Abstract
Purpose: Lycopene belongs to the carotenoids that shows good pharmacological properties including antioxidant, anti-inflammatory and anticancer. However, as a result of very low aqueous solubility, it has a limited systemic absorption, following oral administration.
Methods: Here, we prepared a stable lycopene-loaded solid lipid nanoparticles using Precirol® ATO5, Compritol 888 ATO and myristic acid by hot homogenization method with some modification. The size and morphological characteristics of nanoparticles were evaluated using Scanning Electron Microscopy (SEM). Moreover, zeta potential and dispersity index (DI) were measured using zeta sizer. In addition, encapsulation efficiency (EE%), drug loading (DL) and cumulative drug release were quantified.
Results: The results showed that the size and DI of particles was generally smaller in the case of SLNs prepared with precirol when compared to SLNs prepared with compritol. Scanning electron microscopy (SEM) and particle size analyses showed spherical SLNs (125 ± 3.89 nm), monodispersed distribution, and zeta potential of −10.06 ± 0.08 mV. High EE (98.4 ± 0.5 %) and DL (44.8 ± 0.46 mg/g) were achieved in the case of nanoparticles prepared by precirol. The stability study of the lycopene-SLNs in aqueous medium (4 °C) was showed that after 2 months there is no significant differences seen in size and DI compared with the fresh formulation.
Conclusion: Conclusively, in this investigation we prepared a stable lycopene-SLNs with good physicochemical characteristic which candidate it for the future in vivo trials in nutraceutical industries.
Keywords: Lycopene, Solid Lipid Nanoparticles (SLNs), Myristic acid, Hot homogenization method, Physicochemical characterization
Introduction
With the increase of different incurable diseases, the nutraceutical scientist should pay more attention to man’s feeding system. Lifestyle and nutrition can help preventing cancer. Carotenoids are common natural compounds in food booklet that play a significant role in the prevention of cancers. However, their low bioavailability due to their inadequate intestinal absorption and low solubility in the aqueous medium, and moreover chemical instability are important limiting factors in the food industry. Hence, there is urgent needs to develop new methods to increase their productivity and absorption as well. Carotenoids are fat-soluble pigments mostly appear in plants and microorganisms (algae and some bacteria) which have an essential role in photosynthesis.1 In addition, the carotenoids play important pharmacological effects in animals including cells protection from free radicals,2 coping with cancer cells and inhibition of the lipoxygenase activities,3 and protection of fats against spontaneous oxidation.4 In the food industry carotenoids are used widely in manufacturing of food products and soft/energy drinks as antioxidant, color and flavor modifier (especially bitter).5 Due to the unsaturated structure of carotenoids, the compounds are susceptible to oxidative changes. Factors such as temperature, light and pH can also cause different changes in their color and nutritional value.1 Recently, with the development of nanotechnology in food sciences and technology, investigators try to encapsulate these valuable resources in different nanoparticles to save their nutritional values, bioactivities and antioxidant properties as well as their stability and sustainability. The sparingly solubility of carotenoids hamper good intestinal absorption, thus reduce their bio-efficiency.
In the recent decade many attempts have been directed to overcome the solubility issues of carotenoids using their formulation in the lipid based nanoparticles e.g. liposomes,6-9 micelle,10-12 solid lipid nanoparticles13 and nanostructured lipid carriers.14,15 Solid lipid nanoparticles (SINs) are colloidal drug carrier with nanometer-sized, which contains the solid lipid matrix. SLNs consistent high biodegradability and biocompatibility and are good candidates as carriers for both hydrophilic and lipophilic compounds.16 SLNs construction comprise a simple homogenization and solidification process that would allow successful scale up for industry.17 In addition, compared with nanostructured lipid carriers (NLCs), SLNs display more controlled drug release effectiveness.18 Altogether, SLNs possess different advantages in nutraceutical developments including high stability, protection of incorporated compound against chemical degradation,19 biocompatibility of the carrier,20 and avoidance of organic solvent during formulation.21 Bioactive compounds in the solid core of SLNs have mobility limitation and speed of their distribution to the particle surface are lower than other nano-emulsions.22 These features of SLNs make them suitable for the decrease of decomposition reactions such as oxidation of bioactive compounds e.g carotenoids. Different procedures are used for the preparation of SLNs including hot/cold homogenization, high pressure homogenization (HPH), ultrasonification, and emulsification and solvent evaporation method. Almost in all methods, emulsions are prepared in the first step, and then by mechanical means large droplets are break down into smaller particles.
In this investigation we tried to develop an enhanced lycopene loaded solid lipid nanoparticles (lycopene-SLNs) using simple hot homogenization method with some modification. Although in 2012 Riangjanapatee et al formulated lycopene-NLC and evaluated the surfactant type effects on the stability of the formulation,23 to the best of our knowledge this investigation is a pioneering attempt in the formulation of lycopene in solid lipid nanoparticles composed of Precirol ® ATO 5 and Compritol 888 ATO as a lipid matrix to improve the pharmacokinetic behavior. Moreover, to enhance zeta potential of the formulated nanoparticles, to obtain a stable formulation, myristic acid was used during all formulation. Anionic lycopene-SLNs were physicochemically and morphologically characterized by means of zetaseizer and scanning electron microscopy (SEM). Moreover, we evaluated different physicochemical characterization including encapsulation efficiency (EE%), drug loading (DL) and stability.
Materials and Methods
Chemicals and reagents
All Chemical substances and compounds used in this investigation were pharmaceutical grade. Precirol® ATO5 and Compritol 888 ATO was gifted from Gattefosse (Nanterre, France). Tween 80, poloxamer 407 and myristic acid were purchased from Sigma-Aldrich (Poole, UK). All solvents used in this study were extra pure and purchased from Merck Co (Darmstadt, Germany).
Lycopene resources and extraction
Tomatoes needed to extract lycopene were purchased from a local market and after washing were air dried under the shade. Dried and ground tomatoes were extracted using petroleum ether aiding laboratory mixers for at least 30 minutes. After then the extract was filtrate using paper filter and solvent was removed in vacuo by rotary evaporator at maximum temperature of 40°C. The lycopene content of the extract was isolated using antisolvent precipitation method. For this, the crude extract was dissolved in ethyl acetate. Then methanol was added dropwise to the extract solution to completely precipitate lycopene. The sediment was dissolved in ethyl acetate and precipitation was repeated once again. Finally, the tartar was filtrate and remaining solvent was evaporated by a flow of nitrogen gas. Eventually dried red sediments were used for the production of solid lipid nanoparticles.
Preparation of Lycopene loaded solid lipid nanoparticles
Lycopene-SLNs were prepared by the hot homogenization method according to our previous work with some modification.20 Briefly, lipid phase including lycopene and solid lipid (glyceryl palmitostearate (Precirol® ATO 5) or glyceryl behenate (Compritol® 888 ATO) was simply dispersed by unintended heating at ~10 °C above the lipids melting point. Moreover, myristic acid (< 0.5 % w/w) was added to the lipid phase as zeta enhancer. To prepared aqueous phase, an appropriate concentration of stabilizers (Poloxamer 407) was heated to the same temperature of the oil phase in distilled water. Then the hot aqueous phase was dropped to the oil phase during 30 minutes and homogenization (12 000 rpm and 70 °C). In the next step, the obtained emulsion was further homogenized (at 19 000 rpm) for additional 10 min. Lycopene-SLNs were finally obtained by allowing the hot nanoemulsion to cool down at room temperature, and then were stored at 4 °C. Blank SLNs also were prepared by the same method except instead of lycopene, an equal amount of precirol or compritol was used.
Size, zeta potential, and morphological characteristics of nanoparticles
Getting an appropriate system for lycopene nanoparticles (NPs) preparation, the size of the prepared NPs was measured immediately after fabrication by laser diffraction immediately after preparation (SALD-MS30, Schimadzu, USA). In this context, SLNs was diluted by distilled water to reach appropriate concentration. Moreover, particle size distribution [mean diameter and dispersity index (DI)] and zeta potential of suitable formulation system was determined in pH 8.3 using Malvern zetasizer (3000HS, Malvern Instruments, UK) in the final concentration which recommended by the manufacturer of the instrument. The morphology of the fabricated NPs was observed with Scanning Electron Microscopy (SEM) based on our previous published method.16,21
Encapsulation efficiency (%) and drug loading
The entrapment efficiency (EE) of the NPs were determined as the percentage of lycopene entrapped in the carriers compared to the total dug which was used for the formulation. For the measurement of the (EE), original suspension containing lycopene-SLNs and 0.3% tween 80 was placed in Ultra free tube with a cutoff of 10,000 Da (Ultrafree, MC Millipore, Bedford, USA) and centrifuged for 8 min at 14,000g (3K30, SIGMA Labrorzentrifugen GmbH, Germany). The filtrate was mixed by hexane (1:1) and hanged for at least 10 min and then the hydrophobe phase was separated by a separatory funnel and the quantity of free lycopene was determined using spectrophotometric method (λ max: 471 nm in hexane). To gain a calibration curve, working standard solutions were prepared by serially dilution with hexane. The stock solutions were prepared by dissolving pure lycopene in hexane at the concentrations of 6000 μg/mL. Each calibration curve consisted of 6 calibration points (600, 300, 100, 80, 40 and 20 μg/mL). Calibration curve was plotted by least square linear regression analysis. The drug EE in the SLNs was calculated from Eq. (1) and drug loading (DL) was obtained from Eq. (2).
| Eq. (1) |
| Eq. (2) |
where WLL is the weight of lycopene loaded in nanoparticles and WNP is the weight of nanoparticles solid mass.
In vitro release studies
Cumulative in vitro release of lycopene-SLNs was carried out based on dialysis method. Regarding to this, 1 mL of lycopene-SLNs was charged into dialysis bag (molecular weight cutoff: 12 kDa). The bag was then inserted in a glass holder containing 10 mL of dialysis buffer (0.3% W/V tween 80 in DW).20 Moreover, intact lycopene also was dialyzed similarly to evaluate the permeability of lycopene to the membrane. During dialysis the media was continuously stirred at RT and 100 rpm and at the fixed time periods, 1 mL of medium was removed and 1 mL of fresh media was added to the receptacle. Drug concentration was determined by the spectrophotometric method mentioned earlier.
Physical stability studies
To evaluate the stability of the formulated SLNs, samples were stored for 2 months at a glass tube at refrigerator (4 °C). Then mean diameter, DI and EE were measured and compared with the fresh ones.20
Statistical analyses
All data represent the mean of at least three repeated experiments (error bars represent mean ± standard deviation). Independent Student’s t-test was utilized to compare mean differences between two independent groups and one-way ANOVA was used to multiple comparisons. Post hoc pairwise comparisons were carried out using Tukey multiple comparison tests for those that showed significant mean differences (SPSS; version 13.0). We used Shapiro–Wilk test to compare the shape of sample distribution with the shape of a normal curve. The statistical significance was defined as p<0.05.
Results and Discussion
Preparation and physicochemical characterization of Lycopene-SLNs
SLNs of lycopene was developed by hot homogenization method without using of organic solvents by means of different solid lipids including Precirol® ATO 5 and Compritol® 888 ATO. The SLNs were stabilized using a surfactant, i.e. Tween 80 and Poloxamer 407. Moreover, myristic acid was used to enhance the zeta quantity of the particles surface. It is well known that the carbonyl carbon of the organic acid such as myristic acid is directly linked to, and in conjugation with, a second electronegative oxygen atom bearing a hydrogen atom. This electronic arrangement allows for loss of a proton and ionization because electron density is "pulled" from the hydroxyl hydrogen through the conjugated carboxyl group, and the negative charge formed upon ionization (in the conjugate base) is stabilized by resonance delocalization.24
The method and composition of the SLNs were optimized, and they were further considered in terms of total DL, EE, particle size, zeta potential, morphology, and stability studies. The characteristics of some formulated lycopene-SLNs and blank SLNs in this investigation are listed in Table 1. Our results showed that the size and DI was generally smaller in the case of SLNs prepared with precirol when compared to SLNs prepared with compritol (Table 1).
Table 1. Composition, particle size, and encapsulation efficiency (EE) of lycopene-loaded SLNs and blank SLNs formulation.
| Formulation No | Lipid type and concentration (g) | Lycopene concentration (g) |
Oil phase
surfactant concentration a (g) |
Aqueous phase
surfactant concentration b (g) |
Particle
size(nm) |
Dispersity index (DI) |
Encapsulation
Efficiency (EE%) |
Drug
Loading (DL,mg/g) |
| Blank SLNs | - | - | - | - | - | - | - | - |
| F1 | Precirol (0.55) | - | 0.01 | 0.52 | 153 ± 6.13 | 0.658 ± 0.131 | - | - |
| F2 | Precirol (0.75) | - | 0.05 | 0.65 | 124 ± 5.24 | 0.382 ± 0.142 | - | - |
| F3 | Compritol (0.55) | - | 0.07 | 0.55 | 162 ± 4.24 | 0.832 ± 0.121 | - | - |
| F4 | Compritol (0.75) | - | 0.10 | 0.62 | 139 ± 3.13 | 0.927 ± 0.128 | - | - |
| Lycopene-SLNs | - | - | - | - | - | - | - | - |
| F5 | Precirol (0.59) | 0.053 | 0.01 | 0.48 | 162 ± 7.01 | 0.444 ± 0.109 | 94 ± 0.2 | 51.8 ± 0.45 |
| F6 | Precirol (0.62) | 0.049 | 0.06 | 0.55 | 155 ± 5.34 | 0.502 ± 0.151 | 96.7 ± 0.4 | 46.7 ± 0.39 |
| F7 | Precirol (0.65) | 0.052 | 0.09 | 0.69 | 125 ± 3.89 | 0.159 ± 0.105 | 98.4 ± 0.5 | 44.8 ± 0.46 |
| F8 | Compritol (0.60) | 0.048 | 0.01 | 0.44 | 166 ± 1.83 | 0.177 ± 0.221 | 86.6 ± 0.6 | 49.5 ± 0.63 |
| F9 | Compritol (0.67) | 0.049 | 0.06 | 0.53 | 143 ± 3.89 | 0.253 ± 0.105 | 89.5 ± 0.3 | 47.6 ± 0.29 |
| F10 | Compritol (0.73) | 0.054 | 0.09 | 0.71 | 129 ± 1.83 | 0.177 ± 0.221 | 93.1 ± 0.6 | 44.4 ± 0.33 |
Data are expressed as mean ± SD (n=3).
a: Tween 80 and b: Poloxamer 407 was used as the oil phase and aqueous phase surfactant in all formulations, respectively. 0.3% (w/w) myristic acid also was used in all formulation.
Particle size and morphology
The average size of the particles and their shapes may affect the drug release pattern, entrapment efficiency, cytotoxicity and pharmacokinetic behavior.25 The results here showed that the size of the nanoparticles was generally in direct relation with the oil phase surfactant concentration. Clearly seen that nanoparticle size is reduced by increasing the amount of oil phase surfactant. However, our previous results showed that with the increase of tween 80 the cytotoxicity of NPs will increase.16 Moreover, there is an obvious decrease of nanoparticle size with an increase of homogenizing duration time and speed (data not shown). Of the different formulation, F2 (blank SLNs) and F7 (lycopene-SLNs) at 25 °C exhibited mono dispersed characteristics with a mean diameter of 124 ± 5.24 nm and 125 ± 3.89 nm. Besides, F2 and F7 showed acceptable DI of 0.382 ± 0.142 and 0.253 ± 0.105, respectively. The DI values indicated a narrow particle size distribution. These results were approved more by Scanning Electron Microscopy (SEM) images (Figure 1). Moreover, SEM showed spherical and uniform SLNs. Zeta potential as important criteria can predict the particles long-term stability.
Figure 1.
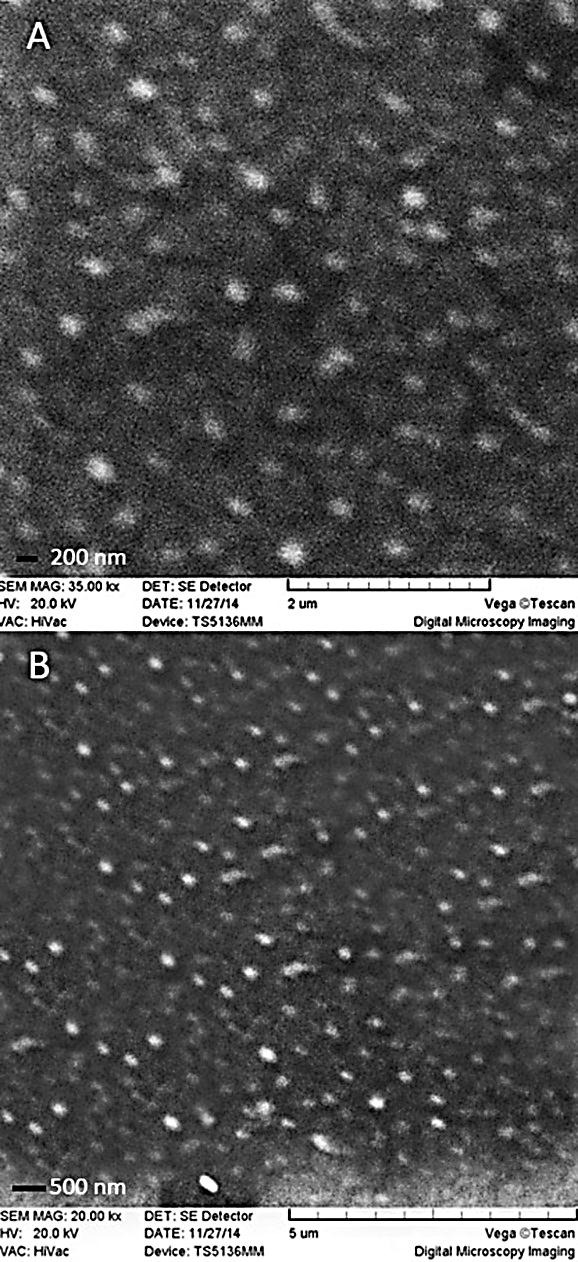
(A) SEM images of blank-SLNs (F2) and (B) lycopene-loaded SLNs (F7)
The zeta potential of higher than −60 mV for a colloidal dispersion forecast physically stable condition for the particles of the dispersion. 26 In this investigation, at pH of distilled water (8.3), zeta potential of the selected formulation, F2 and F7, were -14.21 ± 1.14 and -10.08 ± 2.06, respectively (Figure 2).
Figure 2.
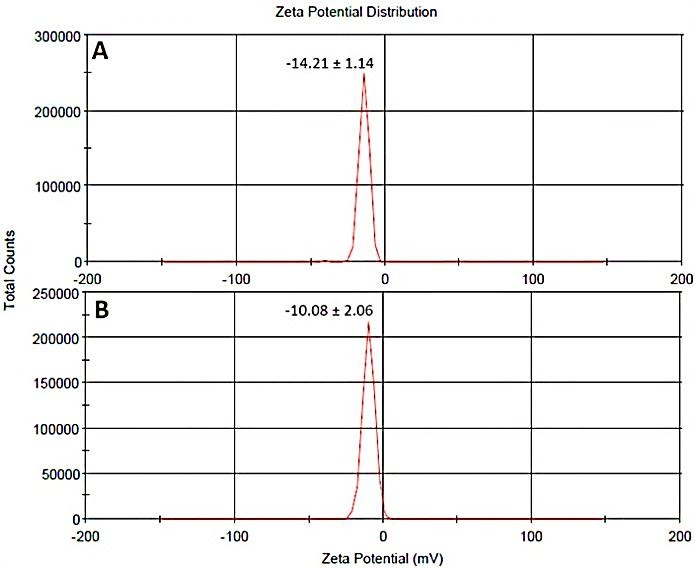
Zeta potential of (A) F2 and (B) F7. (F2) and (F7) represent the formulation code of 2 and 7 for blank SLNs and lycopene-SLNs, respectively.
In vitro release studies
In this study the cumulative drug release was assessed using dialysis method. Figure 3 shows the release profile of lycopene-SLNs in DW containing 0.05% (W/V) tween 80 as co-solubilizer. The release profile showed that lycopene-SLNs exhibited no burst drug release and less than 30% of lycopene was released after 72 h. This type of sustained release profile of sparingly water soluble drug was also reported by others.20,27 It is may be mainly referring to the low diffusion of the drug from the lipid matrix of SLNs into aqueous media. However, as seen in Figure 3 approximately 23% of lycopene drug release was occurred in the first 24 h. This release may be attributed to those drugs which is located around the surface of the SLNs. Thought, all intact lycopene (>95%) was released to the media in the first 24 h which gives us confidence that lycopene could penetrate into the cellulose pores.
Figure 3.
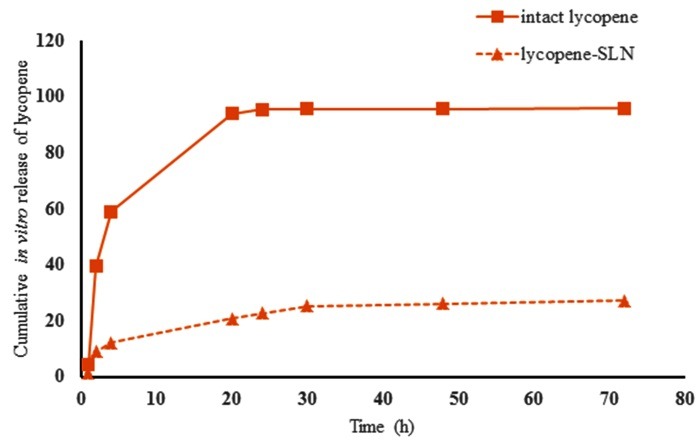
Cumulative release of lycopene. The release of intact lycopene was assessed in a same condition to evaluate the perme ability of the plain drug through the membrane
Physical stability studies
Of different formulations, the most suitable one − the formulation composed of 3.5% (w/w) lycopene, a surfactant consisting of 6.07% tween 80 and 46.55% poloxamer 407 in a solid lipid matrix of 43.85% precirol − was placed on long-term stability at 4 ºC for 3 months. After three months, the NPs was checked for any potential aggregation, and SLNs were evaluated in terms of size, DI, and EE. Stability studies showed that in the usual dispersed aqueous medium (distilled water), coacervation and precipitation of lipid did not occur. The result of the stability studies showed that after three months storage of formulation at 4 °C, the mean diameter, entrapment efficiency and DI of lycopene-SLNs displayed no significant differences, as compared with the fresh preparation (p > 0.05) (Table 2).
Table 2. Stability study of lycopene-SLNs. Data are presented here as mean ± SD (n=3).
| Formulation | Mean diameter (nm) | Dispersity index | Entrapment efficiency (EE%) | Drug leakage |
| Blank SLNs | 133 ± 3.78 | 0.146 ± 0.04 | - | - |
| Fresh lycopene-SLNs (F7) | 125 ± 3.89 | 0.159 ± 0.105 | 98.4±0.5 | - |
| Lycopene-SLNs (F7) in 4 °C after 3 months | 128 ± 7.52 | 0.173 ± 0.05 | 88.7 ± 0.19 | 9.7% |
Conclusion
Here solid lipid nanoparticle containing lycopene successfully fabricated using simple hot homogenization procedure. Lycopene nanoparticles showed good physicochemical characterization in terms of good stability during storage times. The obtained small size of nanoparticles (110 to 130 nm) is hopeful characteristic to candidate lycopene-SLNs formulation to orally usage. There is enough observation revealed that the intestinal absorption of lycopene-SLNs formulated here will be good. Encapsulation efficiency of lycopene-SLNs stored for at least three months showed that an ignorable leakage is occurred in 4 °C. Finally, this investigation could be a pioneer study to propose that using of these types of carotenoid-SLNs (nano-nutraceutical) in the manufacturing of different beverages and dairy products as food supplementary materials.
Acknowledgments
The authors wish to thank Research Center for Pharmaceutical Nanotechnology (RCPN) for their technical and financial support.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- 1.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Su Q, Rowley KG, Balazs ND. Carotenoids: Separation methods applicable to biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781(1-2):393–418. doi: 10.1016/s1570-0232(02)00502-0. [DOI] [PubMed] [Google Scholar]
- 3.Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M. et al. Cancer prevention by natural carotenoids. BioFactors. 2000;13(1-4):89–94. doi: 10.1002/biof.5520130115. [DOI] [PubMed] [Google Scholar]
- 4.Timmons JS, Weiss WP, Palmquist DL, Harper WJ. Relationships among dietary roasted soybeans, milk components, and spontaneous oxidized flavor of milk. J Dairy Sci. 2001;84(11):2440–9. doi: 10.3168/jds.S0022-0302(01)74694-2. [DOI] [PubMed] [Google Scholar]
- 5.Jaswir I, Noviendri D, Hasrini RF, Octavianti F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J Med Plants Res. 2011;5(33):7119–31. doi: 10.5897/JMPRX11.011. [DOI] [Google Scholar]
- 6.Jiang J, Yu Z, Qiu Q, Wu Y. protective effects of beta-carotene liposome against rat neutrophile membrane damage caused by intra- or extra-cellular reactive oxygen species. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1996;18(5):387–91. [PubMed] [Google Scholar]
- 7.Tan C, Feng B, Zhang X, Xia W, Xia S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocolloid. 2016;52:774–84. doi: 10.1016/j.foodhyd.2015.08.016. [DOI] [Google Scholar]
- 8.Tan C, Zhang Y, Abbas S, Feng B, Zhang X, Xia S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surf B Biointerfaces. 2014;123:692–700. doi: 10.1016/j.colsurfb.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Toniazzo T, Berbel IF, Cho S, Fávaro-Trindade CS, Moraes ICF, Pinho SC. β-carotene-loaded liposome dispersions stabilized with xanthan and guar gums: Physico-chemical stability and feasibility of application in yogurt. LWT Food Sci Technol. 2014;59(2):1265–73. doi: 10.1016/j.lwt.2014.05.021. [DOI] [Google Scholar]
- 10.Kotake-Nara E, Nagao A. Effects of mixed micellar lipids on carotenoid uptake by human intestinal caco-2 cells. Biosci Biotechnol Biochem. 2012;76(5):875–82. doi: 10.1271/bbb.110777. [DOI] [PubMed] [Google Scholar]
- 11.White DA, Ornsrud R, Davies SJ. Determination of carotenoid and vitamin a concentrations in everted salmonid intestine following exposure to solutions of carotenoid in vitro. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(3):683–92. doi: 10.1016/s1095-6433(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen YJ, Inbaraj BS, Pu YS, Chen BH. Development of lycopene micelle and lycopene chylomicron and a comparison of bioavailability. Nanotechnology. 2014;25(15):155102. doi: 10.1088/0957-4484/25/15/155102. [DOI] [PubMed] [Google Scholar]
- 13.Gomes GVdL, Borrin TR, Cardoso LP, Souto E, Pinho SCd. Characterization and shelf life of ²-carotene loaded solid lipid microparticles produced with stearic acid and sunflower oil. Braz Arch Biol Techn. 2013;56(4):663–71. [Google Scholar]
- 14.Hejri A, Khosravi A, Gharanjig K, Hejazi M. Optimisation of the formulation of beta-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013;141(1):117–23. doi: 10.1016/j.foodchem.2013.02.080. [DOI] [PubMed] [Google Scholar]
- 15.Riangjanapatee P, Muller RH, Keck CM, Okonogi S. Development of lycopene-loaded nanostructured lipid carriers: Effect of rice oil and cholesterol. Pharmazie. 2013;68(9):723–31. [PubMed] [Google Scholar]
- 16.Eskandani M, Barar J, Dolatabadi JE, Hamishehkar H, Nazemiyeh H. Formulation, characterization, and geno/cytotoxicity studies of galbanic acid-loaded solid lipid nanoparticles. Pharm Biol. 2015;53(10):1525–38. doi: 10.3109/13880209.2014.991836. [DOI] [PubMed] [Google Scholar]
- 17.Muller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (sln and nlc): Present state of development and industrial applications. Curr Drug Discov Technol. 2011;8(3):207–27. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (nlcs) better than solid lipid nanoparticles (slns): Development, characterizations and comparative evaluations of clotrimazole-loaded slns and nlcs? Eur J Pharm Sci. 2012;47(1):139–51. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Tech. 2012;23(1):13–27. [Google Scholar]
- 20.Eskandani M, Nazemiyeh H. Self-reporter shikonin-act-loaded solid lipid nanoparticle: Formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci. 2014;59:49–57. doi: 10.1016/j.ejps.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Ezzati Nazhad Dolatabadi J, Hamishehkar H, Eskandani M, Valizadeh H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2014;117:21–8. doi: 10.1016/j.colsurfb.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Rao J, McClements DJ. Optimization of lipid nanoparticle formation for beverage applications: Influence of oil type, cosolvents, and cosurfactants on nanoemulsion properties. J Food Eng. 2013;118(2):198–204. doi: 10.1016/j.jfoodeng.2013.04.010. [DOI] [Google Scholar]
- 23.Riangjanapatee P, Okonogi S. Effect of surfactant on lycopene-loaded nanostructured lipid carriers. Drug Discov Ther. 2012;6(3):163–8. [PubMed] [Google Scholar]
- 24.B. Pratt W, Taylor P. Principles of Drug Action: The Basis of Pharmacology. 3, illustrated ed. Michigan: Churchill Livingstone; 1990. [Google Scholar]
- 25.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (sln) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 26.Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharmaceut. 1998;168(2):221–9. doi: 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]
- 27.Das S, Ng WK, Kanaujia P, Kim S, Tan RB. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: Effects of process variables. Colloids Surf B Biointerfaces. 2011;88(1):483–9. doi: 10.1016/j.colsurfb.2011.07.036. [DOI] [PubMed] [Google Scholar]


