Abstract
Purpose: The paper tries to assess the protective effect of fisetin against •OH-induced DNA damage, then to investigate the possible mechanism.
Methods: The protective effect was evaluated based on the content of malondialdehyde (MDA). The possible mechanism was analyzed using various antioxidant methods in vitro, including •OH scavenging (deoxyribose degradation), •O2- scavenging (pyrogallol autoxidation), DPPH• scavenging, ABTS•+ scavenging, and Cu2+-reducing power assays.
Results: Fisetin increased dose-dependently its protective percentages against •OH-induced DNA damage (IC50 value =1535.00±29.60 µM). It also increased its radical-scavenging percentages in a dose-dependent manner in various antioxidants assays. Its IC50 values in •OH scavenging, •O2- scavenging, DPPH• scavenging, ABTS•+ scavenging, and Cu2+-reducing power assays, were 47.41±4.50 µM, 34.05±0.87 µM, 9.69±0.53 µM, 2.43±0.14 µM, and 1.49±0.16 µM, respectively.
Conclusion: Fisetin can effectively protect DNA against •OH-induced oxidative damage possibly via reactive oxygen species (ROS) scavenging approach, which is assumed to be hydrogen atom (H•) and/or single electron (e) donation (HAT/SET) pathways. In the HAT pathway, the 3’,4’-dihydroxyl moiety in B ring of fisetin is thought to play an important role, because it can be ultimately oxidized to a stable ortho-benzoquinone form.
Keywords: •OH-induced DNA damage; Antioxidant mechanism; Hydrogen atom transfer; Single electron transfer mechanism; 3’,4’-dihydroxyl
Introduction
Reactive oxygen species (ROS), generated by normal cellular metabolism and exogenous agents (e.g. xenobiotics, ionising and nonionsing radiation), may lead to a condition of oxidative stress if its production overwhelms the antioxidant defences.1,2 It has been considered as a significant promoter of cancer, cardiovascular malfunction, aging, and other diseases. Cellular DNA is a particularly sensitive target because of the potential to create cumulative mutations that can disrupt cellular homeostasis. However, the enzymatic repair in living organisms might be inadequate for protecting against permanent DNA mutations. Therefore, for the past few years, it has become a research focus to search for effective and safe antioxidants from natural sources.3,4
Most of natural antioxidants belong to the family of phenolic or polyphenolic compounds, which show their antioxidant activity via a hydrogen atom transfer (HAT) or single electron transfer (SET) mechanism.5
Fisetin (3,3’,4’,7-tetrahydroxyflavone, Figure 1) is a phenolic flavonoid in various food, such as strawberry, onion and persimmon.6 It has a wide range of pharmacological effects, such as antitumor, antiinflammation,7 anticoagulant and dissolving thrombus.8 In recent years, its protective effect on the liver in human body (especially patients with diabetes) attracted a wide spread attention. Researches showed that, the fisetin's liver protective effect and promoting glucose homeostasis effect were related to its antioxidant activity.9 However, its antioxidant ability and mechanism remain unknown. Therefore, the present study used DNA protection model to investigate its antioxidant activity then further discuss the possible mechanisms.
Figure 1.
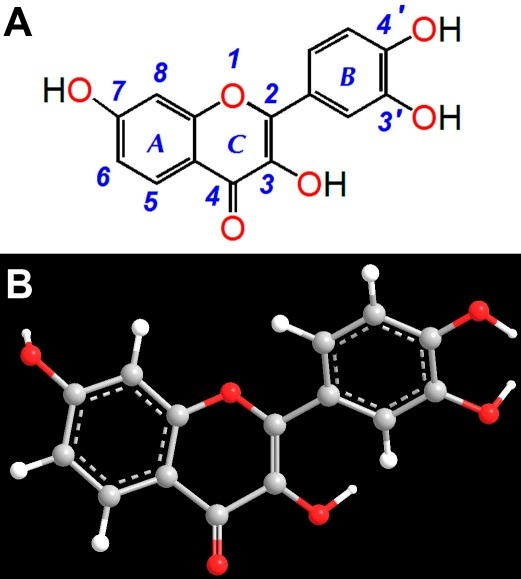
The structure of fisetin (A) and its ball-stick model (B)
Materials and Methods
Chemicals
Fisetin (98%, CAS 528-48-3) and DNA sodium salt (fish sperm) were obtained from Aladdin Co. (Shanghai, China). DPPH• (1,1-diphenyl-2-picryl-hydrazl), ABTS [2,2′-azino-bis(3-ethylbenzo- thiazoline-6-sulfonic acid diammonium salt)], neocuproine, BHA (butylated hydroxyanisole), Trolox [(±)-6-hydroxyl-2,5,7,8 -tetramethlychromane-2-carboxylic acid], pyrogallol and deoxyribise were purchased from Sigma Co. (Sigma-Aldrich Shanghai Trading Co., China). Other chemicals used in this study were of analytical grade and obtained from Guangzhou Chemical Reagent Factory (Guangzhou, China).
Methods
The protective effect of fisetin against •OH-induced DNA damage was determined by our method.10 Mechanistic analysis experiments included •OH scavenging, •O2- scavenging, DPPH• scavenging, ABTS•+ scavenging, and Cu2+-reducing power assays. The •OH scavenging assay was based on the deoxyribose degradation, and improved by our laboratory;11 in the improved deoxyribose degradation assay, all samples were pre-treated before determination. The •O2- scavenging assay was based on pyrogallol autoxidation reaction, and also improved by our laboratory; In the improved pyrogallol autoxidation assay, pH was modified as 7.4.11 The other antioxidant assays were described in our previous paper.10,11 In all these assays, BHA and Trolox were used as the positive controls. The inhibition (or protecting DNA, reducing power) percentages were obtained according to the corresponding calculation formulas. The calculation formulas and experimental protocols were detailed in the Suppl. 1.
Results and Discussion
ROS can lead to the formation of single and double-strand breaks, as well as induce chemical and structural modifications to purine and pyrimidine bases, resulting in millions of lesions per cell each day.4 Deoxythymineglycol (dTG),8-hydroxy-deoxyguanine (8-OH-dG), malondialdehyde (MDA) and formamidopyrimidine (FAPy) constitute the important markers of DNA oxidative damage.12,13 Among these fragments, MDA is usually used to reflect the DNA protective percentage, because MDA can be easily detected via combining with 2-thiobarbituric acid (TBA).10
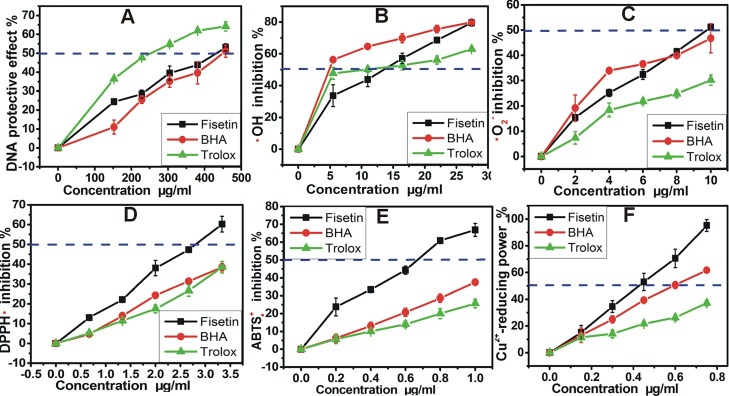
As is seen in Figure 2A, the protective percentages of fisetin from •OH-induced DNA damage increased in a dose-dependent fashion. The IC50 values of fisetin, BHA and Trolox were respectively 1535.00±29.60 µM, 2469.00±96.69 µM and 1084.67±20.23 µM (Table 1). The results suggested that fisetin could protect DNA against •OH-induced damage. However, its protective effect is inferior to that of Trolox but stronger than that of BHA.
Figure 2.
The dose response curves of fisetin in the assays: (A) protective effect against DNA damage; (B) hydroxyl (•OH) radical-scavenging; (C) superoxide anion (•O2-) radical-scavenging assay; (D) DPPH• radical-scavenging assay; (E) ABTS+• radical-scavenging assay; (F) Cu2+-reducing power. Each value is expressed as the mean±SD (n=3). The concentration was the final concentration in the corresponding reaction system.
Table 1. The IC50 values of fisetin and the positive controls (µM) .
| - | Fisetin | Positive controls | |
| BHA | Trolox | ||
| DNA protective effect | 1535.00±29.60b | 2469.00±96.69c | 1084.67±20.23a |
| •OH scavenging | 47.41±4.50c | 2.40±1.19a | 41.84±9.55b |
| •O2− scavenging | 34.05±0.87a | 76.02±5.22c | 66.75±2.25b |
| DPPH• scavenging | 9.69±0.53a | 22.84±0.34c | 19.55±2.20b |
| ABTS+• scavenging | 2.43±0.14a | 7.73±0.43b | 7.54±0.57b |
| Cu2+-reducing | 1.49±0.16a | 3.25±0.17b | 4.31±0.21c |
The IC50 value is defined as the concentration of 50% radical inhibition (or protection percentage, relative reducing percentage). These IC50 values were calculated by linear regression analysis based on the response curves in Figure 2 and converted from µg/mL to µM. Each value in this table is expressed as the mean±SD (n=3). Mean values with different superscripts (a, b, or c) in the same row are significantly different (p < 0.05), while those with same superscripts are not significantly different (p < 0.05).
Some studies have shown that phenolic antioxidants protect DNA possibly through base-excision repair, which may arise from ROS (especially •OH and •O2−) scavenging at high reaction rates.14-16 Therefore, the •OH and •O2− radical-scavenging capacities of fisetin were further measured in the study.
Our data in Figure 2B&C showed that fisetin could efficiently scavenge •OH and •O2- radicals dose-dependently. The IC50 values of fisetin, BHA and Trolox of •OH-scavenging were respectively 47.41±4.50 µM, 2.40±1.19 µM, and 41.84±9.55 µM. In the •O2− radical-scavenging assay, IC50 values of fisetin, BHA and Trolox were 34.05±0.87 µM, 76.02±5.22 µM and 66.75±2.25 µM, respectively (Table 1). This indicated that both •OH and •O2- radicals could be eliminated by fisetin at low concentration, and that ROS scavenging may be a possible approach for fisetin to protect DNA.
To confirm whether HAT and SET might happen in the ROS scavenging by fisetin, we further measured its radical-scavenging on DPPH•. The previous studies suggested that DPPH• may undergo HAT pathway to be scavenged to yield a stable DPPH-H molecule.17 As illustrated in Figure 2D and Table 1, fisetin scavenged DPPH• radical with high efficiency. It clearly suggests a HAT pathway in the ROS scavenging by fisetin. The possible reaction of fisetin with DPPH• radical can be proposed as Figure 3.
Figure 3.
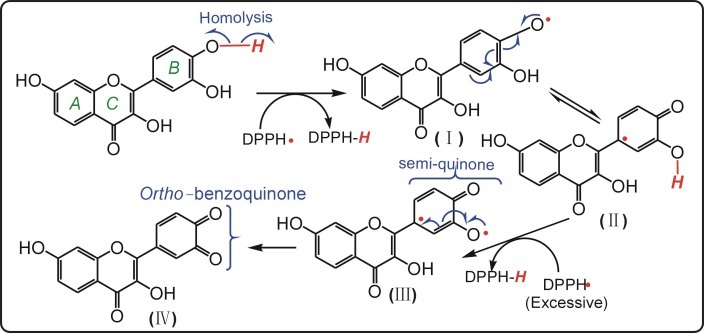
The proposed reaction of fisetin with DPPH• radical.
In the process, since B ring is regarded as the active sites in the antioxidant process of flavonoids,18 phenolic-OH in B ring of fisetin is thought to undergo homolysis prior to either the A or C ring, to produce H• and fisetin• radical (I). Then DPPH-H molecule may be generated through H• combining with DPPH•. And the fisetin• radical might transform into a semi-quinone form (III), which could be further extracted H• by excess DPPH• to form the stable ortho-benzoquinone form. Now it is clear that, the 3’,4’-dihydroxyl moiety in B ring of fisetin played an important role, because it could be ultimately oxidized to a stable ortho-benzoquinone form.
Since the formation of ABTS•+ from ABTS was previously proven to be via one SET oxidation,19 ABTS•+ scavenging was also reported to be a SET mechanism.20 The effective scavenging ABTS•+ by fisetin indicate a SET possibility in its ROS scavenging reaction. The SET possibility was further supported by the Cu2+-reducing power assay, in which fisetin efficiently reduced Cu2+→Cu+ (Figure 2F and Table 1). Cu2+-reducing however is well-known as an electron (e) transfer reaction.
Conclusion
Fisetin can effectively protect DNA against •OH-induced oxidative damage possibly via reactive ROS scavenging approach, which is assumed to be via HAT/SET pathways. In the HAT pathway, the 3’,4’-dihydroxyl moiety in B ring of fisetin is thought to play an important role, because it can be ultimately oxidized to a stable ortho-benzoquinone form.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- 1.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic Biol Med. 2002;32(11):1102–15. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 2.Cooke MS, Henderson PT, Evans MD. Sources of extracellular, oxidatively-modified DNA lesions: Implications for their measurement in urine. J Clin Biochem Nutr. 2009;45(3):255–70. doi: 10.3164/jcbn.SR09-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 4.Silva JP, Gomes AC, Coutinho OP. Oxidative DNA damage protection and repair by polyphenolic compounds in pc12 cells. Eur J Pharmacol. 2008;601(1-3):50–60. doi: 10.1016/j.ejphar.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Xue Y, Zheng Y, An L, Dou Y, Liu Y. Density functional theory study of the structure-antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014;151:198–206. doi: 10.1016/j.foodchem.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 6.Sahu BD, Kumar JM, Sistla R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of nf-kappab signaling. J Nutr Biochem. 2016;28:171–82. doi: 10.1016/j.jnutbio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Pal HC, Sharma S, Elmets CA, Athar M, Afaq F. Fisetin inhibits growth, induces g(2) /m arrest and apoptosis of human epidermoid carcinoma a431 cells: Role of mitochondrial membrane potential disruption and consequent caspases activation. Exp Dermatol. 2013;22(7):470–5. doi: 10.1111/exd.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31(6):478–94. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Prasath GS, Pillai SI, Subramanian SP. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur J Pharmacol. 2014;740:248–54. doi: 10.1016/j.ejphar.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Mai W, Wang L, Han W. A hydroxyl-scavenging assay based on DNA damage in vitro. Anal Biochem. 2013;438(1):29–31. doi: 10.1016/j.ab.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Lin J, Gao Y, Han W, Chen D. Antioxidant activity and mechanism of rhizoma cimicifugae. Chem Cent J. 2012;6(1):140. doi: 10.1186/1752-153X-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng RL, Huang ZY. Free-Radical Biology. 3rd ed. Beijing, China: Higher Education Press; 2007. [Google Scholar]
- 13.Sacheck JM, Milbury PE, Cannon JG, Roubenoff R, Blumberg JB. Effect of vitamin e and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med. 2003;34(12):1575–88. doi: 10.1016/s0891-5849(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Idaboy JR, Galano A. On the chemical repair of DNA radicals by glutathione: Hydrogen vs electron transfer. J Phys Chem B. 2012;116(31):9316–25. doi: 10.1021/jp303116n. [DOI] [PubMed] [Google Scholar]
- 15.Wallace SS. Enzymatic processing of radiation-induced free radical damage in DNA. Radiat Res. 1998;150(5 Suppl):S60–79. [PubMed] [Google Scholar]
- 16.Zheng RL, Shi YM, Jia ZJ, Zhao CY, Zhang Q, Tan XR. Fast repair of DNA radicals. Chem Soc Rev. 2010;39(8):2827–2834. doi: 10.1039/b924875g. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Schaich KM. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (dpph) assay for antioxidant activity. J Agric Food Chem. 2014;62(19):4251–60. doi: 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- 18.Tsimogiannis DI, Oreopoulou V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency: A kinetic approach for the 3’4’-hydroxy substituted members. Innov Food Sci Emerg. 2006;7(4):140–6. doi: 10.1016/j.ifset.2005.09.001. [DOI] [Google Scholar]
- 19.Changha L, Jeyong Y. UV direct photolysis of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) in aqueous solution: Kinetics and mechanism. J Photoch Photobio A. 2008;197(2-3):232–8. doi: 10.1016/j.jphotochem.2007.12.030. [DOI] [Google Scholar]
- 20.Villata LS, Berkovic AM, Gonzalez MC, Martire DO. One-electron oxidation of antioxidants: A kinetic-thermodynamic correlation. Redox Rep. 2013;18(5):205–9. doi: 10.1179/1351000213Y.0000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]