Abstract
Background: The carbohydrate–insulin model of obesity posits that habitual consumption of a high-carbohydrate diet sequesters fat within adipose tissue because of hyperinsulinemia and results in adaptive suppression of energy expenditure (EE). Therefore, isocaloric exchange of dietary carbohydrate for fat is predicted to result in increased EE, increased fat oxidation, and loss of body fat. In contrast, a more conventional view that “a calorie is a calorie” predicts that isocaloric variations in dietary carbohydrate and fat will have no physiologically important effects on EE or body fat.
Objective: We investigated whether an isocaloric low-carbohydrate ketogenic diet (KD) is associated with changes in EE, respiratory quotient (RQ), and body composition.
Design: Seventeen overweight or obese men were admitted to metabolic wards, where they consumed a high-carbohydrate baseline diet (BD) for 4 wk followed by 4 wk of an isocaloric KD with clamped protein. Subjects spent 2 consecutive days each week residing in metabolic chambers to measure changes in EE (EEchamber), sleeping EE (SEE), and RQ. Body composition changes were measured by dual-energy X-ray absorptiometry. Average EE during the final 2 wk of the BD and KD periods was measured by doubly labeled water (EEDLW).
Results: Subjects lost weight and body fat throughout the study corresponding to an overall negative energy balance of ∼300 kcal/d. Compared with BD, the KD coincided with increased EEchamber (57 ± 13 kcal/d, P = 0.0004) and SEE (89 ± 14 kcal/d, P < 0.0001) and decreased RQ (−0.111 ± 0.003, P < 0.0001). EEDLW increased by 151 ± 63 kcal/d (P = 0.03). Body fat loss slowed during the KD and coincided with increased protein utilization and loss of fat-free mass.
Conclusion: The isocaloric KD was not accompanied by increased body fat loss but was associated with relatively small increases in EE that were near the limits of detection with the use of state-of-the-art technology. This trial was registered at clinicaltrials.gov as NCT01967563.
Keywords: body composition, energy expenditure, ketogenic diet, insulin, carbohydrate, fat, macronutrients
INTRODUCTION
Dietary carbohydrates and insulin have been suggested to play causal roles in the pathological accumulation of body fat (1–4). According to this carbohydrate–insulin model of obesity, an increased proportion of the diet as carbohydrates results in elevated insulin secretion that suppresses the release of fatty acids into circulation and directs circulating fat toward storage. Additionally, the decreased availability of fatty acids for use by metabolically active tissues, such as heart, muscle, and liver, is perceived as a state of cellular internal starvation, possibly through an increased ratio of cellular AMP to ATP (4), leading to an adaptive decrease in energy expenditure (EE)7 and an increase in food intake (1, 4–7). Therefore, the positive energy balance associated with development of obesity is hypothesized to be a consequence of the insulin-driven shift in fat partitioning toward storage and away from oxidation resulting from an increased proportion of dietary carbohydrates.
A logical consequence of the carbohydrate–insulin model is that decreasing the proportion of dietary carbohydrate to fat without altering protein or calories will reduce insulin secretion, increase fat mobilization from adipose tissue, and elevate the oxidation of circulating free fatty acids (FFAs). The altered metabolic and endocrine milieu is therefore predicted to relieve the state of cellular internal starvation resulting in decreased hunger, increased body fat loss, and increased EE. In contrast, a more conventional model asserts that a calorie is a calorie, meaning that isocaloric exchanges between dietary carbohydrate and fat will not substantially influence EE or body fat (8).
Testing these competing model predictions is challenging because outpatient diet studies are typically associated with poor adherence, even when all study foods are provided (9). We aimed to avoid this difficulty by confining study volunteers to metabolic wards where they consumed, under supervision, a 4-wk run-in high-carbohydrate baseline diet (BD) followed by an isocaloric low-carbohydrate ketogenic diet (KD) for another 4 wk. Our prespecified primary endpoints were changes in total EE (EEchamber), sleeping EE (SEE), and 24-h respiratory quotient (RQ) measured by using metabolic chambers. Body composition changes were prespecified secondary endpoints of the study.
METHODS
Study protocol
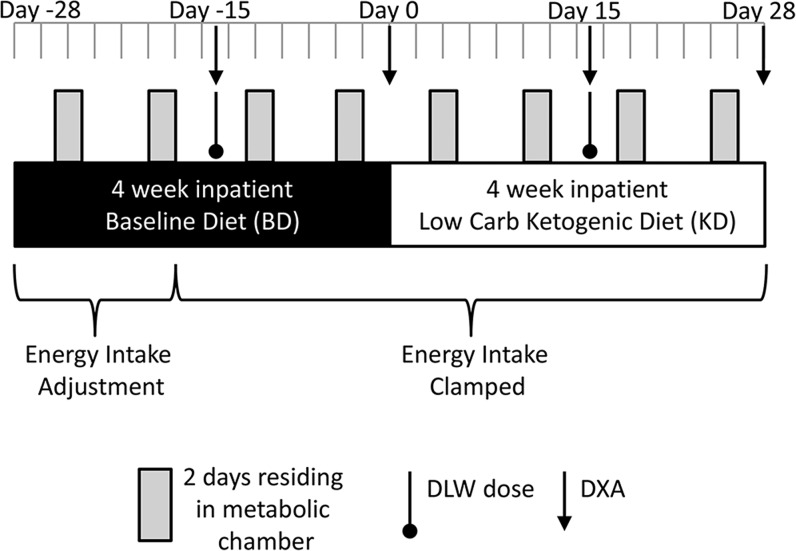
Figure 1 depicts a summary of the study design in which subjects were admitted to metabolic wards and consumed a BD (Table 1) for a 4-wk run-in period (days −28 to 0) followed by a KD (Table 1) for another 4 wk (days 1–28). The study protocol was approved by the Institutional Review Boards of the National Institute of Diabetes and Digestive and Kidney Diseases (NCT01967563), the Pennington Biomedical Research Center (2013-3-PBRC), Columbia University Medical Center (IRB-AAAL7113), and the Translational Research Institute for Metabolism and Diabetes (IRB 493675).
FIGURE 1.
Overview of the study design. Seventeen overweight and obese men were confined to metabolic wards where they consumed a BD designed to represent a habitual high-carbohydrate intake for a 28-d run-in period followed by an isocaloric KD for an additional 28 d. Dietary protein was kept constant throughout, and the subjects were prescribed 90 min of low-intensity daily aerobic exercise. Every week, subjects spent 2 consecutive days residing in metabolic chambers to measure total daily energy expenditure, respiratory quotient, and sleeping energy expenditure. Body composition was measured by DXA, and the average energy expenditure during the last 2 wk of each diet period was measured by the DLW method. BD, high-carbohydrate baseline diet; DLW, doubly labeled water; DXA, dual-energy X-ray absorptiometry; KD, low-carbohydrate ketogenic diet.
TABLE 1.
Daily diet composition of the 7-d, 2400-kcal rotating menus for the BD and KD1
| BD | KD | |
| Energy, kcal | 2398 | 2394 |
| Protein, g | 91 | 91 |
| Carbohydrate, g | 300 | 31 |
| Fat, g | 93 | 212 |
| Sodium, mg | 3060 | 5060 |
| trans Fat, g | 1.2 | 2.3 |
| Monounsaturated fat, g | 31.8 | 100.2 |
| Polyunsaturated fat, g | 19.9 | 32.5 |
| Saturated fat, g | 33.0 | 65.6 |
| Fiber, g | 26 | 12 |
| Total sugar, g | 147 | 10 |
| Protein, % of energy | 15 | 15 |
| Carbohydrate, % of energy | 50 | 5 |
| Fat, % of energy | 35 | 80 |
| Chemical analysis | ||
| Protein, % of energy | 16.1 ± 0.42 | 16.9 ± 0.5 |
| Carbohydrate, % of energy | 48.1 ± 0.7 | 5.9 ± 1.1 |
| Fat, % of energy | 35.6 ± 0.6 | 77.3 ± 1.0 |
| Sodium, mg | 2665 ± 157 | 4910 ± 610 |
| Fiber, g | 23.7 ± 2.2 | 13.2 ± 2.1 |
Diets were analyzed by using NUTRITIONIST PRO version 1.3 (First Databank Inc.; The Hearst Corporation). BD, high-carbohydrate baseline diet; KD, low-carbohydrate ketogenic diet.
Mean ± SE (all such values).
Seventeen men between the ages of 18 and 50 y with a BMI (kg/m2) between 25 and 35 provided informed consent and were admitted to metabolic wards (5 subjects at NIH, and 4 subjects at each of the other sites). Participants were excluded if they were not weight stable (weight change of >5% in the past 6 mo), were <92% of lifetime maximum weight, had blood pressure >140/90 mm Hg, were unable to complete daily bouts of stationary cycling, had evidence of diseases that affect metabolism or eating disorders, were taking medications interfering with study outcomes, or were unwilling or unable to eat the foods provided in the study. We also excluded subjects whose habitual diets were <30% or >65% of total calories from carbohydrate as determined by a food-frequency questionnaire.
Subjects were invited to participate in 3 screening visits designed to ensure acceptability and adherence to the study diets and procedures, assess potential social or psychological barriers to the completion of the study, complete a medical history and physical examination, answer several questionnaires, measure resting EE, and personalize the intensity of the prescribed daily stationary cycling that was subsequently clamped for the study duration. Subjects were prescribed 90 min of daily stationary cycling, and overall physical activity was quantified with small, portable, pager-type accelerometers (GT3XE+, Actigraph Corporation) sampled at 80 Hz.
We required that the average EEchamber measured during the last pair of chamber days during the BD period to be within 5% of the average EEchamber measured the previous week. In 2 cases, this criterion was not met, and an additional week of BD was required to ensure stability of EEchamber before initiating the KD period. All but one of the sites had the metabolic chambers colocated with the metabolic ward. At one site the subjects were transported to and from the metabolic chamber by automobile under supervision.
Diets
The 7-d rotating menus were designed to match the macronutrient targets of a habitual BD and a KD by using NUTRITIONIST PRO software version 1.3 (First Databank Inc.; The Hearst Corporation). The energy and macronutrient composition for each day of both the BD and KD menus was verified by chemical analysis (Covance Laboratories). Most of the food was prepared at the Pennington Biomedical Research Center metabolic kitchen, frozen, and shipped to the study sites, where fresh produce was added and the meals were prepared for consumption according to standardized procedures. Both the BD and KD menus contained minimal quantities of processed food, and, despite the large differences in macronutrient composition and sugar content, the BD did not include large quantities of added or liquid sugars. In that regard, the BD may have differed somewhat from the customary diets of these subjects. Sample menus for 1 d of each diet are included in the Supplemental Materials.
The energy intake was determined weekly for each subject during the initial BD period by using the average EEchamber for the previous week and rounding upward to the nearest 50 kcal. Energy intake adjustments were permitted until day −9 of the BD period to match EEchamber, but adjustments after day −15 were unnecessary, and energy intake was subsequently clamped for the remainder of the study.
Table 1 presents the 7-d average diet compositions during the isocaloric BD and KD periods. The macronutrient proportions determined by chemical analyses were consistent with the values calculated by the nutrient analysis software used to design the diets. The software allowed for a more detailed breakdown of the diet composition and showed that the BD derived ∼25% of total calories from sugars, whereas the KD had <2% from sugars.
All subjects were confined to the metabolic ward throughout the study with no access to outside food. Subjects knew that it was imperative that they eat all of the food provided. If they were not able to eat a study food, they were instructed to notify the study dietitian immediately so that other arrangements could be considered. Dietitians and health technicians met with the subjects regularly to discuss the diet and assess compliance. Visitors were allowed to meet with study subjects in a common area under observation of the nursing and/or research staff to avoid the exchange of food or beverages. Meals were consumed in a common area under observation of the research staff, and participants were not allowed to leave the table during the meals. All meal trays were checked after consumption, and any food that was not consumed was weighed and subsequent meals were adjusted for previously uneaten food.
EE via metabolic chamber
All chamber measurement periods were >23 h, and we extrapolated the data to represent 24-h periods by assuming that the mean of the measured periods was representative of the 24-h period. See the Supplemental Materials for a detailed derivation of the indirect calorimetry equations and their parameterization (Supplemental Table 1) for both the BD and KD periods.
During the BD period, the EE was calculated as shown in Equation 1:
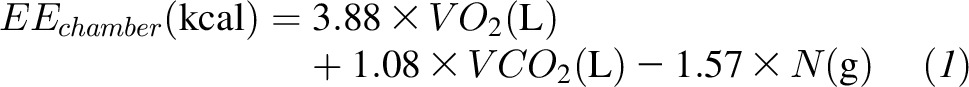 |
where VO2 and VCO2 were the volumes of oxygen consumed and carbon dioxide produced, respectively, and N was the 24-h urinary nitrogen excretion measured by chemiluminescence (Antek MultiTek Analyzer; PAC).
Indirect calorimetry calculations are affected by the end products of protein oxidation (10). Interestingly, unlike prolonged fasting, in which the ratio of nitrogen contained in urea plus creatinine to ammonia can decrease substantially from the standard ratio of 95:5, neither the BD (P = 0.08) nor the KD (P = 0.85) deviated significantly from this standard ratio.
During the KD period, the equations were adjusted as shown in Equation 2 to account for urinary ketone excretion, Kexcr:
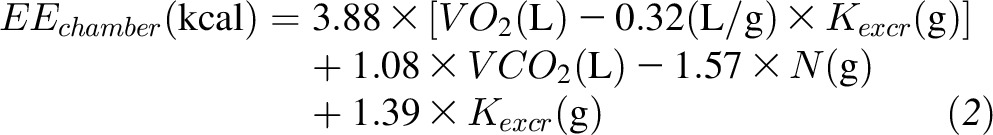 |
SEE was determined by the lowest EE over a continuous 180-min period between the hours of 0000 and 0600 (11). Other components of EE, including energy cost of cycling exercise at a clamped intensity (EEexercise), EE when not moving (EEsedentary), physical activity expenditure on days inside the metabolic chamber (PAEchamber), spontaneous physical activity inside the metabolic chamber (SPA), and awake and fed thermogenesis (AFT), were defined as described in the Supplemental Materials and Supplemental Figures 1 and 2. The procedures used to adjust the primary EE data for measured changes in body weight and composition are also described in the Supplemental Materials.
EE via doubly labeled water
Subjects drank from a stock solution of 2H2O and H218O water in which 1 g 2H2O (99.99% enrichment) was mixed with 19 g H218O (10% enrichment). An aliquot of the stock solution was saved for dilution to be analyzed along with each set of urine samples. The water was weighed to the nearest 0.1 g into the dosing container. The prescribed dose was 1.0 g/kg body weight, and the actual dose amounts were entered in the dose log. Spot urine samples were collected daily. Isotopic enrichments of urine samples were measured by isotope ratio mass spectrometry. The average CO2 production rate (rCO2) can be estimated from the rate constants describing the exponential disappearance of the labeled 18O and D water isotopes (kO and kD) in repeated spot urine samples collected over several days and were corrected for previous isotope doses. We used the parameters of Racette et al. (12) with the weighted dilution space calculation, Rdil, proposed by Speakman (13) as shown in Equation 3:
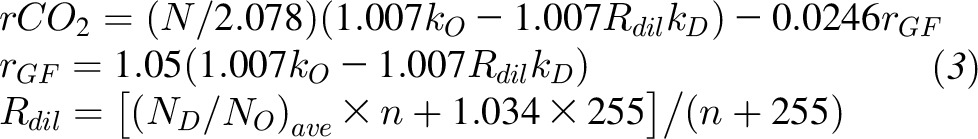 |
where (ND / NO)ave is the mean of the ND / NO values from the n subjects and rGF is the rate of water loss through gaseous routes subject to isotope fractionation.
The Supplemental Materials provide a detailed derivation of the equations used to determine the average total EE (EEDLW) from the doubly labeled water measurement of rCO2. During the BD period, EEDLW was calculated as shown in Equation 4:
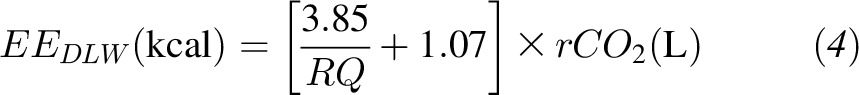 |
where the RQ was calculated as the average 24-h RQ measured during the metabolic chamber days. During the KD period, EEDLW was calculated as shown in Equation 5:
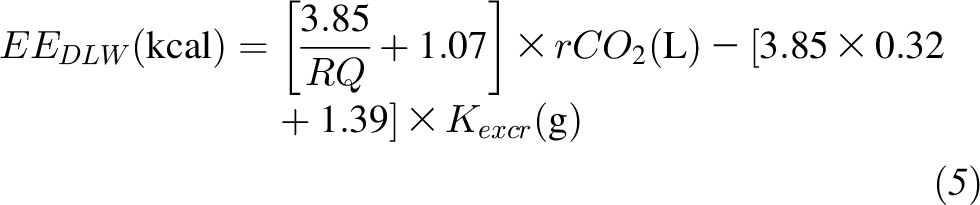 |
PAEchamber is known to be less than physical activity expenditure on days outside the metabolic chamber (PAEnonchamber)(14) and presumably accounts for the main difference between EEDLW and EEchamber. We quantified the physical activity EE on nonchamber days as shown in Equation 6:
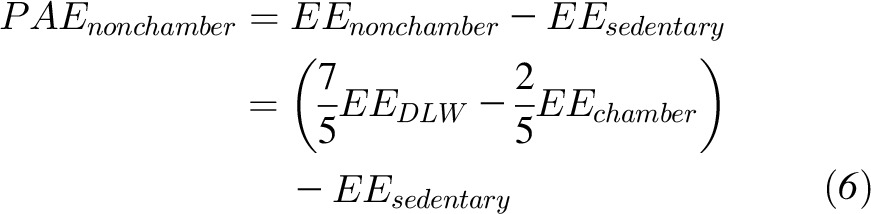 |
where EEsedentary was calculated as the average daily chamber value over the corresponding period.
Anthropometry and body composition
Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with subjects wearing a hospital gown and undergarments and after an overnight fast. Body fat was measured by using dual-energy X-ray absorptiometry (DXA) scanners (Lunar iDXA; GE Healthcare).
Blood and urine measurements
See Supplemental Table 2 for the description of the methods and assay statistics for the various blood and urine measurements. Because acetoacetate could be lost from the urine as a result of non-enzymatic decarboxylation to acetone, it is possible that ketone excretion may have been underestimated because of acetone evaporation from the 24-h urine collection containers. If a substantial amount of acetoacetate had been lost from the urine samples, then the acetoacetate fraction of total ketones would be decreased in urine compared with the blood. This did not happen. Rather, the urine acetoacetate fraction was 62% ± 3%, which was slightly higher than the blood fraction, which was 50% ± 2% (P = 0.004). Furthermore, the measured urinary ketone excretion was commensurate with the circulating ketone concentrations (15), suggesting that the measured total ketone excretion was not severely affected by loss of acetoacetate.
Statistical analyses
This study was powered to detect a change in EEchamber ≥150 kcal/d between the BD and KD periods in 16 subjects by using an endpoint analysis with probability (power) of 0.93 assuming a 120-kcal/d SD in EEchamber (16) and a type I error probability of 0.05. We chose the 150-kcal/d effect size because this was the smallest change in EEchamber that was considered physiologically important.
Statistical analyses were performed with the use of SAS version 9.3 (SAS Institute Inc.). The data tables present least squares means ± SEs and were analyzed by using a repeated-measures mixed model with a covariance structure of compound symmetry (PROC MIXED; SAS). We also tested a first-order autoregressive covariance pattern, and the results were unchanged. Study site was not a significant determinant when included in the mixed model, and its inclusion had no significant effect on the primary endpoints. Data are therefore reported without adjusting for study site. Missing data were not imputed because the repeated-measures mixed-model procedure is robust to data missing at random (17). The figures depict means ± 95% CIs at each time point, and 2-sided t tests were used to compare changes with respect to the day 0 BD. Outliers were identified by Cook’s distance with a cutoff of 4/n, where n is the number of observations. Outlier data points were excluded from the analyses and treated as missing data. Statistical significance was declared at P < 0.05 for 2-sided tests, and comparisons depicted in the figures were Bonferroni-adjusted for multiple comparisons.
RESULTS
Subjects and compliance
Seventeen men (10 black or African American, 5 white, 1 Asian, and 1 Hispanic) successfully completed the screening phase of the study (see Supplemental Figure 3 for the study flow diagram). The subjects were mean ± SE age 33 ± 1.8 y, weighed 87.4 ± 3.7 kg, and had a BMI of 28.8 ± 0.8, and their percent body fat measured on day −15 was 28.9% ± 1.1%.
The diets were well tolerated, and all of the study food was consumed at each meal. Compliance with the daily prescribed cycling exercise was excellent in all but 3 subjects whose accelerometry data indicated that they sometimes failed to perform the exercise on non-chamber days.
Body weight and composition changes
The subjects lost 0.8 ± 0.2 kg (P = 0.002) of body weight over the last 15 d of the BD period (Figure 2A) with 0.5 ± 0.1 kg (P = 0.005) of this unintentional weight loss coming from body fat (Figure 2B). One subject had a body fat mass measurement at day 0 that was >2 kg less than the measurement at day −15, despite losing <0.5 kg of body weight. This was a clear outlier [Cook’s distance = 0.1 > 4/(17 subjects × 4 observations/subject) = 0.06], and the subject was excluded from the analysis of fat mass changes.
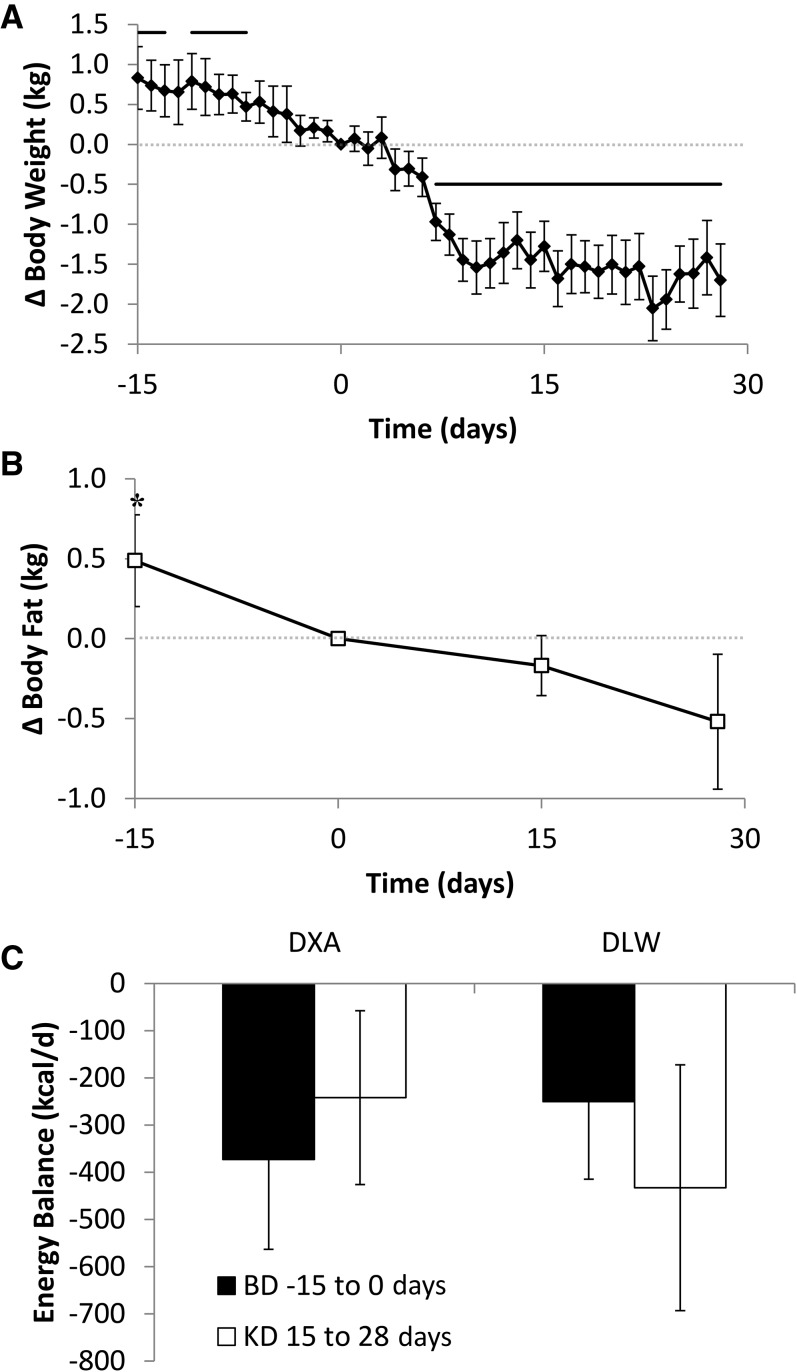
FIGURE 2.
Body composition and overall energy balance. Subjects experienced an unintentional loss of (A) body weight (◆) and (B) fat mass (□) throughout the study, indicating an overall state of negative energy balance. Weight loss was accelerated during the first 2 wk of the KD, but the rate of body fat loss slowed during this period. During the final 2 wk of the KD, both the rates of body weight and fat loss were similar to baseline. (C) The calculated negative energy balance during the last 2 wk of the baseline period (BD −15 to 0 d) and the KD period (15–28 d) was not significantly different whether assessed by the measured body composition changes (DXA; n = 16) or by calculating energy intake minus expenditure as measured by DLW (n = 16). Horizontal solid lines indicate significant weight changes from the final BD day, P < 0.0012 as assessed by a paired, 2-sided t test and Bonferroni adjusted for 43 comparisons. *Significant change in fat mass from final BD day, P < 0.017 as assessed by a paired, 2-sided t test and Bonferroni adjusted for 3 comparisons. Means ± 95% CIs are presented. BD, high-carbohydrate baseline diet; DLW, doubly labeled water; DXA, dual-energy X-ray absorptiometry; KD, low-carbohydrate ketogenic diet.
The body weight and composition changes indicated an overall state of negative energy balance that was calculated to be −373 ± 97 kcal/d (P = 0.002) by using standard coefficients for the energy densities of body fat and fat-free mass (18) as shown in Figure 2C. Introduction of the KD was followed by a rapid additional 1.6 ± 0.2 kg of weight loss (P < 0.0001), likely primarily the result of body water loss because fat mass decreased by only 0.2 ± 0.1 kg (P = 0.09) over the next 15 d. Over the entire 28-d KD period, the total weight lost was 2.2 ± 0.3 kg (P < 0.0001), with 0.5 ± 0.2 kg (P = 0.03) from loss of body fat. The energy imbalance during the last 2 wk of the KD was calculated to be −242 ± 94 kcal/d (P = 0.02; Figure 2C) and was not significantly different from the last 2 wk of the BD period (P = 0.33).
EE and RQ
Because of chamber malfunctions, 1 subject had missing chamber data on days −8 and −7, and 2 other subjects had missing chamber data on days 11 and 12. All other data points from these subjects were retained in the analyses. EEDLW from one subject was a clear outlier [Cook’s distance = 0.4 > 4/(17 subjects × 2 observations/subject) = 0.29] such that, despite gaining 0.2 kg of weight during the KD period, EEDLW was >1000 kcal/d greater than both energy intake and the EEDLW measured during BD. This was not the same subject who was an outlier for the body fat mass changes described above.
Table 2 shows that EEchamber during the last 2 wk of the BD period was 2619 ± 93 kcal/d, which was slightly less than energy intake (2739 ± 108 kcal/d; P < 0.0001). EEDLW during the last 2 wk of the BD period (including the 4 d spent in the metabolic chamber) was 2995 ± 45 kcal/d, which was significantly greater than both EEchamber (P = 0.0005) and energy intake (P = 0.005). Therefore, the doubly labeled water data confirmed a state of overall negative energy balance of −251 ± 84 kcal/d (Figure 2C) and was not significantly different from the value calculated by using the body composition changes (P = 0.42). The negative energy balance was likely due to increased physical activity on the nonchamber days as indicated by a 21% ± 4% increase in hip accelerometer counts during the nonchamber days (P = 0.0002).
TABLE 2.
Energy intake, energy expenditure, and RQ during the BD and KD periods1
| BD (n = 17) | KD (n = 17) | P2 | |
| Carbohydrate intake, g/d | 338 ± 5.1 | 35.8 ± 4.7 | <0.0001 |
| Fat intake, g/d | 108 ± 8.2 | 242 ± 8.2 | <0.0001 |
| Protein intake, g/d | 104 ± 4.1 | 104 ± 4.1 | 0.6285 |
| Energy intake, kcal/d | 2739 ± 108 | 2738 ± 108 | 0.626 |
| EEchamber, kcal/d | 2619 ± 93 | 2676 ± 93 | 0.0004 |
| SEE, kcal/d | 1569 ± 63 | 1658 ± 63 | <0.0001 |
| 24-h RQ | 0.879 ± 0.0066 | 0.778 ± 0.006 | <0.0001 |
| BW adj EEchamber, kcal/d | 2615 ± 62 | 2703 ± 62 | <0.0001 |
| BW adj SEE, kcal/d | 1566 ± 34 | 1677 ± 33 | <0.0001 |
| DXA adj EEchamber,3 kcal/d | 2617 ± 60 | 2713 ± 59 | <0.0001 |
| DXA adj SEE,3 kcal/d | 1567 ± 30 | 1688 ± 30 | <0.0001 |
| EEexercise, kcal/min | 3.12 ± 0.26 | 3.04 ± 0.25 | 0.1506 |
| SPA, kcal/min | 0.2241 ± 0.017 | 0.1963 ± 0.017 | 0.0102 |
| AFT, kcal/min | 0.254 ± 0.019 | 0.248 ± 0.018 | 0.6154 |
| EEsedentary, kcal/min | 1.34 ± 0.055 | 1.4 ± 0.054 | <0.0001 |
| EEDLW,3 kcal/d | 2995 ± 45 | 3146 ± 45 | 0.0309 |
| PAEchamber, kcal/d | 700 ± 20 | 636 ± 20 | 0.0356 |
| PAEnonchamber,3 kcal/d | 1221 ± 66 | 1347 ± 66 | 0.1957 |
All values are least squares mean ± SEs. The data were analyzed by using a repeated-measures mixed model. AFT, awake and fed thermogenesis; BW adj, adjusted for measured changes in body weight; DXA adj, adjusted for measured changes in body composition; EEchamber, total daily energy expenditure measured during repeated stays in metabolic chambers; EEDLW, average energy expenditure measured by doubly labeled water; EEexercise, energy cost of cycling exercise at a clamped intensity; EEsedentary, energy expenditure when not moving; PAEchamber, physical activity expenditure on days inside the chamber; PAEnonchamber, physical activity expenditure on days outside the chamber; SEE, sleeping energy expenditure; SPA, spontaneous physical activity inside the metabolic chamber; 24-h RQ, daily respiratory quotient.
Values refer to the effects of the diet period and were not corrected for multiple comparisons.
n = 16 as a result of 1 outlier removed as described in the text.
Table 2 shows that EEchamber during the KD phase was 2676 ± 93 kcal/d and was 57 ± 13 kcal/d greater than during the baseline period (P = 0.0004). Because EE is known to be proportional to body weight and even more strongly related to fat-free mass (19), we adjusted EEchamber for the observed weight and body composition changes as described in the Supplemental Materials. The weight-adjusted EEchamber was 88 ± 13 kcal/d greater during the KD period than during the BD period (Table 2; P < 0.0001). Adjusting the EEchamber data for body composition changes resulted in the KD period having 96 ± 12 kcal/d greater expenditure than the BD period (Table 2; P < 0.0001).
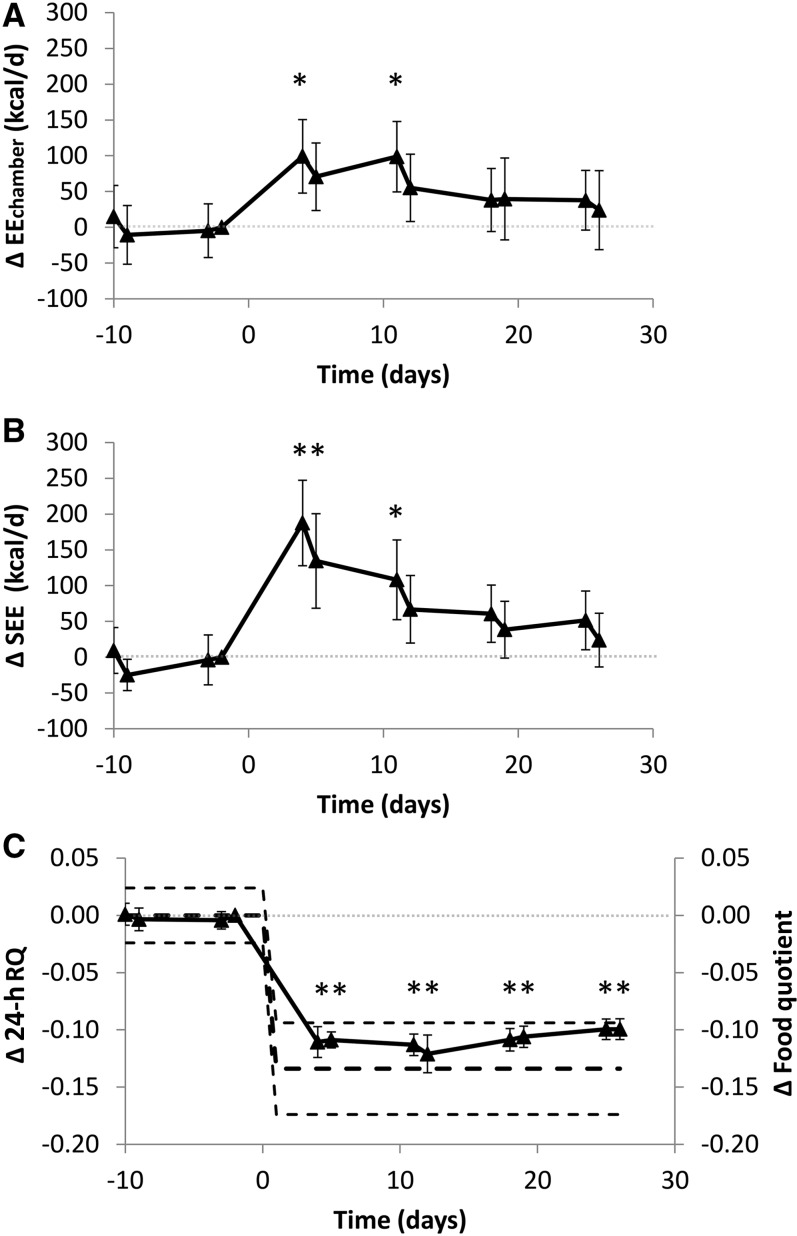
The time course of the unadjusted EEchamber changes is depicted in Figure 3A, illustrating that there was no significant linear trend over time during the BD period (P = 0.76), and introduction of the KD coincided with an increase in EE by ∼100 kcal/d in the first week, after which there was a significant linear decrease over time (P = 0.002). The waning of EEchamber during the KD persisted after adjustment for changes in body weight (P = 0.027) and body composition (P = 0.021).
FIGURE 3.
Changes in total daily energy expenditure, SEE, and RQ. (A) EEchamber significantly increased within the first week of the KD compared with that of the final BD day, but subsequently there was a significant decreasing linear trend (P = 0.002). (B) SEE rapidly increased during the KD, but subsequently there was a significant decreasing linear trend (P < 0.0001). (C) Daily RQ (24-h RQ; n = 17) decreased within the first week of the KD and stayed significantly below that of BD throughout the remainder of the study and was close to the calculated mean change in food quotient (thick dashed black line) and within its 95% CI (thin dashed black lines). Means ± 95% CIs are presented, n = 17. *Significant change from the final BD day, P < 0.0045 as assessed by a paired, 2-sided t test and Bonferroni adjusted for 11 comparisons. Note that the statistical analyses presented in the main text and Table 2 used a repeated-measures mixed model rather than pairwise Bonferroni-adjusted comparisons with the final BD day. BD, high-carbohydrate baseline diet; EEchamber, total daily energy expenditure measured during repeated stays in metabolic chambers; KD, low-carbohydrate ketogenic diet; RQ, respiratory quotient; SEE, sleeping energy expenditure.
SEE extrapolated to the entire day was 1569 ± 63 kcal/d during the baseline period and significantly increased by 89 ± 14 kcal/d during the KD (Table 2; P < 0.0001). Adjusting the SEE data for the measured body weight changes (see Supplemental Materials) resulted in a total increase of 111 ± 13 kcal/d (Table 2; P < 0.0001) during the KD relative to the BD. Adjusting for body composition changes resulted in a total SEE increase of 121 ± 13 kcal/d (Table 2; P < 0.0001) during the KD.
Figure 3B shows the time course of the unadjusted SEE changes, which were stable during the BD period (P = 0.93 testing for a linear trend in time), significantly increased by ∼200 kcal/d within the first week of the KD, and then waned linearly over time (P < 0.0001). The decline of SEE during the KD persisted after adjustment for changes in body weight (P < 0.0001) and body composition (P < 0.0001).
We also explored other components of EE measured during the chamber stays as described in the Supplemental Materials, including the EEexercise, EEsedentary, SPA, PAEchamber, and AFT, which is the thermic effect of food plus the energy expended above SEE as a result of being awake (20). Table 2 shows that PAEchamber and SPA decreased during the KD, whereas EEexercise and AFT were unchanged and EEsedentary increased.
Table 2 and Figure 3C show that the 24-h RQ decreased significantly from 0.879 ± 0.007 during the BD period to 0.775 ± 0.006 at the start of the KD period (P < 0.0001) and remained approximately constant until the end of the study, indicating a rapid and persistent increase in fat oxidation. The changes in 24-h RQ were similar to the changes in daily food quotient calculated by chemical analysis of the diet.
EEDLW was 3146 ± 45 kcal/d during the KD, which was 151 ± 63 kcal/d greater than during the BD period (Table 2; P = 0.03). Although this apparent increase in EEDLW during the KD was likely accounted for by an increase in physical activity on days outside the metabolic chamber, Table 2 shows that PAEnonchamber did not significantly increase during the KD compared with during the BD (126 ± 93 kcal/d; P = 0.2). Physical activity expenditure was 514 ± 107 kcal/d greater on the days outside the chamber during the baseline diet (P = 0.0002) and was 696 ± 172 kcal/d greater during the KD (P = 0.0011). However, there was no significant difference between the diets (P = 0.083).
The energy imbalance calculated by subtracting EEDLW from energy intake was not significantly different from that calculated by using the body composition changes during either the BD (P = 0.42) or the KD (P = 0.29) (Figure 2C). However, the calculated energy imbalance measured by using the doubly labeled water method was 182 ± 66 kcal/d more negative during the KD period than during the BD (P = 0.015 for a simple pairwise comparison between diets). However, this difference in energy imbalance between diet periods calculated by DLW should be interpreted with caution since these exploratory endpoints were also subject to multiple comparisons with the DXA determined energy imbalances during both diet periods.
24-h urinary excretion
Daily insulin secretion, as estimated by 24-h urinary C-peptide excretion, rapidly and persistently decreased by 47% ± 3% after the introduction of the KD (Table 3; P < 0.0001). Total urinary ketone excretion increased >10-fold (Table 3; P < 0.0001) but amounted to <15 kcal/d of excreted energy.
TABLE 3.
24-h urinary excretion during the BD and KD periods1
| BD (n = 17) | KD (n = 17) | P2 | |
| C-peptide, nmol/d | 20.9 ± 1.1 | 11.1 ± 1.0 | <0.0001 |
| Ketones, g/d | 0.31 ± 0.35 | 3.48 ± 0.34 | <0.0001 |
| Nitrogen, g/d | 13.2 ± 0.59 | 14.7 ± 0.56 | 0.0008 |
| Urea, g/d | 23.6 ± 1.44 | 28.2 ± 1.37 | 0.0001 |
| Ammonia, g/d | 0.33 ± 0.12 | 1.02 ± 0.12 | <0.0001 |
| Creatinine, g/d | 1.8 ± 0.083 | 1.79 ± 0.081 | 0.9093 |
| Adrenaline, mg/d | 0.0813 ± 0.0061 | 0.0759 ± 0.0059 | 0.0669 |
| Norepinephrine, mg/d | 0.542 ± 0.043 | 0.448 ± 0.042 | <0.0001 |
All values are least squares means ± SEs. The data were analyzed by using a repeated-measures mixed model. BD, high-carbohydrate baseline diet; KD, low-carbohydrate ketogenic diet.
Values refer to the effects of the diet period and were not corrected for multiple comparisons.
Urinary nitrogen excretion increased by 1.5 ± 0.4 g/d (Table 3; P = 0.0008) during the KD phase and indicated significantly increased protein utilization. The time course of the changes in urinary nitrogen excretion showed that the increased protein utilization occurred within the first week of the KD and persisted until day 11 (not shown). Excretion of both urea and ammonia significantly increased during the KD, whereas creatinine excretion was unchanged (Table 3). Norepinephrine excretion significantly decreased during the KD period and adrenaline excretion also tended to decrease (P = 0.07), indicating decreased sympathetic tone.
Overnight fasted plasma metabolite and hormone concentrations
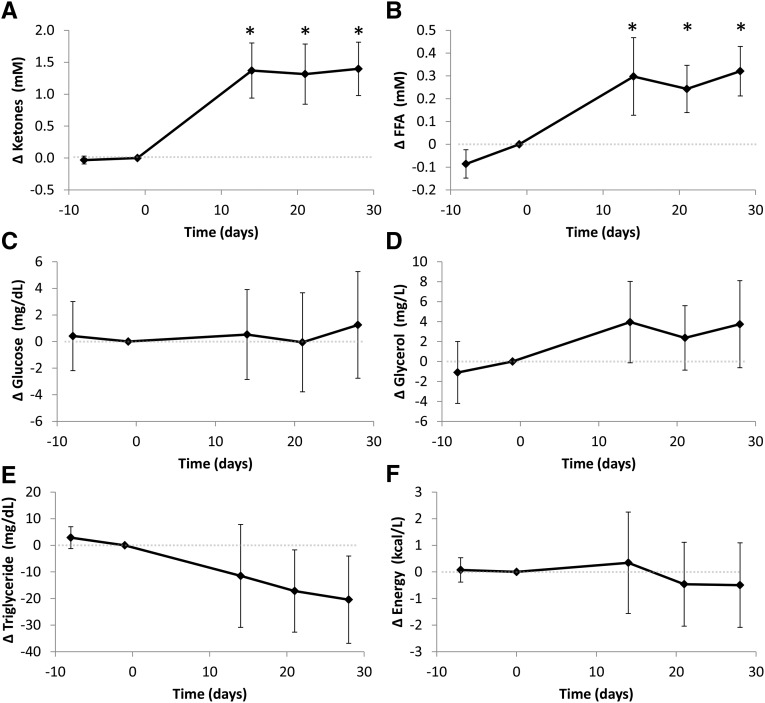
Figure 4 shows the changes in plasma ketones (i.e., the sum of acetoacetate and β-hydroxybutyrate), FFAs, glucose, glycerol, and triglycerides in the overnight fasted state. After the KD, there was an increased availability of circulating ketones and FFAs, a trend towards decreased triglycerides, no significant changes in circulating glucose or glycerol, and no significant change in overall circulating energy. Table 4 presents the overnight fasted plasma concentrations during the BD and KD periods. One subject had plasma insulin and C-peptide values that were abnormally high at the final time point and were clear outliers [Cook’s distance for insulin = 0.29 and Cook’s distance for C-peptide = 0.27, both of which are greater than the threshold 4/(17 subjects × 5 observations/subject) = 0.046]; these data were excluded. As expected, the KD was associated with significant increases in ketones, FFAs, and glycerol. Fasting glucose was not significantly different between the diets, whereas glucagon significantly increased and C-peptide, insulin, and triglycerides significantly decreased during the KD. Leptin was significantly decreased during the KD period. Thyroid-stimulating hormone and free thyroxine concentrations were significantly increased during the KD phase, whereas both free and total tri-iodothyronine were significantly decreased.
FIGURE 4.
Changes in circulating plasma fuel sources in the overnight fasted state. Plasma ketones (calculated as the sum of acetoacetate and beta hydroxybutyrate) (A) and FFAs (B) both significantly increased during the KD compared with those in the final BD day, whereas glucose (C) and glycerol (D) were unchanged from baseline. Plasma triglycerides (E) tended to decrease during the KD, and the overall circulating energy (F) was unchanged. Means ± 95% CIs are presented, n = 17. *Significant change from the final BD day, P < 0.0125 as assessed by a paired, 2-sided t test and Bonferroni adjusted for 4 comparisons. Note that the statistical analyses presented in the main text and Table 4 used a repeated-measures mixed-model rather than pairwise Bonferroni-adjusted comparisons with the final BD day. BD, high-carbohydrate baseline diet; FFA, free fatty acid; KD, low-carbohydrate ketogenic diet.
TABLE 4.
Overnight fasted plasma concentrations measured during the BD and KD periods1
| BD (n = 17) | KD (n = 17) | P2 | |
| Acetoacetate, mmol/L | 0.108 ± 0.075 | 0.781 ± 0.069 | <0.0001 |
| BHB, mmol/L | 0.103 ± 0.071 | 0.758 ± 0.066 | <0.0001 |
| FFAs, mmol/L | 0.479 ± 0.035 | 0.803 ± 0.029 | <0.0001 |
| Glycerol, mg/L | 7.21 ± 0.78 | 11.1 ± 0.66 | 0.0006 |
| Glucose, mg/dL | 81.4 ± 1.5 | 81.9 ± 1.4 | 0.664 |
| Glucagon, pg/mL | 93.6 ± 8.9 | 126 ± 8.6 | <0.0001 |
| C-peptide,3 ng/mL | 1.47 ± 0.11 | 1.15 ± 0.11 | <0.0001 |
| Insulin,3 μU/mL | 7.92 ± 0.93 | 6.27 ± 0.9 | 0.0039 |
| Triglyceride, mg/dL | 104 ± 6.4 | 85.4 ± 6 | 0.001 |
| Leptin, ng/mL | 8.94 ± 1.1 | 7.13 ± 1.1 | <0.0001 |
| TSH, μIU/mL | 1.84 ± 0.19 | 1.98 ± 0.18 | 0.0448 |
| Free thyroxine, ng/dL | 1.18 ± 0.033 | 1.32 ± 0.032 | <0.0001 |
| Total thyroxine, μg/dL | 6.56 ± 0.3 | 6.76 ± 0.3 | 0.109 |
| Free tri-iodothyronine, pg/mL | 2.85 ± 0.097 | 2.5 ± 0.094 | <0.0001 |
| Total tri-iodothyronine, ng/dL | 95.1 ± 3.6 | 75.7 ± 3.4 | <0.0001 |
All values are least squares means ± SEs. The data were analyzed by using a repeated-measures mixed model. BD, high-carbohydrate baseline diet; BHB, β-hydroxybutyrate; FFA, free fatty acid; KD, low-carbohydrate ketogenic diet; TSH, thyroid-stimulating hormone.
Values refer to the effects of the diet period and were not corrected for multiple comparisons.
n = 16 for the only final time point during the KD as a result of a single outlier described in the main text.
DISCUSSION
This study demonstrated that transitioning from the BD to the KD coincided with a substantial decrease in daily insulin secretion and 24-h RQ, increased circulating FFA and ketones, and marginal increases in EEchamber and SEE. These data, although somewhat confounded by ongoing weight loss, suggest that large isocaloric changes in the proportion of dietary carbohydrate to fat transiently increase EE by only ∼100 kcal/d after adjusting for body weight and composition. Furthermore, the body weight and composition adjustments likely overestimated the EE changes during the KD because much of the weight loss was likely from water rather than loss of metabolically active tissues.
Our study adds to the literature addressing the perennial question: is a calorie a calorie? A conventional view is that the proportion of carbohydrate to fat in the diet has a physiologically negligible effect on EE when dietary protein and energy intake are held constant (8). In contrast, the carbohydrate–insulin model predicts that the KD would lead to increased EE, thereby resulting in a metabolic advantage amounting to ∼300–600 kcal/d (21, 22). Our data do not support EE increases of that magnitude.
Several controlled feeding studies have demonstrated significant differences in EE between isocaloric diets with differences in dietary protein (23–25). Unless accompanied by an increase in dietary protein (22, 26), carbohydrate restriction has not previously been observed to increase EE. Rather, studies that use clamped dietary protein and varying carbohydrates from 20% to 75% of total calories have found either small decreases in EE with lower-carbohydrate diets (16, 27–30) or no statistically significant differences (22, 24, 31–38). Mathematical model simulations predicted that cutting dietary carbohydrates to very low amounts would reverse this trend and lead to slightly increased EE (16). This prediction appears to have been borne out in our data, where we observed small increases in SEE, EEchamber, and EEDLW with a KD in which dietary carbohydrate was cut to 5% and protein was clamped at 15% of total calories.
The rapid increase in SEE and EEchamber within the first week of the KD may have been caused by increased hepatic oxygen consumption proportional to the rate of ketogenesis (39). For ketogenesis to fully explain the observed early ∼200-kcal/d increase in SEE requires ∼150 g/d of ketogenesis (16), which is commensurate with both the observed circulating ketone concentrations as well as the urinary excretion rate, and implies a rate of ketogenesis approximately half of that achieved within 1 wk of fasting when ketogenesis reaches a maximum (15). The KD likely also increased the flux through the energy-requiring gluconeogenic pathway as well as the triglyceride fatty acid cycle, both of which would be expected to slightly increase EE (26, 40). EE may have decreased subsequently as gluconeogenesis declined with the brain shifting away from glucose toward ketone oxidation (15, 41, 42). Decreased insulin secretion per se may also result in an adaptive suppression of EE (43). Furthermore, the overall state of negative energy balance, decreased circulating concentrations of thyroid hormones, and decreased 24-h catecholamine secretion all favor decreased EE.
Although the primary EE endpoints determined by the metabolic chamber indicated small transient increases during the KD period amounting to <100 kcal/d, the exploratory EEDLW endpoint increased by ∼150 kcal/d. Because physical activity typically increases on days outside the metabolic chamber (14), EEDLW was greater than EEchamber, and any additional increment in EE detected by EEDLW during the KD must be explained by differences in PAEnonchamber. Indeed, PAEnonchamber during the KD increased by ∼130 kcal/d compared with BD, but this was not statistically significant. Nevertheless, we cannot rule out the possibility that a KD might increase physical activity in free-living subjects and that we failed to observe this effect because the subjects’ physical activities were limited by the metabolic ward setting.
The carbohydrate–insulin model predicts a greater rate of body fat loss during the KD period. Our data do not support this prediction because body fat loss slowed on transition to the KD, possibly because of augmented utilization of body protein, as indicated by the increased urinary nitrogen excretion that persisted until day 11 of the KD period. The rate of fat loss during the final 2 wk of the KD was similar to that of the baseline period. We suspect that the increased dietary fat resulted in elevated circulating postprandial triglyceride concentrations throughout the day, which may have stimulated adipose tissue fat uptake (44) and/or inhibited adipocyte lipolysis (45, 46). These mechanistic questions deserve further study, but it is clear that regulation of adipose tissue fat storage is multifaceted and that insulin does not always play a predominant role (16).
A major limitation of our study is the unintentional weight loss. Despite slight positive energy balance during the chamber days, the overall negative energy balance amounted to ∼300 kcal/d and was likely due to greater spontaneous physical activity on nonchamber days. This occurred despite confining the subjects to metabolic wards and our best efforts to maintain constant activity levels by prescribing 90 min of fixed intensity stationary cycling exercise every day. Nevertheless, similar to a previous metabolic ward study (14), physical activity on nonchamber days was substantially greater than on chamber days.
In addition to the lack of weight maintenance, there are other limitations of our study. We did not measure fecal energy content, which may have differed between the diets. We did not include a control group that did not receive the KD or a group that had the diets delivered in the reverse order. Therefore, although the timing of the observed changes in EEchamber, SEE, and 24-h RQ are highly suggestive, we cannot definitively claim that the KD per se was the cause. Nor do our observations necessarily translate to women or men classified as normal weight or underweight or having class II obesity or above.
In summary, we found that a carefully controlled isocaloric KD coincided with small increases in EE that waned over time. Despite rapid, substantial, and persistent reductions in daily insulin secretion and RQ after introducing the KD, we observed a slowing of body fat loss. Therefore, our data do not support the carbohydrate–insulin model predictions of physiologically relevant increases in EE or greater body fat loss in response to an isocaloric KD. However, it is possible that dietary carbohydrate restriction might result in decreased ad libitum energy intake—a prediction of the carbohydrate-insulin model that was not tested in the current study but deserves further investigation.
Acknowledgments
We thank Jon Moon at MEI Research for his assistance in coordinating the calibration of metabolic chambers with the Biomedical Engineers at each of the study sites: Chris Bock, Robert Brychta, Jeremy Huff, and Russell Rising. Jeff Volek and Brittanie Volk from Beyond Nutrition Inc. helped design the study diets in collaboration with the investigators and the study dietitians: Courtney Brock, Amber Courville, Wahida Karmally, Pamela Legowski, Renee Puyau, and Janet Schebendach. Serge Cremers, Mary Walter, and Peter Walter assayed the blood and urine samples. Crystal Brown, Emma Crayner, Lilian Howard, Karen Jones, Elinor Naor, Stacy Shankleton, Monica Skarulis, Celeste Waguespack, and Laura Yannai were study coordinators and research support staff. We thank Dympna Gallagher for her assistance with body composition measurements and Dale Schoeller and Jennifer Rood for their assistance with the doubly labeled water analyses.
The authors’ responsibilities were as follows—KDH, KYC, YYL, RLL, LESM, MLR, MR, SRS, BTW, and ER: designed the study and conducted the research; KDH and JG: analyzed the data; KDH, KYC, RLL, LESM, MLR, MR, SRS, BTW, and ER: wrote the manuscript; and KDH had primary responsibility for the final content. The authors declared no conflicts of interest. Nutrition Sciences Initiative (NuSI) convened the research team, helped formulate the hypothesis, and provided partial funding. NuSI and its scientific advisors were given the opportunity to comment on the study design and the manuscript, but the investigators retained full editorial control.
Footnotes
Abbreviations used: AFT, awake and fed thermogenesis; BD, high-carbohydrate baseline diet; DXA, dual-energy X-ray absorptiometry; EE, energy expenditure; EEchamber, total daily energy expenditure measured during repeated stays in metabolic chambers; EEDLW, average energy expenditure measured by doubly labeled water; EEexercise, energy cost of cycling exercise at a clamped intensity; EEsedentary, energy expenditure when not moving; FFA, free fatty acid; KD, low-carbohydrate ketogenic diet; PAEchamber, physical activity expenditure on days inside the metabolic chamber; PAEnonchamber, physical activity expenditure on days outside the metabolic chamber; rCO2, CO2 production rate; RQ, respiratory quotient; SEE, sleeping energy expenditure; SPA, spontaneous physical activity inside the metabolic chamber.
REFERENCES
- 1.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA 2014;311:2167–8. [DOI] [PubMed] [Google Scholar]
- 2.Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab 2006;2:447–58. [DOI] [PubMed] [Google Scholar]
- 3.Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ 2013;346:f1050. [DOI] [PubMed] [Google Scholar]
- 4.Wells JC, Siervo M. Obesity and energy balance: is the tail wagging the dog? Eur J Clin Nutr 2011;65:1173–89. [DOI] [PubMed] [Google Scholar]
- 5.Astwood EB. The heritage of corpulence. Endocrinology 1962;71:337–41. [DOI] [PubMed] [Google Scholar]
- 6.Pennington AW. Obesity. Med Times 1952;80:389–98. [PubMed] [Google Scholar]
- 7.Pennington AW. A reorientation on obesity. N Engl J Med 1953;248:959–64. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz AC, Schoeller DA. Is a calorie a calorie? Am J Clin Nutr 2004;79:899S–906S. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85:1023–30. [DOI] [PubMed] [Google Scholar]
- 10.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 1988;47:608–28. [DOI] [PubMed] [Google Scholar]
- 11.Schoffelen PF, Westerterp KR. Intra-individual variability and adaptation of overnight- and sleeping metabolic rate. Physiol Behav 2008;94:158–63. [DOI] [PubMed] [Google Scholar]
- 12.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol 1994;267:E585–90. [DOI] [PubMed] [Google Scholar]
- 13.Speakman JR. Doubly labelled water: theory and practice. London: Chapman & Hall; 1997. [Google Scholar]
- 14.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991;53:1368–71. [DOI] [PubMed] [Google Scholar]
- 15.Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 1989;5:247–70. [DOI] [PubMed] [Google Scholar]
- 16.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, Goodwin S, Guo J, Howard L, Knuth ND, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab 2015;22:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004;61:310–7. [DOI] [PubMed] [Google Scholar]
- 18.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 2006;61:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes 2013;62:4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012;307:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab 2013;98:2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:1281–98. [DOI] [PubMed] [Google Scholar]
- 26.Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am J Clin Nutr 2009;90:519–26. [DOI] [PubMed] [Google Scholar]
- 27.Astrup A, Buemann B, Christensen NJ, Toubro S. Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am J Physiol 1994;266:E592–9. [DOI] [PubMed] [Google Scholar]
- 28.Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, Jequier E, Tappy L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord 2000;24:1413–8. [DOI] [PubMed] [Google Scholar]
- 29.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr 1995;62:19–29. [DOI] [PubMed] [Google Scholar]
- 30.Shepard TY, Weil KM, Sharp TA, Grunwald GK, Bell ML, Hill JO, Eckel RH. Occasional physical inactivity combined with a high-fat diet may be important in the development and maintenance of obesity in human subjects. Am J Clin Nutr 2001;73:703–8. [DOI] [PubMed] [Google Scholar]
- 31.Davy KP, Horton T, Davy BM, Bessessen D, Hill JO. Regulation of macronutrient balance in healthy young and older men. Int J Obes Relat Metab Disord 2001;25:1497–502. [DOI] [PubMed] [Google Scholar]
- 32.Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK, Sharp TA, Francis CC, Hill JO. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr 2006;83:803–8. [DOI] [PubMed] [Google Scholar]
- 33.Hill JO, Peters JC, Reed GW, Schlundt DG, Sharp T, Greene HL. Nutrient balance in humans: effects of diet composition. Am J Clin Nutr 1991;54:10–7. [DOI] [PubMed] [Google Scholar]
- 34.Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr 1991;53:430–6. [DOI] [PubMed] [Google Scholar]
- 35.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr 1997;66:276–82. [DOI] [PubMed] [Google Scholar]
- 36.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood JC, Windhauser MM, Bray GA. Fat and carbohydrate balances during adaptation to a high-fat diet. Am J Clin Nutr 2000;71:450–7. [DOI] [PubMed] [Google Scholar]
- 37.Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF. Metabolic adaptation to high-fat and high-carbohydrate diets in children and adolescents. Am J Clin Nutr 2003;77:479–89. [DOI] [PubMed] [Google Scholar]
- 38.Yerboeket-van de Venne WP, Westerterp KR. Effects of dietary fat and carbohydrate exchange on human energy metabolism. Appetite 1996;26:287–300. [DOI] [PubMed] [Google Scholar]
- 39.Scholz R, Schwabe U, Soboll S. Influence of fatty acids on energy metabolism. 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and oleate in perfused rat liver. Eur J Biochem 1984;141:223-30. [DOI] [PubMed] [Google Scholar]
- 40.Elia M, Zed C, Neale G, Livesey G. The energy cost of triglyceride-fatty acid recycling in nonobese subjects after an overnight fast and four days of starvation. Metabolism 1987;36:251–5. [DOI] [PubMed] [Google Scholar]
- 41.Cahill GF., Jr Starvation in man. N Engl J Med 1970;282:668–75. [DOI] [PubMed] [Google Scholar]
- 42.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 43.Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Gluer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 2015;102:807–19. [DOI] [PubMed] [Google Scholar]
- 44.Sniderman AD, Cianflone K, Summers L, Fielding B, Frayn K. The acylation-stimulating protein pathway and regulation of postprandial metabolism. Proc Nutr Soc 1997;56:703–12. [DOI] [PubMed] [Google Scholar]
- 45.Evans K, Clark ML, Frayn KN. Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol 1999;276:E241–8. [DOI] [PubMed] [Google Scholar]
- 46.Samra JS, Giles SL, Summers LK, Evans RD, Arner P, Humphreys SM, Clark ML, Frayn KN. Peripheral fat metabolism during infusion of an exogenous triacylglycerol emulsion. Int J Obes Relat Metab Disord 1998;22:806–12. [DOI] [PubMed] [Google Scholar]