Abstract
Purpose: Inflammation and oxidative stress can lead to different chronic diseases including cancer and atherosclerosis. Many medicinal plants have the potential to show as anti-inflammatory activity. Present investigation was performed to investigate anti-inflammatory, antioxidant activity, and quantification of selected bioactive plant polyphenols of the ethanol (EAH) and aqueous (AAH) extracts of Acalypha hispida (Euphorbiaceae) leaves.
Methods: Anti-inflammatory activity was evaluated by carragenan and histamine induced rat paw edema models while antioxidant capacity was evaluated by DPPH free radical scavenging, Fe+2 chelating ability, reducing power, NO scavenging, total phenolic and total flavonoid content assay. Identification and quantification of bioactive polyphenols was done by HPLC.
Results: At the doses of 200 and 400 mg/kg, both EAH and AAH showed statistically significant inhibition of paw volume in the anti-inflammatory activity test. Both the extracts showed DPPH scavenging (IC50: 14 and 17 µg/ml, respectively), Fe+2 ion chelating (IC50: 40 and 46 µg/ml, respectively), NO scavenging activity (65.49 and 60.66% inhibition at 100 µg/ml), and concentration dependent reducing power ability. For EAH and AAH, flavonoid content was 126.30 and 149.72 mg QE/g dry extract, while phenolic content was 130.51 and 173.80 mg GAE/g dry extract, respectively. HPLC analysis of EAH and AAH indicated the presence of high content of ellagic acid along with other phenolic constituents.
Conclusion: High content of ellagic acid along with other phenolic constituents might have played an important role in the observed anti-inflammatory and antioxidant activity.
Keywords: Carrageenan, Histamine, anti-inflammatory, DPPH, Ellagic acid
Introduction
Acalypha hispida Burm.f. (Euphorbiaceae) is an erect, sparsely branched shrub, locally known as sibjhul, sibjota or jotamangshi in Bangladesh. It grows in the coastal regions of Bangladesh. The plant is native to New Guinea, the Malay Archipelago and other islands in the East Indies.1 It is commonly used as an ornamental plant in the garden and house. The leaves are laxative, diuretic and used in the treatment of leprosy and gonorrhea. Different part of the plant is also used in infectious diarrhoea, pulmonary problems, and as an expectorant in asthma.2 The plant contains ellagitannins namely, acalyphidins M1, M2, and D1, anthocyanins namely, cyanidin 3-O-(2″-galloyl-β-galactopyranoside), cyanidin 3-O-(2″-galloyl-β-galactopyranoside), and cyanidin 3-O-β-galactopyranoside.3,4 Previous phytochemical screening of A. hispida leaves extract indicated the presence of reducing sugars, glycosides, steroids, flavonoids, and saponins.3,4,5 The leaves of A. hispida has been reported to possess cytotoxic, antibacterial,5 antileprotic,6 antimicrobial,7 and antifungal8 properties, while the flower extract was reported to have DPPH free radical scavenging and cytotoxic activity.9 As a part of the continuation of our research on bioactivity screening of Bangladeshi medicinal plants,10,11,12 here we report the anti-inflammatory, antioxidant activity and quantification of the major polyphenols of ethanol and water extracts of A. hispida leaves.
Materials and Methods
Plant collection and extraction
The leaves of A. hispida was collected from Khulna, Bangladesh during July, 2012 and identified by the experts at Bangladesh National Herbarium (voucher specimen no.: DACB 34471). Shade-dried leaves were ground into coarse powder with the help of a grinder. The powdered plant material was kept in an airtight container and preserved in a cool, dark and dry place until the extraction commenced. Powdered plant material (150 g) was soaked in 900 ml of ethanol and kept for 72 h with occasional shaking and stirring. The solvent was filtered through a cotton plug. The filtrate was evaporated to dryness using a rotary vacuum evaporator to get the ethanol extract (EAH). The plant material was dried from the residual solvent and macerated with MilliQ water. Upon filtration, the filtrate was freeze dried to get the aqueous extract (AAH).
Test animals
For the in vivo anti-inflammatory activity study, male rats of Wister strain weighing 179-205 g were used. The animals were housed at the Pharmacology Research Laboratory of Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong under standard laboratory conditions maintained at 25±1°C and fed with rodent food and water ad libitum. Protocols approved by BCSIR Ethics Committee on research in animals were followed during all experimental procedures that involve animals.
Chemicals and drug
Indomethacin, carrageenan, histamine phosphate, (+)-catechin hydrate, gallic acid, vanillic acid, caffeic acid, p-coumaric acid, (-)-epicatechin, rutin hydrate, quercetin, ellagic acid, ascorbic acid, DPPH, and Folin-Ciocalteu’s reagent were purchased from Sigma–Aldrich (St. Louis, MO, USA). Tween 80, chloroform (analytical grade), ethanol (analytical grade), acetonitrile (HPLC grade), methanol (HPLC grade), acetic acid (HPLC grade), trichloroacetic acid, phosphate buffer (pH 6.6), potassium ferricyanide, ferric chloride, sodium phosphate, EDTA, ammonium molybdate, and sodium carbonate were purchased from Merck (Darmstadt, Germany).
Tests for anti-inflammatory activity
Carrageenan-induced rat paw edema test
The rats were divided into control, positive control and test groups, consisting of five animals in each. Control and positive control groups were orally administered with 1% Tween-80 in normal saline (10 ml/kg), and indomethacin (10 mg/kg), respectively. Test groups received EAH and AAH orally at the doses of 200 and 400 mg/kg, respectively. Acute inflammation was induced in all the rats by injecting 0.1 ml of carrageenan (1% w/v in 1% Tween-80 in normal saline) in the right hind paw of the rats, 1 h post exposure of the treatments. The paw volume was determined with a micrometer screw gauge at 1, 2, 3, 4 and 5 h after carrageenan administration.13
Histamine-induced rat paw edema test
The rats were divided into groups, and treated in the same fashion as that of carrageenan-induced edema test. Acute inflammation was initiated in all test animals by injecting 0.1 ml of histamine (1% w/v in 1% Tween-80 in normal saline) in the right hind paw of the rats. The paw volume was measured with a micrometer screw gauge at 1, 2, 3, 4 and 5 h after histamine administration.14
Tests for antioxidant activity
DPPH free radical scavenging assay
Aliquots (1 ml) of EAH, AAH and ascorbic acid at different concentrations were added to 3 ml of a 0.004% w/v DPPH solution. The mixture was allowed to stand in the dark at 25°C for 30 min and the absorbance of the mixture was recorded against blank at 517 nm using a double beam UV/Visible spectrophotometer (Analykjena, Model 205, Jena, Germany). The IC50 was determined from the absorbance versus concentration plot.15
Fe2+ ion chelating assay
Aliquots of (5 ml) different concentrations (5-100 μg/ml) of EAH, AAH and standard (EDTA) were taken and 0.1 ml solution of 2 mM ferrous chloride was added to it, followed by the addition of 0.2 ml of 5 mM ferrozine. After an interval of 10 min to complete the reaction, the absorbance of the solution was measured at 562 nm.16
Reducing power assay
Different concentrations of EAH, AAH and standards (ascorbic acid and BHA) (5–100 μg/ml) in 1 ml of distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1 %). The mixture was incubated at 50°C for 20 min. A 10 % solution of trichloroacetic acid (2.5 ml) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1 %) and the absorbance of the mixture was measured at 700 nm.17
Nitric oxide scavenging assay
Sodium nitroprusside in phosphate buffer was mixed with different concentrations of EAH and AAH (5-100 μg/ml), and incubated at 25°C for 30 min. The incubated solution (1.5 ml) was diluted with 1.5 ml of Griess reagent (1% sulphanilamide, 2% phosphoric acid, and 0.1% naphthylethylenediamine dihydrochloride). The absorbance of the resulting reaction mixture was measured at 546 nm.18
Total phenolic content assay
Aliquots (0.5 ml) of the extracts (1 mg/ml) and various concentrations of gallic acid solutions (500-15.62 mg/L) were mixed with 5 ml of Folin-Ciocaltu’s reagent (1:10 v/v in distilled water) and 4 ml of sodium carbonate. The mixture was then vortexed for 15 sec and kept for 30 min at 40°C for the reaction to complete and the absorbance was measured at 765 nm. Total phenol content of the extracts were determined from the standard curve and expressed as mg of gallic acid equivalent (GAE) per gram extract.19
Total flavonoid content assay
The extracts (5 ml, 1 mg/ml) were mixed with 2.5 ml of aluminium chloride reagent (133 mg of aluminium chloride and 400 mg of sodium acetate in 100 ml of de-ionised water) and kept for 30 min at room temperature. The absorbance of the reaction mixture was measured at 430 nm. Total flavonoids content was determined from the standard curve and expressed as mg of quercetin equivalent (QE) per gram extract.20
Quantification of polyphenolic compounds by HPLC
HPLC chromatographic analysis was done using a Thermo Scientific Dionex UltiMate 3000 Rapid Separation LC (RSLC) system (Thermo Fisher Scientific Inc., MA, USA), coupled to a quaternary rapid separation pump (LPG-3400RS), rapid separation diode array detector (DAD-3000RS) and ultimate 3000RS auto sampler (WPS-3000). Separation was accomplished on Acclaim® C18 (4.6 x 250 mm; 5µm) column (Dionex, USA), controlled at a constant temperature of 30°C. The separation was carried out using a gradient elution programme (0-10 min, 5%A/95%B; 10-20 min, 10%A/80%B/10%C; 20-30 min, 20%A/60%B/20%C; and 30 min, 100%A, where A is acetonitrile, B is acetic acid solution in water at pH 3.0, and C is methanol). The flow rate was maintained at 1 ml/min, and the injection volume was 20 µl. For the detection, UV detector was set to 280 nm and held for 18 min, changed to 320 nm and held for 6 min, finally to 380 nm and continued for the rest of the analysis while the diode array detector was set at an acquisition range of 200-700 nm. The standard solution was made by diluting the standard stock solutions in methanol to give a concentration of 20 µg/ml for each polyphenols, except for caffeic acid (8 µg/ml) and quercetin (6 µg/ml). Solution of the test extracts were prepared in ethanol having a concentration of 5 mg/ml. Spiking the sample solution with phenolic standards was done for further confirmation of individual polyphenols.21
Statistical analysis
Data were presented as mean ± standard deviation (SD). One-way ANOVA followed by Dunnett’s test was performed and the results were considered statistically significant when p<0.05.
Results
Anti-inflammatory activity test
Carrageenan-induced paw edema
At the doses of 200 and 400 mg/kg, both EAH and AAH significantly decreased the volume of carrageenan-induced rat paw edema. At the above dose levels, the maximum decrease in paw volume was 26 and 36%, respectively for EAH, in contrast to 29 and 40%, respectively for AAH as compared to control. Indomethacin showed statistically significant anti-inflammatory activity with the highest inhibition (64%) of paw edema at 5 h after carrageenan injection (Table 1).
Table 1. Effect of ethanol and aqueous extracts of A. hispida leaves on carrageenan-induced rat paw edema volume.
| Treatment | Dose (mg/kg) | Right hind paw volume (% Inhibition) | ||||
| 1 h | 2 h | 3 h | 4 h | 5 h | ||
| Vehicle | 10 | 0.95 ± 0.15 | 1.11 ± 0.21 | 1.28 ± 0.19 | 1.45 ± 0.27 | 1.51 ± 0.24 |
| Indomethacin | 10 | 0.51 ± 0.12 (46)* | 0.53 ± 0.18 (52)* | 0.55 ± 0.25 (57)* | 0.59 ± 0.16 (59)** | 0.60 ± 0.17 (60)** |
|
Ethanol
extract |
200 | 0.82 ± 0.17 (14)* | 0.95 ± 0.19 (14)* | 1.04 ± 0.21 (19)* | 1.13 ± 0.10 (22)* | 1.12 ± 0.15 (26)** |
| 400 | 0.78 ± 0.17 (18)* | 0.85 ± 0.19 (23)* | 0.91 ± 0.13 (29)* | 0.97 ± 0.20 (33)* | 0.96 ± 0.14 (36)** | |
|
Aqueous
extract |
200 | 0.81 ± 0.18 (15) | 0.92 ± 0.16 (17)* | 1.01 ± 0.19 (21) | 1.07 ± 0.21 (26)* | 1.08 ± 0.14 (29)** |
| 400 | 0.73 ± 0.19 (23) | 0.77 ± 0.16 (31)* | 0.84 ± 0.15 (34)* | 0.92 ± 0.17 (37)* | 0.90 ± 0.13 (40)** | |
Value presented as the mean ± SE (n=5); *p<0.05, **p<0.01 versus control (Dunnett’s test).
Histamine-induced paw edema
Rats pre-treated with EAH at 200 and 400 mg/kg, showed decrease in the histamine-induced paw edema volume with the maximum inhibition of 27 and 39%, respectively, while that of AAH was 31 and 45% inhibition, respectively as compared to control. Indomethacin showed a maximum inhibition of 64% at 5 h after histamine administration, and the results were statistically significant (Table 2).
Table 2. Effect of ethanol and aqueous extracts of A. hispida leaves on histamine-induced rat paw edema volume.
| Treatment | Dose (mg/kg) | Right hind paw volume (% Inhibition) | ||||
| 1 h | 2 h | 3 h | 4 h | 5 h | ||
| Vehicle | 10 | 1.10 ± 0.13 | 1.27 ± 0.11 | 1.36 ± 0.17 | 1.41 ± 0.22 | 1.48 ± 0.27 |
| Indomethacin | 10 | 0.54 ± 0.17 (51)* | 0.58 ± 0.13 (54)* | 0.59 ± 0.18 (57)* | 0.56 ± 0.15 (60)** | 0.53 ± 0.14 (64)** |
|
Ethanol
extract |
200 | 0.92 ± 0.15 (16)* | 1.01 ± 0.13 (20)* | 1.04 ± 0.21 (24) | 1.07 ± 0.12 (24)* | 1.08 ± 0.16 (27)** |
| 400 | 0.82 ± 0.18 (26) | 0.91 ± 0.15 (28)* | 0.92 ± 0.19 (32) | 0.89 ± 0.17 (37)* | 0.90 ± 0.13 (39)** | |
|
Aqueous
extract |
200 | 0.87 ± 0.21 (21) | 0.96 ± 0.13 (24)* | 0.99 ± 0.20 (27) | 1.01 ± 0.15 (28)* | 1.02 ± 0.11 (31)** |
| 400 | 0.81 ± 0.19 (26) | 0.88 ± 0.13 (31)* | 0.89 ± 0.17 (35)* | 0.84 ± 0.18 (40)* | 0.82 ± 0.17 (45)** | |
Value presented as the mean ± SE (n=5); *p<0.05, **p<0.01 versus control (Dunnett’s test).
Antioxidant activity test
DPPH free radical scavenging assay
In the quantitative DPPH free radical scavenging assay, EAH and AAH showed strong antioxidant activity with IC50 values of 14 and 17 µg/ml, respectively, while that of ascorbic acid was 10 µg/ml.
Fe2+ ion chelating ability
Both EAH and AAH showed Fe2+ ion chelating ability with IC50 values of 40 and 46 µg/ml, respectively. The IC50 value of EDTA, used as the standard in this assay showed an IC50 of 17 µg/ml.
Reducing power assay
Concentration dependent reducing power was observed for both EAH and AAH with the maximum absorbance of 1.13 and 0.88, respectively at the highest concentration tested (100 ug/ml) (Figure 1).
Figure 1.
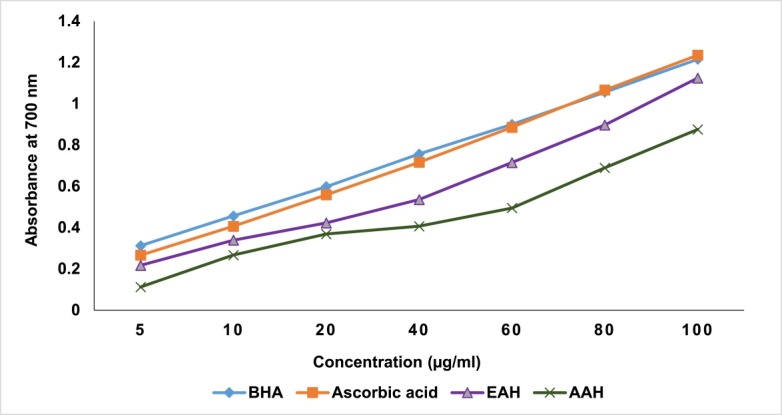
Reducing power of ethanol and aqueous extracts of A. hispida leaves.
Nitric oxide scavenging assay
Both the extracts EAH and AAH significantly scavenged NO radical. The IC50 value for EAH and AAH were found to be 43 and 50 µg/ml, respectively and were comparable to that of ascorbic acid (17 µg/ml) (Figure 2).
Figure 2.
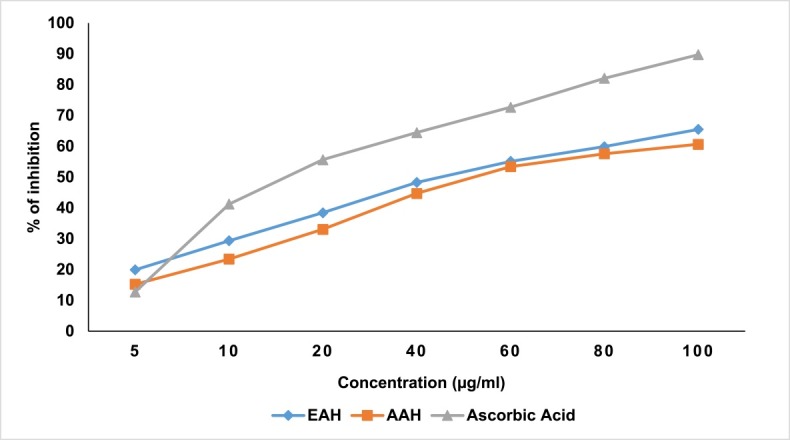
Nitric oxide (NO) scavenging activity of ethanol and aqueous extracts of A. hispida leaves.
Total phenolic content assay
The total phenolic content of EAH and AAH were 130.5 and 173.8 mg GAE/g of dry extract, respectively (Figure 3).
Figure 3.
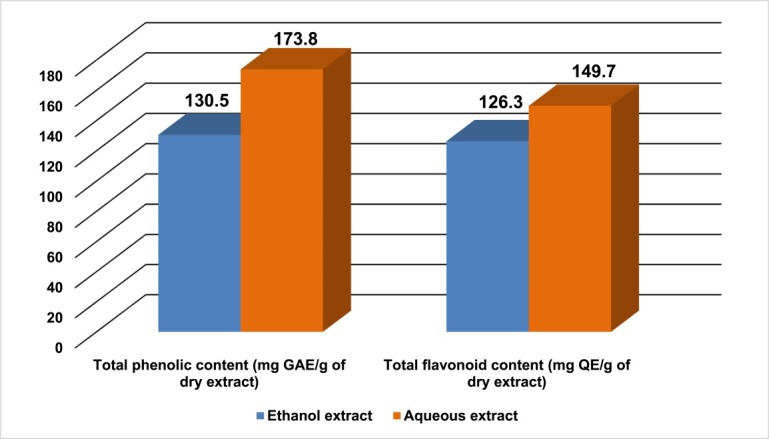
Total phenolic and flavonoid content of ethanol and aqueous extracts of A. hispida leaves.
Total flavonoid content assay
The total flavonoid content for EAH and AAH were 126.3 and 149.7 mg QE/g of dry extract, respectively (Figure 3).
Quantification of polyphenolic compounds by HPLC
The HPLC analysis indicated that both EAH and AAH are rich in ellagic acid content (119.40 and 540.90 mg/100 g dry extract). Gallic acid and quercetin were detected in both extracts but concentration was in lower amount (31.60 and 5.80 mg of gallic acid/100 g EAH extract, 0.50 and 0.60 mg of quercetin/100 g AAH extract, respectively). p-Coumaric acid and rutin were detected only in AAH (0.90 and 14.30 mg/100 g AAH, respectively) (Figure 4, Figure 5 and Figure 6).
Figure 4.
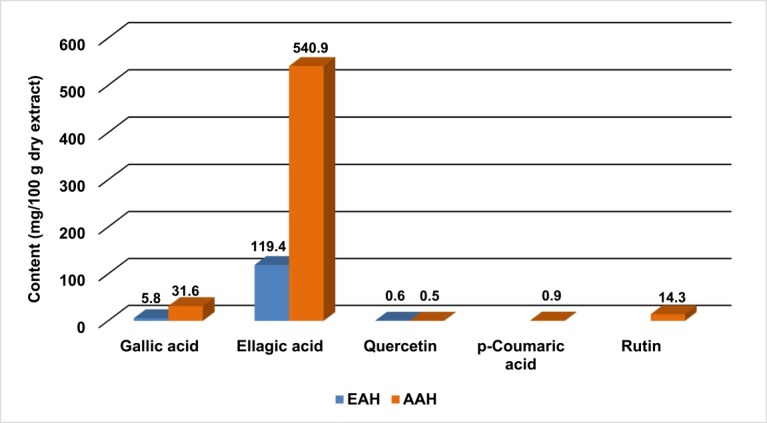
Polyphenolic contents of ethanol and aqueous extracts of A. hispida leaves.
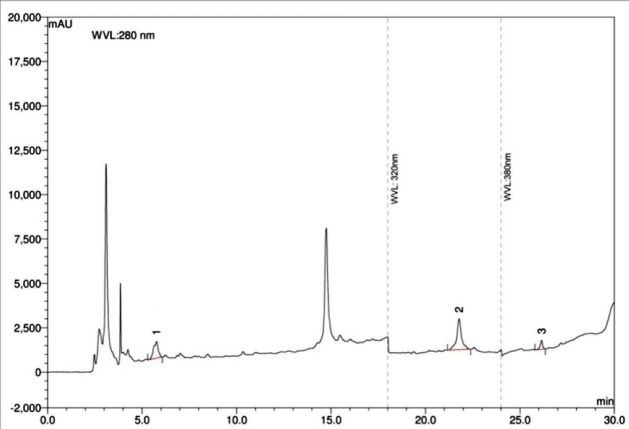
Figure 5.
HPLC chromatogram of ethanol extract of A. hispida leaves. Peaks 1: gallic acid, 2: ellagic acid, 3: quercetin.
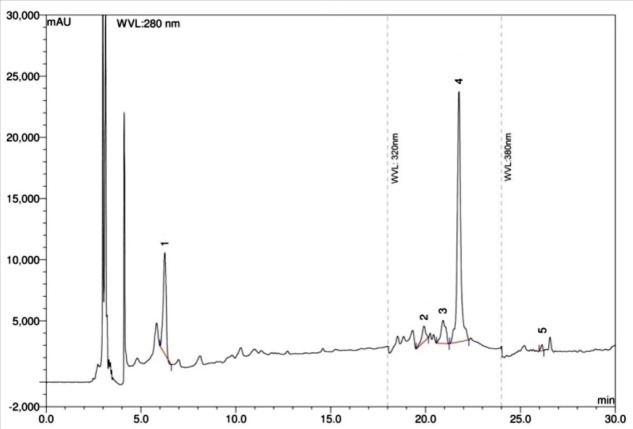
Figure 6.
HPLC chromatogram of aqueous extract of A. hispida leaves. Peaks 1: gallic acid, 2: p-coumaric acid, 3: rutin, 4: ellagic acid, 5: quercetin.
Discussion
The inflammatory reaction induced by carrageenan in rats is recognised by a biphasic response. The key feature of marked edema formation is mediated by histamine, serotonin and bradykinins in the first phase, while the second phase is associated with the release of prostaglandins and nitric oxide, which is generated by cyclooxygenase-2 (COX-2), and inducible isoform of nitric oxide synthase (iNOS).22,23 Histamine is an important mediator of inflammation, and act as a potent vasodilator, and vascular permeability facilitator.24 Administration of histamine in test animals results in an increase in the vascular penetrability of the host capillary venules at the site of injection. Agents that oppose the activity of histamine receptors reduce the swelling triggered by inflammation. In the present investigation, both the ethanol and aqueous extracts of A. hispida leaves significantly reduced the edema formation in carrageenan, as well as in histamine induced rat paw edema. Thus the anti-inflammatory activity of the extracts might involve antihistaminic activity. In addition, inhibition of other inflammatory pathway cannot be ruled out, since the extracts also inhibited carrageenan induced inflammatory response, which involves several inflammatory pathways.
Plant derived compounds with antioxidant activity often show anti-inflammatory activity.25 The ability of antioxidants in protecting the cells from oxidative stress can contribute towards the anti-inflammatory activity.26 Commonly occurring plant phenolics and flavonoids are often associated with antioxidant activity.27 Thus a number of assays were performed to investigate the antioxidant capacity of A. hispida leaves. In the DPPH radical scavenging assay, A. hispida leaves extracts showed a concentration dependent free radical scavenging activity. Saponins, flavonoids and tannins, which are also present in this plant, are known to show DPPH radical scavenging activity by donating proton to neutralise the free radicals.28,29 Agents that form σ bond with a metal are active as secondary antioxidants, because they decrease the redox potential and steadying the oxidised form of the metal ion.30 Results demonstrated that A. hispida leaf extracts have the capacity to reduce ferric ion, a further proof to the antioxidant potential of the extracts. Nitric oxide (NO) works as a neural modulator, which is involved in neurotransmitter release, neuronal excitability and other intellectual activities including learning and memory.31 Though it has a role in physiologic processes, it also participates in pathogenic pathways underlying a large group of disorders including inflammation, inflammatory bowel disorder, primary headaches and stroke.32 A. hispida leaf extracts showed NO scavenging activity suggesting the ability to ameliorate inflammatory response associated with NO.
With a view to justify the correlation between pharmacological activity and phenolic constituents of A. hispida leaf extracts, HPLC analysis was conducted to identify some of the biologically important antioxidant principles from plants. The HPLC analysis showed a high content of ellagic acid in both extracts. Previous studies revealed that ellagic acid possesses strong antioxidant and anti-inflammatory activities.33,34 The anti-inflammatory activity of ellagic acid can be attributed due to its ability to inhibit one or more inflammatory pathways including inhibition of COX-2 and NO synthase expression.35 Presence of phenolic hydroxyl group in the structure of ellagic acid enables the donation of proton to neutralise free radicals.36 Trace amount of gallic acid, rutin and quercetin were also present in the leaf extracts. In addition to their antioxidant property, aforementioned compounds are also reported to have anti-inflammatory activity.37-39
The anti-inflammatory activity observed for the aqueous extract was higher than that of the ethanol extract. In the HPLC analysis, ellagic acid content, along with other phenolics were found higher for aqueous extract than the ethanol extract and it is also reflected by the total phenol and total flavonoid content assay. Therefore, stronger anti-inflammatory activity of the aqueous extract may be due its higher phenol and flavonoid content.
Conclusion
The ethanol and aqueous extracts of A. hispida leaf exhibited significant anti-inflammatory activity through the reduction of carrageenan and histamine induced edema formation in test rats. The extracts also showed potent antioxidant activity in a number of methods. The observed anti-inflammatory activity may be due to the presence of high concentration of ellagic acid in the plant extracts. In addition, other phenolic constituents present in the plant might have assisted towards the anti-inflammatory activity.
Acknowledgments
Authors are grateful to the Chemical Research Division of Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh for allowing them to use their research facilities to perform these research works.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Meyer S. Phytochemical methods A guide to modern techniques of plant analysis. USA: Champan and Hall; 1982. [Google Scholar]
- 2.Seebaluck R, Gurib-Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae)--a review of their ethnopharmacology and phytochemistry. J Ethnopharmacol. 2015;159:137–57. doi: 10.1016/j.jep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Amakura Y, Miyake M, Ito H, Murakaku S, Araki S, Itoh Y. et al. Acalyphidins M1, M2 and D1, ellagitannins from Acalypha hispida. Phytochemistry. 1999;50(4):667–75. doi: 10.1016/s0031-9422(98)00579-2. [DOI] [Google Scholar]
- 4.Reiersen B, Kiremire BT, Byamukama R, Andersen MO. Anthocyanins acylated with gallic acid from chenille plant, Acalypha hispida. Phytochemistry. 2003;64(4):867–71. doi: 10.1016/S0031-9422(03)00494-1. [DOI] [PubMed] [Google Scholar]
- 5.Bokshi B, Sayeed MAS, Ahmed MI, Karmakar UK, Sadhu SK. Assessment of antimicrobial and cytotoxic activities of ethanolic extract of leaves of Acalypha hispida. IJPSR. 2012;3(6):1705–8. [Google Scholar]
- 6.McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf J. 1999;32:513–24. [Google Scholar]
- 7.Adesina SK, Idowu O, Ogundaini AO, Oladimeji H, Olugbade TA, Onawunmi GO. et al. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Aacalypha hispida. Phytother Res. 2000;14(5):371–4. doi: 10.1002/1099-1573(200008)14:5<371::aid-ptr625>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Ejechi BO, Souzey JA. Inhibition of biodeterioration of yam tuber Dioscorea rotundata Poir in storage with phenolic extract of Acalypha hispida Burm. f. leaves. J Stored Prod Res. 1999;35(2):127–34. doi: 10.1016/s0022-474x(98)00038-1. [DOI] [Google Scholar]
- 9.Onocha PA, Oloyede GK, Afolabi QO. Chemical composition, cytotoxicity and antioxidant activity of essential oils of Acalypha hispida flowers. Int J Pharmacol. 2011;7(1):144–8. doi: 10.3923/ijp.2011.144.148. [DOI] [Google Scholar]
- 10.Ahmed F, Selim MS, Shilpi JA. Antibacterial activity of Ludwigia adscendens. Fitoterapia. 2005;76(5):473–5. doi: 10.1016/j.fitote.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Rouf R, Uddin SJ, Shilpi JA, Toufiq-Ur-Rahman M, Ferdous MM, Sarker SD. Anti-diarrhoeal properties of Diospyros peregrina in the castor oil-induced diarrhoea model in mice. Ars Pharm. 2006;47:81–9. [Google Scholar]
- 12.Saha S, Shilpi JA, Mondal H, Gofur R, Billah M, Nahar L. et al. Bioactivity studies on Musa seminifera Lour. Pharmacogn Mag. 2013;9(36):315–22. doi: 10.4103/0973-1296.117827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeghi H, Mostafazadeh M, Sadeghi H, Naderian M, Barmak MJ, Talebianpoor MS. et al. In vivo anti-inflammatory properties of aerial parts of Nasturtium officinale. Pharm Biol. 2014;52(2):169–74. doi: 10.3109/13880209.2013.821138. [DOI] [PubMed] [Google Scholar]
- 14.Perianayagam JB, Sharma SK, Pillai KK. Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J Ethnopharmacol. 2006;104(3):410–4. doi: 10.1016/j.jep.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 15.Dehshiri MM, Aghamollaei H, Zarini M, Nabavi SM, Mirzaei M, Loizzo MR. et al. Antioxidant activity of different parts of Tetrataenium lasiopetalum. Pharm Biol. 2013;51(8):1081–5. doi: 10.3109/13880209.2013.775594. [DOI] [PubMed] [Google Scholar]
- 16.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315(1):161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 17.Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites. 2009;60(4):405–12. doi: 10.3989/gya.010109. [DOI] [Google Scholar]
- 18.Govindarajan R, Rastogi S, Vijayakumar M, Shirwaikar A, Rawat AK, Mehrotra S. et al. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003;26(10):1424–7. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–14. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 20.Hossain H, Karmakar UK, Biswas SK, Shahid-Ud-Daula AF, Jahan IA, Adnan T. et al. Antinociceptive and antioxidant potential of the crude ethanol extract of the leaves of Ageratum conyzoides grown in Bangladesh. Pharm Biol. 2013;51(7):893–8. doi: 10.3109/13880209.2013.770535. [DOI] [PubMed] [Google Scholar]
- 21.Mondal H, Saha S, Awang K, Hossain H, Ablat A, Islam MK. et al. Central-stimulating and analgesic activity of the ethanolic extract of Alternanthera sessilis in mice. BMC Complement Altern Med. 2014;14:398. doi: 10.1186/1472-6882-14-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W. et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;91(25):12013–7. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sommer CV, Parth CM. Inflammation, tissue repair and fever. In: Porth C, editor. Essential of Pathophysiology- Concepts of Altered Health Sciences. 3rd ed. New York: Lippincott Williams and Wilkins; 2006. P. 495-514.
- 24.Cuman RKN, Bersani-Amadio CA, Fortes ZB. Influence of type 2 diabetes on the inflammatory response in rat. Inflamm Res. 2001;50(9):460–5. doi: 10.1007/pl00000271. [DOI] [PubMed] [Google Scholar]
- 25.Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian species. Food Chem. 2007;104(4):1372–8. doi: 10.1016/j.foodchem.2007.01.064. [DOI] [Google Scholar]
- 26.González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martinez-Augustin O. et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51(4):331–62. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami B, Rahmani Z. Antioxidant and antihaemolytic activities of the leaves of Kefe cumin (Laser trilobum L) Umbelliferae. Trop J Pharm Res. 2010;9(5):441–9. doi: 10.4314/tjpr.v9i5.61053. [DOI] [Google Scholar]
- 28.Ponou BK, Teponno RB, Ricciutelli M, Quassinti L, Bramucci M, Lupidi G. et al. Dimeric antioxidant and cytotoxic triterpenoid saponins from Terminalia ivorensis A. Chev. Phytochemistry. 2010;71(17-18):2108–15. doi: 10.1016/j.phytochem.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical. Biochem Pharmacol. 1998;56(2):213–22. doi: 10.1016/S0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 30.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harng Jyur (Chrysanthemum morifolium Ramat) LWT-Food Sci Technol. 1999;32(5):269–77. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- 31.Fossier P, Blanchard B, Ducrocq C, Leprince C, Tauc L, Baux G. Nitric oxide transforms serotonin into an inactive form and this affects neuromodulation. Neuroscience. 1999;93(2):597–603. doi: 10.1016/S0306-4522(99)00165-7. [DOI] [PubMed] [Google Scholar]
- 32.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42. [PubMed] [Google Scholar]
- 33.Guruvayoorappan C, Kuttan G. (+)-Catechin inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-alpha in LPS-stimulated macrophages. Innate Immun. 2008;14(3):160–74. doi: 10.1177/1753425908093295. [DOI] [PubMed] [Google Scholar]
- 34.Iñiguez-Franco F, Soto-Valdez H, Peralta E, Ayala-Zavala JF, Auras R, Gámez-Meza N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly(L-lactic acid) J Agric Food Chem. 2012;60(26):6515–23. doi: 10.1021/jf300668u. [DOI] [PubMed] [Google Scholar]
- 35.Gainok J, Daniels R, Golembiowski D, Kindred P, Post L, Strickland R. et al. Investigation of the anti-inflammatory, antinociceptive effect of ellagic acid as measured by digital paw pressure via the Randall-Selitto meter in male Sprague-Dawley rats. AANA J. 2011;79(4 Suppl):S28–34. [PubMed] [Google Scholar]
- 36.Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T. et al. Effects of the Interaction of Tannins with Co-existing Substances. VI. : Effects of Tannins and Related Polyphenols on Superoxide Anion Radical, and on 1, 1-Diphenyl-2-picrylhydrazyl Radical. Chem Pharm Bull. 1989;37(8):2016–21. doi: 10.1248/cpb.37.2016. [DOI] [Google Scholar]
- 37.Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY. et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Kroes BH, Van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58(6):499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 39.Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003;54(4):313–8. doi: 10.1078/0940-2993-00260. [DOI] [PubMed] [Google Scholar]