Abstract
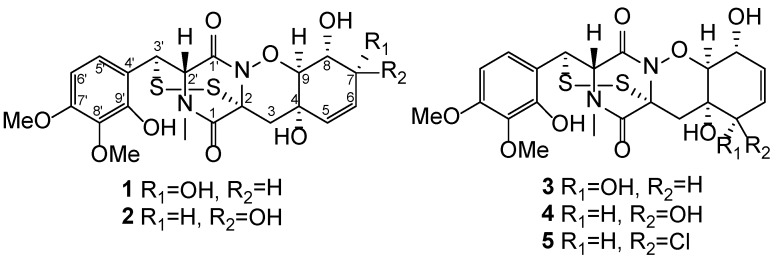
Three new epidithiodiketopiperazines pretrichodermamides D–F (1–3), together with the known N-methylpretrichodermamide B (4) and pretrichodermamide С (5), were isolated from the lipophilic extract of the marine algae-derived fungus Penicillium sp. KMM 4672. The structures of compounds 1–5 were determined based on spectroscopic methods. The absolute configuration of pretrichodermamide D (1) was established by a combination of modified Mosher′s method, NOESY data, and biogenetic considerations. N-Methylpretrichodermamide B (5) showed strong cytotoxicity against 22Rv1 human prostate cancer cells resistant to androgen receptor targeted therapies.
Keywords: marine-derived fungus, secondary metabolites, diketopiperazine, oxazadecaline, cytotoxicity
1. Introduction
Epithiodiketopiperazines with 1,2-oxazadecaline moiety are rare in nature. To date, only eleven such compounds have been reported [1,2,3,4]. Structural differences in compounds of this class consist in N-methylation, C-4–C-5-epoxidation [5,6] and substituent at C-5. In addition, first mono- and trithioderivatives have recently been reported [3,4]. The fungi of genus Trichoderma were producers of most of these alkaloids. However, pretrichodermamide A has also been reported to be synthesized by Aspergillus sp. [7], while N-methylated compounds methylgliovirin (the first described compound from this class) [5], N-methylpretrichodermamide B (adametizine A) [8,9], and pretrichodermamide C (adametizine B) [8,9] have only been isolated from Penicillium species. Many oxazadecaline thiodiketopiperazines have been reported and patented as antibiotics, and chloroderivatives have shown cytotoxic activity against murine lymphoma and Jurkat cells with IC50 of 2–5 µM [2,8,9,10]. During our ongoing search for structurally novel and bioactive metabolites from marine-derived fungi, we investigated the fungus Penicillium sp. KMM 4672 isolated from Vietnamese brown alga Padina sp. A chemical study resulted in the isolation and identification of three new 1,2-oxazadecaline epidithiodiketopiperazines pretrichodermamides D–F (1–3), together with the known pretrichodermamide C (4) and N-methylpretrichodermamide B (5) (Figure 1). Herein, we report the isolation, structure elucidation, and biological assay results of the new compounds 1–3 produced by the marine fungus Penicillium sp.
Figure 1.
Chemical structures of isolated compounds 1–5.
2. Results
The EtOAc extract of the culture of the fungus was suspended in H2O–EtOH (4:1) and successively partitioned with hexane, EtOAc, and n-BuOH. The EtOAc portion was subjected to column chromatography over silica gel and by HPLC to yield individual compounds 1–5 as white powders.
The molecular formula of compound 1 was determined to be C21H24N2O9S2 from a HRESIMS peak at m/z 511.0857 [M − H]− and was in accordance with 13C NMR data. A thorough analysis of the 1H and 13C NMR data (Table 1) of 1 with DEPT and HSQC techniques revealed the presence of two methoxyls, one N-methyl, one methylene, four sp2-methines, and five sp3-methines together with two sp3-quaternary carbons. The remaining functionalities, corresponding to the carbon signals at δC 165.4 (C), 164.2 (C), 153.0 (C), 147.6 (C), 135.9 (C), and 116.3 (С), suggested the presence of two amide carbonyl carbons, three oxygenated, and one C-substituted sp2-carbons.
Table 1.
NMR spectroscopic data (DMSO-d6) for pretrichodermamides D–F (1–3).
| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δС, mult | δH (J in Hz) | δС, mult | δH (J in Hz) | δС, mult | δH (J in Hz) | |
| 1 | 165.4, C | - | 165.5, C | - | 165.4, C | - |
| 2 | 68.0, C | - | 67.7, C | - | 69.1, C | - |
| 3 | 38.4, CH2 | α: 2.17, d (15.3) β: 2.33, d (15.3) |
39.0, CH2 | α: 2.22, d (15.7) β: 2.27, d (15.5) |
35.6, CH2 | α: 1.93, brd (15.5) β: 2.06, dd (15.4, 1.5) |
| 4 | 66.9, C | - | 67.0, C | - | 67.4, C | - |
| 5 | 133.8, CH | 5.56, d (10.1) | 131.8, CH | 5.54, dd (10.1, 2.2) | 69.2, CH | 3.69, d (5.5) |
| 6 | 127.4, CH | 5.60, dd (10.0, 4.4) | 129.7, CH | 5.43, dd (10.2, 2.2) | 126.9, CH | 5.69, ddd (10.0, 5.1, 2.3) |
| 7 | 65.8, CH | 4.03, q (4.5) | 72.2, CH | 3.96, tt (7.7, 2.2) | 131.2, CH | 5.56, dd (9.9, 2.5) |
| 8 | 66.2, CH | 3.74, ddd (9.4, 6.6, 4.6) | 71.0, CH | 3.56, ddd (10.7, 7.7, 5.7) | 64.6, CH | 4.16, m |
| 9 | 81.9, CH | 4.12, d (9.4) | 83.4, CH | 3.83, d (10.7) | 83.5, CH | 3.97, dd (7.1, 1.5) |
| 1′ | 164.2, C | - | 164.4, C | - | 163.8, C | - |
| 2′ | 66.0, CH | 4.56, d (2.6) | 65.7, CH | 4.57, d (2.6) | 65.4, CH | 4.57, d (2.6) |
| 3′ | 41.4, CH | 4.55, d (2.5) | 41.5, CH | 4.58, d (2.5) | 41.0, CH | 4.59, d (2.5) |
| 4′ | 116.3, C | - | 116.3, C | - | 116.3, C | - |
| 5′ | 122.6, CH | 7.32, d (8.8) | 122.6, CH | 7.32, d (8.8) | 122.7, CH | 7.35, d (8.8) |
| 6′ | 103.3, CH | 6.55, d (8.8) | 103.3, CH | 6.55, d (8.8) | 103.2, CH | 6.54, d (8.8) |
| 7′ | 153.0, C | - | 153.0, C | - | 152.8, C | - |
| 8′ | 135.9, C | - | 135.9, C | - | 135.8, C | - |
| 9′ | 147.6, C | - | 147.5, C | - | 147.5, C | - |
| 10′ | 32.6, CH3 | 2.96, s | 32.6, CH3 | 2.96, s | 32.5, CH3 | 2.96, s |
| 7′-OMe | 55.7, CH3 | 3.78, s | 55.6, CH3 | 3.78, s | 55.6, CH3 | 3.78, s |
| 8′-OMe | 60.2, CH3 | 3.68, s | 60.2, CH3 | 3.68, s | 60.2, CH3 | 3.68, s |
| 9′-OH | - | 9.43, s | - | 9.42, s | - | 9.38, s |
| 4-OH | - | 5.26, s | - | 5.29, s | - | 4.96, brs |
| 5-OH | - | - | - | - | - | 5.19, d (5.6) |
| 7-OH | - | 4.89, d (5.4) | - | 5.02, d (6.9) | - | |
| 8-OH | - | 4.35, d (6.6) | - | 4.64, d (5.7) | - | 5.15, d (6.7) |
a 1H NMR and 13C NMR spectroscopic data were measured at 500.13 MHz and 125.77 MHz, respectively; b 1H NMR and 13C NMR spectroscopic data were measured at 700.00 MHz and 176.04 MHz, respectively.
A direct comparison of 1H and 13C NMR spectra of 1 (Table 1) with those of pretrichodermamide C (4) [8] (S21–S22, Supplementary data) showed similarities including signals of two methoxyls (δH 3.68, 3.78; δC 55.7, 60.2), N-methyl (δH 2.96; δC 32.6), one phenolic hydroxyl group (δH 9.43), two aromatic methine (δH 6.55, 7.32; δC 103.3, 122.6), and two amide carbonyls (δC 164.2, 165.4), suggesting that 1 has a framework similar to that of 4.
The HMBC correlations from both H-3 (δH 2.17, 2.33) to C-4 (δC 66.9), C-5 (δC 133.8), and C-9 (δC 81.9), from 4-OH (δH 5.26) to C-3 (δC 38.4), C-4, C-5, and C-9, from H-9 (δH 4.12) to C-8 (δC 66.2), and from H-7 (δH 4.03) to C-5, C-6 (δC 127.4), and C-8 established the cyclohexene ring with a C-5–C-6 double bond placement. The location of secondary hydroxyl groups at C-7 and C-8 was proven by HMBC correlations from 7-OH (δH 4.89) to C-7 and from 8-OH (δH 4.35) to C-8. The planar structure of 1 was thus elucidated.
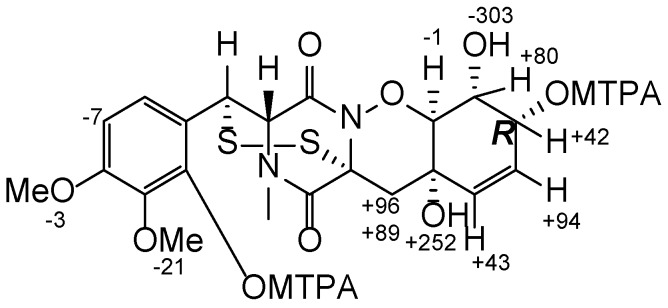
Esterification of the C-7 and C-9′ hydroxy moieties of 1 with (R)- and (S)-MTPA chloride afforded the (S)- and (R)-bis-MTPA-esters, respectively. The observed chemical shift differences Δδ (δS − δR) (Figure 2) indicated 7R configuration [11]. The absolute configurations of the remaining stereocentres in cyclohexene ring were established as 4S, 8R, 9S, the same as in adametizine B (pretrichodermamide C) and adametizine A (N-methylpretrichodermamide B) (Figure 3) based on the ROESY-correlations 7-OH with H-9, and H-9 with 4-OH and 8-OH, together with the coupling constants 3JH8-H9 (9.4 Hz) and 3JH7-H8 (4.6 Hz), which were in accordance with calculated dihedral angles (177° and 46°, respectively). The absolute configurations at C-2, C-2′, and C-3′ were assigned to be the same as known adametizine A and adametizine B on the basis of the similarity of C-2, C-2′, and C-3′ chemical shifts for these biogenetically related compounds [9]. Compound 1 was named pretrichodermamide D.
Figure 2.
∆δ (δS−δR) values (in Hz) for the MTPA ester of 1.
Figure 3.
Energy-minimized 3D models of 1–3 with selected ROESY correlations.
The molecular formula of compound 2 was determined as C21H24N2O9S2, the same as 1, by a HRESIMS peak at m/z 511.0869 [M − H]− and by 13C NMR analysis. The general features of the 1H and 13C NMR spectra (Table 1) of 2 resembled those of 1 with the exception of the proton and carbon signals at C-7 and C-8. The HMBC correlations from H-7 (δH 3.96) to C-6 (δC 129.7) and C-8 (δC 71.0), from H-9 (δH 3.83) to C-4 (δC 67.0) and C-8, and from 4-OH (δH 5.29) to C-3 (δC 39.0), C-5 (δC 131.8), and C-9 (δC 83.4) proved that the planar structure of 2 was identical with pretrichodermamide D (1). The vicinal coupling constant JH7-H8 (7.7 Hz) and JH8-H9 (10.7 Hz) according to Karplus equation (calculated dihedral angles are 168° and 174°, respectively) indicated the axial stereolocations of H-7, H-8 (δH 3.56), and H-9. These relative configurations were additionally proved by ROESY’s (Figure 3) H-7 with 8-OH (δH 4.64) and H-9. The absolute stereoconfigurations of 2 were assigned as for pretrichodermamide D (1) according to biogenetic considerations. Thus, compound 2 was determined as the C-7 epimer of pretrichodermamide D and named pretrichodermamide E.
The molecular formula of compound 3 was determined as C21H24N2O9S2 (the same as 1 and 2) on the basis of HRESIMS and 13C NMR spectra. The NMR data for this compound were very similar to those obtained for pretrichodermamide C (4) with the exception of proton and carbon signals at C-3, C-4, C-5, C-6, and C-9. The HMBC correlations from H-5 (δH 3.69) to C-3 (δC 35.6), C-4 (δC 67.4), C-6 (δC 126.9), and C-7 (δC 131.2), from H-8 (δH 4.16) to C-7 and C-9 (δC 83.5), and from H-9 (δH 3.97) to C-4 and C-8 (δC 64.6) established a planar structure of the cyclohexene ring with a double bond between C-6 and C-7. The mutual ROESY correlations (Figure 3) from H-9 to 4-OH (δH 4.96), 5-OH (δH 5.19), and 8-OH (δH 5.15) showed α-orientation of 5-OH and detected 3 as the С-5-epimer of pretrichodermamide C (4). The relative configurations of the 1,2-oxazadecaline fragment were additionally suggested by a W-type coupling constant between H-9 (δH 3.97, dd, 7.3, 1.5) and H-3β (δH 2.06, dd, 15.4, 1.5). The absolute configurations in compound 3 were proposed based on biogenetic relationships with compounds 1, 2, 4, and 5. Compound 3 was named pretrichodermamide F.
Besides the new pretrichodermamides D–F (1–3), the known pretrichodermamide C (4) and N-methylpretrichodermamide B (5) were also isolated from this fungus. For the first time these compounds were found in Egyptian hyper saline lake fungus Penicillium sp. [8] and were later isolated from sponge-derived Penicillium adametzioides and published as new adametizines A and B, respectively. The absolute stereochemistry for adametizines were determined based on X-ray and ECD data [9]. The structures of 4 and 5 were established on the basis of 1D and 2D NMR data and high resolution ESIMS analysis (Supplementary data S20–S26). The absolute structures of compounds 4 and 5 were determined the same as for adametizines B and A, respectively, based on identity of their ECD spectra.
In a next step, we investigated the effects of compounds 1–5 on viability and the apoptosis induction of human prostate cancer cells. It should be noted that, in a recently published study, N-methylpretrichodermamide B did not show any cytotoxic effect against a number of different cancer cells up to 10 µM [9]. MTT assays revealed N-methylpretrichodermamide B (5) to be highly cytotoxic in 22Rv1, PC-3, and LNCaP cells with IC50 0.51, 5.11, and 1.76 µM, respectively, while revealed IC50s of 0.013, 0.015, and 0.004 µM were determined for docetaxel (positive control). Remarkably, 5 induces apoptosis in human prostate cancer 22Rv1 cells (31.3% ± 8.2% apoptosis after treatment with 1 µM for 48 h), which are highly resistant to androgen receptor (AR)-targeted therapies due to a loss of the ligand-binding domain of the AR receptor [12]. Сompounds 1–4 did not exhibit cytotoxic activity against human prostate cancer cells at concentrations up to 100 µM. No significant effect on cell cycle progression was observed for any of the compounds at concentrations up to 100 µM. 22Rv1 cells are known to be resistant to the hormone therapy due to the presence of androgen receptor splice variant AR-V7, while LNCaP cells bearing w/t AR are sensitive to the hormone deprivation [12]. Remarkably, 5 was mostly active in AR-V7-positive 22Rv1 cells with IC50 at nanomolar concentrations (MTT test). In addition, the effect of compounds 1–5 was tested on non-malignant murine cells (splenocytes and erythrocytes). N-methylpretrichodermamide B (5) did not show hemolitic activity up to 100 µM and was cytotoxic for splenocytes only at high doses (ED50 62.1 µM).
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 343 polarimeter (Perkin Elmer, Waltham, MA, USA). UV spectra were recorded on a Specord UV VIS spectrometer (Carl Zeiss, Jena, Germany) in MeOH. IR spectra were determined on a Specord M 82 (Carl Zeiss, Jena, Germany) in CHCI3. NMR spectra were recorded in DMSO-d6 on a Bruker DPX-500 (Bruker BioSpin GmbH, Rheinstetten, Germany) (500.13/125.77 MHz) and Bruker DRX-700 (Bruker BioSpin GmbH, Rheinstetten, Germany) (700.00/176.04 MHz) spectrometer, using TMS as an internal standard. HRESIMS spectra were measured on an Agilent 6510 Q-TOF LC mass spectrometer (Agilent Technologies, Santa Clara, CA, USA).
Low pressure liquid column chromatography was performed using silica gel (50/100 μm, Imid, Russia). Plates (4.5 × 6.0 cm) precoated with silica gel (5–17 μm, Imid) were used for thin layer chromatography. Preparative HPLC was carried out on a Shimadzu LC-20 chromatograph (Shimadzu USA Manufacturing, Canby, OR, USA) using a YMC ODS-AM (YMC Co., Ishikawa, Japan) (5 µm, 10 × 250 mm) and YMC SIL (YMC Co., Ishikawa, Japan) (5 µm, 10 × 250 mm) columns with an Shimadzu RID-20A refractometer (Shimadzu Corporation, Kyoto, Japan).
The energy-minimized structure models for 1–3 have been calculated based on crystallographic data (CCDC 1040973) for adametizine A (N-methylpretrichodermamide, compound 5) [9] by the MM2 force field calculation method using ChemBio3D Ultra 12.0, CambridgeSoft Corporation (Cambridge, MA, USA).
3.2. Fungal Strain
The strain was isolated from brown algae Padina sp. (South China Sea, Vietnam) by the plating method using malt extract agar and identified on the basis of morphological and molecular features. For DNA extraction, the culture was grown on malt extract agar under 25 °C for 7 d. DNA extraction was performed by HiPurATM Plant DNA Isolation kit (CTAB Method) (HiMedia Laboratories Pvt. Ltd., Mumbai, India) according to the manufacturer′s instructions. Fragments containing the ITS regions were amplified using primers ITS1 and ITS4 [13]. Amplification of the partial calmodulin gene was performed using Cmd5 and Cmd6 primers [14]. The newly obtained sequences were checked visually and compared to available sequences of GenBank by using BLAST-n. According to BLAST analysis of the ITS1-5.8S-ITS2 and partial calmodulin datasets, the strain Penicillium sp. KMM 4672 is related to P. citrinum-group and displays the most similarity with P. steckii (99% and 97%, respectively). The sequences were deposited in GenBank nucleotide sequence database under KU 695807 and KU 695808. The strain was deposited in the Collection of Marine Microorganisms under the code KMM 4672.
3.3. Cultivation of Fungus
The fungus was grown stationary at 22 °С for three weeks in 60 × 500 mL Erlenmeyer flasks, each containing 60 g of the solid nutrient medium of the following composition: rice (20.0 g), yeast extract (20.0 mg), KH2PO4 (10 mg), and natural sea water (40 mL).
3.4. Extraction and Isolation
The fungal mycelia with the medium were extracted for 24 h with 12 L of EtOAc. Evaporation of the solvent under reduced pressure yielded a brown oil (9.2 g), to which 250 mL of H2O–EtOH (4:1) was added, and the combination was thoroughly mixed to yield a suspension. It was extracted successively with hexane (150 mL × 2), EtOAc (150 mL × 2) and n-BuOH (150 mL × 2). The EtOAc fraction was concentrated in vacuo to give a residue (6.0 g), which was separated on a silica gel column (30 × 3cm) eluted with a hexane-EtOAc gradient (1:0–0:1). The hexane-EtOAc fraction PS-101-64 (65:35, 210 mg) was purified by RP HPLC on a YMC ODS-AM column eluting with MeOH–H2O (65:35) to yield 5 (170 mg). The hexane-EtOAc fraction PS-101-87 (50:50, 73 mg) was separated by RP HPLC on a YMC ODS-AM column eluting with MeOH–H2O (65:35) to yield the 4 (16 mg), 1 (4.6 mg) and PS-103-4 fractions (7.8 mg). The PS-103-4 fraction was purified by HPLC on a YMC Sil column eluting with CHCl3–MeOH (95:5) to yield 2 (3.4 mg) and 3 (3.2 mg).
Pretrichodermamide D (1): white powder; −205 (c 0.17, MeOH); UV (MeOH) λmax (log ε) 205 (4.54) nm; ECD (0.17 mM, MeOH) λmax (Δε) 218 (−20.33), 258 (−6.98), 301 (+0.83) nm; 1H and 13C NMR data, see Table 1, Supplementary data S1–S6; HRESIMS m/z 511.0859 [M − H]− (calcd for C21H23N2O9S2, 511.0850).
Pretrichodermamide E (2): white powder; −85 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 205 (4.51) nm; ECD (0.21 mM, MeOH) λmax (Δε) 218 (−17.64), 258 (−6.04), 300 (+0.80) nm; IR (CHCl3) νmax 3514, 3000, 2842, 1694, 1617, 1509, 1466, 1346, 1274, 1097, 1056, 1029 cm−1; 1H and 13C NMR, see Table 1, Supplementary data S7–S13; HRESIMS m/z 511.0869 [M − H]− (calcd for C21H23N2O9S2, 511.0850).
Pretrichodermamide F (3): white powder; −114 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 206 (4.45) nm; ECD (0.17 mM, MeOH) λmax (Δε) 217 (−23.27), 260 (−6.64), 301 (+0.49) nm; IR (CHCl3) νmax 3515, 3001, 2842, 1692, 1617, 1509, 1466, 1347, 1277, 1098, 1054, 1036 cm−1; 1H and 13C NMR, see Table 1, Supplementary data S14–S20; HRESIMS m/z 535.0815 [M + Na]+ (calcd for C21H24N2O9S2Na, 535.0815).
Pretrichodermamide C (4): white powder; −166.9 (c 0.09, MeOH) [lit. −167.0 (c 0.12, MeOH)] [8]; UV (MeOH) λmax (log ε) 206 (4.34) nm; ECD (0.20 mM, MeOH) λmax (Δε) 217 (−27.17), 262 (−7.03), 303 (+0.69) nm; 1H and 13C NMR data, see Supplementary data S21–S22; HRESIMS m/z 535.0830 [M + Na]+ (calcd for C21H24N2O9S2Na, 535.0815).
N-methylpretrichodermamide B (5): white powder; −232.0 (c 0.14, MeOH) [lit. −102.0 (c 0.07, MeOH)] [8]; UV (MeOH) λmax (log ε) 205 (4.56) nm; ECD (0.32 mM, MeOH) λmax (Δε) 217 (−42.10), 262 (−9.18), 303 (+0.87) nm; 1H and 13C NMR data, see Supplementary data S23–S26; HRESIMS m/z 553.0479 [M + Na]+ (calcd for C21H23N2O8S2ClNa, 553.0477).
3.5. Preparation of (S)-MTPA and (R)-MTPA Esters of Pretrichodermamide D (1)
4-dimethylaminopyridine (a few crystals) and (R)-MTPA-Cl (5 μL) was added to a solution of the pretrichodermamide D (1.8 mg) in pyridine at room temperature and stirred for 4 h. After evaporation of the solvent, the residue was passed through a silica gel column (20% EtOAc-hexane) to afford the (S)-MTPA ester (1.0 mg). The (R)-MTPA ester (1.2 mg) was prepared in a similar manner using (S)-MTPA-Cl.
(S)-MTPA ester of 1: 1H NMR (DMSO-d6, 500.13 MHz) δ: 7.16 (1H, d, J = 9.4 Hz, H-6′), 6.04 (1H, s, 4-OH), 5.93 (1H, d, J = 9.8 Hz, H-5), 5.80 (1H, dd, J = 9.8; 5.2 Hz, H-6), 5.73 (1H, t, J = 4.9, H-7), 4.67 (1H, d, J = 3.7, 8-OH), 4.30 (1H, dt, J = 11.0; 4.5 Hz, H-8), 3.89 (3H, s, 8′-OMe), 3.68 (3H, s, 7′-OMe), 3.68 (3H, s, OMe), 3.55 (3H, s, OMe), 2.90 (3H, s, Me-10′), 2.47 (1H, d, J = 15.3 Hz, H2-3), 2.38 (1H, d, J = 15.3 Hz, H2-3), 7.45–7.75 (11H, m, 2Ph, H-5′). The signals of H-2′ and H-3′ overlapped with solvent signals. HRESIMS m/z 979.1438 [M + Cl]– (calcd for C41H38N2O13S2F6Cl, 979.1414).
(R)-MTPA ester of 1: 1H NMR (DMSO-d6, 500.13 MHz) δ: 7.66 (1H, d, J = 8.9 Hz, H-5′), 7.17 (1H, d, J = 8.9 Hz, H-6′), 5.54 (1H, s, 4-OH), 5.85 (1H, d, J = 9.7 Hz, H-5), 5.64 (1H, dd, J = 9.7; 5.0 Hz, H-6), 5.65 (1H, t, J = 5.0, H-7), 5.27 (1H, d, J = 5.0, 8-OH), 4.15 (1H, dt, J = 10.3; 5.0 Hz, H-8), 3.89 (3H, s, 8′-OMe), 3.73 (3H, s, 7′-OMe), 3.72 (3H, s, OMe), 3.59 (3H, s, OMe), 2.28 (1H, d, J = 15.3 Hz, H2-3), 2.21 (1H, d, J = 15.3 Hz, H2-3), 7.40–7.68 (11H, m, 2Ph). The signals of H-2′, H-3′, and Me-10′ overlapped with solvent signals. HRESIMS m/z 979.1430 [M + Cl]– (calcd for C41H38N2O13S2F6Cl, 979.1414).
3.6. Cell Culture
The human prostate cancer cells lines 22Rv1, PC-3, and LNCaP were purchased from ATCC. Cell lines were cultured according to the manufacturers instructions in 10% FBS/RPMI media (Invitrogen) with (for LNCaP) or without (for 22Rv1 and PC-3) 1 mM sodium pyruvate (Invitrogen). Cells were continuously kept in culture for a maximum of 3 months and were routinely inspected microscopically for stable phenotype and regularly checked for contamination with mycoplasma. Cell line authentication was performed by DSMZ (Braunschweig, Germany) using highly polymorphic short tandem repeat loci [15].
3.7. Cytotoxicity Assay
The in vitro cytotoxicity of individual substances was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, which was performed as previously described [16]. Cytotoxicity against CD-I mouse splenocytes was determined according to Freshney [17]. Docetaxel was used as a reference substance.
3.8. Cell Cycle and Apoptosis Induction Analysis
The cell cycle distribution was analyzed by flow cytometry using PI staining as described before with slight modifications [18]. In brief, cells were pre-incubated overnight in 6-well plates (2 × 105 cells/well in 2 mL/well). The medium was changed to fresh medium containing different concentrations of the substances. After 48 h of treatment, cells were harvested with a trypsin-EDTA solution, fixed with 70% EtOH, stained, and analyzed by FACS. The results were quantitatively analyzed using Cell Quest Pro software (Version 5.2.1., BD Bioscience, Bedford, MA, USA). Cells appeared at sub-G1 peak were assumed as apoptotic.
3.9. Hemolytic Activity
The hemolytic activity was evaluated using CD-I mouse erythrocytes as previously described [19,20].
4. Conclusions
Three new epidithiodiketopiperazines, named pretrichodermamides D–F (1–3) were isolated from the lipophilic extract of marine algae-derived fungus Penicillium sp. KMM 4672. Each new compound contains rare 1,2-oxazadecaline moieties [1]. Compounds 1 and 2 are the first isomers at oxazadecaline moiety among the related alkaloids. N-methylpretrichodermamide B (5), highly cytotoxic in 22Rv1 human prostate cancer cells, is resistant to androgen receptor-targeted therapies. At the same time, N-methylpretrichodermamide B was found to be cytotoxic for non-malignant cells (splenocytes and erythrocytes) only at high doses (ED50 62.1 and >100 µM). Therefore, this compound may be a promising candidate for the therapy of human drug-resistant prostate cancer.
Acknowledgments
This study was supported by the Russian Science Foundation (grant No 14-14-00030).
Supplementary Materials
1H, 13C, DEPT, COSY-45, HSQC, HMBC, and ROESY spectra of new compounds 1–3, 1H, 13C, and DEPT spectra of compounds 4 and 5 are available online at www.mdpi.com/1660-3397/14/6/122/s1.
Author Contributions
Anton N. Yurchenko wrote a manuscript, designing the experiments, and discussed the results; Olga F. Smetanina and Elena V. Ivanets isolated, purified, and chemically modified compounds; Anatoly I. Kalinovsky performed NMR analysis; Yuliya V. Khudyakova and Natalya N. Kirichuk identified and cultivated of fungus; Roman S. Popov performed MS analysis; Carsten Bokemeyer raised financial support for biological experiments, discussed the results, and helped write the manuscript; Gunhild von Amsberg design bioassays, raised financial support for biological experiments, discussed the results, and help write the manuscript; Ekaterina A. Chingizova performed cytotoxicity experiments with murine cells; Shamil Sh. Afiyatullov supervised research; Sergey A. Dyshlovoy performed and designed bioassays with human cells, discussed the results, and helped write a manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mfuh A.M., Zhang Y., Stephens D.E., Vo A.X.T., Arman H.D., Larionov O.V. Concise total synthesis of trichodermamides A, B, and C enabled by an efficient construction of the 1,2-oxazadecaline core. J. Am. Chem. Soc. 2015;137:8050–8053. doi: 10.1021/jacs.5b05205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamazaki H., Rotinsulu H., Narita R., Takahashi R., Namikoshi M. Induced production of halogenated epidithiodiketopiperazines by a marine-derived Trichoderma cf. brevicompactum with sodium halides. J. Nat. Prod. 2015;78:2319–2321. doi: 10.1021/acs.jnatprod.5b00669. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki H., Takahashi O., Murakami K., Namikoshi M. Induced production of a new unprecedented epitrithiodiketopiperazine, chlorotrithiobrevamide, by a culture of the marine-derived Trichoderma cf. brevicompactum with dimethyl sulfoxide. Tetrahedron Lett. 2015;56:6262–6265. doi: 10.1016/j.tetlet.2015.09.113. [DOI] [Google Scholar]
- 4.Kajula M., Ward J.M., Turpeinen A., Tejesvi M.V., Hokkanen J., Tolonen A., Häkkänen H., Picart P., Ihalainen J., Sahl H.G., et al. Bridged epipolythiodiketopiperazines from Penicillium raciborskii, an endophytic fungus of Rhododendron tomentosum Harmaja. J. Nat. Prod. 2016;79:685–690. doi: 10.1021/np500822k. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto C., Yokose K., Furumai T., Maruyama H.B. A new epidithiodiketopiperazine group antibiotic, FA-2097. J. Antibiot. 1982;35:374–377. doi: 10.7164/antibiotics.35.374. [DOI] [PubMed] [Google Scholar]
- 6.Stipanovic R.D., Howell C.R. The structure of gliovirin, a new antibiotic from Gliocladium virens. J. Antibiot. 1982;35:1326–1330. doi: 10.7164/antibiotics.35.1326. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y., Debbab A., Mándi A., Wray V., Schulz B., Müller W.E.G., Kassack M., Lin W., Kurtán T., Proksch P., et al. Alkaloids from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2013;894:894–906. doi: 10.1002/ejoc.201201220. [DOI] [Google Scholar]
- 8.Orfali R.S., Aly A.H., Ebrahim W., Abdel-Aziz M.S., Müller W.E.G., Lin W., Daletos G., Proksch P. Pretrichodermamide C and N-methylpretrichodermamide B, two new cytotoxic epidithiodiketopiperazines from hyper saline lake derived Penicillium sp. Phytochem. Lett. 2015;11:168–172. doi: 10.1016/j.phytol.2014.12.010. [DOI] [Google Scholar]
- 9.Liu Y., Li X.M., Meng L.H., Jiang W.L., Xu G.M., Huang C.G., Wang B.G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015;78:1294–1299. doi: 10.1021/acs.jnatprod.5b00102. [DOI] [PubMed] [Google Scholar]
- 10.Yokose K., Nakayama N., Miyamoto C., Furumai T., Maruyama H.B., Stipanovic R.D., Howell C.R. Structure of Fa-2097, a new member of the dioxopiperazine antibiotics. J. Antibiot. 1984;37:667–669. doi: 10.7164/antibiotics.37.667. [DOI] [PubMed] [Google Scholar]
- 11.Kusumi T., Ooi T., Ohkubo Y., Yabuuchi T. The modified Mosher’s method and the sulfoximine method. Bull. Chem. Soc. Jpn. 2006;79:965–980. doi: 10.1246/bcsj.79.965. [DOI] [Google Scholar]
- 12.Liu C., Lou W., Zhu Y., Nadiminty N., Schwartz C.T., Evans C.P., Gao A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014;20:3198–3210. doi: 10.1158/1078-0432.CCR-13-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White T.J., Bruns T., Lee S., Taylor J.W. Amplificaion and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; London, UK: 1990. pp. 315–322. [Google Scholar]
- 14.Hong S.B., Go S.J., Shin H.D., Frisvad J.C., Samson R.A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia. 2005;97:1316–1329. doi: 10.3852/mycologia.97.6.1316. [DOI] [PubMed] [Google Scholar]
- 15.Dyshlovoy S.A., Menchinskaya E.S., Venz S., Rast S., Amann K., Hauschild J., Otte K., Kalinin V.I., Silchenko A.S., Avilov S.A., et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer. 2016;138:2450–2465. doi: 10.1002/ijc.29977. [DOI] [PubMed] [Google Scholar]
- 16.Dyshlovoy S.A., Venz S., Shubina L.K., Fedorov S.N., Walther R., Jacobsen C., Stonik V.A., Bokemeyer C., Balabanov S., Honecker F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014;96:223–239. doi: 10.1016/j.jprot.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Freshney R.I. Culture of Animal Cells: A Manual of Basic Technique. Wiley-Liss; New York, NY, USA: 1994. p. 486. [Google Scholar]
- 18.Dyshlovoy S.A., Hauschild J., Amann K., Tabakmakher K.M., Venz S., Walther R., Guzii A.G., Makarieva T.N., Shubina L.K., Fedorov S.N., et al. Marine alkaloid monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget. 2015;6:17328–17341. doi: 10.18632/oncotarget.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malagoli D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebr. Surviv. J. 2007;4:92–94. [Google Scholar]
- 20.Taniyama S., Arakawa O., Terada M., Nishio S., Takatani T., Mahmud Y., Noguchi T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon. 2003;42:29–33. doi: 10.1016/S0041-0101(03)00097-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.