vitro. 2012;26(5):759–65. doi: 10.1016/j.tiv.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed MA, Ahmed HI, El-Morsy EM. Melatonin protects against diazinon-induced neurobehavioral changes in rats. Neurochem Res. 2013;38(10):2227–36. doi: 10.1007/s11064-013-1134-9. [DOI] [PubMed] [Google Scholar]
- 10.Lecoeur S, Videmann B, Mazallon M. Effect of organophosphate pesticide diazinon on expression and activity of intestinal P-glycoprotein. Toxicol Lett. 2006;161(3):200–9. doi: 10.1016/j.toxlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Guizzetti M, Pathak S, Giordano G, Costa LG. Effect of organophosphorus insecticides and their metabolites on astroglial cell proliferation. Toxicology. 2005;215(3):182–90. doi: 10.1016/j.tox.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Calviello G, Piccioni E, Boninsegna A, Tedesco B, Maggiano N, Serini S. et al. DNA damage and apoptosis induction by the pesticide mancozeb in rat cells: Involvement of the oxidative mechanism. Toxicol Appl Pharmacol. 2006;211(2):87–96. doi: 10.1016/j.taap.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Carlson K, Jortner BS, Ehrich M. Organophosphorus compound-induced apoptosis in sh-sy5y human neuroblastoma cells. Toxicol Appl Pharmacol. 2000;168(2):102–13. doi: 10.1006/taap.2000.8997. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H. et al. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol. 2012;34(3):959–68. doi: 10.1016/j.etap.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salminen S, Nybom S, Meriluoto J, Collado MC, Vesterlund S, El-Nezami H. Interaction of probiotics and pathogens--benefits to human health? Curr Opin Biotechnol. 2010;21(2):157–67. doi: 10.1016/j.copbio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira Silva E, Cruz de Carvalho T, Parshikov IA, Alves dos Santos R, Silva Emery F, Jacometti Cardoso Furtado NA. Cytotoxicity of lapachol metabolites produced by probiotics. Lett Appl Microbiol. 2014;59(1):108–14. doi: 10.1111/lam.12251. [DOI] [PubMed] [Google Scholar]
- 17.Uccello M, Malaguarnera G, Basile F, D'Agata V, Malaguarnera M, Bertino G. et al. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12 Suppl 1:S35. doi: 10.1186/1471-2482-12-S1-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014;44(1):1–8. doi: 10.1016/j.medmal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Nowak A, Libudzisz Z. Ability of probiotic lactobacillus casei dn 114001 to bind or/and metabolise heterocyclic aromatic amines in vitro. Eur J Nutr. 2009;48(7):419–27. doi: 10.1007/s00394-009-0030-1. [DOI] [PubMed] [Google Scholar]
- 20.Oatley JT, Rarick MD, Ji GE, Linz JE. Binding of aflatoxin B1 to bifidobacteria in vitro. J Food Prot. 2000;63(8):1133–6. doi: 10.4315/0362-028x-63.8.1133. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Mendoza A, Guzman-de-Pena D, Garcia HS. Key role of teichoic acids on aflatoxin b binding by probiotic bacteria. J Appl Microbiol. 2009;107(2):395–403. doi: 10.1111/j.1365-2672.2009.04217.x. [DOI] [PubMed] [Google Scholar]
- 22.Carasi P, Trejo FM, Perez PF, De Antoni GL, Serradell Mde L. Surface proteins from lactobacillus kefir antagonize in vitro cytotoxic effect of clostridium difficile toxins. Anaerobe. 2012;18(1):135–42. doi: 10.1016/j.anaerobe.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Drisko J, Bischoff B, Giles C, Adelson M, Rao RV, McCallum R. Evaluation of five probiotic products for label claims by DNA extraction and polymerase chain reaction analysis. Dig Dis Sci. 2005;50(6):1113–7. doi: 10.1007/s10620-005-2931-z. [DOI] [PubMed] [Google Scholar]
- 24.Dubernet S, Desmasures N, Gueguen M. A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Lett. 2002;214(2):271–5. doi: 10.1111/j.1574-6968.2002.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 25.Haghshenas B, Abdullah N, Nami Y, Radiah D, Rosli R, Khosroushahi AY. Different effects of two newly-isolated probiotic lactobacillus plantarum 15HN and lactococcus lactis subsp. Lactis 44lac strains from traditional dairy products on cancer cell lines. Anaerobe. 2014;30:51–9. doi: 10.1016/j.anaerobe.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Deng W, Xi D, Mao H, Wanapat M. The use of molecular techniques based on ribosomal RNA and DNA for rumen microbial ecosystem studies: A review. Mol Biol Rep. 2008;35(2):265–74. doi: 10.1007/s11033-007-9079-1. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18(6):499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2015;31(4):367–76. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Daim MM. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology. 2016;68(2):279–89. doi: 10.1007/s10616-014-9779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colovic MB, Vasic VM, Avramovic NS, Gajic MM, Djuric DM, Krstic DZ. In vitro evaluation of neurotoxicity potential and oxidative stress responses of diazinon and its degradation products in rat brain synaptosomes. Toxicol Lett. 2015;233(1):29–37. doi: 10.1016/j.toxlet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Aluigi MG, Guida C, Falugi C. Apoptosis as a specific biomarker of diazinon toxicity in NTera2-D1 cells. Chem Biol Interact. 2010;187(1-3):299–303. doi: 10.1016/j.cbi.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Slotkin TA, Seidler FJ. Developmental neurotoxicity of organophosphates targets cell cycle and apoptosis, revealed by transcriptional profiles in vivo and in vitro. Neurotoxicol Teratol. 2012;34(2):232–41. doi: 10.1016/j.ntt.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappab and mapk signalling. Cell Microbiol. 2008;10(7):1442–52. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 34.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: Recent advances. Inflamm Bowel Dis. 2009;15(2):300–10. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 35.Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L. et al. Probiotics and health: An evidence-based review. Pharmacol Res. 2011;63(5):366–76. doi: 10.1016/j.phrs.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Oelschlaeger TA. Mechanisms of probiotic actions - a review. Int J Med Microbiol. 2010;300(1):57–62. doi: 10.1016/j.ijmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: Chlorpyrifos, chlorpyrifos oxon, and diazinon. Environ Health Perspect. 2007;115(1):65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in wistar and norway rats. Toxicol Mech Methods. 2012;22(8):638–47. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- 39.Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol. 2010;48(10):2803–8. doi: 10.1016/j.fct.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Verma A, Shukla G. Synbiotic (lactobacillus rhamnosus+lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in sprague-dawley rats: A long-term study. Eur J Cancer Prev. 2014;23(6):550–9. doi: 10.1097/CEJ.0000000000000054. [DOI] [PubMed] [Google Scholar]