Abstract
Purpose: The physicochemical properties of free films made from different mixtures of sustained release polymers were investigated, and an optimum formulation coating on drug containing pellets, based on the study of free film was evaluated.
Methods: In order to determine the effect of different variables on the permeability and swelling of films and procedure optimization, the experimental design was fulfilled based on the statistical method of a 33 full factorial design, and according to this method 27 formulations were prepared. The films were prepared using casting-solvent evaporation method. Water vapor permeability, the swelling and permeability of free films in both acidic and buffer media, were carried out. Then, the pellets containing theophylline were coated with the optimum formulation.
Results: The results of this study demonstrated that an increase in the free film thickness and Eurdragit RS ratio in films lowered the water vapor transmission (WVT), the swelling and the permeability of all formulations, while an increase in the quantity of ethylcellulose, up to a specific ratio (approximately 40%), decreased the permeability and swelling. The most optimum free film formulation was made up of 60% Eudragit RS and 40% ethylcellulose.
Conclusion: Pellets coated with a 10% coating level of ethylcellulose and Eudragit RS (4:6) showed suitable release properties and could serve as a favorable sustained release system for theophylline.
Keywords: Sustained release, Permeability, Swelling, Theophylline, Ethylcellulose, Eudragit
Introduction
Controlled release technology, which is used in the formulation of pharmaceutical product, has become increasingly important due to its significant role in recent pharmaceutical technology. A controlled release system has the advantages, including more constant or prolonged therapeutic effects, improved patient compliance, decreased side effects, and decreased costs. Film coating, based on polymeric substances, are widely used in oral dosage forms, with the aim of obtaining a sustained1,2 and targeted drug delivery,3,4 moisture barriers5 and masking of the bitter taste of active ingredients in some drugs.6 Several natural and synthetic polymers have proven to be suitable coating agents by providing different drug release kinetics.7 However, it is often difficult to obtain the desired efficacy from each polymer alone, thus there is the need to combine the physicochemical characteristics of different macromolecules in order to achieve optimum response.
Ethyl cellulose (EC) is one of the most widely used hydrophobic polymers as a matrix former or coating material in sustained release dosage forms.8,9 It offers moderate film forming properties, enabling suitable coatings to be produced. On the other hand, Eudragit RL (ERL) and Eudragit RS (ERS) are acrylic and methacrylic acid esters, respectively, having some hydrophilic properties due to the presence of quaternary ammonium groups; where ERL possesses higher amount (50 mEq/100 g polymer) of such groups than ERS (25 mEq/100 g polymer).10 They are mainly used as film coatings in tablets, granules and other small particles, The release characteristics of film coated controlled release formulations are strongly dependent on the swelling ability and permeability of the film. From another point of view, the study on drug permeation through free films of polymeric materials could be useful in understanding and predicting the properties of coatings on the surface of a dosage form. A few number of studies focused on both permeation and release of drug from coated dosage forms. In a study, Ye et al. prepared mixed films of EC/hydroxypropyl methylcellulose and investigated the permeability of metoprolol tartrate through the films. Also, the drug release from pellets coated with the same formulation of polymer blends was evaluated. The authors included that the diffusion of drug through isolated plasticized polymer films was predictive for the release of film coated dosage forms.11
An experimental design approach allows for a reduction in the number of experiments, with a complete exploration of the experimental domain to be studied; where all the variables are studied at the same time. On the other hand, response surface methodology has been reported as an effective tool for optimizing a process when the independent variables have a combined effect on the desired response.12
Therefore, the objective of this study was to evaluate the properties of free films made from blends of three water insoluble polymers, Eudragit RS, Eudragit RL and ethylcellulose in different ratios, for the control of drug release, for the purpose of investigating the effect of each polymer on the swelling and permeation characteristics of the other polymers, and finally to predict the suitable formulation for coating of pellets in order to achieve the optimum controlled release dosage form. Theophylline was chosen as a model drug. This drug has long been used as a treatment for diseases, including bronchial asthma. However, due to its narrow therapeutic concentration range and meal dependent absorption, managing the dose of this drug has been rather difficult. The absorption of drug from a conventional matrix tablet is strongly influenced by its transition rate in the gastrointestinal tract. Also, the bioavailability of conventional matrix tablets varies widely13 but a multiple-unit dosage form can overcome this problem. On the other hand, theophylline has been widely used as a model drug due to its ready availability, relatively low cost, ease of assay and chemical stability.14
Materials and Methods
Materials
Eudragit RS (ERS) (Rohm Pharma, Germany), Eudragit RL (ERL) (Rohm Pharma, Germany), ethylcellulose (EC) (Darusazi Arya, Iran), tributyl citrate (Merck, Germany), theophylline monohydrate (Cooper Rhone, France), talc (Merck, Germany), magnesium nitrate hexahydrate (Mg(NO3)2.6H2O) (Merck, Germany), polyvinyl pyrrolidone (PVP K30) (Fluka, Switzerland), and non-pareil seeds (NP Pharm, France) were obtained from indicated sources. The other materials used were of analytical grade.
Methods
Experimental design
For the purpose of this study, a 33 full factorial design was used to investigate the effect of independent variables on the responses. The independent variables included film thickness (X1), the ratio of EC to total polymer content (X2), and the ratio of ERS: ERL in free films (X3). The levels of independent factors and dependent variables are listed in Table 1.
Table 1. The levels of independent variables and responses .
| Independent variables | Levels | Responses | ||
| -1 | 0 | 1 | ||
| X 1 : film thickness (µ) | 60 | 130 | 200 | Y1: Water Vapor Transmission of free films (mg/hm2KPa) |
| Y2: swelling index in acidic media (%) | ||||
| Y3: swelling index in buffer media (%) | ||||
| X 2 : the ratio of EC to total polymer content | 0 | 40 | 80 | Y4: permeability in acidic media (cm/s) |
| X 3 : the ratio of ERS: ERL | 50 | 75 | 100 | Y5: permeability in buffer media (cm/s) |
| - | - | - | - | Y6: ratio of permeability in acid:permeability in buffer |
Preparation of free films
Free films comprising different amounts of polymers were prepared using casting-solvent evaporation method. Briefly, A 10% (w/v) solution of Eudragit RS 100, Eudragit RL 100 and/or ethylcellulose was prepared by dissolving granules or powders of these polymers in given ratios of isopropyl alcohol:distilled water (9:1; 4.5:1, and 3.5:1 ratio for films containing 0, 40, and 80% EC, respectively). Then, a fixed amount of triethyl citrate (1:6 ratio related to total polymer content) was added as a plasticizer. The resultant solution with a suspension volume of volume of 25 mL in each plate was transferred on to Teflon plates. The plates were then placed in an oven at a temperature of 50°C for 24 h until drying was completed. After keeping the plates in a desiccator of 100% relative humidity (RH) for 24 h, the films were cut into different special pieces with a scalpel for various experiments. The thickness of the films was measured at five different places using a micrometer. The free films were finally stored in a desiccator of 50% RH made from a saturated solution of magnesium nitrate hexahydrate at room temperature, until use. Different free films were prepared based on the experimental design. Table 2 summarizes the resultant formulations.
Table 2. Characteristics of investigated free films .
| Run | X 1 (µ) | X 2 (%) | X 3 (%) |
| F1 | 60 | 0 | 50 |
| F2 | 60 | 0 | 75 |
| F3 | 60 | 0 | 100 |
| F4 | 60 | 40 | 50 |
| F5 | 60 | 40 | 75 |
| F6 | 60 | 40 | 100 |
| F7 | 60 | 80 | 50 |
| F8 | 60 | 80 | 75 |
| F9 | 60 | 80 | 100 |
| F10 | 130 | 0 | 5 |
| F11 | 130 | 0 | 75 |
| F12 | 130 | 0 | 100 |
| F13 | 130 | 40 | 50 |
| F14 | 130 | 40 | 75 |
| F15 | 130 | 40 | 100 |
| F16 | 130 | 80 | 50 |
| F17 | 130 | 80 | 75 |
| F18 | 130 | 80 | 100 |
| F19 | 200 | 0 | 50 |
| F20 | 200 | 0 | 75 |
| F21 | 200 | 0 | 100 |
| F22 | 200 | 40 | 50 |
| F23 | 200 | 40 | 75 |
| F24 | 200 | 40 | 100 |
| F25 | 200 | 80 | 50 |
| F26 | 200 | 80 | 75 |
| F27 | 200 | 80 | 100 |
Water vapor transmission study
Water vapor transmission (WVT) of films was performed gravimetrically at a temperature of 25°C. Free films, with appropriate dimensions, were sealed in WVT cups containing 10 mL of distilled water. The cups were accurately weighed and placed in a desiccator containing silica gel and appropriate amounts of calcium chloride in order to create a climate of low relative humidity (approximately 0%). Then, reweighing of the cups was done again at designated intervals (24, 48, 72, 96 and 120 h), and the profile of mass change versus time was plotted for each free film. WVT was calculated using the following equation:
WVT=w/tAP0(RH1-RH2) Equation 1
Where w/t is the change in mass (flux, mg/h) resulting from the slope of profile of the mass change versus time, A is the area of the film surface exposed to the permeant (m2), P0 is the vapor pressure of pure water (kPa), and (RH1−RH2) is the relative humidity gradient. At a temperature of 25°C, the obtained P0 was 3.159 kPa.15
Water uptake experiments
Water uptake tests of free films were conducted in the media with a pH of 1.2 and 6.8 for acidic and buffer media, respectively. 1 cm2 area of each free film was dried at 50°C for 24 h, and after accurate weighing it was immersed in a dissolution flask containing 250 ml of different media at 37°C. The swollen sample was withdrawn at specific intervals, with its surface water wiped off using a filter paper, and finally accurately reweighed. The swelling index, Is, was calculated using the following expression:16
Is(%)=[(Ws–Wd)/Wd]×100 Equation 2
Where Wd and Ws are the weights of the dried and swollen free films, respectively. All experiments on water uptake were applied in triplicate.
Permeability experiments
Samples of free films were retained between compartments of a side-by-side diffusion cell with a diffusion area of 3.46 cm2. Each cell was continuously stirred and the temperature was maintained at 37°C. Permeability experiments were conducted in the acidic and buffer media, respectively for 3 h. The donor compartment was also composed of theophyllineas with an initial concentration of 3 g/L. Samples of 10 ml were taken from the acceptor cells at predetermined intervals and replaced together with the fresh medium. Then, the samples were assayed for theophylline spectrophotometrically at 272 nm. The permeability was calculated using the following equation:17
P=dM/dtSCd Equation 3
Where M is the amount of drug diffused (mg) through free films at time t, S is the effective diffusion area (cm2), Cd is the concentration of drug in the donor cell and P is the permeability of free film (cm/s).
Preparation of drug containing pellets
Drug containing pellets were prepared by coating the theophylline onto the non-pareil beads (850 to 1180 μm) using fluidized bed coater (Wurster insert, Werner Glatt, Germany). A 30% w/v aqueous suspension of theophylline (<90 μm) was prepared by dispersing theophylline in a 9% w/v PVP K30 solution, and finally passing it through a 140 mesh sieve. The final suspension was coated onto non-pareils using fluidized bed coater. Table 3 presents a list of the coating conditions. The coating process continued until pellets having about 20% w/w drug load were produced. After coating, the pellets were extra fluidized for about 5 min, followed by storage in an oven at 40°C, for 2 h.
Table 3. WVT, swelling and theophylline permeability of the formulations .
| Run | WVT index (mg/hm 2 KPa)(mean±SD) | Swelling index in acid (%)(mean±SEM) | Swelling index in buffer (%)(mean±SEM) | Permeability in acid (cm/sec) (mean±SEM)*10-5 | Permeability in buffer (cm/sec) (mean±SEM)*10-5 | Ratio of permeability in acid:permeability in buffer (mean±SEM) |
| F1 | 43.545±0.500 | 39.81±2.06 | 48.77±4.72 | 4.433±0.031 | 5.363±0.015 | 0.827±0.005 |
| F2 | 31.589±0.572 | 14.66±4.16 | 33.44±4.00 | 2.303±0.023 | 2.567±0.006 | 0.897±0.011 |
| F3 | 21.279±0.687 | 12.93±2.36 | 19.92±3.21 | 0.125±0.007 | 0.133±0.008 | 0.947±0.020 |
| F4 | 41.790±0.658 | 16.45±4.73 | 18.56±0.28 | 0.674±0.005 | 0.910±0.005 | 0.741±0.005 |
| F5 | 38.280±0.762 | 15.63±4.68 | 27.27±2.60 | 0.529±0.007 | 0.705±0.003 | 0.751±0.188 |
| F6 | 33.738±0.381 | 13.96±4.42 | 18.23±2.18 | 0.213±0.003 | 0.237±0.005 | 0.912±0.299 |
| F7 | 43.106±1.432 | 14.07±3.54 | 21.46±2.60 | 0.504±0.008 | 0.467±0.023 | 1.081±0.179 |
| F8 | 44.532±0.830 | 16.80±2.36 | 27.00±5.88 | 0.540±0.009 | 0.400±0.007 | 1.348±0.057 |
| F9 | 43.213±0.681 | 13.49±2.44 | 20.72±3.26 | 0.417±0.007 | 0.331±0.006 | 1.259±0.051 |
| F10 | 38.607±1.154 | 33.85±5.26 | 47.13±6.84 | 2.450±0.010 | 3.310±0.006 | 0.739±0.002 |
| F11 | 26.980±0.660 | 17.85±1.26 | 27.79±6.98 | 1.220±0.006 | 1.920±0.010 | 0.634±0.004 |
| F12 | 18.097±0.985 | 14.28±0.14 | 16.22±2.39 | 0.044±0 | 0.058±0 | 0.762±0 |
| F13 | 33.233±1.138 | 10.46±2.25 | 19.43±4.72 | 0.376±0 | 0.480±0.007 | 0.783±0.11 |
| F14 | 31.920±0.873 | 13.59±1.01 | 18.66±3.88 | 0.386±0.005 | 0.414±0.003 | 0.932±0.017 |
| F15 | 29.947±0.980 | 9.62±4.22 | 15.85±7.81 | 0.112±0.002 | 0.116±0.002 | 0.966±0.034 |
| F16 | 38.060±0.687 | 17.29±4.65 | 14.43±3.20 | 0.242±0.002 | 0.116±0.002 | 2.087±0.035 |
| F17 | 39.606±0.832 | 18.41±1.28 | 22.09±2.19 | 0.233±0.002 | 0.254±0.002 | 0.918±0.009 |
| F18 | 36.747±1.244 | 12.26±3.59 | 17.68±1.15 | 0.218±0.009 | 0.186±0.008 | 1.187±0.087 |
| F19 | 29.387±0.685 | 28.02±4.45 | 37.87±3.24 | 1.456±0.006 | 1.970±0.010 | 0.739±0.019 |
| F20 | 23.253±0.504 | 18.71±4.45 | 25.71±5.16 | 0.735±0.011 | 1.067±0.006 | 0.689±0.011 |
| F21 | 13.382±0.687 | 6.78±2.43 | 13.74±8.21 | 0.018±0 | 0.013±0 | 1.353±0 |
| F22 | 30.283±0.560 | 11.49±5.21 | 18.16±0.63 | 0.270±0.009 | 0.293±0.005 | 0.921±0.044 |
| F23 | 27.202±0.687 | 15.35±2.70 | 20.22±9.09 | 0.193±0.003 | 0.233±0.002 | 0.831±0.018 |
| F24 | 24.017±1.184 | 12.40±3.69 | 15.16±4.59 | 0.059±0.003 | 0.062±0.004 | 0.957±0.107 |
| F25 | 34.770±1.159 | 10.41±1.72 | 18.94±10.26 | 0.159±0.005 | 0.188±0.003 | 0.848±0.038 |
| F26 | 33.013±1.154 | 13.46±6.72 | 19.98±5.31 | 0.154±0.005 | 0.131±0.003 | 1.177±0.068 |
| F27 | 31.370±1.008 | 8.13±2.59 | 19.36±5.44 | 0.146±0.034 | 0.117±0.006 | 1.261±0.342 |
Content uniformity
Two hundred milligrams of theophylline pellets were ground and transferred into 250 ml volumetric flasks containing phosphate buffer at a pH of 6.8. The flasks were shaken in a shaking water bath at 25°C for 3 h. The concentration of the drug was determined spectrophotometrically in filtered solutions at 272 nm. All assays were carried out in triplicate.
Polymer coating
The two most optimum free film formulations (ERS-EC in the ratio of 60:40 and ERS) were chosen according to the contour plots and optimization of formulations. Then, 10% (w/w) solutions of the optimum formulations were prepared in isopropyl alcohol:water in ratios of 4.5:1 and 9:1, respectively. The solution was plasticized with triethyl citrate (in the ratio of 1:6 related to dry polymer) with talc added as a glidant (5% w/w, related to dry polymer). The final dispersion was coated onto 100 g of drug loaded pellets with a fluidized bed coating apparatus (Wurster insert, Werner Glatt). The process conditions were as follows: spray rate of 10 g min-1, atomization pressure of 2 bars, nozzle diameter of 1 mm, inlet temperature between 40 and 45°C and outlet temperature between 30 and 35°C. Samples of coated pellets were removed from the apparatus upon reaching the coating loads of 5, 10, 15 and 20% (w/w), respectively.
Dissolution studies
Dissolution studies were conducted on a 900 ml medium at 37°C using a USP dissolution apparatus I (Pharmatest, PTWS, Germany) at a rotation speed of 50 rpm. Accurately weighed (100 mg) pellets were transferred into the dissolution medium and at predetermined intervals, the samples were taken from the vessel and ultra violetly (UV) assayed spectrophotometrically at 272 nm. Dissolution test was performed for 1 h in the medium with pH 1.2 (HCl 0.1 N), followed by 7 h in the medium with pH 6.8 (phosphate buffer). All experiments on dissolution were applied in triplicate.
Scanning electron microscopy
The surface characteristics of the powder samples were observed using scanning electron microscopy (SEM) (LEO 1450 VP, UK). The samples were sputter coated with silver for 1 min in a sputter coater (Polaron E5400, UK).
Statistical analysis of data
The effects of the independent variables on the responses were modelled using a second order polynomial equation:
Y=c+b1X1+b2X2+b3X3+b4X12+b5X22+b6X32+b7X1X2+b8X1X3+b9X2X3+b10X1X2X3 Equation 4
The modeling was performed using SPSS (Version 16.0), with a backward, stepwise linear regression technique, and significant terms (P < 0.05) were chosen for the final equations. The resultant response surface plots and contour plots from the equations were obtained using Statgraphics 5.
Results and Discussion
The results of WVT experiments are shown in Table 3. From the results there was a decrease in WVT with increasing film thickness, and this could have resulted from the prolonged pathway of water vapor molecules through the films. The addition of ERS to the free films lowered the WVT index. From the comparison of the structure of ERS and ERL, it is obvious that ERS is more hydrophobic than ERL due to the less amounts of quaternary ammonium groups in its structure18 and therefore gives rise to the less tendency of water vapor permeation through the free films.
The results of WVT were consistent with the data of another investigation in which WVT index of free films containing ERS was 13 mg/m2hKPa, and the addition of EC increased the water vapor permeability of the films.19 In the present study, the WVT indices for free films compromising 100% ERS, with thicknesses of 60, 130 and 200 μ, were 21.729, 18.097 and 13.382 mg/m2hKPa, respectively, which were the lowest amounts among all the given formulations. On the other hand, an increase in the ratio of EC to the other polymers in the formulations enhanced WVT, such that in a ratio of 80% EC, the WVT lowering effect of ERS was not significant. This outcome could have resulted from the less integration of free films, as illustrated in the scanning electron microscopy (SEM) (Figure 1) due to the presence of three different polymers, followed by a decrease in the resistance to water vapor transmission of the free films.
Figure 1.
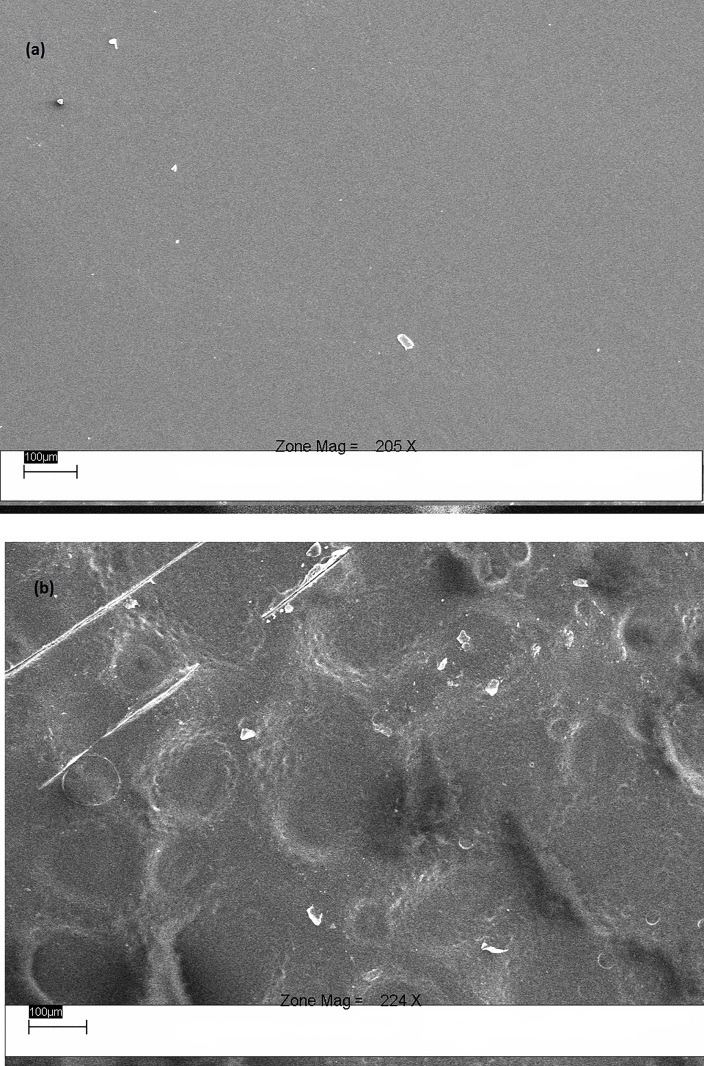
Scanning electron micrographs of the surface of free films; (a) ERS:ERL (50:50) (F10) and (b) ERS:ERL:EC (10:10:80) (F16) (magnification 200×).
The swelling index of formulations is presented in Table 3. As shown, formulations F15, F21 and F27 had the lowest swelling index in the acidic media. None of the three mentioned formulations had ERL in their compositions. It has been demonstrated in other studies that the swelling of ERS is partially restricted in the presence of chloride ion18,20 which explains the small amounts of swelling of free films containing ERS. On the other hand, maximum amount of swelling in acidic and buffer media occurred in formulations containing ERS-ERL, with the ratio of 1:1 (F1, F10 and F19). A major decrease in swelling was observed when the film thickness increased in the presence of ERS. This effect diminished when ERS was partially substituted with ERL in free films. As a matter of fact, less amounts of ionizable quaternary ammonium groups in the structure of ERS restricted the accessibility of the functional groups, and swelling could be more influenced by variation in thickness compared with free films containing ERL. The presence of EC in the formulations compartment, in amounts up to 40%, lowered the swelling index in SGF and SIF. The larger amounts of EC showed no significant swelling controlling effect, especially in the presence of higher ratios of ERS, and this demonstrated that ERS was the most restrictive polymer on the swelling of free films.
The results of the permeability of theophylline for all formulations are shown in Table 3. Accordingly, formulations F1, F10 and F19, containing high elements of ERL in their compositions, exhibited minimum resistances to drug permeation in both acidic and buffer media. On the contrary, minimum amounts of permeability was attributed to formulations F12, F21, F24 (p<0.01) in which ERL was not present and the film thicknesses were either 130 or 200 µ. The latter formulation was made up of 40% EC, and could be assumed as a suitable ratio of EC to Eudragit for sustaining drug release.
The selection of accurate independent variables and dependent factors is a key parameter in an experimental design for optimization of process. The aim of the current study was to achieve an appropriate ratio of different time dependent polymers and to predict a suitable coating system for controlling the drug release from thepohylline pellets. Therefore, the ratios of ERS:ERL and EC:total polymer contents were assumed as the independent variables. With regards to the important effect of coating thickness on drug release, film thickness was selected as the third variable. Water vapor permeation of free films was selected as a response due to the importance of the protective effect of coating films against moisture permeation.21 Also, according to the close relationship between drug release and the permeability of free films, the latter characteristic was the second candidate as a response for optimization. On the other hand, due to the need to predict a suitable formulation for controlled release product, the similar permeability in two simulated media, could be assumed as the sustained and uniform drug release in the gastro intestinal (GI) tract. Thus, the ratio of permeability in acidic: buffer media (Pa:Pb) was chosen as the other response for optimization (Table 3).
Mathematical equations were obtained for each response using the statistical program SPSS based on the relationship between the independent and dependent factors. The equations are listed as follows:
Y1=72.663 – 0.142X1 – 0.367X2 – 0.479X3+0.001X1X2 + 0.001X1X3+0.007X2X3–0.00001X1X2X3 Equation 5
Y2=52.122-0.026X1-0.664X2-0.373X3+0.003X2X2+0.005X2X3 Equation 6
Y3=54.676-0.037X1-0.994X2+0.004X2X2+0.008X2X3-0.003X3X3 Equation 7
Y4=99.998–0.429X1–1.542X2–0.930X3+0.005X1X2+0.004X1X3+0.003X2X2+0.013X2X3–0.00005X1X2X3 Equation 8
Y5=117.498–0.413X1–1.890X2–1.139X3+0.006X1X2+0.004X1X3+0.004X2X2+0.016X2X3–0.000057X1X2X3 Equation 9
Y6=1.487–0.025X3+0.000016X1X3+0.0001X2X2+0.00018X3X3 Equation 10
Analysis of variance (ANOVA) confirmed the significance of the assumed regression models for the responses. To prove the validity of the equations, values of X1, X2 and X3 were substituted in the equations in order to obtain the predicted values of responses. Accordingly, the similarity between the results of the observed and predicted responses could be indicative of the accurate selection of the factorial design, variables and levels.
The selection of appropriate constraints for the responses was required for the optimization of the process and also to determine the optimum formulation(s). As the WVT of free films was indicative of the protection against moisture permeation, amounts less than 30 mg/m2hKPa were assumed as the optimum Y1 response. Also, the constraint used for Y6 ranged from 0.95 to 1.05, levels in which the drug release from the formulations could be equal in both the acidic and buffer media. Contour plots were drawn for Y1 and Y6 (Figure 2). The best area for formulations was selected according to the contour plots and constraints. It was discovered that formulations F12, F15, F21 and F24 exhibited theoretical optimum responses. Of these, F15 and F24 (containing 40% EC and 60% ERS), which only differed in the film thickness also resulted in practical optimum responses. The two other formulations had only ERS in their compositions. Therefore, the coating systems containing ERS:EC (6:4) or ERS were selected as the optimum formulations for coating of theopylline pellets. The drug release from the pellets was examined after preparation of the pellet. Figure 3 shows the drug release from the pellets containing optimum coating formulations with different coating thicknesses. As shown in the Figure, an increase in the coating thickness managed a slower release from the pellets, which was in direct relation with the higher tortuosity and path of drug molecules.22 On the other hand, the addition of 40% EC to the coating formulations resulted in more sustained and controlled drug release, and the data were compatible with the data of permeability. Pellets coated with ERS:EC (6:4), and having coating thickness of 10%, met the suitable sustained drug release condition from a sustained release dosage form of theophylline defined by USP,23 and such pellets could be selected as the optimum pellets.
Figure 2.
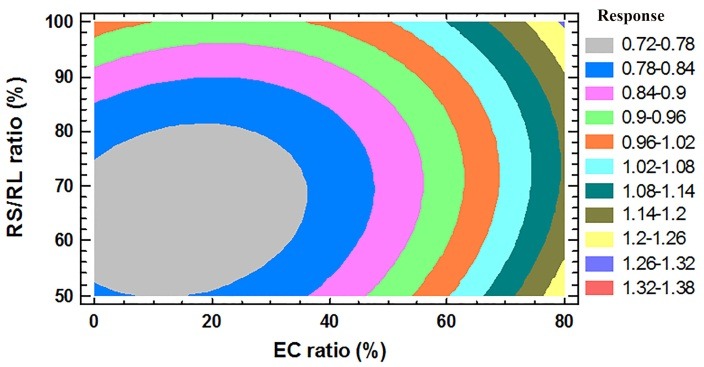
Contour plot for Y6 response (ratio of permeability in acid:permeability in buffer).
Figure 3.
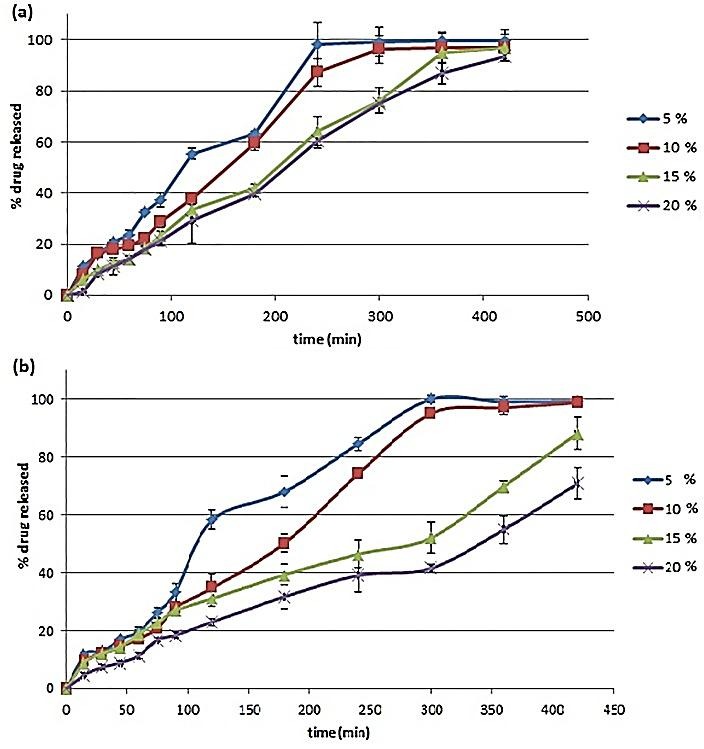
Drug release from theophylline pellets with coating thickness of 5, 10, 15 and 20% and coated with (a) ERS and (b) ERS:EC(6:4).
Conclusion
The results of this investigation demonstrated that the primary studies on free films could be valuable for the prediction of drug release from pellets. Factorial design was the appropriate method for the evaluation of different parameters in the process of free film preparation and optimization of the coating system.
According to the data, the formulation containing Eudragit RS:Ethylcellulose (in the ratio of 6:4) exhibited suitable conditions necessary for controlling drug release, and the pellets coated with this formulation could be selected as a theophylline controlled release dosage form.
Acknowledgments
This work is the Pharm. D thesis of Mr. A. Tavakol which is supported by a grant from research chancellor of Ahvaz Jundishapur University of Medical Sciences. The authors would like to thank Arya and NP Pharm for their collaboration and providing samples used in this paper.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- 1.Alnaief M, Antonyuk S, Hentzschel CM, Leopold CS, Heinrich S, Smirnova I. A novel process for coating of silica aerogel microspheres for controlled drug release applications. Micropor Mesopor Mat. 2012;160:167–73. doi: 10.1016/j.micromeso.2012.02.009. [DOI] [Google Scholar]
- 2.Haaser M, Karrout Y, Velghe C, Cuppok Y, Gordon KC, Pepper M. et al. Application of terahertz pulsed imaging to analyse film coating characteristics of sustained-release coated pellets. Int J Pharm. 2013;457(2):521–6. doi: 10.1016/j.ijpharm.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Maroni A, Del Curto MD, Zema L, Foppoli A, Gazzaniga A. Film coating for oral colon delivery. Int J Pharm. 2013;457(2):372–94. doi: 10.1016/j.ijpharm.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Survase S, Kumar N. Dual coating of swellable and rupturable polymers on glipizide loaded mcc pellets for pulsatile delivery: Formulation design and in vitro evaluation. Int J Pharm. 2011;419(1-2):121–30. doi: 10.1016/j.ijpharm.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Kugge C, Vanderhoek N, Bousfield DW. Oscillatory shear response of moisture barrier coatings containing clay of different shape factor. J Colloid Interface Sci. 2011;358(1):25–31. doi: 10.1016/j.jcis.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 6.Miyadai N, Higashi K, Moribe K, Yamamoto K. Optimization and characterization of direct coating for ibuprofen particles using a composite fluidized bed. Adv Powder Technol. 2012;23(1):40–5. doi: 10.1016/j.apt.2010.12.004. [DOI] [Google Scholar]
- 7.Van Savage G, Rhodes CT. The sustained release coating of solid dosage forms: a historical review. Drug Dev Ind Pharm. 1995;21(1):93–118. doi: 10.3109/03639049509048098. [DOI] [Google Scholar]
- 8.Desai J, Alexander K, Riga A. Characterization of polymeric dispersions of dimenhydrinate in ethyl cellulose for controlled release. Int J Pharm. 2006;308(1-2):115–23. doi: 10.1016/j.ijpharm.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Lokhande AB, Mishra S, Kulkarni RD, Naik JB. Influence of different viscosity grade ethylcellulose polymers on encapsulation and in vitro release study of drug loaded nanoparticles. J Pharm Res. 2013;7(5):414–20. doi: 10.1016/j.jopr.2013.04.050. [DOI] [Google Scholar]
- 10. Lehmann K. Chemistry and application properties of polymethacrylate coating systems. In: Mc Ginity GW, editors. Aqueous polymeric coating for pharmaceutical dosage form. USA: Marcel Dekker; 1997.
- 11.Ye ZW, Rombout P, Remon JP, Vervaet C, Van den Mooter G. Correlation between the permeability of metoprolol tartrate through plasticized isolated ethylcellulose/hydroxypropyl methylcellulose films and drug release from reservoir pellets. Eur J Pharm Biopharm. 2007;67(2):485–90. doi: 10.1016/j.ejpb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasa PC, Ravi R, Tharanathan RN. Effect of storage conditions on the tensile properties of eco-friendly chitosan films by response surface methodology. J Food Eng. 2007;80:184–9. doi: 10.1016/j.jfoodeng.2006.05.007. [DOI] [Google Scholar]
- 13.Abrahamsson B, Alpsten M, Bake B, Jonsson UE, Eriksson-Lepkowska M, Larsson A. Drug absorption from nifedipine hydrophilic matrix extended-release (er) tablet-comparison with an osmotic pump tablet and effect of food. J Control Release. 1998;52(3):301–10. doi: 10.1016/s0168-3659(97)00267-8. [DOI] [PubMed] [Google Scholar]
- 14.Sriamornsak P, Thirawong N, Weerapol Y, Nunthanid J, Sungthongjeen S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur J Pharm Biopharm. 2007;67(1):211–9. doi: 10.1016/j.ejpb.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Akhgari A, Farahmand F, Afrasiabi Garekani H, Sadeghi F, Vandamme TF. Permeability and swelling studies on free films containing inulin in combination with different polymethacrylates aimed for colonic drug delivery. Eur J Pharm Sci. 2006;28(4):307–14. doi: 10.1016/j.ejps.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Blanchon S, Couarraze G, Rieg-Falson F, Cohen G, Puisieux F. Permeability of progesterone and a synthetic progestin through methacrylic films. Int J Pharm. 1991;72(1):1–10. doi: 10.1016/0378-5173(91)90374-W. [DOI] [Google Scholar]
- 17.Lin WJ, Lu CH. Characterization and permeation of microporous poly (ɛ-caprolactone) films. J Membrane Sci. 2002;198(1):109–18. doi: 10.1016/S0376-7388(01)00652-4. [DOI] [Google Scholar]
- 18.Bodmeier R, Guo X, Sarabia RE, Skultety PF. The influence of buffer species and strength on diltiazem hcl release from beads coated with the aqueous cationic polymer dispersions, eudragit rs, rl 30d. Pharm Res. 1996;13(1):52–6. doi: 10.1023/a:1016021115481. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Sauer D, McGinity JW. Influence of hydroxyethylcellulose on the drug release properties of theophylline pellets coated with eudragit rs 30 d. Eur J Pharm Biopharm. 2005;59(1):147–54. doi: 10.1016/j.ejpb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Wagner K, McGinity J. Influence of chloride ion exchange on the permeability and drug release of eudragit rs 30 d films. J Control Release. 2002;82(2-3):385–97. doi: 10.1016/s0168-3659(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 21.Elsabee MZ, Abdou ES. Chitosan based edible films and coatings: A review. Mater Sci Eng C Mater Biol Appl. 2013;33(4):1819–41. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Fan TY, Wei SL, Yan WW, Chen DB, Li J. An investigation of pulsatile release tablets with ethylcellulose and eudragit l as film coating materials and cross-linked polyvinylpyrrolidone in the core tablets. J Control Release. 2001;77(3):245–51. doi: 10.1016/s0168-3659(01)00508-9. [DOI] [PubMed] [Google Scholar]
- 23. United States Pharmacopeia 34/National Formulary 29. Rockville, MD, USA: United States Pharmacopeial Convention; 2011.


