Abstract
Background: Twenty-four–hour urine sodium excretion is recommended for monitoring population sodium intake. Because of concerns about participation and completion, sodium excretion has not been collected previously in US nationally representative surveys.
Objective: We assessed the feasibility of implementing 24-h urine collections as part of a nationally representative survey.
Design: We selected a random half sample of nonpregnant US adults aged 20–69 y in 3 geographic locations of the 2013 NHANES. Participants received explicit instructions, started and ended the urine collection in a urine study mobile examination center, and answered questions about their collection. Among those with a complete 24-h urine collection, a random one-half were asked to collect a second 24-h urine sample. Sodium, potassium, chloride, and creatinine excretion were analyzed.
Results: The final NHANES examination response rate for adults aged 20–69 y in these 3 study locations was 71%. Of those examined (n = 476), 282 (59%) were randomly selected to participate in the 24-h urine collection. Of these, 212 persons [75% of those selected for 24-h urine collection; 53% (equal to 71% × 75% of those selected for the NHANES)] collected a complete initial 24-h specimen and 92 persons (85% of 108 selected) collected a second complete 24-h urine sample. More men than women completed an initial collection (P = 0.04); otherwise, completion did not vary by sociodemographic characteristics, body mass index, education, or employment status for either collection. Mean 24-h urine volume and sodium excretion were 1964 ± 1228 mL and 3657 ± 2003 mg, respectively, for the first 24-h urine sample, and 2048 ± 1288 mL and 3773 ± 1891 mg, respectively, for the second collection.
Conclusion: Given the 53% final component response rate and 75% completion rate, 24-h urine collections were deemed feasible and implemented in the NHANES 2014 on a subsample of adults aged 20–69 y to assess population sodium intake. This study was registered at clinicaltrials.gov as NCT02723682.
Keywords: 24-hour urine, sodium excretion, NHANES, biomarker, hypertension, sodium intake
INTRODUCTION
Excess dietary sodium intake increases blood pressure and the risk of hypertension (1–4). Sodium reduction is recommended in the United States to reduce the risk of hypertension and projected death and health care costs from heart disease and stroke (5). Sodium intake has been monitored through 24-h dietary recalls (DRs)8 for many years, and DRs have been used to identify foods that contribute sodium in the diet. However, there are limitations to self-reported dietary intake, and updating nutrient databases is a challenge, because food products are reformulated constantly (5). To assess the effectiveness of current efforts to reduce sodium in the food supply, it is critical to monitor the sodium intake of the US population with the use of objective measures. Twenty-four–hour urine sodium excretion is the recommended method for assessing population sodium intake (5, 6), yet low participation and difficulty in completing collection potentially can bias estimates (6, 7). As with sodium intake, urinary sodium excretion varies from day to day, which complicates the use of sodium excretion to estimate excess sodium intake (8–10). To take within-individual variation into account, >1 nonconsecutive day is needed to estimate the distribution of sodium intake, and, in particular, the percentage of the population with excess sodium intake. Because of the potential burden and bias from lack of participation and low completion, 24-h urine specimens were not collected previously in nationally representative US surveys.
The objective of the current study (NCT02723682) was to assess the feasibility of implementing a 24-h urine collection in the NHANES, specifically to determine whether >70% of those selected would provide a complete specimen for an initial and second urine collection without affecting their participation in other NHANES components. Specific aims included 1) evaluating all procedures related to the 24-h urine collection, including laboratory analyses; 2) estimating completion rates overall and in population subgroups; 3) evaluating completeness of the urine collection; and 4) determining whether 24-h urine collection affected the completion rates of other NHANES postexamination components. Furthermore, although it was hypothesized that starting and ending urine collection in person, at a study site, would enhance the completeness of the collection, it was not known how this would affect participation. Thus, we also evaluated whether starting and ending collection at home or in person at a urine study mobile examination center (UMEC) might affect participation or result in significant differences in mean total urine volume and excretion of urinary sodium, potassium, chloride, and creatinine.
METHODS
The NHANES is a cross-sectional survey with a nationally representative sample of the US civilian noninstitutionalized population. In 2013, the sample design included an oversample of certain demographic groups: persons ≥80 y of age and non-Hispanic black, non-Hispanic Asian, Hispanic, and low-income persons (11). Participants initially were interviewed at home to obtain information on health history, health behaviors, and risk factors, followed by a physical examination at a mobile examination center (MEC). During the MEC examination, participants were recruited to participate in several post-MEC examination components, including the 24-h urine feasibility study. The general procedures of the NHANES have been described elsewhere (12).
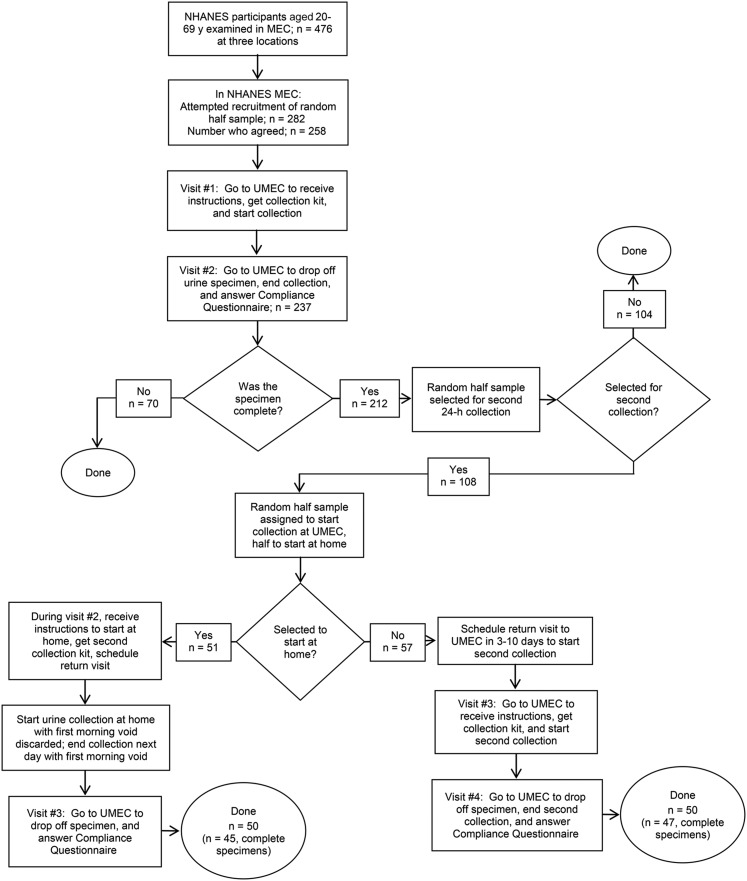
The 24-h urine feasibility study was conducted in 2013 at 3 NHANES locations that included rural, suburban, and urban populations. For the feasibility study, we selected a random half sample of nonpregnant participants aged 20–69 y examined in the MEC who were able to respond for themselves (n = 282; Figure 1). Participants who arrived considerably late to the MEC or left considerably early were not offered participation in the feasibility study but were instead assigned to NHANES examinations.
FIGURE 1.
Participation protocol for the NHANES 24-h urine collection feasibility study in adults aged 20–69 y. MEC, mobile examination center; UMEC, urine study mobile examination center.
Participants were invited to join the urine feasibility study during their MEC examination. Individuals who agreed were scheduled to return to the UMEC for 2 visits. During the first visit, participants received explicit verbal and written instructions and a urine collection kit. They also were asked to empty their bladder before leaving the UMEC to mark the start of the 24-h collection. The second visit was scheduled 24 h later when participants returned to the UMEC, voided one last time to end their urine collection, and answered questions on the completeness of the urine collection. A reminder call was made before the scheduled visits when appointments were scheduled >1 d in advance. When participants did not show up at the time of their appointment, ≤5 telephone calls were made in an attempt to reschedule. The number of telephone calls depended on whether contact was made with the participant or another household member, or the contact attempt was unsuccessful.
Participants were given a urine collection kit to take home. Items in the kit included 2 insulated bags (one for travel), ice packs, a urine collection hat for women, a collection cup for men, three 2-L urine containers, a funnel, 3 large storage bags (to store urine containers optionally in the refrigerator), and written instructions. Participants were instructed to keep the urine specimen cold during the 24-h collection period. The collection start and end times were written on a label on the containers at the time of the initial and final void. The 24-h urine specimen was considered to be complete if 1) the collection start and end time was recorded, 2) the duration of collection was ≥22 h, 3) the total urine volume was ≥500 mL, 4) the participant reported that no more than a few drops of urine were missed during collection, and 5) if the participant was a woman, she reported not menstruating during the urine collection. The completeness of the urine specimen was assessed through a standard questionnaire (Supplemental Material). Participants received a $100 token of appreciation for each urine specimen they returned. Participants with an incomplete specimen were not asked to redo the collection.
One-half of participants who returned a complete specimen were randomly selected and invited to collect a second 24-h urine specimen (Figure 1). The second collection was scheduled 3–10 d later, but not on the same day of the week as the first urine collection. When this was not possible, participants were scheduled on days they were available. In addition, because traveling to the UMEC to start and end the urine collection could be burdensome, especially when a long commute was involved, we assessed whether a protocol that involved 3 visits, allowing the participant to start and end the collection at home rather than going to the UMEC, would yield a similar completion rate. To do this, we randomly assigned one-half of the participants selected for the second 24-h urine collection to begin and end the collection at the UMEC and one-half to begin and end the collection at home.
Those selected for the UMEC were scheduled to return to the UMEC for a third and fourth visit to start and end the second urine collection by voiding at the UMEC. Participants selected to start and end the urine collection at home received instructions during their second visit (when returning the initial 24-h urine specimen). They were instructed to begin their second collection at home by discarding the first morning void (i.e., not collect it) on the collection day, and to end the collection on the following morning, after collecting the first morning void. A return appointment to the UMEC (visit no. 3) was made to drop off the second 24-h urine specimen on the day the urine collection was scheduled to end, or ≤48 h after the start of the urine collection.
We attempted to obtain urine collections on all days of the week, because sodium intake may vary from day to day. In addition, we anticipated that a large proportion of participants would opt for a weekend if not encouraged to collect the urine specimen on a weekday. Thus, to increase urine collection on weekdays, a random one-half of the study participants were asked to collect on a weekday and the other one-half on a weekend. Participants who stated that it was impossible to collect during the assigned interval (either weekday or weekend) were allowed to be scheduled during an alternative time interval.
We also monitored the effect of the 24-h urine collection on other NHANES 2013 post-MEC examination components, specifically, a physical activity monitor (PAM), received during the MEC examination and worn and mailed back 8 d after the examination, and a second DR conducted over the telephone 3–10 d after the MEC examination.
Conducting a 24-h urine collection was costly. In addition to outfitting 2 UMECs for this study, purchasing equipment and supplies, remunerating participants, and setting up a laboratory to process the urine specimens, 4 technicians were hired to staff 2 UMECs for 7 d/wk, 12 h/d. The time involved also was substantial. The mean times in the MEC and UMEC were 4 min to recruit participants, 11 min for the first visit, and 9 min for the second visit. We estimated 90 min to collect the urine over 24 h. Those who collected a second urine specimen spent up to an additional 103 min, on average. Staff made telephone calls to remind participants of their appointments and to reschedule them when participants did not show for their appointments, and they portioned urine specimens into aliquots and shipped them to 2 laboratories.
The 24-h urine feasibility study protocol was approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent.
Criteria for evaluating feasibility
We established the following criteria a priori to evaluate the feasibility of implementing the 24-h urine collection to the 2014 NHANES: 1) ≥70% of eligible participants agreed to participate and collected a complete specimen for the first 24-h urine collection; 2) ≥70% of those asked to collect a second 24-h urine specimen provided a complete specimen for the second 24-h urine collection; and 3) PAM or DR completion rates did not decrease by >10 percentage points.
For the collection to be considered feasible for the main study, all 3 criteria had to be met. The 70% completion rate was chosen in consideration of high respondent burden and the logistic complexity of the component. This is higher than reported completion rates in recent national studies of 24-h urine collections in other industrialized countries (10). With the use of the final MEC response rate for adults aged 20–69 y in the NHANES 2011–2012 (67%) as an estimate of what would be expected in 2013, we estimated that our target completion rate would obtain a final component response rate of 47% (67% × 70%). This meant that if we implemented this component in the NHANES, we anticipated that it would yield complete specimens for 47% of the persons originally screened into the nationally representative sample. The overall NHANES examination nonresponse has been assessed previously (13). To explore potential component-specific selection bias, we examined whether participants who agreed to collect 24-h urine samples differed from those who did not agree to collect 24-h urine samples by age, sex, race and ethnicity, education level, and employment status. To evaluate changes in the postexamination component completion rates (PAM and DR), NHANES participants not selected to participate in the 24-h urine collection served as a control group.
Urine processing and laboratory measurements
Urinary sodium, potassium, chloride, and creatinine were measured in the feasibility study. In the UMEC laboratory, trained technicians weighed the individual urine containers to obtain the total urine volume. They then combined and mixed the specimens from all containers and took aliquots of the combined specimen. The aliquots were stored at −30°C in the UMEC for ≤6 d and shipped frozen overnight on dry ice to laboratories for analysis. Urinary sodium, potassium, and chloride were measured with the use of ion-selective electrodes on a Hitachi Modular P clinical analyzer (Roche Diagnostics) at the CDC’s National Center for Environmental Health. Urinary creatinine was measured with the use of the Roche creatinine enzymatic assay on the Roche Cobas 6000 Analyzer at the University of Minnesota.
Other measurements
Self-reported information on race and ethnicity was categorized as non-Hispanic white, non-Hispanic black, and other. Because of the small sample size of the “other” group, no separate estimates were presented for persons classified as “other,” but they are included in the total study population estimates. Although it was not part of the feasibility criteria, we also examined participation by BMI. Measured weight and height were used to calculate BMI, rounded to one decimal point. Based on their BMI, participants were classified as normal or underweight [BMI (in kg/m2) <25], overweight (BMI 25 to <30), or obese (BMI ≥30) (14). Employment status was identified by the following questions: “Which of the following were you doing last week? Working at a job or business, with a job or business but not at work, looking for work, or not working at a job or business?” (15). Participants who reported working at a job or with a job but not at work were categorized as employed. Education status was based on responses to the question, “What is the highest grade or level of school you have completed or the highest degree you have received?” (15).
Statistical analyses
We evaluated participation rates overall and by sociodemographic characteristics. The amount of analyte (e.g., sodium) in each urine specimen was calculated by multiplying the concentration of the analyte by the corresponding volume of the 24-h urine collection. The volume of the 24-h urine collection was adjusted for the collection time as (total volume collected/collection time) × 24. Thus, all volume and analyte excretion results shown are adjusted for 24 h. We calculated means and SDs of total urine volume and analytes overall by sociodemographic subgroup, and, for participants with a second urine collection, by whether the collection started and ended at home or the UMEC. Differences in means were determined with the use of t tests at the P < 0.05 level. Within-person CVs were calculated between the initial and second 24-h urine collections as the square root of the within-person variance divided by the mean of each study analyte. Analyses were performed with the use of SAS version 9.3.
RESULTS
Completion rates
In 2013, 282 adults, or 59% of 476 NHANES participants aged 20–69 y examined in the MEC, were selected for the 24-h urine feasibility study. Among those, 91% agreed to participate, 84% returned a urine specimen, and 75% collected a complete 24-h urine specimen (Table 1). Of those participants asked to collect a second 24-h urine specimen, 85% collected a complete 24-h urine specimen.
TABLE 1.
Participation of adults aged 20–69 y in the NHANES 24-h urine collection feasibility study
| Initial 24-h urine collection |
Second 24-h urine collection |
|||
| Participation status | n | % | n | % |
| Total number sampled | 282 | 108 | ||
| Agreed to participate | 258 | 91 | 102 | 94 |
| Returned a urine specimen | 238 | 84 | 100 | 93 |
| Weekend collection1 | 103 | 432 | 39 | 393 |
| Weekday collection | 134 | 562 | 59 | 593 |
| Collected complete specimen | 212 | 75 | 92 | 85 |
| Weekend collection | 93 | 444 | 36 | 395 |
| Weekday collection | 119 | 564 | 56 | 615 |
Information on day of the week was not available for 1 participant in the initial 24-h collections and for 2 participants in the second urine collections.
Value presented was the percentage of returned initial 24-h urine collections (n = 237).
Value presented was the percentage of returned second 24-h urine collections (n = 100).
Value presented was the percentage of completed initial 24-h urine collections (n = 212).
Value presented was the percentage of completed second 24-h urine collections (n = 92).
The initial 24-h urine collection was not offered to 5 individuals who arrived late to the MEC or left early. Participants refused the urine collection because there was no time in their schedule (n = 7), they did not want to participate (n = 7), they were going to be traveling (n = 3), or the UMEC was too far (n = 2). Among those who agreed to the 24-h urine collection, 20 individuals cancelled or did not show for their UMEC appointment to receive instructions or to return their urine specimen. Two participants were not offered the second urine collection because the study location was closing and there were <3 d left in the schedule. Four participants did not agree to collect a second 24-h urine because they had no time (n = 2), thought it was too far to travel to the UMEC (n = 1), or did not want to do another collection (n = 1). Among those who agreed, 2 participants did not show for their appointment to receive instructions or return a specimen.
Among the 238 individuals who returned their initial 24-h urine specimen, there was no information on day of the week for 1 participant, 103 (43%) collected their initial 24-h urine on a weekend, 39 (39%) of those who returned a second urine specimen (n = 100) collected their urine on a weekend, and there was no information on day of the week for 2 participants for the second urine collection (Table 1). There was no significant difference in completion rates between weekday and weekend collection for the first and second 24-h urine collections. For the first urine collection, 93 of 103 persons (90%) who collected their initial 24-h urine on a weekend met criteria for a complete specimen, whereas 119 of 134 (89%) who collected their urine on a weekday had a complete specimen; and 36 of 39 (92%) who collected their second 24-h urine on a weekend had a complete specimen, whereas 56 of 59 (95%) who collected their urine on a weekday had a complete specimen. Among the participants who were employed, most collected their urine specimen on a day they did not work; 151 of 218 (69%) participants collected an initial urine specimen on a day they did not work. Another 15 individuals were unemployed and 5 did not respond to the employment question. For the second collection, among participants who were employed, 66 of 94 (70%) collected a second 24-h urine specimen on a day they did not work. Another 4 individuals were unemployed, and 2 did not respond to the employment question.
Missing more than a few drops of urine was the most common reason for an incomplete specimen (18 of 26 participants with an incomplete initial specimen and 5 of 8 participants with an incomplete second specimen). Four women had incomplete specimens (3 for the initial collection and 1 for the second collection) because they began their menstrual periods during the 24-h urine collection time frame. Other reasons a specimen was incomplete included the following: total volume of urine <500 mL (n = 4), the urine specimen was collected over <22 h (n = 1), and the collection start and/or end time could not be ascertained (n = 2).
For the first 24-h urine collection, men had a significantly higher completion rate than women: 81% compared with 70% (P < 0.05; Table 2). The proportion of participants with a complete 24-h urine specimen did not vary significantly across categories of age, race and ethnicity, BMI, education, or employment status for participants selected for the initial or second collection or by sex for participants selected for the second collection.
TABLE 2.
Completion rates for 24-h urine collections by characteristics of study participants in the NHANES 24-h urine collection feasibility study1
| Initial 24-h collection |
Second 24-h collection |
|||||
| Completed |
Completed |
|||||
| Sampled, n | n | % | Sampled, n | n | % | |
| All2 | 282 | 212 | 75 | 108 | 92 | 85 |
| Age, y | ||||||
| 20–29 | 43 | 34 | 79 | 15 | 13 | 87 |
| 30–39 | 60 | 44 | 73 | 16 | 15 | 94 |
| 40–49 | 73 | 53 | 73 | 31 | 26 | 84 |
| 50–59 | 60 | 48 | 80 | 30 | 24 | 80 |
| 60–69 | 46 | 33 | 72 | 16 | 14 | 88 |
| Sex | ||||||
| M | 139 | 112 | 81* | 54 | 47 | 87 |
| F | 143 | 100 | 70 | 54 | 45 | 83 |
| Race and ethnicity | ||||||
| Non-Hispanic white | 178 | 131 | 74 | 65 | 55 | 85 |
| Non-Hispanic black | 83 | 64 | 77 | 33 | 28 | 85 |
| BMI, kg/m2 | ||||||
| Underweight or normal weight (<25) | 85 | 66 | 77 | 36 | 28 | 78 |
| Overweight (25–29.9) | 91 | 64 | 70 | 35 | 30 | 86 |
| Obese (≥30) | 104 | 81 | 78 | 36 | 33 | 92 |
| Education | ||||||
| <High school | 42 | 34 | 81 | 15 | 14 | 93 |
| High school graduate | 82 | 60 | 73 | 27 | 21 | 78 |
| Some college | 86 | 65 | 76 | 37 | 32 | 87 |
| College graduate | 72 | 53 | 74 | 29 | 25 | 86 |
| Employment status | ||||||
| Employed | 177 | 130 | 73 | 64 | 53 | 83 |
| Not employed | 105 | 82 | 78 | 44 | 39 | 89 |
*Significantly different from women, P < 0.05 (chi-square test).
Includes race and ethnicity groups other than non-Hispanic black and non-Hispanic white.
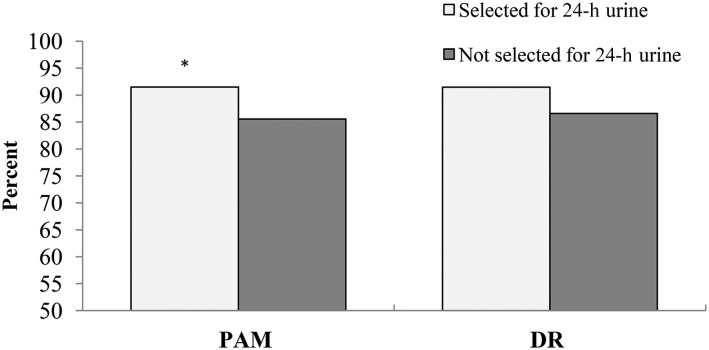
Participants selected to collect a 24-h urine specimen had significantly higher completion rates for the PAM than did those not selected (91% compared with 86%, P < 0.05; Figure 2). Completion rates for the DR also were slightly higher among participants selected for the feasibility study; however, the difference was not significant (91% compared with 87%, P = 0.098).
FIGURE 2.
Completion rates for the NHANES postexamination components PAM and second DR in participants aged 20–69 y by eligibility for the 24-h urine collection feasibility study. Participants selected to collect a 24-h urine sample had significantly higher completion rates for the PAM than did those not selected, *P < 0.05 (chi-square test). No significant differences in DR completion rate were observed between participants selected for 24-h urine collection and those not selected. DR, dietary recall; PAM, physical activity monitor.
Urine volume and analyte excretion
We assessed total urine volume and urinary sodium, potassium, chloride, and creatinine excretion for participants with a complete sample. One additional participant was excluded from the analyte analysis because the total urine volume was not recorded. Overall adjusted mean ± SD 24-h urine volume and sodium excretion were 1964 ± 1228 mL and 3657 ± 2003 mg, respectively, for the first 24-h urine collection (n = 211) and 2048 ± 1288 mL and 3774 ± 1891 mg, respectively, for the second collection (n = 92).
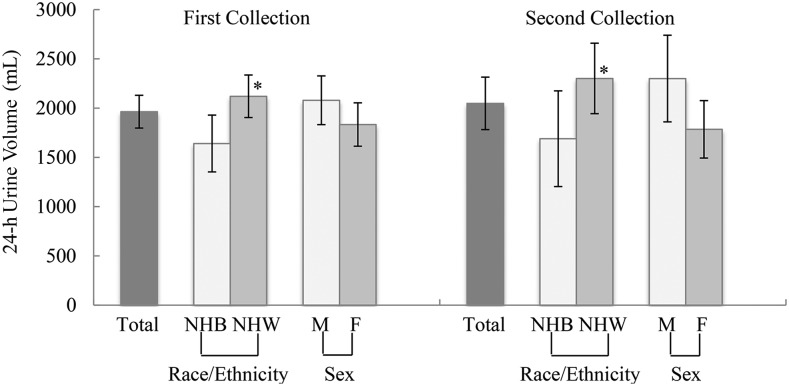
Mean total urine volume appeared to be slightly higher in men than women, but the difference in volume was not statistically significant (first collection, P = 0.146; second collection, P = 0.053; Figure 3). In contrast, mean total urine volume was significantly higher in non-Hispanic white participants than in non-Hispanic black participants (first collection, P = 0.011; second collection, P = 0.046). Mean total urine volume for the first and second collection was 1641 ± 1155 mL (n = 64) and 1690 ± 1253 mL (n = 28), respectively, in non-Hispanic black participants compared with 2120 ± 1245 mL (n = 130) and 2302 ± 1323 mL (n = 55), respectively, in non-Hispanic white participants.
FIGURE 3.
Adjusted mean (95% CI) 24-h urine volume for adults aged 20–69 y in the NHANES 24-h urine collection feasibility study with a complete specimen, by sex and race. Mean volume is adjusted for duration of collection. One participant was excluded from the initial 24-h urinary analysis because the total urine volume was unknown. The number of complete specimens for the first 24-h urine collection was as follows: total, n = 211; men, n = 112; women, n = 99; non-Hispanic blacks, n = 64; and non-Hispanic whites, n = 130. The number of complete specimens for the second 24-h urine collection was as follows: total, n = 92; men, n = 47; women, n = 45; non-Hispanic blacks, n = 28; and non-Hispanic whites, n = 55. The “total” category includes race and ethnicity groups other than non-Hispanic black or non-Hispanic white. Significant difference in mean urine volume observed between non-Hispanic black and non-Hispanic white participants for both first and second 24-h urine collection, *P < 0.05 (t test). NHB, non-Hispanic black; NHW, non-Hispanic white.
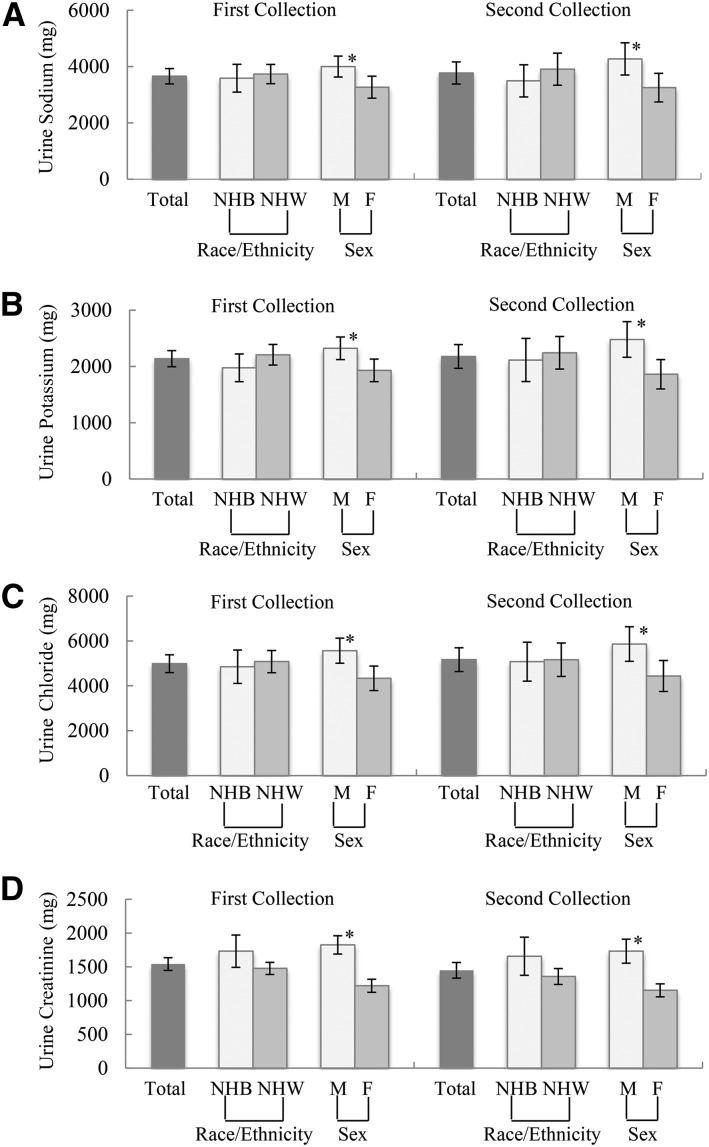
Mean urine sodium excretion was significantly higher in men than in women for the first (men, 4001 ± 1990 mg compared with women, 3268 ± 1956 mg, P < 0.05; Figure 4) and second (men, 4273 ± 1954 mg compared with women, 3252 ± 1690 mg, P < 0.05) urine collections, but did not differ by race (P = 0.627 and P = 0.302 for the first and second collections, respectively). On average, urinary potassium, chloride, and creatinine excretions were significantly higher in men than in women but did not differ by race for both the first and second urine collections. Mean total urine volume and urinary sodium, potassium, chloride, and creatinine did not differ significantly between the first and second 24-h urine collections, overall, by sex, or by race. Among participants with 2 urine collections, within-person day-to-day variances are shown in Supplemental Table 1 for the study analytes, by sex and race group. Overall, the within-person day-to-day variances were 16% for urine volume, 20% for sodium, 13% for potassium, 23% for chloride, and 9% for creatinine. The within-person day-to-day variances in 24-h urinary sodium, potassium, and chloride, but not creatinine, appeared to be somewhat greater for men than for women and for non-Hispanic blacks than for non-Hispanic whites.
FIGURE 4.
Mean (95% CI) total 24-h urine sodium (A), potassium (B), chloride (C), and creatinine (D) for adults aged 20–69 y in the NHANES 24-h urine collection feasibility study with a complete urine specimen, by sex and race. One participant was excluded from the initial 24-h urinary analysis because the total urine volume was unknown. The number of complete specimens for the first 24-h urine collection was as follows: total, n = 211; men, n = 112; women, n = 99; non-Hispanic blacks, n = 64; and non-Hispanic whites, n = 130. The number of complete specimens for the second 24-h urine collection was as follows: total, n = 92; men, n = 47; women, n = 45; non-Hispanic blacks, n = 28; and non-Hispanic whites, n = 55. The “total” category includes other race and ethnicity groups. Mean 24-h sodium, potassium, chloride, and creatinine excretions were significantly higher in men than in women, *P < 0.05 (t test), but did not differ by race, for both the first and second urine collections. NHB, non-Hispanic black; NHW, non-Hispanic white.
Starting and ending the 24-h urine collection at home compared with in person at the UMEC
We assessed completion rates for participants who started and ended their second 24-h urine collection at the UMEC compared with at home. Of the 108 individuals selected for a second urine specimen, one-half were randomly assigned to start their second 24-h urine collection at home. The percentage of participants who returned a urine specimen was significantly lower for those who started and ended collection in person at the UMEC (88%) compared with those who started and ended at home (98%, P-difference = 0.04). Although the percentage of individuals with a complete specimen who started and ended collection in person at the UMEC was also lower than the percentage of those who started and ended collection at home, the difference in completion rate was not statistically significant (Table 3). Mean total urine volume appeared to be somewhat higher for participants who started and ended the second urine collection at home than that for those who started and ended the collection at the UMEC (Table 3); however, the difference in mean urine volume was not statistically significant whether they started at the UMEC or at home.
TABLE 3.
Participation in and mean total urine volumes of the initial and second collections, by protocol, for participants aged 20–69 y who were selected for the second 24-h urine collections in the NHANES 24-h urine collection feasibility study1
| UMEC | Home | |
| Participation status | ||
| Total sampled, n | 57 | 51 |
| Returned a urine specimen | 50 (88) | 50 (98)* |
| Collected a complete specimen | 47 (82) | 45 (88) |
| Total urine volume,2 mL | ||
| Initial collection | 1875 (1577, 2172) | 2353 (1877, 2829) |
| Second collection | 1855 (1544, 2165) | 2250 (1807, 2694) |
Values are means (95% CIs) or n (%), unless otherwise indicated. A random one-half of participants selected to collect a second 24-h urine were instructed to start and end collection at the UMEC, whereas the other half were asked to start and end at home. *Significantly different from those who started and ended collection at the UMEC, P < 0.05 (chi-square test). UMEC, urine study mobile examination center.
In participants with a complete second 24-h urine collection (UMEC, n = 47; home, n = 45).
There was also no significant difference in mean urinary sodium, potassium, chloride, or creatinine excretion in those who started and ended the urine collection at the UMEC compared with those who started and ended the collection at home, overall or by sex (data not shown).
DISCUSSION
Results from 3 locations in the NHANES 2013 indicated that 24-h urine collection in the NHANES is feasible in US adults aged 20–69 y. Across different age, sex, and race and ethnic groups, >70% of the participants selected for the feasibility study collected a complete 24-h urine specimen. Similar completion rates were obtained in participants with different education levels, employment statuses, and BMI categories. Overall, 85% of those who were invited to collect a second 24-h urine specimen returned another complete specimen. In addition, the completion rates in other NHANES postexamination components were unaffected by the 24-h urine collection. The NHANES MEC examination response rate for adults aged 20–69 y in the 3 study locations was 71%. Therefore, the 75% completion rate for the initial 24-h urine collection was equivalent to a final component response rate of 53%, which exceeded the expected 47% target component response rate. The final component response rate indicated the need to further adjust NHANES examination weights for component nonresponse if 24-h urine collection is implemented in the survey. In the current study, we observed similar completion rates across age or race and ethnicity subgroups, which suggests that it is feasible to use adjusted sample weights to produce national estimates from 24-h urine data.
Mean 24-h urine volume in the feasibility study (1964 mL) was at the high end of the mean urine volume expected compared with those from the US and Canadian sites of the International Cooperative Study on Salt, Other Factors, and Blood Pressure (730–2010 mL) and those with complete urine assessed by para-aminobenzoic acid (1094–1788 mL), whereas creatinine excretion (1541 mg) was within the range of these studies (1011–1818 mg) (16–20). Mean sodium excretion from our study also was comparable with results from a systematic review of US studies dating from between 1957 and 2003 (3526 mg overall; 3911 mg for men and 3084 mg for women), which were conducted well before our urine feasibility study (21); a calibration study that compared timed urine spots with 24-h urine collections (3.54 g for men and 3.09 g for women) (22); the Observing Protein and Energy Nutrition study (4502 mg for men and 3310 mg for women) (23); and the Energetics study (3692 mg for men and 2555 mg for women) (23). Urinary sodium excretion also is comparable with estimates from dietary intake data collected in 2009–2012 (3552 mg dietary sodium/d, ≥19 y) (24). The consistency of our results with previous findings supports the quality of the urine collections in the feasibility study and the external validity of our collection protocol.
Completion rates for our feasibility study were higher than those in recent national studies of 24-h urine collections in other industrialized countries, which generally were <70%, although differences in definitions and protocols between studies limit these comparisons (10). NHANES participants were recruited from the ongoing survey, and their 24-h urine collections were scheduled immediately after the study’s physical examination. We offered appointment times throughout the day on both weekdays and weekends, and we offered an incentive of $100 for each urine specimen returned.
More women than men missed urine during the 24-h collection (15 women compared with 8 men for the first and second collections combined), possibly because of anatomical differences. Some participants forgot to collect their void, particularly the first void of the collection. Although instructions can be enhanced to help participants collect their specimens properly, helping individuals to remember to collect all of their urine is challenging.
There is no standard method to evaluate whether a 24-h urine collection is complete (11), and each method has its strengths and limitations. Although the recovery of para-aminobenzoic acid is considered to be the standard for assessing completeness and was used in the United Kingdom, it is costly and requires participants to take supplements at prescribed times during a specific period, increasing burden and decreasing participation, thus limiting its use in general population surveys (25, 26). Similar to our study, other national surveys have used self-report, duration of urine collection, total urine volume, and/or creatinine criteria (post hoc analysis) (10, 25). We chose to adapt the protocol used in the International Cooperative Study on Salt, Other Factors, and Blood Pressure, in which completion of the 24-h urine collection was enhanced through explicit instructions and starting and ending the collection in person to ensure accurate timing.
Substantial within-person variation in sodium excretion observed in the current study illustrated the importance of accounting for day-to-day variability of dietary intake in the surveillance system. We were successful in obtaining 24-h urine samples collected on weekdays and weekends; however, the majority of participants who collected a urine sample and were employed did so on a day on which they did not go to work (69% for the first collection; 70% for the second collection), which may have affected the representativeness of the estimates. Although we tried to schedule the second collection on a day of the week that was different from the first urine collection to assess within-person variability from day-to-day intake, it may not have accounted for the potential effect of alternative work schedules. Further analysis is needed to assess sodium intake on workdays compared with nonworkdays.
Participants who started and ended the second 24-h urine collection at home had higher completion rates than those who started and ended at the UMEC. However, among those with a complete specimen, there was no significant difference in mean urine volume, sodium, potassium, chloride, and creatinine excretion between the 2 groups. Although these results may encourage national surveys to include fewer trips to the examination sites, the small sample and the observed wide range of CIs for the estimates command further investigation in other surveys and populations.
NHANES participants may refuse any component of the survey; however, we did not collect data on the reasons participants completed various portions of the survey. Thus, we can only speculate on the reasons for higher completion rates for other postexamination components in NHANES participants who were selected compared with those not selected to collect a 24-h urine sample for the feasibility study. Perhaps participants who collected a urine specimen were motivated after completing portions of the survey and were more likely to continue with other sections.
We were limited in the selection of 3 locations for the feasibility study, because they needed to occur without disrupting NHANES operations. The demographic composition of the sample was almost entirely non-Hispanic white and non-Hispanic black, which yielded an insufficient number of Hispanic and Asian adults to assess participation in these groups. The high cost to conduct the feasibility study also limited the sample size. Consequently, the results of this feasibility study are not generalizable to the US population.
Given the success of the feasibility study, a 24-h urine collection was implemented in the NHANES 2014 on a random half subsample of adults aged 20–69 y to serve as a baseline for more objective monitoring of population sodium intake in US adults. All participants were asked to start and end their urine collection at the UMEC, if possible, and an incentive of $100 was provided for each 24-h urine collection. Minor modifications in the NHANES 2014 protocol included the use of a larger urine container, increasing the assignment of weekday collections to 60% of the participants, and a lower, but more conventional, completion criterion of 400 mL in total urine volume (27). Collection of 24-h urine in 2014 was successfully completed on the basis of the same criteria used in the feasibility study (i.e., >70% of those selected for 24-h urine collection completed an initial collection). Data on 24-h urine analytes are available for analysis through the NHANES Research Data Center (28).
Acknowledgments
The authors’ responsibilities were as follows—ALT, MEC, CML, JDW, RKM, and BAB: designed the 24-h urine feasibility study; ALT: managed the 24-h urine feasibility study; drafted the manuscript, which was modified based on comments from all coauthors; and had primary responsibility for the final content; ALT, T-CC, and XZ: analyzed the data; and all authors: designed the study and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DR, dietary recall; MEC, mobile examination center; PAM, physical activity monitor; UMEC, urine study mobile examination center.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. , for the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 2.Cutler JA, Follmann D, Allender PS. Randomized trials of sodium reduction: an overview. Am J Clin Nutr 1997;65(Suppl):643S–51S. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 4.Elliott P, Walker LL, Little MP, Blair-West JR, Shade RE, Lee DR, Rouquet P, Leroy E, Jeunemaitre X, Ardaillou R, et al. Change in salt intake affects blood pressure of chimpanzees: implications for human populations. Circulation 2007;116:1563–8. [DOI] [PubMed] [Google Scholar]
- 5.Food and Nutrition Board, Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington (DC): National Academies Press; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization/World Health Organization. Strategies to monitor and evaluate population sodium consumption and sources of sodium in the diet: report of a joint technical meeting convened by WHO and the Government of Canada. Washington (DC): PAHO/WHO; 2010. [Google Scholar]
- 7.Hawkes C, Webster J. National approaches to monitoring population salt intake: a trade-off between accuracy and practicality? PLoS One 2012;7:e46727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Nutrition Board, Institute of Medicine. Sodium intake in populations: assessment of evidence. Washington (DC): National Academies Press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K, Dyer AR, Cooper RS, Stamler R, Stamler J. Can overnight urine replace 24-hour urine collection to assess salt intake? Hypertension 1979;1:529–36. [DOI] [PubMed] [Google Scholar]
- 10.Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr 2015;35:349–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011–2014. Vital Health Stat 2 2014;(162):1–33. [PubMed] [Google Scholar]
- 12.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1 2013;(56):1–37. [PubMed] [Google Scholar]
- 13.Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, Curtin LR. National Health and Nutrition Examination Survey: Estimation Procedures, 2007–2010. National Center for Health Statistics. Vital Health Stat 2 (159):1–17. 2013 [cited 2015 Jul 22]. Available from: http://www.cdc.gov/nchs/data/series/sr_02/sr02_159.pdf. [PubMed]
- 14.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Washington (DC): NIH publication no. 98-4083, September 1998. [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Demographic background questions [cited 2015 Jun 10]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/DMQ_Family_H.pdf.
- 16.INTERSALT Co-operative Research Group. INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24-hour urinary sodium and potassium excretion. BMJ 1988;297:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DR, Bingham SA. Sodium and potassium intakes in a representative population sample: estimation from 24 h urine collections known to be complete in a Cambridgeshire village. Br J Nutr 1986;55:13–22. [DOI] [PubMed] [Google Scholar]
- 18.Murakami K, Sasaki S, Takahashi Y, Uenishi K, Watanabe T, Kohri T, Yamasaki M, Watanabe R, Baba K, Shibata K, et al. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition 2008;24:16–22. [DOI] [PubMed] [Google Scholar]
- 19.Leclercq C, Maiani G, Polito A, Ferro-Luzzi A. Use of PABA test to check completeness of 24-h Urine collections in elderly subjects. Nutrition 1991;7:350–4. [PubMed] [Google Scholar]
- 20.Bingham SA, Murphy J, Waller E, Runswick SA, Neale G, Evans D, Cummings JH. Para-amino benzoic acid in the assessment of completeness of 24-hour urine collections from hospital outpatients and the effect of impaired renal function. Eur J Clin Nutr 1992;46:131–5. [PubMed] [Google Scholar]
- 21.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr 2010;92:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CY, Cogswell ME, Loria CM, Chen T-C, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr 2013;143:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol 2015;181:473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson SL, King SM, Zhao L, Cogswell ME. Prevalence of excess sodium intake in the United States—NHANES 2009–2012. MMWR Morb Mortal Wkly Rep 2016;64:1393–7. [DOI] [PubMed] [Google Scholar]
- 25.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subar AF, Midthune D, Tasevska N, Kipnis V, Freedman LS. Checking for completeness of 24-h urine collection using para-amino benzoic acid not necessary in the Observing Protein and Energy Nutrition study. Eur J Clin Nutr 2013;67:863–7. [DOI] [PubMed] [Google Scholar]
- 27.National Health and Nutrition Examination Survey 24-hour urine study procedures manual [Internet]. Jan 2014 [cited 2016 Feb 12]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/24_Hour_Urine_Study_Procedures_Manual.pdf.
- 28.National Center for Health Statistics. Research Data Center [Internet] [cited 2016 Feb 25]. Available from: http://www.cdc.gov/rdc/.