Abstract
Background: To prevent cognitive impairment, phenylketonuria requires lifelong management of blood phenylalanine (Phe) concentration with a low-Phe diet. The diet restricts intake of Phe from natural proteins in combination with traditional amino acid medical foods (AA-MFs) or glycomacropeptide medical foods (GMP-MFs) that contain primarily intact protein and a small amount of Phe.
Objective: We investigated the efficacy and safety of a low-Phe diet combined with GMP-MFs or AA-MFs providing the same quantity of protein equivalents in free-living subjects with phenylketonuria.
Design: This 2-stage, randomized crossover trial included 30 early-treated phenylketonuria subjects (aged 15–49 y), 20 with classical and 10 with variant phenylketonuria. Subjects consumed, in random order for 3 wk each, their usual low-Phe diet combined with AA-MFs or GMP-MFs. The treatments were separated by a 3-wk washout with AA-MFs. Fasting plasma amino acid profiles, blood Phe concentrations, food records, and neuropsychological tests were obtained.
Results: The frequency of medical food intake was higher with GMP-MFs than with AA-MFs. Subjects rated GMP-MFs as more acceptable than AA-MFs and noted improved gastrointestinal symptoms and less hunger with GMP-MFs. ANCOVA indicated no significant mean ± SE increase in plasma Phe (62 ± 40 μmol/L, P = 0.136), despite a significant increase in Phe intake from GMP-MFs (88 ± 6 mg Phe/d, P = 0.026). AA-MFs decreased plasma Phe (−85 ± 40 μmol/L, P = 0.044) with stable Phe intake. Blood concentrations of Phe across time were not significantly different (AA-MFs = 444 ± 34 μmol/L, GMP-MFs = 497 ± 34 μmol/L), suggesting similar Phe control. Results of the Behavior Rating Inventory of Executive Function were not significantly different.
Conclusions: GMP-MFs provide a safe and acceptable option for the nutritional management of phenylketonuria. The greater acceptability and fewer side effects noted with GMP-MFs than with AA-MFs may enhance dietary adherence for individuals with phenylketonuria. This trial was registered at www.clinicaltrials.gov as NCT01428258.
Keywords: inborn errors of amino acid metabolism, phenylalanine, tyrosine, threonine, medical food, sapropterin dihydrochloride, executive function
INTRODUCTION
Phenylketonuria (Online Mendelian Inheritance in Man 261600 is caused by a deficiency of hepatic phenylalanine hydroxylase activity (PAH8; Enzyme Commission number 1.14.16.1) that catalyzes the conversion of Phe to Tyr in a reaction dependent on the essential PAH cofactor tetrahydrobiopterin (sapropterin dihydrochloride) (1). Tyr is an indispensable amino acid (AA) in phenylketonuria. Normal intake of dietary protein in untreated phenylketonuria causes Phe to accumulate in blood, leading to toxic concentrations of Phe in the brain and the devastating phenotype of profound cognitive impairment (2). The primary therapy for phenylketonuria is lifelong adherence to a low-Phe diet that limits Phe intake from natural foods that contain protein, and traditionally, supplements with AA-based medical foods (AA-MFs) to provide the majority of dietary nitrogen and micronutrients (3, 4). Lifelong compliance with the phenylketonuria diet is poor (5), and there is evidence of suboptimal health outcomes in patients using AA-MFs including neurocognitive impairment (6), skeletal fragility (7–9), and impaired renal function (10). New options are needed to provide an alternative to AA-MFs and improve lifelong compliance with the low-Phe diet for those with phenylketonuria (11, 12).
Glycomacropeptide is uniquely suited to the nutritional management of phenylketonuria because it is the only known dietary protein that in its pure form contains no Phe and can be made into a variety of palatable glycomacropeptide medical foods (GMP-MFs) that are low in Phe and high in protein content (13, 14). Glycomacropeptide, or caseinomacropeptide, is a polar glycophosphopeptide that is isolated from cheese whey and shows prebiotic properties (15). It demonstrates several unique biological properties and is currently used as a food ingredient with applications to infant formula, gastrointestinal health, and obesity, as summarized in reviews (16, 17). Glycomacropeptide is not a complete protein and contains limiting amounts of the following indispensable AAs for phenylketonuria: Arg, His, Leu, Trp, and Tyr. Concentrations of Ile and Thr in glycomacropeptide are 2- to 3-fold greater, respectively, than those found in other dietary proteins (13).
Our preclinical studies in the phenylketonuria mouse model established that glycomacropeptide supplemented with limiting AAs supports growth and reduces concentrations of Phe in plasma and brain (18), improves bone status (8), and attenuates metabolic stress (19) compared with an AA diet. Clinical evaluation of glycomacropeptide in 11 phenylketonuria subjects who participated in an inpatient metabolic study demonstrated safety, acceptability, improved satiety, and greater protein retention with GMP-MFs than with AA-MFs (20, 21), and provided evidence to optimize supplementation of glycomacropeptide with limiting AAs (20). On the basis of these preclinical and clinical investigations conducted during 2004–2009, GMP-MFs first became available in the United States in 2010.
There is a paucity of evidence from controlled clinical studies to support the nutritional management of phenylketonuria with the use of AA-MFs (22). Our objective was to investigate the efficacy and safety of a low-Phe diet combined with either GMP-MFs or AA-MFs providing the same quantity of protein equivalents in free-living subjects with phenylketonuria.
METHODS
Participants
Participants with phenylketonuria were recruited from the Biochemical Genetics Program, Waisman Center, University of Wisconsin-Madison; from the Division of Genetics and Genomics, Boston Children’s Hospital, Harvard Medical School; and by national advertisement within the phenylketonuria community between November 2010 and July 2015. The inclusion criteria for subject participation include the following: 1) individuals with phenylketonuria aged ≥12 y treated shortly after birth with a low-Phe diet; 2) diagnosis with classical or variant phenylketonuria based on plasma Phe concentration of >600 μmol/L; 3) current prescribed diet includes >50% of daily protein needs from AA-MFs; and 4) ability to consume both AA-MFs and GMP-MFs. Potential subjects consuming GMP-MFs before the study were eligible to participate if they returned to AA-MFs for 3 wk before the first study visit to allow for a washout of any effects from consuming GMP-MFs before the study; one subject elected to washout to enroll in the study. Individuals taking sapropterin dihydrochloride, a synthetic form of the tetrahydrobiopterin cofactor for PAH (KUVAN; BioMarin Pharmaceutical Inc.), were eligible to participate if their Phe tolerance was stable and they remained on the same dose of KUVAN throughout the study. Optimal control of plasma Phe concentrations was not required for participation in the study. Exclusion criteria were pregnancy or other concerns deemed to interfere with participation in the study protocol. The University of Wisconsin-Madison Health Sciences Institutional Review Board approved the study protocol, and all subjects provided written informed consent. This trial was registered at www.clinicaltrials.gov as NCT01428258.
Study design and protocol
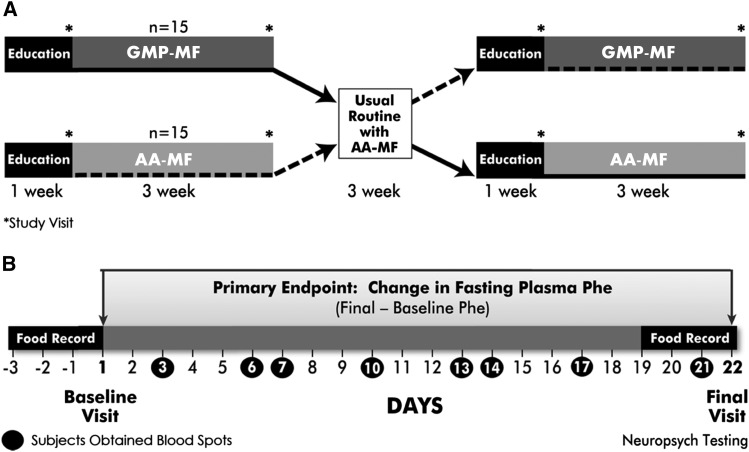
This study was a 2-stage, randomized, crossover intervention trial comparing a low-Phe diet in combination with either AA-MFs or GMP-MFs in 30 free-living subjects with early-treated phenylketonuria. Equal randomization of the diet treatment order was achieved by using a computer-generated scheme. Subjects completed the protocol at either the Waisman Center (n = 19) or Boston Children’s Hospital (n = 11). The 11-wk protocol is shown in Figure 1A, and the timeline for protocol activities is detailed in Figure 1B. The registered dietitian (RD) study coordinator maintained frequent contact with subjects to encourage compliance with the protocol.
FIGURE 1.
Study design and protocol. Thirty subjects with early-treated phenylketonuria completed a 2-stage randomized crossover trial in which they consumed, in random order for 3 wk each, a low-Phe diet combined with GMP-MFs or AA-MFs at home. Each medical food treatment was preceded by 1 wk of education regarding the protocol with a 3-wk washout period between treatments; subjects consumed AA-MFs during these times (A). The protocol for each dietary treatment included 2 study visits with venipuncture to obtain blood samples, one at baseline (day 1) and one at final (day 22), collection of 8 blood spot specimens on filter paper during each 3-wk treatment period, completion of daily medical food logs, 3-d food records, and diet acceptability questionnaires (day 7 and day 21), and neuropsychological testing at the final study visit for each diet (B). AA-MF, amino acid medical food; GMP-MF, glycomacropeptide medical food; Neuropsych, neuropsychological.
Each subject completed 4 study visits, one at the beginning (day 1, baseline) and one at the end of each 3-wk dietary treatment (day 22, final). Each study visit included a physical examination in the Clinical Research Unit by a doctor or nurse practitioner, venipuncture to obtain a fasting blood sample for determination of the plasma AA profile and chemistry panel analyses, and an interview with the study RD to review food records and discuss compliance with the protocol.
Neuropsychological testing to assess executive function, known to be affected by phenylketonuria (23), was conducted by research staff trained in administration of standard psychological assessments under the supervision of a licensed psychologist at the end of each dietary treatment period (visits 2 and 4). The Wechsler Abbreviated Scale of Intelligence test was performed at the first study visit if information regarding intelligence quotient was not available in the medical record. The standardized tests that were used included the Beck Anxiety Inventory (BAI), Behavior Rating Inventory of Executive Function (BRIEF) (24), the Delis-Kaplan Executive Function system, and the Cambridge Neuropsychological Test Automated Battery. Results from the BAI and BRIEF tests are reported; Delis-Kaplan Executive Function and Cambridge Neuropsychological Test Automated Battery demonstrated similar results and are not reported in this article.
Amino acid and glycomacropeptide medical foods
A baseline prescription for AA-MFs was provided for all subjects by their home metabolic clinic based on their typical daily intake of protein equivalents (PEs) from AA-MFs. The mean ± SE prescribed dose was 0.85 ± 0.03 g PEs from AA-MFs · kg body weight−1 · d−1. Each subject was counseled on how to follow their low-Phe diet with emphasis on avoiding high-Phe foods within their daily Phe allowance and consuming sufficient amounts of AA-MFs in ≥3 servings/d. Subjects consumed their preferred AA-MFs resulting in the use of 15 different types of Phe-free AA-MFs as shown in Supplemental Table 1.
The primary change for the glycomacropeptide treatment was to substitute the prescribed daily intake of PEs from AA-MFs with GMP-MFs. The GMP-MFs were donated by Cambrooke Therapeutics from 2010 to 2015 and provided PEs as a proprietary formulation of glycomacropeptide (Glytactin) comprising ∼70% glycomacropeptide (cGMP-20; Arla Foods Ingredients) and 30% supplemental AAs (25). Each subject sampled a variety of low-Phe GMP-MFs during the screening process before enrollment and chose which products to include in their 3-wk glycomacropeptide treatment as summarized in Supplemental Table 1. Because GMP-MFs provide ∼1.8 mg Phe/g PEs, subjects were instructed to reduce their intake of natural foods that contain Phe to offset the Phe intake in GMP-MFs and maintain constant Phe intake. Many of the GMP-MFs provide higher caloric intake than the AA-MFs.
Assessment of nutrient intake from food records and medical food logs
Subject dietary intake was determined by 24-h food records for 3 consecutive days before each study visit in addition to daily documentation of medical food intake by using the medical food logs. The nutrient estimations that were calculated from the food records include total energy and macronutrients (calories, fat, carbohydrate, protein), selected AAs, and micronutrients. Nutrient calculations were performed by an RD skilled in standardized diet entry by using Food Processor SQL (version 10.12.0; ESHA) and reviewed by a second RD experienced in phenylketonuria diet assessment. Additional information regarding the Phe content of processed and restaurant foods was obtained from manufacturers and Virginia Schuett’s Low Protein Food List for PKU (26) and added to the database within Food Processor SQL.
Assessment of dietary compliance
Compliance with the dietary interventions was assessed by using the medical food logs to determine the amount and frequency of intake (number of servings per day) of AA-MFs and GMP-MFs during each 3-wk treatment. The change in plasma Thr concentration was used as an objective measure of compliance with GMP-MFs because of the known association between higher Thr intake and expansion of the plasma Thr pool (20, 27–29). The Thr content of GMP-MFs is twice that of AA-MFs ingested by subjects in this study (106 ± 4 mg Thr/g PEs compared with 59 ± 1 mg Thr/g PEs, P < 0.0001) because of a high concentration of Thr within the glycomacropeptide peptide. A reduction in plasma Thr concentration when comparing final with baseline Thr concentration for the glycomacropeptide treatment was assumed to indicate low intake of GMP-MFs in the 72 h preceding the final study visit. We were unable to identify a biomarker that reflected consumption of AA-MFs.
Clinical measurements
Serum chemistry profiles were analyzed by using standard techniques at the clinical laboratories of the University of Wisconsin Hospital and Clinics or Boston Children’s Hospital. The plasma concentrations of free AAs and the concentrations of Phe and Tyr in dried blood spots obtained by subjects were determined in the Wisconsin State Laboratory of Hygiene. The plasma AA profile was determined by using a Hitachi L-8900 amino acid analyzer with post–column ninhydrin derivatization (30). The interassay CV for analysis of plasma Phe was 1.6%. The standard newborn screening, nonderivatized flow-injection analysis tandem mass spectrometry method was used for determination of Phe and Tyr concentrations in dried blood spots by using duplicate samples (31). The interassay CV for analysis of Phe and Tyr in dried blood spots was 11.7% and 12.1%, respectively.
Statistical analysis
The primary outcome measure was the change in plasma concentration of Phe in subjects ingesting GMP-MFs compared with the change when ingesting AA-MFs, calculated as the difference between the final Phe concentration at day 22 and the baseline Phe concentration at day 1. The trial was powered at 80% (β = 0.20) based on a previously reported SD of 150 μmol/L for plasma Phe (20, 32) to detect a change in plasma Phe concentration of 120 μmol/L at α = 0.05 (2-sided test). This provided a sample size of 25 subjects, and we aimed to recruit 30 subjects with an estimated dropout rate of 15–20%.
All statistical analyses were performed by using SAS version 9.4 and most analyses involved PROC MIXED (SAS Institute Inc). After confirmation that the data were normally distributed, ANOVA was conducted to test for the main effects for treatment, sequence of diets, and treatment–sequence interaction. When there was evidence of a sequence effect, individual stages were examined separately by using repeated-measures ANOVA or unpaired t tests. Statistical significance was set at P < 0.05.
For the primary endpoint, ANOVA showed a significant treatment effect without significant effects of dietary sequence. However, baseline Phe concentration and dietary Phe intake were determined to be significant predictors of the change in plasma Phe concentration. Thus, ANCOVA was used in the final analysis to compare the mean change in plasma Phe concentration between the AA-MF and GMP-MF treatments by using covariates for baseline Phe (P = 0.001) and Phe intake (P = 0.0212) in the model. The proportion of subjects consuming AA-MFs and GMP-MFs whose plasma Phe concentration increased from day 1 to day 22 was compared by using McNemar’s test in SAS PROC FREQ (SAS Institute Inc.).
A secondary endpoint was the time course of changes in blood Phe and Tyr concentrations assessed in dried blood spots collected by subjects during each of the 3-wk AA-MF and GMP-MF treatments. These data and additional data from the daily medical food logs were analyzed in SAS by using PROC MIXED and repeated-measures analysis, where day was the repeated factor and diet was the treatment factor; random effects for subjects were included. A compound symmetry model was used for the repeated measures, i.e., day effect.
For the acceptability questionnaire, a paired t test pairing within subjects was used to compare AA-MFs with GMP-MFs; a signed rank test was used where data were not normally distributed. For the BRIEF evaluation, a paired t test pairing within subjects was used to compare AA-MFs with GMP-MFs; when sequence effects were observed, an unpaired t test across subjects was used for visit 2 or visit 4. The relation between plasma Thr concentration and plasma Phe concentration was measured by using Pearson’s correlation coefficient and associated t test.
RESUTS
Characteristics of the participants
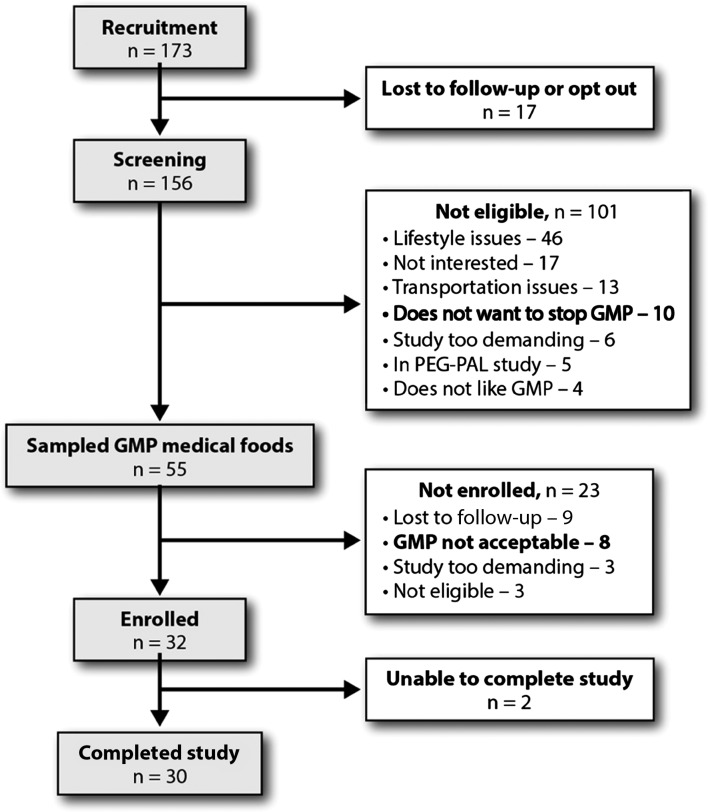
Of the 156 patients with phenylketonuria who were screened for participation in the study, 32 met eligibility criteria and were enrolled, shown in Figure 2. Two subjects dropped out because they were unable to complete requirements of the protocol because of time constraints. The final sample size of 30 subjects (18 females and 12 males) included 5 minors (aged 15–17 y) and 25 adults (aged 18–49 y). The baseline characteristics of the study population are summarized in Table 1. Twenty subjects had a PAH genotype and/or lack of response to the PAH cofactor sapropterin dihydrochloride consistent with classical phenylketonuria, and 10 subjects had a PAH genotype and/or response to sapropterin dihydrochloride consistent with a milder or variant form of phenylketonuria associated with a higher tolerance to dietary Phe. Baseline fasting plasma Phe concentrations at the first study visit for subjects with classical phenylketonuria were significantly higher than the Phe concentrations for subjects with variant phenylketonuria (mean ± SEM concentration 867 ± 73 μmol/L, n = 20 for classical phenylketonuria compared with 461 ± 59 μmol/L, n = 10 for variant phenylketonuria; P < 0.001). In contrast, baseline fasting plasma Tyr concentrations at the first study visit did not differ between subjects with classical and variant phenylketonuria (33.5 ± 2.1 μmol/L compared with 32.9 ± 2.9 μmol/L, respectively). Two of the 30 subjects scored below average (1.5 SD below the normative range) on the Wechsler Abbreviated Scale of Intelligence test but followed the protocol carefully with support. The mean ± SD BMI (in kg/m2) for the 15 adult females was 27.0 ± 5.3 and for the 10 adult males was 25.2 ± 3.6. The BMI-for-age percentile for the 5 adolescent subjects aged 15–17 y ranged from the 30th to 93rd percentile; mean ± SD BMI was 23.2 ± 2.4. Subjects maintained stable weight during the study.
FIGURE 2.
Flow diagram of the study selection process. GMP, glycomacropeptide; PEG-PAL, pegylated phenylalanine ammonia lyase.
TABLE 1.
Characteristics of enrolled participants1
| Subject no. | BMI, kg/m2 | Classical/ variant2 | Mutation3 | Phe, μmol/L | Tyr, μmol/L |
| 1 | 21.7 | Variant4 | L348V; 165T | 201 | 25 |
| 2 | 20.3 | Variant | R68S; IVS12+1G>T | 224 | 36 |
| 3 | 39.7 | Variant | L48S; 195_K96delinsK | 297 | 53 |
| 4 | 25.1 | Classical | R408W; V338M | 301 | 26 |
| 5 | 27.3 | Classical | R408W; IVS12+1G>T | 367 | 36 |
| 6 | 21.1 | Variant4 | R241H; Q304Q | 380 | 29 |
| 7 | 24.6 | Variant | 165T; IVS10-11G>A | 461 | 34 |
| 8 | 24.6 | Classical | R158Q; R261Q | 481 | 30 |
| 9 | 25.3 | Classical | E280K; L348V | 520 | 56 |
| 10 | 34.1 | Classical | R408W; IVS7+3G>C | 535 | 42 |
| 11 | 29.2 | Variant | E280K; E390G | 541 | 20 |
| 12 | 22.3 | Classical | MIV; A246fsde1C | 606 | 36 |
| 13 | 26.8 | Variant4 | R158Q; R408W | 640 | 33 |
| 14 | 23.8 | Variant4 | R157N; L348V | 643 | 36 |
| 15 | 23.5 | Classical | R408W; IVS12 | 649 | 23 |
| 16 | 18.4 | Variant4 | P416Q; 664delGA | 704 | 26 |
| 17 | 23.4 | Classical | Y356X; R408W | 743 | 31 |
| 18 | 27.0 | Classical | R408W; R261Q | 754 | 47 |
| 19 | 31.7 | Classical | IVS1+5G>T; IVS12+1G>A | 800 | 38 |
| 20 | 25.3 | Variant | R408W; IVS12+1G>A | 821 | 37 |
| 21 | 26.9 | Classical | L242F; R408W | 829 | 29 |
| 22 | 28.1 | Classical | R408W; Y356X | 843 | 38 |
| 23 | 26.3 | Classical | E280K; IVS12+1G>T | 852 | 20 |
| 24 | 29.5 | Classical | Y277D; IVS10-11G>A | 893 | 17 |
| 25 | 32.0 | Classical | E280K; L348V | 937 | 40 |
| 26 | 23.9 | Classical | R261Q; IVS10-11G>A | 1059 | 30 |
| 27 | 23.8 | Classical | F55>Lfs; IVS5+1G>A | 1086 | 39 |
| 28 | 20.1 | Classical | F55>Lfs; R408W | 1160 | 30 |
| 29 | 26.9 | Classical | R408W; IVS12+1G>A | 1360 | 26 |
| 30 | 23.0 | Classical | IVS4+5G>T; IVS12+1G>A | 1418 | 37 |
Values were obtained at the first study visit when participants were consuming their usual low-Phe amino acid diet, and a fasting blood sample was obtained for baseline analysis of amino acids in plasma.
Subjects classified as having a variant form of phenylketonuria displayed a phenylalanine hydroxylase genotype and/or response to the drug sapropterin dihydrochloride, a synthetic form of the tetrahydrobiopterin cofactor for phenylalanine hydroxylase, that was consistent with a milder or variant form of phenylketonuria.
Mutation names are defined at http://www.pahdb.mcgill.ca and http://www.biopku.org.
Stable dose of sapropterin dihydrochloride (KUVAN, BioMarin Pharmaceutical Inc.) throughout the study.
Nutrient profiles of the diets
The nutrient profiles of the AA and glycomacropeptide treatments were generally constant except for changing the source of PEs in the medical foods from free AAs to primarily intact protein from glycomacropeptide as summarized in Table 2. AA-MFs or GMP-MFs provided 66–68% of total protein intake or 0.74–0.76 g protein · kg–1 · d–1. As expected, GMP-MFs provided a significantly higher percentage of energy than AA-MFs, 32% compared with 23%, P < 0.002. Dietary intake of protein from AA-MFs and GMP-MFs for subjects with classical phenylketonuria and variant phenylketonuria was not significantly different. Independent of diet, subjects with classical phenylketonuria tended to consume less protein from natural food (P = 0.0561) and significantly less Phe (P < 0.05) than subjects with variant phenylketonuria (classical: mean ± SE 0.34 ± 0.04 g protein from natural food · kg–1 · d–1, 15 ± 2 mg Phe · kg–1 · d–1; variant: 0.50 ± 0.07 g protein from natural food · kg–1 · d–1, 22 ± 3 mg Phe · kg–1 · d–1).
TABLE 2.
Nutrient profile of the low-Phe diets in combination with AA-MFs and GMP-MFs1
| Nutrient |
|||
| AA-MF | GMP-MF | P | |
| Energy | |||
| kcal/d | 2113 ± 87 | 2265 ± 121 | 0.253 |
| kcal · kg−1 · d−1 | 31 ± 1 | 33 ± 2 | 0.255 |
| Energy from MF/d, % | 23 ± 2 | 32 ± 2 | 0.002 |
| Protein | |||
| g/d | 80 ± 3 | 79 ± 4 | 0.892 |
| g · kg−1 · d−1 | 1.15 ± 0.05 | 1.14 ± 0.06 | 0.950 |
| g protein from natural foods/d | 26 ± 3 | 27 ± 3 | 0.651 |
| MF | |||
| g protein from MFs/d | 54 ± 3 | 52 ± 3 | 0.527 |
| g protein from MFs · kg−1 · d−1 | 0.76 ± 0.05 | 0.74 ± 0.04 | 0.576 |
| Protein intake from MF/d, % | 68 ± 4 | 66 ± 3 | 0.603 |
| Servings/d at visit 1–22 | 2.99 ± 0.27 | 3.26 ± 0.27 | 0.476 |
| Servings/d at visit 3–42 | 2.43 ± 0.24 | 3.74 ± 0.24 | 0.001 |
| Macronutrient profile (%) | |||
| Energy from protein | 16 ± 4 | 15 ± 4 | 0.216 |
| Energy from fat | 28 ± 2 | 27 ± 1 | 0.600 |
| Energy from carbohydrate | 58 ± 2 | 60 ± 1 | 0.228 |
Values are means ± SEs based on the final 3-d food records. n = 30. Statistical analysis included ANOVA with effects for treatment, sequence, and treatment–sequence interaction. AA-MF, amino acid medical food; GMP-MF, glycomacropeptide medical food; MF, medical food.
Data were obtained from daily MF logs. These data were analyzed with repeated-measures ANOVA and showed a significant effect for sequence (P = 0.044) and treatment (P = 0.003). Thus, data for visits 1–2 and visits 3–4 were analyzed separately, n = 15/group.
Compliance with the AA and glycomacropeptide diets
Compliance with AA-MFs and GMP-MFs was deemed adequate based on comparison of the prescribed intake of medical food (0.85 ± 0.03 g PEs · kg–1 · d–1) with actual intakes derived from the daily medical food logs completed by subjects (Table 2). The frequency of medical food intake recorded by subjects on daily medical food logs was higher for GMP-MFs during both stages of the study and significantly higher during visits 3 and 4 than it was with AA-MFs (3.74 servings GMP-MFs/d compared with 2.43 servings AA-MFs/d; P = 0.001) (Table 2). Six of 30 subjects demonstrated a reduction in final plasma Thr concentration compared with baseline, suggesting reduced intake of GMP-MFs in the 72 h preceding the final blood draw; all 6 subjects showed an increase in plasma Phe concentration with GMP-MFs. Analysis for the primary endpoint with omission of data from these 6 subjects did not change the outcome.
Diet acceptability
Acceptability of GMP-MFs and AA-MFs was assessed with an acceptability questionnaire as shown in Table 3. The glycomacropeptide diet was rated more acceptable based on significantly higher mean scores than with the AA diet at day 21 for likeability of glycomacropeptide products, ease of staying on the phenylketonuria diet, and comfort and convenience of using GMP-MFs away from home. The positive response regarding greater convenience of GMP-MFs than AA-MFs likely reflects differences in product packaging because 8 of the 15 AA-MFs were in powder form and required measurement and mixing, whereas the GMP-MFs were available as powders in serving-size packets, ready-to-drink liquids, or meal replacement bars (see Supplemental Table 1).
TABLE 3.
Acceptability of AA-MFs and GMP-MFs1
| Question | AA-MFs | GMP-MFs | P |
| How much do you like your AA-MF formula/GMP-MF products? | 3.97 ± 0.24 (69) | 4.90 ± 0.18 (93) | 0.001 |
| How easy is it to prepare your AA-MF formula/GMP-MF products? | 4.79 ± 0.22 (83) | 5.07 ± 0.16 (97) | 0.366 |
| How willing are you to take AA-MF formula/GMP-MF products 3 times a day? | 4.50 ± 0.25 (79) | 4.86 ± 0.19 (86) | 0.172 |
| How easy is it to stay on your phenylketonuria diet when you are using AA-MF formula/GMP-MF products? | 4.19 ± 0.18 (79) | 4.69 ± 0.16 (90) | 0.019 |
| How comfortable are you eating AA-MF formula/GMP-MF products in social situations? | 3.83 ± 0.26 (59) | 4.72 ± 0.27 (79) | 0.003 |
| Overall, how convenient is it to take & consume AA-MF formula/GMP-MF products away from home? | 3.34 ± 0.31 (45) | 4.47 ± 0.23 (83) | 0.001 |
Values are means ± SEs (% subjects selecting a positive score of 4, 5, or 6 for each question). Subjects responded to each question on day 21 of both the AA-MF and GMP-MF treatments by selecting a response on a 6-point scale where 1 = dislike extremely, 2 = dislike, 3 = somewhat dislike, 4 = somewhat like, 5 = like, and 6 = like extremely. Comparisons were conducted by using a paired t test (n = 29) on the scores for the 6 questions in response to each diet. A signed rank test was used where data were not normally distributed. Similar statistical significance was obtained when subjects completed the questionnaire on day 7 of each dietary treatment. [Following are subjects’ comments written on the questionnaire in response to “please write additional comments below” and/or comments mentioned to the registered dietitian study coordinator during study visits. The numbers in parentheses indicate the number of subjects making this comment. AA-MF diet: gastrointestinal symptoms including diarrhea, constipation, heartburn, nausea, and abdominal pain (n = 10); higher awareness of lingering hunger after transition from GMP-MFs to AA-MFs diet (n = 3); and hunger, headaches, fatigue, ate less food due to gastrointestinal distress (n = 1–2 for each). GMP-MF diet: liked convenience, flexibility, and variety of products (n = 11); felt full and made better food choices with less snacking (n = 11); preferred taste (n = 6); perceived improved performance with exercise, work, or school (n = 6); had improved gastrointestinal symptoms with less nausea, heartburn, and abdominal pain and regular bowel movements (n = 6); had concern about high caloric content of some GMP-MF products (n = 5); reported gastrointestinal symptoms and low aesthetic appeal related to the precipitate in Restore (n = 5); still craved AA-MF formula (n = 5); and perceived greater emotional stability (n = 3).] AA-MF, amino acid medical food; GMP-MF, glycomacropeptide medical food.
Information about acceptability of GMP-MFs was also garnered during subject recruitment and screening (Figure 2). Eight of 55 individuals who sampled GMP-MFs during the screening process stated that GMP-MFs were not acceptable and were therefore not enrolled. However, 10 individuals using GMP-MFs before study recruitment were unwilling to stop taking GMP-MFs and return to using AA-MFs as required in our protocol to enroll in the study.
Subject comments about the side effects associated with GMP-MFs and AA-MFs reflect information written by subjects on the acceptability questionnaires as well as comments made to the RD study coordinator during study visits (see Table 3). For AA-MFs, subjects noted a variety of gastrointestinal symptoms including diarrhea, constipation, heartburn, nausea, and abdominal pain. A sensation of hunger, especially after transitioning back from GMP-MFs to the AA-MFs, was also noted. For GMP-MFs, subjects noted the convenience, flexibility, and variety of glycomacropeptide products; felt full and made better food choices throughout the day with GMP-MFs; preferred the taste of glycomacropeptide compared with AA medical foods; and perceived improved performance and reduced gastrointestinal symptoms. There was concern expressed by adult female subjects about the high caloric content of some GMP-MFs; teens participating in sports felt the high caloric content to be advantageous. The Restore GMP-MF sports drink had low aesthetic appeal and invoked gastrointestinal symptoms related to the precipitate in this product. Although several comments were made by groups of 10–11 subjects, we cannot conclude that these comments apply to our entire subject population because the information was not prompted by a structured survey of all 30 subjects.
Physical examination and blood chemistry
Annual meetings of a Data Safety Monitoring Board reviewed subject results and concluded that there were no adverse events associated with the dietary protocol. There were no concerns detected on physical examination at each study visit to indicate negative effects on health status when subjects consumed AA-MFs or GMP-MFs as the primary protein source for 3 wk. Mean blood chemistry values were within the normal range for both diets. Blood chemistry panels indicated no significant differences between concentrations of blood urea nitrogen (BUN), ammonia, creatinine, and estimated glomerular filtration rate as indicators of renal status measured at the last study visit for AA-MFs compared with GMP-MFs (Table 4). Likewise, alkaline phosphatase, alanine amino transferase, aspartate amino transferase, and bilirubin as indicators of liver function were not significantly different with the AA-MFs and GMP-MFs (data not shown). Electrolytes showed no significant differences, except for carbon dioxide, which was significantly higher with GMP-MFs during the second phase of the study (visit 4) than with AA-MFs. Albumin concentration, but not total protein and prealbumin, was significantly higher with GMP-MFs than with AA-MFs.
TABLE 4.
Chemistry panel results after 3 wk following the low-Phe diets in combination with AA-MFs and GMP-MFs1
| Test | AA-MFs | GMP-MFs | P |
| Electrolytes, mmol/L | |||
| Sodium | 139 ± 0.4 | 139 ± 0.5 | 0.851 |
| Potassium | 3.94 ± 0.05 | 3.94 ± 0.04 | 0.970 |
| Chloride | 105 ± 0.5 | 105 ± 0.5 | 0.707 |
| CO2 visit 2 | 26 ± 0.5 | 25 ± 0.6 | 0.083 |
| CO2 visit 4 | 24 ± 0.6 | 26 ± 0.6 | 0.019 |
| Renal | |||
| Blood urea nitrogen, mg/dL | 11.2 ± 0.6 | 10.6 ± 0.6 | 0.947 |
| Ammonia, μmol/L | 24 ± 1.9 | 24 ± 2.0 | 0.594 |
| Creatinine, mg/dL | 0.77 ± 0.03 | 0.77 ± 0.02 | 0.701 |
| Estimated glomerular filtration rate, mL · min−1 · 1.73 m−2 | 98 ± 4.8 | 100 ± 4.9 | 0.540 |
| Nutrient status | |||
| Total protein, g/dL | 7.35 ± 0.08 | 7.42 ± 0.07 | 0.291 |
| Albumin, g/dL | 4.24 ± 0.04 | 4.35 ± 0.04 | 0.027 |
| Prealbumin, mg/dL | 27 ± 1.0 | 27 ± 1.1 | 0.828 |
| Glucose, mg/dL | 84 ± 1.3 | 85 ± 1.1 | 0.443 |
| Hematocrit, % | 42 ± 0.3 | 41 ± 0.3 | 0.222 |
Values are means ± SEs. n = 30. All values are within normal range. Statistical analysis included ANOVA with effects for treatment, baseline, sequence, and treatment–sequence interaction. CO2 showed treatment–sequence interaction (P = 0.0161), and data were analyzed separately for final visits 2 and 4 with unpaired t test, n = 15. Albumin and hematocrit were analyzed by ANCOVA with baseline concentrations as a covariate. For all other tests, baseline was not significantly different. AA-MF, amino acid medical food; CO2, carbon dioxide; GMP-MF, glycomacropeptide medical food.
Neuropsychological evaluation of executive function and anxiety
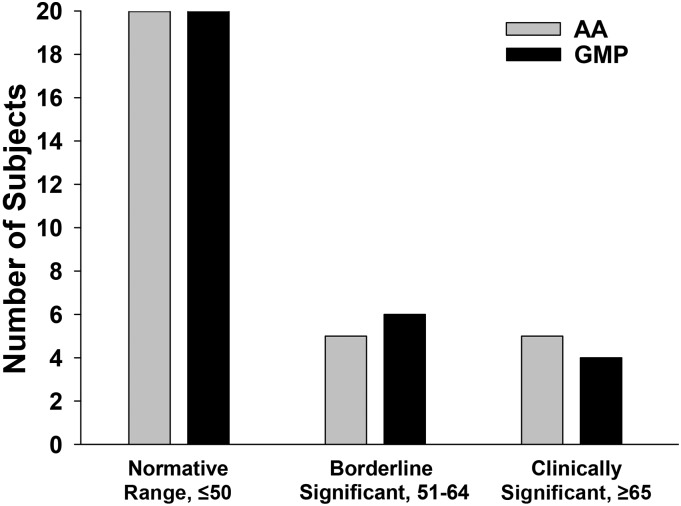
The BRIEF assessment scores for the overall Global Executive Composite (GEC) are shown in Table 5. The mean T-score for GEC based on subject self-report was within the normative T-score range of <50 (SD = 10) (24) and identical for the GMP-MF and AA-MF treatments. The number of subjects with GEC scores within the normative (n = 20), borderline significant (n = 4–5), and clinically significant (n = 4–5) ranges was not significantly different for AA-MFs and GMP-MFs as shown in Figure 3. The BAI self-report scores showed no differences in perceived anxiety when comparing GMP-MFs and AA-MFs (data not shown). Overall, 75% of subjects expressed minimal anxiety, and 25% expressed mild-to-moderate anxiety. In summary, there was no evidence that subjects perceived negative effects on executive function and anxiety when comparing AA-MFs and GMP-MFs.
TABLE 5.
Behavior Rating Inventory of Executive Function assessments for the low-Phe diets in combination with AA-MFs and GMP-MFs1
| Assessment | AA-MFs | GMP-MFs |
| Global Executive Composite2 | 48.8 ± 2.1 (35–71) | 49.0 ± 1.8 (35–70) |
| Metacognition Index | 50.1 ± 2.0 (36–83) | 49.7 ± 1.6 (37–68) |
| Behavior Regulation Index | 48.1 ± 2.3 (35–84) | 48.6 ± 2.0 (35–82) |
Values are T-score means ± SEs (range). n = 30. The Behavior Rating Inventory of Executive Function assessment is based on self-report after subjects followed each diet for 3 wk. Standard T-scores have a mean of 50 points and a SD of 10 points. There was no significant treatment effect for all 3 measures regardless of method of analysis, i.e., paired t test or unpaired t test for visit 2 or visit 4 when a sequence effect was observed (P = 0.46–0.99). AA-MF, amino acid medical food; GMP-MF, glycomacropeptide medical food.
The Global Executive Composite score represents the total score from 8 domains that are grouped into 2 indices: Behavior Regulation Index, which includes the inhibition, shift, and emotional control domains, and the Metacognition Index, which includes the initiation, working memory, planning/organizing, organizing materials, and monitoring domains.
FIGURE 3.
The number of phenylketonuria subjects with T-scores for the Behavioral Rating Inventory of Executive Function Global Executive Composite in the normative, borderline significant, and clinically significant categories after following a low-phenylalanine diet in conjunction with AA medical foods and GMP medical foods for 3 wk at home, n = 30 subjects. A T-score of 1.5 SD (15 points) above the mean (50 points) is indicative of reduced executive function. AA, amino acid; GMP, glycomacropeptide.
Change in plasma concentrations of Phe and Tyr
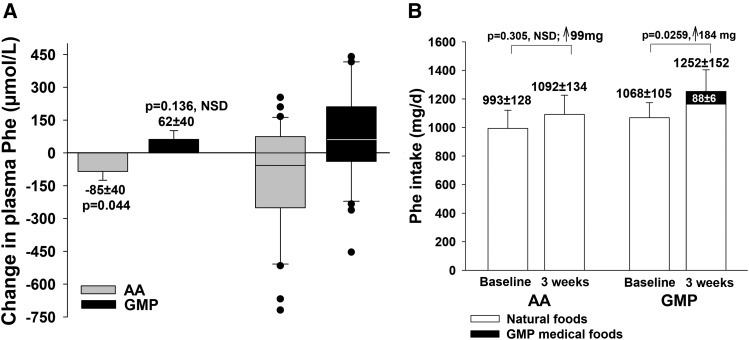
Results for the primary endpoint, change in fasting plasma concentration of Phe with the AA-MFs compared with the GMP-MFs, are shown in Figure 4. The ANCOVA with adjustment for covariates for baseline Phe concentration (P = 0.0001) and dietary Phe intake (P = 0.0212) indicated a significant treatment effect (mean ± SEM difference: −147 ± 39, P = 0.0008) without significant effects for sequence (P = 0.0561) or treatment–sequence interaction (P = 0.2301). GMP-MFs showed an increase in plasma Phe concentration that was not significant (62 ± 40 μmol/L, P = 0.136), and AA-MFs showed a modest but significant decrease in plasma Phe concentration (−85 ± 40 μmol/L, P = 0.044). There was considerable variation in Phe response to both AA-MFs and GMP-MFs.
FIGURE 4.
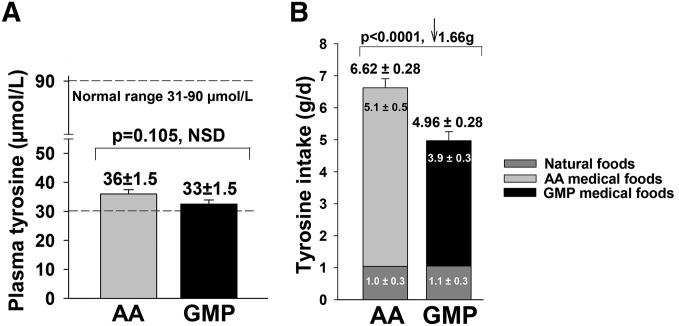
Change in fasting plasma concentration of Phe (A) and dietary intake of Phe (B) in participants with phenylketonuria who followed a low-Phe diet in conjunction with AA medical foods and GMP medical foods for 3 wk at home. Plasma data were analyzed by ANCOVA to compare the change in plasma Phe concentrations with AA medical foods and the GMP medical foods with adjustment for covariates for baseline Phe concentration (P = 0.0001) and dietary Phe intake (P = 0.0212) based on the final 3-d food records. Results indicate a significant treatment effect (mean difference, −147 ± 39, P = 0.0008) without significant sequence or treatment–sequence effects. The GMP treatment showed no significant increase in plasma Phe concentration (62 ± 40, P = 0.136), and the AA treatment showed a modest but significant decrease in plasma Phe concentration (−85 ± 40, P = 0.044). The box and whisker figure illustrates the variation in Phe response with consumption of AA and GMP medical foods; dots indicate individual values outside the 10th–90th percentiles. The box represents the middle 50% of all 30 subjects (25th–75th percentile) with a line showing the median value; the whiskers indicate the 10th and 90th percentiles. Dietary Phe intake was compared for the AA treatment and the GMP treatment by paired t test. Dietary Phe intake did not increase significantly with the AA treatment, but it did increase significantly with the GMP treatment (P = 0.0259) because of an additional intake of 88 ± 6 mg Phe/d from the GMP medical foods. AA medical foods do not contain Phe. Intake of Phe from natural foods was not significantly different for the AA and GMP treatments. Values are least-squares means (plasma Phe) or means (Phe intake) ± SEs, n = 30. AA, amino acid; GMP, glycomacropeptide; NSD, not significantly different; ↑, increase in Phe intake.
Subgroup analysis of the 20 subjects with classical phenylketonuria for change in fasting plasma Phe concentration showed a response similar to the full dataset with 30 subjects. In contrast, subgroup analysis of the 10 subjects with variant phenylketonuria did not show a significant treatment effect for change in plasma Phe in ANCOVA (GMP-MFs: 24 ± 50 μmol/L, P = 0.648; AA-MFs: −12 ± 50 μmol/L, P = 0.813), perhaps because of reduced power for this subset. Irrespective of analysis as classical or variant subgroups or the total sample of 30 subjects, there was no evidence that GMP-MFs induced a significant change in plasma Phe concentration.
The proportion of subjects who showed an increase in plasma Phe concentration with AA-MFs and GMP-MFs was not significantly different based on McNemar’s test (P = 0.267). There were 18 subjects (60%) who showed an increase and 12 subjects (40%) who showed a decrease in plasma Phe with GMP-MFs. There were 13 subjects (43%) who showed an increase and 17 subjects (57%) who showed a decrease in plasma Phe with AA-MFs. Interestingly, there were 9 subjects who showed an increase in plasma Phe with both AA-MFs and GMP-MFs and 8 subjects who showed a decrease in plasma Phe with both AA-MFs and GMP-MFs.
Dietary Phe intake is a major determinant of plasma Phe concentration in phenylketonuria and of importance in interpreting the current study because GMP-MFs contain 1.8 mg Phe/g PEs, whereas the AA-MFs used in this study were Phe free. Dietary Phe intake did not increase significantly with the AA-MFs, but it did increase significantly with the GMP-MFs (P = 0.0259) because of an additional intake of 88 ± 6 mg Phe/d from the GMP-MFs (Figure 4B). This increase in Phe intake with GMP-MFs occurred despite counseling subjects to reduce their intake of natural foods to offset the Phe contained in GMP-MFs. Intake of Phe from natural foods was not different and did not change significantly compared with baseline for either treatment.
The change in plasma Tyr concentrations was not significantly different when comparing AA-MFs and GMP-MFs (P = 0.105 ANCOVA with baseline Tyr as covariate, P = 0.0001). Interestingly, similar final plasma Tyr concentrations were maintained with both diets despite significantly greater Tyr intake with AA-MFs than with the GMP-MFs, Figure 5.
FIGURE 5.
Fasting plasma Tyr concentrations after treatment with the AA and GMP medical foods for 3 wk were NSD (P = 0.105), despite significantly greater Tyr intake with AA medical foods than with GMP medical foods (P = 0.0001). Statistical analysis for final plasma Tyr concentration used ANCOVA with a covariate for baseline Tyr concentration; values are least-squares means ± SEs, n = 30. Statistical analysis for Tyr intake from the final 3-d food records was ANOVA; values are means ± SEs, n = 30. AA, amino acid; GMP, glycomacropeptide; NSD, not significantly different.
Concentrations of Phe and Tyr measured in dried blood spots across time
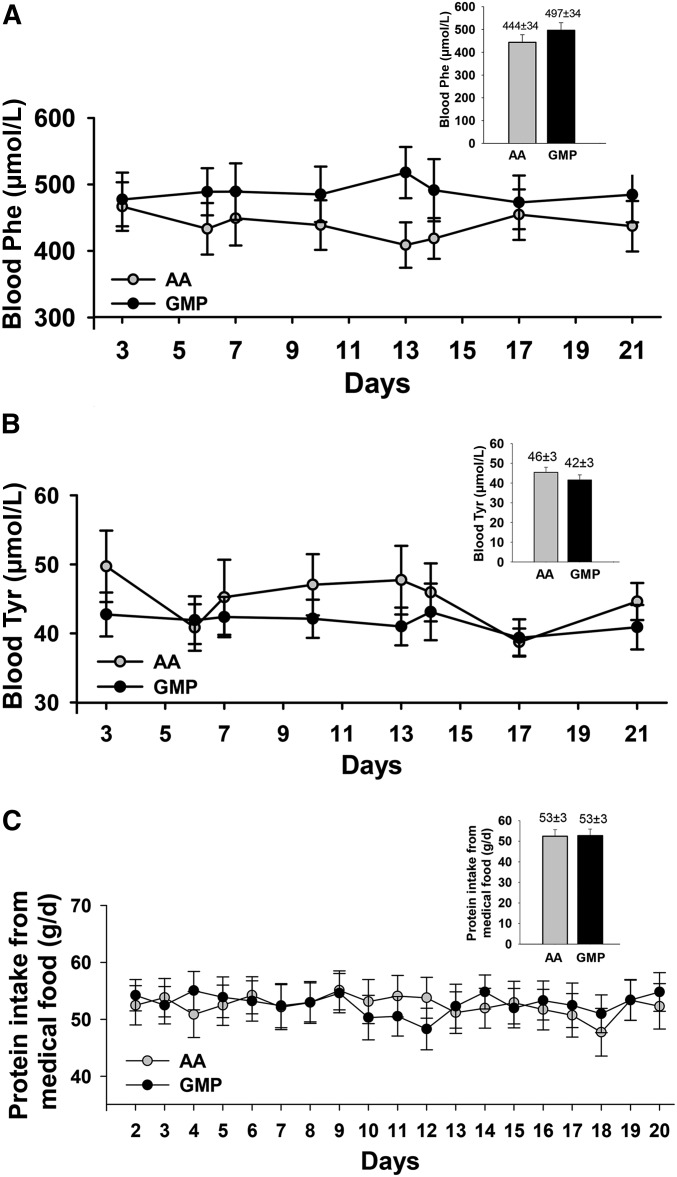
Clinical monitoring of Phe concentrations in phenylketonuria often includes patients spotting finger prick blood samples on filter paper cards that they mail to their clinic for analysis, frequently through the use of the newborn screening methodology of tandem mass spectrometry (30). To simulate clinical practice, we used a similar approach wherein subjects mailed in 8 filter paper cards over the 3-wk treatment period for each diet, as shown in Figure 6. There were no significant changes in blood concentrations of Phe and Tyr across the 3-wk treatment periods with either AA-MFs or GMP-MFs. Intake of PEs from medical food across the 3-wk treatment period was also not significantly different for AA-MFs and GMP-MFs (Figure 6C).
FIGURE 6.
Fasting concentrations of Phe (A) and Tyr (B) in blood were based on analysis of dried blood spots collected by subjects and analyzed with tandem mass spectrometry. (C) Daily intake of protein equivalents from medical food was based on daily medical food logs completed by subjects. Repeated-measures ANOVA indicated there was no significant treatment effect due to ingestion of AA and GMP medical foods on blood concentrations of Phe and Tyr and daily intake of protein equivalents from medical food over the 3-wk treatment period (P = 0.175–0.9522). Values are means ± SEs, n = 26–30 for each of the 8 time points for blood Phe and Tyr concentrations. The insert shows the average blood concentrations of Phe and Tyr and average daily intake of protein from medical foods across time (least-squares means ± SEs). AA, amino acid; GMP, glycomacropeptide.
The ratio of the concentrations of Phe:Tyr in plasma and blood
The ratio of the concentrations of Phe:Tyr in plasma or blood is often used clinically as a measure of Phe control in phenylketonuria, although there is little evidence to support this approach (33). The change in the ratio of Phe:Tyr in plasma was consistent with changes observed separately for plasma concentrations of Phe and Tyr. Analysis of the change in the plasma ratio of Phe:Tyr with ANCOVA with a covariate for baseline ratio (P = 0.0045) showed a significant treatment effect (P = 0.0006) with a trend for GMP-MFs to increase the ratio (2.42 ± 1.38; P = 0.0889) and for AA-MFs to significantly decrease the ratio (−3.65 ± 1.37; P = 0.0128). The ratio of Phe:Tyr in dried blood spots was deemed inconclusive because of low precision of measurement and high variance as previously reported (30).
Plasma AA profiles
The full profile of AA concentrations in fasting plasma samples at the baseline visit and the final visit for the AA-MF and GMP-MF treatments is shown in Table 6. Except for Phe, all mean concentrations of AAs were within the normal range for both AA-MFs and GMP-MFs (34). The baseline concentration of Phe was higher for AA-MFs than for GMP-MFs and shown to be a significant predictor in the ANOVA model (P = 0.0001) for change in plasma Phe concentration.
TABLE 6.
Effect of low-Phe diets in combination with AA-MFs and GMP-MFs on baseline and final concentrations of amino acids in plasma1
| AA-MF diet |
GMP-MF diet |
|||||||
| Amino acid | Baseline, μmol/L | Final, μmol/L | P4 | Baseline, μmol/L | Final, μmol/L | P4 | Change with AA-MF vs. GMP-MF diet,2 P | Normal range,3 μmol/L |
| Ala | 299 ± 17 | 307 ± 16 | 0.522 | 306 ± 17 | 302 ± 18 | 0.765 | 0.460 | 200–579 |
| Arg | 48 ± 2 | 53 ± 2 | 0.031 | 53 ± 3 | 53 ± 2 | 0.752 | 0.068 | 32–120 |
| Asp | 2 ± 0.2 | 2 ± 0.2 | 0.529 | 2 ± 0.4 | 2 ± 0.3 | 0.883 | 0.554 | <7 |
| Citrulline | 34 ± 2 | 34 ± 1 | 0.821 | 34 ± 2 | 36 ± 1 | 0.316 | 0.579 | 17–46 |
| Cys | 19 ± 2 | 19 ± 2 | 0.644 | 17 ± 2 | 18 ± 2 | 0.163 | 0.190 | 3–95 |
| Glu | 22 ± 3 | 25 ± 3 | 0.264 | 23 ± 2 | 26 ± 3 | 0.151 | 0.815 | 13–113 |
| Gln | 477 ± 13 | 491 ± 12 | 0.129 | 487 ± 11 | 480 ± 12 | 0.441 | 0.108 | 371–957 |
| Gly | 282 ± 21 | 279 ± 21 | 0.625 | 283 ± 17 | 265 ± 17 | 0.006 | 0.048 | 126–490 |
| His | 73 ± 2 | 76 ± 2 | 0.075 | 71 ± 2 | 73 ± 2 | 0.251 | 0.615 | 39–123 |
| Ile | 47 ± 2 | 48 ± 2 | 0.244 | 47 ± 2 | 47 ± 2 | 0.807 | 0.318 | 36–107 |
| Leu | 90 ± 3 | 94 ± 4 | 0.038 | 89 ± 3 | 91 ± 3 | 0.450 | 0.325 | 68–183 |
| Lys | 143 ± 6 | 150 ± 6 | 0.072 | 143 ± 6 | 128 ± 4 | 0.001 | <0.001 | 103–255 |
| Met | 16 ± 1 | 17 ± 0.5 | 0.268 | 17 ± 1 | 19 ± 1 | 0.025 | 0.385 | 4–44 |
| Ornithine | 43 ± 2 | 45 ± 2 | 0.286 | 43 ± 2 | 43 ± 2 | 0.791 | 0.346 | 38–130 |
| Phe | 763 ± 64 | 655 ± 59 | 0.017 | 691 ± 58 | 777 ± 65 | 0.057 | 0.001 | 35–80 |
| Pro | 122 ± 7 | 130 ± 9 | 0.291 | 123 ± 6 | 116 ± 9 | 0.336 | 0.136 | 97–368 |
| Ser | 94 ± 4 | 96 ± 4 | 0.501 | 94 ± 4 | 95 ± 3 | 0.887 | 0.701 | 63–187 |
| Taurine | 93 ± 6 | 87 ± 7 | 0.298 | 93 ± 7 | 92 ± 6 | 0.934 | 0.491 | 42–156 |
| Thr | 106 ± 5 | 112 ± 6 | 0.377 | 103 ± 4 | 149 ± 10 | <0.001 | <0.001 | 85–231 |
| Trp | 26 ± 2 | 26 ± 1 | 0.558 | 26 ± 1 | 25 ± 2 | 0.406 | 0.221 | 29–77 |
| Tyr | 34 ± 2 | 36 ± 2 | 0.180 | 33 ± 2 | 32 ± 1 | 0.548 | 0.172 | 31–90 |
| Val | 198 ± 7 | 201 ± 7 | 0.632 | 192 ± 9 | 159 ± 5 | <0.001 | <0.001 | 136–309 |
Values are means ± SEs. n = 30. Statistical analysis by ANOVA with effects for treatment, sequence, and treatment–sequence interaction to compare change in amino acid concentrations with the AA-MF treatment compared with the GMP-MF treatment calculated as the difference between final and baseline amino acid concentrations. A P value of <0.05 is considered significant. All values were within the normal range. AA-MF, amino acid medical food; GMP-MF, glycomacropeptide medical food.
Treatment from overall ANOVA; sequence effects were not significant except for alanine, which showed significant sequence–treatment interaction, P = 0.0012. However, when data were analyzed separately for the first and second phases of the study (n = 15 subjects/diet), there were no significant differences in the change in plasma alanine concentration for the AA-MF and GMP-MF treatments. Final alanine concentrations for the amino acid and glycomacropeptide treatments were also not significantly different.
Mayo Clinic Medical Laboratories, plasma amino acid reference values (34).
Change in amino acid concentration from baseline for the AA-MF treatment or for the GMP-MF treatment.
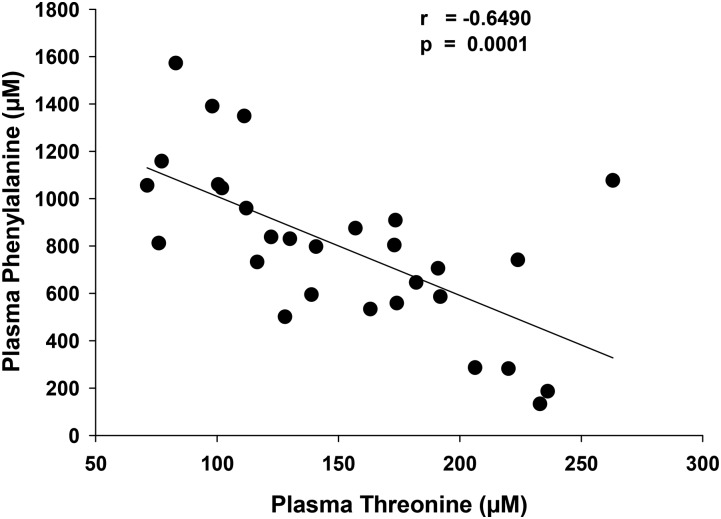
As expected and previously reported for our inpatient glycomacropeptide study (20), Thr mean ± SE concentration showed a significant increase with GMP-MFs from 103 ± 4 μmol/L to 149 ± 10 μmol/L (P < 0.001). There was a significant negative correlation between plasma Thr concentration in the range of 75–250 μmol/L and plasma Phe concentration in the range of 1100–200 μmol/L (r = −0.649, P < 0.0001) (Figure 7). There were no significant differences in the change from baseline in concentrations of the AAs that are supplemented in glycomacropeptide (Arg, His, Leu, Trp, and Tyr). The relative plasma concentrations of the branched-chain AAs with GMP-MFs were Val:Leu:Ile, ∼3:2:1. This is consistent with the normal reference range and indicates that there was no evidence of an imbalance in the plasma concentrations of branched-chain AAs as a result of the Leu supplementation in GMP-MFs (34, 35).
FIGURE 7.
Negative correlation of plasma Thr concentration and plasma Phe concentration as measured by Pearson’s correlation coefficient for the glycomacropeptide medical foods treatment, n = 29.
DISCUSSION
The cornerstone of phenylketonuria management to prevent cognitive impairment is lifelong adherence to a low-Phe diet, which limits intake of natural protein found in foods, in combination with medical foods that provide a source of low-Phe protein and/or AAs (3, 4). In this study, we investigated the effects of substituting primarily intact protein from GMP-MFs (i.e., formula, bars, and pudding) for the protein equivalents provided by AA-MFs (i.e., 15 different formula products) in free-living subjects with phenylketonuria following a low-Phe diet. Ingestion of GMP-MFs did not induce a significant increase in plasma Phe concentration despite higher Phe intake because of the Phe content of GMP-MFs. Ingestion of AA-MFs was associated with a significant ∼10% decrease in plasma Phe concentration (P = 0.044). However, blood Phe concentrations did not change significantly across the 3-wk treatment period for either AA-MFs or GMP-MFs. We conclude that GMP-MFs show efficacy in controlling blood Phe concentrations across time similar to that observed with AA-MFs, but demonstrate advantages in taste, convenience, and reduced gastrointestinal side effects.
We observed no evidence of health concerns with GMP-MFs compared with AA-MFs in this 3-wk study. Consistent with the higher dietary acid load provided by AA-MFs relative to GMP-MFs, carbon dioxide, which is primarily bicarbonate, was significantly higher with GMP-MFs for the second stage of the study (20, 36). Supplementation of glycomacropeptide with limiting AAs based on our inpatient study (20)—notably, adding Arg, omitting Met, and increasing Tyr and Leu supplementation—resulted in plasma AA concentrations that were within normal limits and not significantly different from those noted at baseline with AA-MFs. This supports the adequacy of the AA supplementation scheme for GMP-MFs used in Glytactin (25).
Given that Tyr is an indispensable AA in phenylketonuria and shows poor solubility, low plasma concentrations of Tyr are a consistent observation in phenylketonuria. Interestingly, final plasma Tyr concentrations were not significantly different for the AA and glycomacropeptide treatments despite intake of ∼1.5 g additional Tyr/d (33% increase) with AA-MFs. The explanation may reflect lower bioavailability of Tyr from AA-MFs or metabolism of Tyr to phenolic and indolic compounds by the intestinal microbiota (37), as noted in our studies with phenylketonuria mice (38). Potential differences in Tyr bioavailability and the reduced gastrointestinal side effects noted by subjects consuming GMP-MFs are supported by evidence that glycomacropeptide is a prebiotic (15). Moreover, distinct alterations in the intestinal microbiota were recently reported in children with phenylketonuria fed AA-MFs compared with control subjects (39).
The degree of dietary compliance strongly determines the efficacy of studies comparing dietary interventions (40), and changes in dietary behavior are difficult to achieve when established over a long period (41). Phenylketonuria is an extreme example of a well-established eating pattern with initiation of AA-MFs shortly after birth and prescribed for life. This suggests the potential that subjects with phenylketonuria who are compliant with consuming AA-MFs, as was required for this study and for whom glycomacropeptide was a new diet, as applies to most of our subjects, are imprinted with a preference for the taste and emotional components associated with lifelong consumption of AA-MFs. Indeed, several subjects commented that GMP-MFs tasted better but that they still craved AA-MFs. Thus, it is striking that we observed a similar response in plasma Phe concentrations with GMP-MFs compared with AA-MFs as reported for our inpatient study (20)—i.e., a nonsignificant mean ± SD increase in plasma Phe concentration of 57 ± 172 μmol/L (n = 11) (20) when all food was provided to subjects in the clinical research unit compared with a nonsignificant increase of 62 ± 219 μmol/L (n = 30) when subjects were asked to follow a low-Phe diets supplemented with AA-MFs and GMP-MFs at home. Taken together, this supports adequate compliance with a demanding dietary intervention and reliability of our study results.
Several explanations may account for a higher dietary Phe intake without a significant increase in plasma Phe concentration, suggesting an apparent increase in Phe tolerance, with ingestion of GMP-MFs. High concentrations of the large, neutral AAs Ile, Leu, and Thr in glycomacropeptide may reduce intestinal absorption of Phe through the shared B0AT1 transport system, which includes the intestinal LAT2 transporter, and for which Met, Leu, Ile, and Val show the highest affinity (42–44). The greater daily frequency of intake of GMP-MFs than AA-MFs may improve protein utilization resulting in lower circulating Phe concentrations (45). Our inpatient study showed improved protein retention with GMP-MFs compared with AA-MFs as reflected in delayed intestinal absorption of AAs and a significant reduction in postprandial BUN (20). In the current study in which fasting blood samples were used, BUN was not altered; however, plasma albumin concentration was significantly higher with GMP-MFs in support of improved protein synthesis or reduced inflammation (19) than with AA-MFs. The Leu supplementation of GMP-MFs (∼200 mg Leu/g PEs), which provides 50% more Leu than AA-MFs and balances the higher Ile content of the glycomacropeptide peptide, may also contribute to improved protein retention as noted in the elderly and in athletes fed whey protein with Leu supplementation (46, 47).
Expansion of the plasma Thr pool with greater intake of Thr from GMP-MFs may improve Phe tolerance. Sanjurjo et al. (32) reported a significant decrease in plasma Phe concentration in phenylketonuria subjects given a dietary Thr supplementation of 50 mg · kg−1 · d−1; Thr intake from GMP-MFs was ∼80 mg · kg−1 · d−1 in the current study. The observation of a significant negative correlation between increasing plasma Thr concentration and decreasing plasma Phe concentration suggests an association between Thr and Phe metabolism in phenylketonuria subjects who consumed GMP-MFs and a putative effect of Thr to reduce Phe concentrations. In support of this concept, the 6 subjects who showed a decrease in Thr concentration with GMP-MFs, suggesting poor compliance before the final visit, all showed an increase in plasma Phe concentration.
Strengths of our study include the controlled dietary crossover design without evidence of significant sequence or carryover effects, evidence of adequate dietary compliance, and a heterogeneous population of subjects with both classical and variant phenylketonuria from across the United States. Limitations include a relatively short dietary treatment period of 3 wk, evidence of subject bias toward a preference for AA-MFs, and inclusion of 15 different brands of AA-MFs to accommodate subject preferences.
In conclusion, this is the first clinical trial to our knowledge to compare medical foods containing primarily intact protein from glycomacropeptide with traditional AA-MFs. We demonstrate that GMP-MFs are a safe and acceptable option for the nutritional management of phenylketonuria, which may improve lifelong adherence with the requisite low-Phe diet for individuals with phenylketonuria. Reduction in the current Phe content of GMP-MFs (∼1.8 mg Phe/g PEs) is desirable and may lead to reduced blood Phe concentrations. Development of GMP-MFs for infant and toddler products warrants consideration. Further research is needed to understand how ingestion of GMP-MFs affects Thr metabolism and the intestinal microbiota.
Acknowledgments
We thank Sandra van Calcar, who initially helped design and implement the protocol at the Waisman Center; Ann Wessel, who helped with data collection at the Boston site; and Sally Gleason, who provided assistance with recruiting subjects and analyzing the food records.
The authors’ responsibilities were as follows—DMN, MKC, and HLL: designed the research; BMS, GMR, FR, and HLL: collected the data; DMN, BMS, MKC, and SGM: analyzed the data; DMN: wrote the manuscript with critical input and comments from all other authors and had primary responsibility for the final content; and BMS, MKC, SGM, GMR, FR, and HLL: read and approved the final version of the manuscript. DMN is a co-inventor on US Patent 8,604,168 B2, “Glycomacropeptide Medical Foods for Nutritional Management of Phenylketonuria and other Metabolic Disorders,” which is held by the Wisconsin Alumni Research Foundation and licensed to Cambrooke Therapeutics, LLC, and is a consultant to Arla Foods Ingredients and Davisco Foods International. None of the other authors reported a conflict of interest related to this study. Cambrooke Therapeutics Inc. had no role in the design or conduct of the study or in the collection, analysis, or interpretation of the data.
Footnotes
Abbreviations used: AA, amino acid; AA-MF, amino acid medical food; BAI, Beck Anxiety Inventory; BRIEF, Behavior Rating Inventory of Executive Function; BUN, blood urea nitrogen; GEC, Global Executive Composite; GMP-MF, glycomacropeptide medical food; PAH, phenylalanine hydroxylase; PE, protein equivalent; RD, registered dietitian.
REFERENCES
- 1.Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life 2013;65:341–9. [DOI] [PubMed] [Google Scholar]
- 2.Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, Mitchell J, Smith WE, Thompson BH, Berry SA, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med 2014;16:188–200. [DOI] [PubMed] [Google Scholar]
- 3.Macleod EL, Ney DM. Nutritional management of phenylketonuria. Ann Nestle Eng 2010;68:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RH, Rohr F, Frazier D, Cunningham A, Mofidi S, Ogata B, Splett PL, Moseley K, Huntington K, Acosta PB, et al. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med 2014;16:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health 2004;16:41–5. [DOI] [PubMed] [Google Scholar]
- 6.Antenor-Dorsey JA, Hershey T, Rutlin J, Shimony JS, McKinstry RC, Grange DK, Christ SE, White DA. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab 2013;109:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groot MJ, Hoeksma M, van Rijn M, Slart RH, van Spronsen FJ. Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients. Mol Genet Metab 2012;105:566–70. [DOI] [PubMed] [Google Scholar]
- 8.Solverson P, Murali SG, Litscher SJ, Blank RD, Ney DM. Low bone strength is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet. PLoS One 2012;7:e45165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen KE, Ney D. A systematic review of bone mineral density and fractures in phenylketonuria. J Inherit Metab Dis 2014;37:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennermann JB, Roloff S, Gellermann J, Vollmer I, Windt E, Vetter B, Plockinger U, Monch E, Querfeld U. Chronic kidney disease in adolescent and adult patients with phenylketonuria. J Inherit Metab Dis 2013;36:747–56. [DOI] [PubMed] [Google Scholar]
- 11.van Calcar SC, Ney DM. Food products made with glycomacropeptide, a low-phenylalanine whey protein, provide a new alternative to amino acid-based medical foods for nutrition management of phenylketonuria. J Acad Nutr Diet 2012;112:1201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ney DM, Blank RD, Hansen KE. Advances in the nutritional and pharmacological management of phenylketonuria. Curr Opin Clin Nutr Metab Care 2014;17:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzel MR. Manufacture and use of dairy protein fractions. J Nutr 2004;134:996S–1002S. [DOI] [PubMed] [Google Scholar]
- 14.Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM. Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab 2007;92:176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawin EA, De Wolfe TJ, Aktas B, Stroup BM, Murali SG, Steele JL, Ney DM. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol 2015;309:G590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr 2000;84(Suppl 1):S39–46. [DOI] [PubMed] [Google Scholar]
- 17.Thoma-Worringer C, Sorensen J, Lopez-Findino R. Health effects and technological features of caseinomacropeptide. Int Dairy J 2006;16:1324–33. [Google Scholar]
- 18.Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr 2008;138:316–22. [DOI] [PubMed] [Google Scholar]
- 19.Solverson P, Murali SG, Brinkman AS, Nelson DW, Clayton MK, Yen CL, Ney DM. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab 2012;302:E885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Calcar SC, Macleod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, Ney DM. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr 2009;89:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLeod EL, Clayton MK, van Calcar SC, Ney DM. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol Genet Metab 2010;100:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi SH, Singh RH. Protein substitute for children and adults with phenylketonuria. Cochrane Database Syst Rev 2015;2:CD004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, Levy H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab 2007;92:63–70. [DOI] [PubMed] [Google Scholar]
- 24.Guy SC, Isquith, PK, Gioia, GA. Behavior rating inventory of executive function: self-report version. Lutz (FL): Psychological Assessment Resources, Inc; 2004.
- 25.Ney DM, Etzel MR, inventors. Glycomacropeptide medical foods for nutritional management of phenylketonuria and other metabolic disorders. US patent 8,604,168 B2. 2013 Dec 10.
- 26.Schuett V. Low protein food list for PKU. 2nd ed. Seattle (WA): National PKU News; 2002. [Google Scholar]
- 27.Ney DM, Gleason ST, van Calcar SC, Macleod EL, Nelson KL, Etzel MR, Rice GM, Wolff JA. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis 2009;32:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao XH, Wen ZM, Meredith CN, Matthews DE, Bier DM, Young VR. Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr 1986;43:795–802. [DOI] [PubMed] [Google Scholar]
- 29.Darling PB, Grunow J, Rafii M, Brookes S, Ball RO, Pencharz PB. Threonine dehydrogenase is a minor degradative pathway of threonine catabolism in adult humans. Am J Physiol Endocrinol Metab 2000;278:E877–84. [DOI] [PubMed] [Google Scholar]
- 30.Stroup BM, Held PK, Williams P, Clayton MK, Murali SG, Rice GM, Ney DM. Clinical relevance of the discrepancy in phenylalanine concentrations analyzed using tandem mass spectrometry compared with ion-exchange chromatography in phenylketonuria. Mol Genet Metab Rep 2016;6:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem 2003;49:1797–817. [DOI] [PubMed] [Google Scholar]
- 32.Sanjurjo P, Aldamiz L, Georgi G, Jelinek J, Ruiz JI, Boehm G. Dietary threonine reduces plasma phenylalanine levels in patients with hyperphenylalaninemia. J Pediatr Gastroenterol Nutr 2003;36:23–6. [DOI] [PubMed] [Google Scholar]
- 33.Lindegren M, Krishnaswami S, Fonnesbeck C, Reimschisel T, Fisher J, Jackson K, Shields T, Sathe N, McPheeters M. Adjuvant treatment for phenylketonuria (PKU). Comparative effectiveness review no.56. AHRQ Publication No 12-EHC035-EF. Rockville (MD): Agency for Healthcare Research and Quality; 2012. [PubMed]
- 34.Mayo Clinic [Internet]. Rochester (MN): Mayo Medical Laboratories; c1995–2016 [cited 2016 Mar 15]. Test ID: AAQP. Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/9265.
- 35.Elango R, Chapman K, Rafii M, Ball RO, Pencharz PB. Determination of the tolerable upper intake level of leucine in acute dietary studies in young men. Am J Clin Nutr 2012;96:759–67. [DOI] [PubMed] [Google Scholar]
- 36.Manz F, Schmidt H, Scharer K, Bickel H. Acid-base status in dietary treatment of phenylketonuria. Pediatr Res 1977;11:1084–7. [PubMed] [Google Scholar]
- 37.Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 1996;81:288–302. [DOI] [PubMed] [Google Scholar]
- 38.Sawin E, Stroup B, Murali S, Ney D. Metabolomics analysis of phenylketonuria and wild type mice fed casein, amino acid and glycomacropeptide diets. FASEB J 2015;29Suppl 1:745 1. Abstract [Google Scholar]
- 39.de Oliveira FPMR, Dobbler PT, Mai V, Pylro VS, Waugh SG, Vairo F, Refosco LF, Roesch LFW, Schwartz IVD. Phenylketonuria and gut microbiota: a controlled study based on next generation sequencing. PLoS One 2016 Jun 23; DOI:10.1371/journal.pone.0157513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr 2016;103:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53. [DOI] [PubMed] [Google Scholar]
- 42.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008;88:249–86. [DOI] [PubMed] [Google Scholar]
- 43.Schindeler S, Ghosh-Jerath S, Thompson S, Rocca A, Joy P, Kemp A, Rae C, Green K, Wilcken B, Christodoulou J. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab 2007;91:48–54. [DOI] [PubMed] [Google Scholar]
- 44.Hidalgo IJ, Borchardt RT. Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta 1990;1028:25–30. [DOI] [PubMed] [Google Scholar]
- 45.MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW. Administration of protein substitute and quality of control in phenylketonuria: a randomized study. J Inherit Metab Dis 2003;26:319–26. [DOI] [PubMed] [Google Scholar]
- 46.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 2014;99:276–86. [DOI] [PubMed] [Google Scholar]
- 47.Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 2016;103:830–40. [DOI] [PubMed] [Google Scholar]