Abstract
Background: Sugar-sweetened beverage (SSB) consumption and low-grade chronic inflammation are both independently associated with type 2 diabetes and cardiovascular disease. Fructose, a major component of SSBs, may acutely trigger inflammation, which may be one link between SSB consumption and cardiometabolic disease.
Objective: We sought to determine whether beverages sweetened with fructose, high-fructose corn syrup (HFCS), and glucose differentially influence systemic inflammation [fasting plasma C-reactive protein and interleukin-6 (IL-6) as primary endpoints] acutely and before major changes in body weight. Secondary endpoints included adipose tissue inflammation, intestinal permeability, and plasma fetuin-A as potential mechanistic links between fructose intake and low-grade inflammation.
Design: We conducted a randomized, controlled, double-blind, crossover design dietary intervention (the Diet and Systemic Inflammation Study) in 24 normal-weight to obese adults without fructose malabsorption. Participants drank 4 servings/d of fructose-, glucose-, or HFCS-sweetened beverages accounting for 25% of estimated calorie requirements while consuming a standardized diet ad libitum for three 8-d periods.
Results: Subjects consumed 116% of their estimated calorie requirement while drinking the beverages with no difference in total energy intake or body weight between groups as reported previously. Fasting plasma concentrations of C-reactive protein and IL-6 did not differ significantly at the end of the 3 diet periods. We did not detect a consistent differential effect of the diets on measures of adipose tissue inflammation except for adiponectin gene expression in adipose tissue (P = 0.005), which was lowest after the glucose phase. We also did not detect consistent evidence of a differential impact of these sugars on measures of intestinal permeability (lactulose:mannitol test, plasma zonulin, and plasma lipopolysaccharide-binding protein).
Conclusion: Excessive amounts of fructose, HFCS, and glucose from SSBs consumed over 8 d did not differentially affect low-grade chronic systemic inflammation in normal-weight to obese adults. This trial was registered at clinicaltrials.gov as NCT01424306.
Keywords: sugar-sweetened beverages, systemic inflammation, adipose tissue inflammation, fructose, intestinal permeability
INTRODUCTION
The consumption of sugar-sweetened beverages (SSBs)7 is associated with type 2 diabetes mellitus and cardiovascular disease (1–3), both independently and through the increased risk conferred by excess body fat. Chronic low-grade inflammation is associated with both of these diseases, especially obesity. Thus, the association between SSB consumption and the increased risk of chronic disease may be partly mediated by low-grade chronic inflammation.
Numerous lines of evidence from both animal and human studies suggest that the fructose component of SSBs may be largely responsible for the observed associations between SSB intake and risk factors for metabolic disease such as low-grade chronic inflammation (4–7), dyslipidemia (8), and decreased insulin sensitivity (9). We hypothesized that fructose- but not glucose-sweetened beverages may acutely trigger low-grade chronic inflammation in the systemic circulation as well as in adipose tissue in a manner independent of weight change through 1 of 2 different mechanisms. First, high fructose consumption, even under short-term isocaloric conditions, promotes the translocation of microbial material from the gut lumen to the portal circulation in rodents, thereby triggering low-grade hepatic and systemic inflammation (7, 10). Because systemic inflammation can trigger the activation of inflammatory pathways in adipose tissue (11), we hypothesized that hepatic and systemic inflammation triggered by increased intestinal permeability and endotoxinemia may also result in changes in adipose tissue inflammation. Second, even under isocaloric conditions, substituting liquid fructose in place of solid carbohydrate foods resulted in considerably increased de novo lipogenesis (DNL) in the liver within just 9 d (12). DNL is thought to be a major determinant of fetuin-A production by the liver (13, 14), and fetuin-A in return has been hypothesized to be a chaperone for free fatty acids (FFAs) in activating inflammatory pathways in adipose tissue macrophages (ATMs) (15). Thus, it is plausible that fructose-induced DNL may increase plasma fetuin-A concentrations, thereby triggering an increase in low-grade chronic inflammation in adipose tissue as well as systemically.
We previously reported the results from our controlled crossover design dietary intervention study, DASI (Diet and Systemic Inflammation) (NCT01424306), on ad libitum energy intake (16). We describe herein the second set of analyses from this study, in which we evaluated whether consuming high amounts of fructose in the form of SSBs for 8 d promotes low-grade chronic systemic and adipose tissue inflammation in normal-weight to obese adults. We further sought to determine whether this high dose of fructose in the form of SSBs affects intestinal permeability and plasma fetuin-A concentrations, both of which could be mechanisms linking excessive fructose intake to low-grade chronic inflammation in adipose tissue and the systemic circulation.
METHODS
Subjects
The DASI study was carried out at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, Washington, between December 2011 and April 2014. A detailed description of the study has been published previously (16). Twenty-five men and women aged 18–65 y with a BMI (in kg/m2) between 20 and 40 were recruited by newspaper advertisements and fliers posted in the Seattle area. Subjects were recruited into 2 categories based on BMI: normal weight (BMI: 20.0–24.9; n = 13) and overweight/obese (BMI: 25.0–39.9; n = 12).
Subjects were required to have had a stable weight of ±4.5 kg of their lifetime maximum weight and had to be willing to consume only food and beverages provided by the research kitchen for 3 periods of 8 d each. Potential subjects were required to be available for admission to the clinic on ≤6 occasions. Other exclusion criteria included smoking or the use of recreational drugs; alcohol abuse (>2 drinks/d); a history of cardiovascular disease or diabetes mellitus; the presence or history of any chronic inflammatory, autoimmune, or metabolic disease; recent (within 12 mo) pregnancy or breastfeeding; the presence of phenylketonuria, hereditary fructose intolerance, or other malabsorption syndromes; the presence or recent (within 2 mo) history of anemia; and the current or recent (within 3 mo) use of insulin, antidiabetics, β-blockers, glucocorticoids, anabolic steroids, warfarin, antibiotics, probiotics, or nonsteroidal anti-inflammatory drugs (daily and high dose). Before being enrolled, subjects underwent telephone and in-person screening interviews to assess medical history, obtain a plasma biochemistry panel, and conduct a fructose malabsorption test (17) to assess eligibility. Briefly, during the in-person screening visit, each subject consumed a beverage sweetened with fructose in an amount equivalent to 6.25% of estimated daily calorie intake (representing 1 serving of the study beverage) as estimated by the Mifflin formula (18) and a standardized activity factor of 1.5. The hydrogen content of the exhaled breath was measured at baseline and 0, 30, 60, 90, 120, 150, and 180 min after consumption of the beverage. An increase of >20 parts per million above baseline for 2 subsequent time points was considered indicative of fructose malabsorption, which led to exclusion from the study. Written informed consent was obtained from all subjects, and the study was approved by the FHCRC institutional review board.
Study design, diets, and clinic visits
The primary aim of the DASI study was to assess whether beverages sweetened with glucose, high-fructose corn syrup (HFCS), and fructose differentially influence markers of systemic inflammation, as assessed by measuring the fasting plasma concentrations of C-reactive protein (CRP) and IL-6. Ad libitum energy intake was a predetermined secondary endpoint of this study along with fasting plasma adiponectin, zonulin, and LPS-binding protein (LBP), urinary lactulose:mannitol ratio, and adipose tissue inflammation. Results of the energy intake endpoint have been published previously (16).
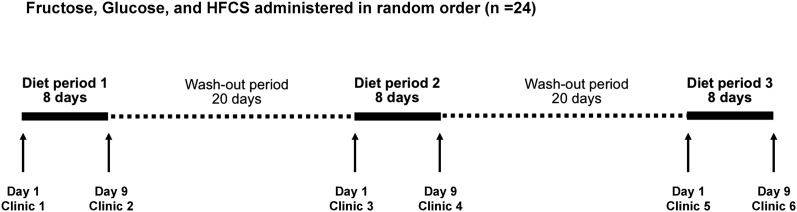
The study design is presented in Figure 1. On day 1 of each intervention period, subjects were admitted to the FHCRC prevention center after a 10-h fast. After ruling out pregnancy in women of child-bearing potential, anthropometrics and vital signs were recorded. A health questionnaire was administered to determine whether subjects had been sick over the previous 20-d period. Blood was then collected by venous puncture into chilled tubes containing EDTA, spun immediately at 3000 × g for 10 min, separated into aliquots, and stored at −70°C.
FIGURE 1.
Study design. Participants completed each of the 3 diet periods, during which they consumed standardized solid foods ad libitum as well as 4 mandatory daily servings of beverages sweetened with fructose, glucose, or HFCS. The order in which the beverages were consumed was randomized, and the intervention periods were separated by washout periods of 20 d each. All foods and beverages were provided, and ad libitum energy intake was assessed by weighing all foods that were not consumed and subtracting those calories from the number of calories in the foods provided. HFCS, high-fructose corn syrup.
After the clinical procedures, subjects were provided with detailed instructions regarding the diet. For a period of 8 d, subjects were required to consume 25% of their estimated daily calorie needs in the form of a beverage sweetened with either 100% fructose, 100% HFCS (55% fructose, 41% glucose, and 4% higher saccharides), or 100% glucose. Aspartame was included in the glucose and HFCS beverages to match the sweetness of the fructose beverage. This was achieved with the use of a concentration of aspartame in the glucose- and HFCS-sweetened beverages that, on average, received the same sweetness rating as the fructose-sweetened beverage by a panel of volunteers. The order in which subjects consumed the beverages was randomized, and subjects, kitchen staff, and all study staff, including the study coordinators, the principal investigator, the laboratory staff carrying out the outcome assessments, and the statistician, were blinded as to the order in which subjects received the beverages. The randomization scheme was generated by the principal investigator with the use of block randomization and stratified for sex and adiposity group (normal weight compared with overweight/obese). A random number generator produced blocks of 6 numbers consisting of the numbers 1–6 in random orders. These blocks were then randomly grouped and ordered to generate randomization lists for the 4 strata defined by sex and adiposity group. The beverages were prepared by individuals who did not communicate with participants or members of the study or kitchen teams to ensure blinding was maintained until the end of the study.
In addition to the beverages, subjects were provided with 125% of their estimated daily calorie needs as a standardized diet that they consumed ad libitum. In total, subjects were provided with 150% of their estimated energy needs, with 25% mandatory consumption as the SSB and 125% provided as solid foods to be consumed ad libitum. All foods were prepared and packaged specifically for each subject by the FHCRC Human Nutrition Laboratory. The solid food diet was designed as a 4-d rotating menu patterned after the average US diet (50% carbohydrate, 34% fat, and 16% protein) and was identical in all 3 phases of the study. Subjects were provided solid food in an amount equal to 125% of their estimated daily calorie needs as calculated by the Mifflin formula to determine their estimated resting energy expenditure, with an activity factor based on their habitual physical activity calculated from a physical activity questionnaire modified from Blair et al. (19) administered during the screening visit. Subjects were asked to eat from the provided solid foods until they were comfortably full and to return any uneaten foods to the kitchen staff for back weighing. They were also required to complete a daily checklist of foods consumed, including an estimation of the uneaten portions to be returned. Because subjects visited the research kitchen ≥2 times/diet period to pick up food and drop off leftovers, any discrepancy between the checklist and kitchen back weight was resolved with the subject immediately. As such, subject compliance with the diet and protocol was confirmed.
On day 9 of each intervention, clinic procedures identical to the day 1 visit were performed with the addition of a physical activity questionnaire modified from Blair et al. (19) to assess physical activity during the preceding 8-d diet period and a lactulose:mannitol test to assess intestinal permeability. For this test, subjects who had fasted were asked to void their bladders and then consume 1 final serving of the beverage they had received for the previous 8 d. In addition to the specific sugar (fructose, HFCS, or glucose), the beverage also contained 5 g lactulose and 2 g mannitol. Urine was collected for the subsequent 5 h. The urine was mixed, the total volume was recorded, and aliquots were frozen at −70°C. The amount of each sugar recovered from the initial dose was used to calculate the lactulose:mannitol ratio and the percentage of lactulose recovered from urine. This is the most frequently used clinical method for assessing intestinal permeability because these sugar probes are passively absorbed, are not metabolized, and pass into the urine unchanged (20).
All subjects were invited to undergo a voluntary adipose tissue biopsy procedure as an ancillary component of the larger study. Fourteen subjects elected to participate in the procedure, which has been described previously (21). Briefly, after locally injecting 2% lidocaine in a saline solution, a small incision was made lateral to the umbilicus. An additional lidocaine solution was injected under the surface, and a 14-gauge needle was used to extract ∼1–2 g adipose tissue. A small amount of tissue (3 pieces of ∼100 mg each) was frozen immediately on dry ice and stored at −70°C for gene expression analysis. The remainder was used to quantify and characterize the tissue leukocytes by flow cytometry as discussed in detail in the sections that follow.
Outcomes
The primary endpoints of this trial were fasting plasma concentrations of CRP and IL-6, both of which are biomarkers of systemic inflammation. The secondary endpoints included measures of intestinal permeability (lactulose:mannitol ratio and lactulose recovery based on a urinary lactulose:mannitol test, fasting plasma zonulin, and fasting plasma LBP—all interpreted together); measures of adipose tissue inflammation [whole-adipose tissue gene expression of adiponectin, TNF-α, IL-1β, IL-6, IL-10, chemokine (C-C motif) ligand 2 (CCL2), and interferon γ (IFN-γ) and adipose tissue content of macrophages, CD11c+ macrophages, neutrophils, dendritic cells, CD4+ and CD8+ T cells, and the adipose tissue CD4+:CD8+ T cell ratio—all interpreted together]; and fasting plasma adiponectin. Secondary endpoints that were not defined a priori included fasting plasma fetuin-A and FFA concentrations. An additional secondary endpoint was ad libitum energy intake during the 3 study diets, the results of which we have published previously (16).
Plasma, urine, and adipose tissue analyses
The concentrations of plasma IL-6 and fetuin-A (R&D Systems), adiponectin and zonulin (ALPCO), and LBP (Cell Sciences) were measured by ELISA on day 9 samples. These assays were performed in triplicate, with all samples from one participant run on the same plate. Intra- and interassay CVs within our laboratory for these assays were 7.8% and 13.7% for IL-6, 9.2% and 8.8% for fetuin-A, 5.3% and 6.5% for adiponectin, 1.9% and 9.2% for zonulin, and 8.3% and 32.2% for LBP, respectively. Because of the high interassay variability for LBP, we normalized across plates with the use of internal standards that were run twice in triplicate on each plate.
Measurements of high-sensitivity CRP (immunonephelometry) and FFA (colorimetric microdetermination) were completed by Northwest Lipid Laboratories. Lactulose and mannitol concentrations in 5-h urine samples were measured by gas chromatography (columns supplied by Agilent Technologies) in the FHCRC Biomarker Laboratory with the use of a previously published method (22). Briefly, lactulose and mannitol were added to pooled urine from healthy volunteers to create a 6-level standard curve with concentrations from 0.02 to 5.00 mg/mL. The mean recovery rate was 96.0% when pure sugar standards were added to the pooled urine in this manner, and the intra- and interassay CVs in the biomarker laboratory were 4.2% and 5.6%, respectively.
Collagenase digestion of adipose tissue
Adipose tissue was processed as described previously (23). Briefly, a final concentration of 0.5 mg collagenase I/mL (Worthington Biochemical Corp.) in phosphate-buffered saline (PBS) with 50 U DNase I/mL was used to digest the tissue for 60 min at 37°C. After digestion, the tissue slurry was passed through a 180-μm mesh filter and rinsed twice with 5 mL PBS + 1% bovine serum albumin before centrifugation for 5 min at 1200 rpm. The cell pellet containing the stromal vascular cell fraction (SVF) was then resuspended in 1× red cell lysis solution (BD Pharm Lyse) and incubated for 2 min at room temperature. After washing twice with cold staining buffer (0.2% bovine serum albumin/0.09% NaN3/PBS), the cell pellet was resuspended in 200 μL staining buffer.
Immunophenotyping of adipose tissue stromavascular cells by flow cytometry
Multiparameter flow cytometry was performed on the SVF freshly isolated from adipose tissue. Stromal vascular cells were labeled with the use of a combination of ≥9 directly conjugated primary antibodies all purchased from either BD Pharmagen, BioLegend, or Beckman-Coulter. They included allophycocyanin-conjugated CD4 and CD206; allophycocyanin-Cy7–conjugated CD3 and CD14; Alexa Fluor 700–conjugated CD8 and CD11c; fluorescein isothiocyanate–conjugated CD40 and CD80; Krome Orange–conjugated CD45; peridinin chlorophyll protein complex-Cy5.5–conjugated CD15 and CD20; phycoerythrin-conjugated CD152 and CD163; phycoerythrin-Cy7–conjugated CD1c; and phycoerythrin-Texas Red–conjugated CD16 and CD45RA. Samples were analyzed immediately after labeling with the use of a Becton Dickinson LSRII flow cytometer to collect ≥30,000 events in a broadly defined lymphocyte-monocyte-granulocyte gate defined by forward- and side-scatter attributes. Analyses were carried out with FlowJo software version 9.4.1 (TreeStar) with the use of histogram and dot plot analyses on the live cell gate. Live cells were defined by the absence of fluorescence associated with the uptake of 4′,6-diamidino-2-phenylindole dihydrochloride (EMD Chemicals and Molecular Probes), a reactive dye that binds to cellular amines of membrane-compromised (i.e., dead) cells. The expression of various combinations of these markers was used to identify and quantify specific subpopulations of leukocytes.
Gene expression analyses
Total RNA was extracted from whole adipose tissue with the use of the RNeasy Lipid Tissue Kit (Qiagen) and quantified with the use of RiboGreen (Invitrogen). cDNA synthesis was carried out on ∼1 mg total RNA with the use of the RETROscript Kit (Applied Biosystems), and polymerase chain reaction was performed with the use of predesigned TaqMan gene expression assays (Applied Biosystems) on an ABI Prism 7900HT sequence detection system. Gene targets included adiponectin, IL-6, IL-10, TNF-α, CCL2, IL-1β, and IFN-γ. We measured β-glucuronidase as a housekeeping gene because it is relatively stably expressed in adipose tissue across different conditions (24). A normalization factor was then calculated with the use of this housekeeping gene and applied to all target genes.
Statistical analysis
All statistical analyses were performed with the use of SPSS version 20.0 (IBM). The study was powered to detect clinically relevant differences between diet groups in the primary endpoints: CRP and IL-6. In a pilot study [study A in Kuzma et al. (16)], fasting plasma CRP and IL-6 concentrations were 0.92 ± 0.64 mg/L and 0.60 ± 0.40 pg/mL, respectively, and were higher after subjects had consumed 25% of their estimated calorie requirements in the form of a fructose-sweetened beverage than when they had consumed a glucose-sweetened beverage (n = 10). Accordingly, it was determined that if 24 subjects completed the DASI study, we would have 80% power to detect a difference between any 2 groups of 0.41 mg/L for CRP and 0.26 ng/L for IL-6, assuming the same variability in response seen in the pilot study and an α-error level of 2.5% (adjusted to account for the 2 primary endpoint measures). We powered the study to detect smaller differences in both endpoints because smaller differences than those observed would still be clinically relevant. Furthermore, the variability in response may be greater than that observed in the pilot study, and a larger sample size improves our power for subgroup analyses (normal-weight compared with overweight/obese individuals). Significance was set at P < 0.05 for all analyses except for CRP and IL-6, which was set at P < 0.025 as described previously. Secondary endpoint measures were not adjusted for multiple testing because all were related to the primary outcomes, and because adjusting the numerous outcomes was judged to overly inflate the β-error, i.e., reduce power to detect the effects of the diets. The outcomes related to intestinal permeability and adipose tissue inflammation are different measures of these biological processes and have previously been considered, interpreted, and reported together (25).
The primary analysis used repeated-measures (RM) ANOVA to assess whether the within-subject variable diet explained any variation in mean plasma CRP or IL-6 measured on day 9 of each diet period. Similarly, this analysis was repeated on the remaining biomarkers of systemic and adipose tissue inflammation and intestinal permeability: LBP, adiponectin, zonulin, fetuin-A, FFA, and the urinary lactulose:mannitol ratio as well as adipose tissue messenger RNA (mRNA) and immune cell populations, which were also measured on day 9 of each diet period. We used RM ANOVA to assess whether there were any changes in body weight or CRP between days 1 and 9 of each diet period and whether this change differed by diet period. A paired t test with Bonferroni adjustment was conducted post hoc to determine which 2 groups differed from each other if the RM ANOVA indicated an overall difference. The data were then stratified by adiposity category in the RM ANOVA to assess whether biomarkers of systemic inflammation and intestinal permeability were differentially affected by the 3 diet periods within these subgroups. Stratified analyses were not conducted on biomarkers of adipose tissue inflammation because the sample size within subgroups was too small. All variables and residuals from RM ANOVA were tested for normality by conducting a Shapiro-Wilk test and by checking histograms and normal plots. Variables were log10-transformed if they were not normally distributed. This was the case for CRP; IL-6; LBP; FFA; fetuin-A; the lactulose:mannitol ratio; the percentage of lactulose and mannitol recovered from urine; IL-1β, IL-6, IL-10, CCL2, and IFN-γ mRNA; and neutrophil, dendritic cell, 11c+ macrophage, and the ratio of CD4+:CD8+ T cell populations in adipose tissue.
For all endpoints, we ran follow-up sensitivity analyses that excluded subjects who reported a minor illness (such as a cold) during ≥1 diet period to assess whether the relation between diet and any of the measured biomarkers was affected by including these subjects.
Our a priori statistical analysis plan was a per-protocol analysis. After the study was completed, we conducted all analyses first as intent-to-treat analyses and included all 25 subjects who initiated the study, with the last observation carried forward for the missing data points. The analysis was then repeated per protocol with the exclusion of the 1 subject who did not complete all 3 diet periods (n = 24). This exclusion did not alter the results in any way; therefore, only the results of the per-protocol analyses are presented herein.
RESULTS
We conducted 63 in-person screening visits. Thirty-five participants did not meet eligibility criteria; 23 participants suffered from fructose malabsorption, and 3 individuals declined to participate. Twenty-five participants were enrolled starting in January 2012; the final subject completed the study in April 2014. One subject was dismissed from the study after completing the first diet period for failing to complete the second and third diet periods. The detailed baseline characteristics for the 24 participants who completed all study procedures have been described (16). Briefly, the 24 subjects were either normal weight (n = 12; 3 women and 9 men) with a mean ± SD age of 33 ± 11 y, BMI of 23.7 ± 1.0, fasting glucose of 87 ± 10 mg/dL, and baseline CRP of 1.1 ± 1.0 mg/L or overweight/obese (n = 12; 6 women and 6 men) with a mean ± SD age of 39 ± 12 y, BMI of 31.0 ± 4.3, fasting glucose of 96 ± 8 mg/dL, and baseline CRP of 2.5 ± 1.8 mg/L (16).
A detailed description of the effect of fructose-, HFCS-, and glucose-sweetened beverages on overall energy intake has been published (16). In summary, subjects consistently consumed >16% total calories than their estimated energy requirement predicted they would need when 25% of their energy was consumed in the form of a sweetened beverage; however, overall energy intake was not significantly different between the 3 diet periods (16). Despite the excessive energy intake, body weight did not change within the 8-d diet periods to a statistically significant degree, and no significant difference in weight change was seen between diet periods. Physical activity also was not significantly different between the 3 diet periods (16).
Biomarkers of systemic inflammation and intestinal permeability
For our primary outcome measures, there was no effect of diet on day 9 plasma concentrations of IL-6 (Table 1) or CRP. There was also no significant change in CRP within the diet periods or a difference in the change between diet periods.
TABLE 1.
Biomarkers of systemic inflammation in all subjects on day 9 of each dietary period unless otherwise noted1
| Fructose | HFCS | Glucose | P-time2 | P-diet2 | P-time × P-diet2 | |
| CRP, mg/L | 0.353 | 0.403 | ||||
| Day 1 | 0.91 (0.45, 2.35) | 1.18 (0.38, 2.49) | 1.67 (0.36, 2.91) | |||
| Day 9 | 1.07 (0.48, 2.10) | 0.84 (0.49, 2.48) | 1.09 (0.38, 3.04) | |||
| IL-6, pg/mL | 0.97 (0.62, 1.90) | 0.96 (0.61, 1.79) | 1.14 (0.61, 1.95) | 0.933 | ||
| Adiponectin, ng/mL | 4635 ± 2545 | 4514 ± 2195 | 4353 ± 2198 | 0.196 |
Values are means ± SDs or medians (IQRs) if nonnormally distributed data. n = 24. CRP, C-reactive protein; HFCS, high-fructose corn syrup.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA.
For our secondary outcome measures, there was no effect of diet on day 9 plasma adiponectin concentrations (Table 1). There was also no effect of diet on plasma zonulin or LBP (Table 2); however, there was a significant effect of diet on the urinary lactulose:mannitol ratio and the percentage of lactulose recovered from urine on day 9 (P < 0.001 for the diet group in overall RM ANOVA) (Table 2). Post hoc paired t tests with Bonferroni adjustment revealed that the lactulose:mannitol ratio after both fructose and glucose diet periods was significantly higher than the ratio after the HFCS diet period (P < 0.003), whereas the fructose and glucose diet periods did not differ from each other. The percentage of lactulose recovered from urine after the glucose diet period was significantly higher than that recovered after the HFCS diet period. There was also no effect of diet on circulating FFAs or fetuin-A (Table 2). In sensitivity analyses, excluding the 6 subjects who reported a minor illness in ≥1 diet period did not change any of the dietary effects on any of the biomarkers (data not shown).
TABLE 2.
Measures of plasma FFAs, fetuin-A, and intestinal permeability markers on day 9 of each dietary period1
| Fructose | HFCS | Glucose | P-diet2 | |
| Zonulin, ng/mL | 12.78 ± 1.54 | 12.92 ± 1.49 | 12.69 ± 1.70 | 0.366 |
| LBP, μg/mL | 27.7 (19.8, 34.2) | 29.8 (21.5, 36.0) | 26.1 (21.7, 40.0) | 0.387 |
| Lactulose:mannitol ratio | 0.047 (0.031, 0.054)a | 0.031 (0.026, 0.035)b | 0.043 (0.031, 0.048)a | <0.001 |
| Lactulose recovery, % | 0.25 (0.19, 0.32)a,b | 0.22 (0.19, 0.25)a | 0.29 (0.24, 0.32)b | <0.0013 |
| FFAs, mEq/L | 0.28 (0.20, 0.38) | 0.30 (0.17, 0.47) | 0.40 (0.24, 0.51) | 0.121 |
| Fetuin-A, mg/L | 490 (404, 567) | 467 (418, 581) | 496 (414, 570) | 0.602 |
Values are means ± SDs or medians (IQRs) if nonnormally distributed data. n = 24. Values in the same row with different superscript letters are significantly different from each other, P < 0.05 (post hoc paired t tests with Bonferroni correction). FFA, free fatty acid; HFCS, high-fructose corn syrup; LBP, LPS-binding protein.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA.
P value determined by the nonparametric Friedman test.
Secondary analyses stratified by adiposity showed that overweight/obese subjects had significantly higher plasma CRP (P = 0.029), IL-6 (P = 0.001), and LBP (P = 0.005) than normal-weight participants (data not shown). However, in analyses conducted to assess whether a significant interaction between adiposity and diet existed, we detected no differential impact of the diets on any of the biomarkers of inflammation in normal-weight compared with overweight/obese individuals (data not shown).
Adipose tissue inflammation
Fourteen subjects opted to undergo a voluntary abdominal subcutaneous adipose tissue biopsy at the end of each diet period. Their baseline characteristics were not significantly different from that of the overall study population. Four subjects (1 man and 3 women) were normal weight (BMI: 23.8 ± 1.0), aged 36 ± 15 y, with a fasting glucose of 93 ± 5 mg/dL and baseline CRP of 0.84 ± 1.1 mg/L. The other 10 subjects (4 men and 6 women) were overweight/obese (BMI: 30.5 ± 3.4), aged 41 ± 13 y, with a fasting glucose of 97 ± 9 mg/dL and baseline CRP of 2.7 ± 1.9 mg/L. Gene expression analyses of samples from these 14 subjects revealed that there was a significant effect of diet on day 9 adipose tissue expression of adiponectin mRNA (P = 0.005). In post hoc t tests, the expression after the fructose diet period was significantly greater than after both the HFCS (P = 0.048) and glucose (P = 0.012) diet periods after Bonferroni correction for multiple testing. The adipose tissue adiponectin expression did not differ after the HFCS compared with the glucose period. There was no effect of diet on mRNA expression of any of the other genes measured except for a trend toward a significant difference in CCL2 mRNA expression (Table 3).
TABLE 3.
Tissue mRNA expression in subcutaneous adipose tissue on day 9 of each diet period in subjects who underwent optional adipose tissue biopsy1
| Fructose | HFCS | Glucose | P-diet2 | |
| Adiponectin | 6288 ± 1840a | 5370 ± 1586b | 5107 ± 1574b | 0.005 |
| TNF-α | 1.40 ± 0.58 | 1.24 ± 0.67 | 1.44 ± 0.89 | 0.476 |
| IL-1β | 0.41 (0.26, 0.54) | 0.32 (0.27, 0.84) | 0.33 (0.25, 0.41) | 0.596 |
| IL-6 | 0.53 (0.35, 0.74) | 0.51 (0.38, 0.75) | 0.49 (0.37, 0.58) | 0.492 |
| IL-10 | 0.66 (0.34, 1.14) | 0.95 (0.34, 1.44) | 0.90 (0.65, 1.19) | 0.149 |
| CCL2 | 31.5 (22.9, 47.6) | 25.3 (19.3, 34.4) | 27.2 (22.6, 33.6) | 0.056 |
| IFN-γ | 0.23 (0.15, 0.44) | 0.23 (0.17, 0.38) | 0.20 (0.15, 0.27) | 0.520 |
Data are in copy number/ng total RNA normalized to the housekeeping gene GUSB. Values are means ± SDs or medians (IQRs) if nonnormally distributed data. n = 14. Values in the same row with different superscript letters are significantly different from each other, P < 0.05 (post hoc paired t tests with Bonferroni correction). CCL2, chemokine (C-C motif) ligand; GUSB, glucuronidase β HFCS, high-fructose corn syrup; IFN-γ, interferon γ.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA.
There was no effect of diet on day 9 tissue populations of neutrophils (CD15+CD16+), total ATMs (CD14+CD206+), CD11c+ ATMs (CD14+CD206+CD11c+), CD4+ T cells (CD3+CD4+), or CD8+ T cells (CD3+CD8+) when data were normalized to cells per gram of adipose tissue or as a percentage of the CD45+ cell fraction (Table 4). There was also no effect of diet on the CD4+:CD8+ T cell ratio. When cells were normalized as a percentage of the CD45+ cell fraction, there was a significant effect of diet on the percentage of CD1c+CD11c+ dendritic cells on day 9 (P = 0.017 for diet group in overall RM ANOVA) (Table 4). In post hoc t tests, dendritic cell numbers after the HFCS diet period were significantly greater than after the glucose diet period (P = 0.012) after Bonferroni correction for multiple testing. Dendritic cell numbers did not differ after the HFCS compared with the fructose period or the glucose compared with the fructose period. No unexpected adverse events or adverse events that were more severe than mild occurred in this study.
TABLE 4.
Subcutaneous adipose tissue cell populations on day 9 of each diet period in subjects who underwent optional adipose tissue biopsy1
| Fructose | HFCS | Glucose | P-diet2 | |
| CD15+CD16+ neutrophils | ||||
| 103 cells SQAT/g | 187 (37, 349) | 69 (16, 347) | 190 (34, 601) | 0.244 |
| CD45+ fraction, % | 28.5 ± 21.7 | 18.0 ± 15.9 | 26.9 ± 20.8 | 0.254 |
| CD1c+CD11c+ dendritic cells | ||||
| 103 cells SQAT/g | 16 ± 6.2 | 21 ± 15 | 14 ± 7.3 | 0.210 |
| CD45+ fraction, % | 1.95 (1.43, 3.85)a,b | 2.95 (1.93, 4.03)a | 1.80 (0.90, 2.78)b | 0.017 |
| CD14+CD206+ ATM | ||||
| 103 cells SQAT/g | 53 ± 39 | 62 ± 37 | 50 ± 35 | 0.546 |
| CD45+ fraction, % | 8.33 ± 6.77 | 9.90 ± 3.92 | 7.38 ± 4.88 | 0.320 |
| CD14+CD206+CD11c+ ATM3 | ||||
| 103 cells SQAT/g | 5.5 (3.3, 13) | 14 (2.7, 21) | 6.1 (3.2, 12) | 0.920 |
| CD45+ fraction, % | 0.78 (0.56, 1.96) | 1.93 (0.57, 3.00) | 0.79 (0.31, 1.98) | 0.346 |
| CD3+CD4+ T cells | ||||
| 103 cells SQAT/g | 42 ± 14 | 50 ± 31 | 57 ± 26 | 0.401 |
| CD45+ fraction, % | 6.51 ± 2.83 | 7.07 ± 3.09 | 6.91 ± 2.51 | 0.813 |
| CD3+CD8+ T cells | ||||
| 103 cells SQAT/g | 39 (25, 54) | 30 (20, 106) | 45 (30, 58) | 0.580 |
| CD45+ fraction, % | 6.75 ± 4.18 | 6.82 ± 3.81 | 6.52 ± 4.20 | 0.949 |
| CD4+:CD8+ T cell ratio | 0.93 (0.61, 1.31) | 1.01 (0.79, 1.39) | 1.07 (0.72, 1.47) | 0.275 |
Data were normalized per gram of tissue and to the percentage of CD45+ leukocytes for each cell population. Unless otherwise noted, values are means ± SDs or medians (IQRs) if nonnormally distributed data. n = 12. Values in the same row with different superscript letters are significantly different from each other, P < 0.05 (post hoc paired t tests with Bonferroni correction). ATM, adipose tissue macrophage; HFCS, high-fructose corn syrup; SQAT, subcutaneous adipose tissue.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA.
n = 11.
DISCUSSION
The major finding from this study is that consuming beverages sweetened with fructose, HCFS, or glucose for 8 d did not differentially affect our primary outcome, fasting plasma CRP and IL-6 as measures of systemic inflammation, in normal-weight to obese adults. We also did not detect any differences between diet periods in measures of adipose tissue inflammation. Together, our data suggest that neither the short- to medium-term consumption of excess energy nor excessive amounts of fructose per se trigger low-grade chronic inflammation.
Our study was motivated by strong evidence from rodent models in which high fructose but not glucose consumption led to increased intestinal permeability and the translocation of bacterial LPS into the portal vein. Elevated portal vein LPS triggered inflammation in the liver via a mechanism that was dependent on the LPS receptor (toll-like receptor 4) as well as the presence of bacteria in the gut (10). Furthermore, treating fructose-fed rodents with antibiotics reduced lipid accumulation and inflammation in the liver, suggesting that both may result directly from exposure to intestinally derived endotoxin (7).
Contrary to these findings, and consistent with a lack of a differential effect on biomarkers of inflammation, we saw no difference in the plasma markers of endotoxin exposure (LBP) or gut tight-junction integrity (zonulin) after the 3 diet periods. We also found no clear evidence that fructose compared with glucose increases intestinal permeability, as measured by the lactulose:mannitol test. A higher urinary lactulose:mannitol ratio suggests that lactulose, a large-diameter molecule that is normally not absorbed in the healthy human gut, has passed paracellularly, or unregulated, into the portal circulation. The elevation of this ratio indicates increased intestinal permeability and suggests that other lumenal contents, such as bacteria-derived molecules, could pass unregulated into the portal circulation as well. Although the reduced lactulose:mannitol ratio at the end of the HFCS phase may seem notable, several considerations lead us to reject our hypothesis that fructose has specific effects on intestinal permeability that differ from those of glucose. First, neither the lactulose:mannitol ratio nor the lactulose recovery differed between the fructose and glucose periods. Second, we detected not even the slightest trend toward differences in the other measures of intestinal permeability (plasma zonulin and LBP). Third, albeit statistically significantly different, the lactulose:mannitol ratio was within the range that is considered normal and well below that observed in clinical conditions associated with intestinal permeability such as celiac disease (26). Finally, for any difference in intestinal permeability to be clinically relevant (at least in the context of our hypothesis), differences would need to be associated with differences in measures of systemic inflammation, which was not the case herein.
Few intervention studies to our knowledge have been carried out in humans investigating SSBs and systemic inflammation, and the results have differed. In line with our findings, Cox et al. (27) observed no change in plasma CRP or IL-6 in subjects who consumed fructose compared with glucose beverages as 25% of calories for 10 wk. However, monocyte chemoattractant protein 1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1), and E-selectin, which are also proinflammatory mediators, all increased considerably from baseline after fructose- but not glucose-sweetened beverage consumption (27). Total body weight and subcutaneous adipose tissue increased slightly but similarly in both groups, but visceral adipose tissue increased in the fructose-sweetened beverage group only (9). Visceral, rather than subcutaneous, adipose tissue is the primary determinant of circulating MCP-1 and PAI-1 concentrations (28, 29). Hence, it is possible that the expansion of visceral adipose tissue in individuals who consumed fructose-sweetened beverages in that study was the primary driver of an increase in these specific markers of inflammation rather than a direct effect of fructose. Further supporting this hypothesis, Silbernagel et al. (30) conducted a 4-wk parallel design study that compared high-glucose and high-fructose consumption on plasma PAI-1, MCP-1, E-selectin, and CRP in healthy adults. Subjects consumed 150 g glucose/d or 150 g fructose/d dissolved in water, a dose similar to the one used in our study. After 4 wk, there were no relevant changes in plasma PAI-1, MCP-1, E-selectin, or CRP after either intervention; there were also no differences between treatments (30). It is worth noting that subjects in both the fructose and glucose groups gained a similarly small amount of weight, yet visceral adipose tissue mass did not change (30). In a randomized crossover study, Aeberli et al. (31) tested whether 80 g compared with 40 g glucose, fructose, or sucrose/d differentially influenced plasma CRP compared with dietary recommendations to consume a low-fructose diet. Compared with preintervention baseline concentrations, fasting CRP was significantly increased after all intervention phases, including the low-fructose diet control phase (31). Although it remains unclear why CRP concentrations increased in all study periods, these data are consistent with our finding of no differential effect of fructose compared with glucose on systemic inflammation.
We also investigated a novel mechanism of adipose tissue inflammation involving the FFA chaperone protein fetuin-A (32). We hypothesized that adipose tissue inflammatory pathways could be triggered directly by fructose-induced systemic inflammation (11) or indirectly by fetuin-A activation of inflammatory pathways in adipose tissue (15). We measured several genes in adipose tissue known to be involved in the inflammatory process and quantified and characterized the activation state of immune cell populations isolated from the SVF in a comprehensive effort to detect any differential effect of the beverages on adipose tissue inflammation. We saw no consistent differences in the markers of adipose tissue inflammation that would suggest that fructose has specific proinflammatory effects on adipose tissue. Although we detected substantially lower numbers of dendritic cells in adipose tissue after subjects had consumed the glucose- compared with HFCS-sweetened beverages, there was no difference between glucose and fructose periods, and we found no consistent changes in any other measure of adipose tissue inflammation. One finding that may be notable is that adiponectin gene expression was considerably greater after the fructose diet period than both the HFCS and glucose diet periods. Adiponectin is an insulin-sensitizing anti-inflammatory adipokine that is inversely correlated with adiposity. It seems possible that the overexposure of adipocytes to glucose may directly or indirectly suppress adiponectin gene expression. The relevance of this finding is unclear, however, because this difference in subcutaneous adipose tissue adiponectin expression was not associated with a similar difference in plasma adiponectin concentrations. This may have been because plasma adiponectin is preferentially derived from intra-abdominal adipose tissue, which may not have been differentially affected by the 3 diets.
The crossover design is a major strength of our study because the influence of the substantial interindividual differences in biomarkers of inflammation and gene expression was minimized by this approach. Furthermore, this is the first study to our knowledge to screen for and enroll only those subjects with normal fructose absorption. The relatively short duration of the intervention was potentially both a strength and limitation of the study. Because subjects did not gain weight, we were able to measure the effects of the beverages on inflammation in a body weight–independent fashion. However, it is also possible that the markers we chose to study do not change that much within this relatively short time period. It is also important to emphasize that our study design is stronger in comparing the data generated at the end of each diet period than it is in assessing changes from baseline during each dietary period.
In conclusion, our data show no differential effects of excessive amounts of fructose, HFCS, or glucose from SSBs consumed over 8 d on low-grade chronic systemic inflammation in normal-weight to obese adults.
Acknowledgments
We thank Pamela Y Yang, Xiaoling Song, and Michelle A Wurscher for their excellent technical assistance; Peggie Bates and Sara Bennett for preparing the diet and beverages; and Linda Glockling and David Hatten for assistance with the randomization and blinding procedures.
The authors’ responsibilities were as follows—JNK: completed the laboratory procedures, statistical analysis of the data, and the first draft of the manuscript; JNK and GC: collected the data; DKH: provided technical assistance; KLB: oversaw the preparation of all study meals; CLR: provided input on the study design and data analysis and interpretation; KEF-S: served as the physician of record for the study; SEH: provided statistical guidance; DSW: assisted in the design of the studies as well as data analysis and interpretation; MK: initiated the studies and had overall responsibility for the design and conduct of the studies and the data analyses; and all authors: contributed to the preparation of the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ATM, adipose tissue macrophage; CCL2, chemokine (C-C motif) ligand 2; CRP, C-reactive protein; DASI, Diet and Systemic Inflammation; DNL, de novo lipogenesis; FFA, free fatty acid; FHCRC, Fred Hutchinson Cancer Research Center; HFCS, high-fructose corn syrup; IFN-γ, interferon γ LBP, LPS-binding protein; MCP-1, monocyte chemoattractant protein 1; mRNA, messenger RNA; PAI-1, plasminogen activator inhibitor type 1; PBS, phosphate-buffered saline; RM, repeated measures; SSB, sugar-sweetened beverage; SVC, stromavascular cell fraction.
REFERENCES
- 1.Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio-Lopez MT, Martinez-Gonzalez MA, Zhou D. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr 2015;113:709–17. [DOI] [PubMed] [Google Scholar]
- 2.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet 2013;113:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, Cullen JM. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr 2013;98:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008;48:983–92. [DOI] [PubMed] [Google Scholar]
- 8.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094–104. [DOI] [PubMed] [Google Scholar]
- 11.Ekström M, Halle M, Bjessmo S, Liska J, Kolak M, Fisher R, Eriksson P, Tornvall P. Systemic inflammation activates the nuclear factor-kappaB regulatory pathway in adipose tissue. Am J Physiol Endocrinol Metab 2010;299:E234–40. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz JM, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, Bergeron N, Bersot TP, et al. . Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 2015;100:2434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006;29:853–7. [DOI] [PubMed] [Google Scholar]
- 14.Haukeland JW, Dahl TB, Yndestad A, Gladhaug IP, Loberg EM, Haaland T, Konopski Z, Wium C, Aasheim ET, Johansen OE, et al. . Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol 2012;166:503–10. [DOI] [PubMed] [Google Scholar]
- 15.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 2012;18:1279–85. [DOI] [PubMed] [Google Scholar]
- 16.Kuzma JN, Cromer G, Hagman DK, Breymeyer KL, Roth CL, Foster-Schubert KE, Holte SE, Callahan HS, Weigle DS, Kratz M. No difference in ad libitum energy intake in healthy men and women consuming beverages sweetened with fructose, glucose, or high-fructose corn syrup: a randomized trial. Am J Clin Nutr 2015;102:1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. [Clinically relevant breath tests in gastroenterological diagnostics—recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases.] Z Gastroenterol 2005;43:1071–90. [DOI] [PubMed] [Google Scholar]
- 18.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 20.Dastych M, Dastych M Jr, Novotna H, Cihalova J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn’s disease. Dig Dis Sci 2008;53:2789–92. [DOI] [PubMed] [Google Scholar]
- 21.Campbell KL, Makar KW, Kratz M, Foster-Schubert KE, McTiernan A, Ulrich CM. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev Res (Phila) 2009;2:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2006;836:63–8. [DOI] [PubMed] [Google Scholar]
- 23.Hagman DK, Kuzma JN, Larson I, Foster-Schubert KE, Kuan LY, Cignarella A, Geamanu E, Makar KW, Gottlieb JR, Kratz M. Characterizing and quantifying leukocyte populations in human adipose tissue: impact of enzymatic tissue processing. J Immunol Methods 2012;386:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalán V, Gomez-Ambrosi J, Rotellar F, Silva C, Rodriguez A, Salvador J, Gil MJ, Cienfuegos JA, Fruhbeck G. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res 2007;39:495–500. [DOI] [PubMed] [Google Scholar]
- 25.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102:721–8. [DOI] [PubMed] [Google Scholar]
- 26.Smecuol E, Sugai E, Niveloni S, Vazquez H, Pedreira S, Mazure R, Moreno ML, Label M, Maurino E, Fasano A, et al. . Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 2005;3:335–41. [DOI] [PubMed] [Google Scholar]
- 27.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, et al. . Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab 2011;96:E2034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, et al. . Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 1996;2:800–3. [DOI] [PubMed] [Google Scholar]
- 29.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 2005;90:2282–9. [DOI] [PubMed] [Google Scholar]
- 30.Silbernagel G, Machann J, Haring HU, Fritsche A, Peter A. Plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, e-selectin and C-reactive protein levels in response to 4-week very-high-fructose or -glucose diets. Eur J Clin Nutr 2014;68:97–100. [DOI] [PubMed] [Google Scholar]
- 31.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–85. [DOI] [PubMed] [Google Scholar]
- 32.Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013;9:144–52. [DOI] [PubMed] [Google Scholar]