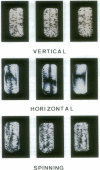
Abstract
Reduction-diffusion theories can account for both morphogenesis and the sensitivity of biological systems to weak fields. They predict that gravity can cause the symmetry breaking that is necessary for pattern formation. Microtubules play an important role in organizing the cell, and recent studies hae shown that they can form in vitro dissipative structures. We have found that these structures show patterns of microtubular orientation that are gravity dependent and that the gravitational field causes symmetry breaking. This behavior, which cannot be explained by convection, is in accordance with the theory of dissipative structures. These results suggest that microtubular dissipative structures may play an important role both in morphogenesis and in accounting for the sensitivity of biological systems to weak fields. They aso provide another explanation for biological gravitropism.
Full text
PDF
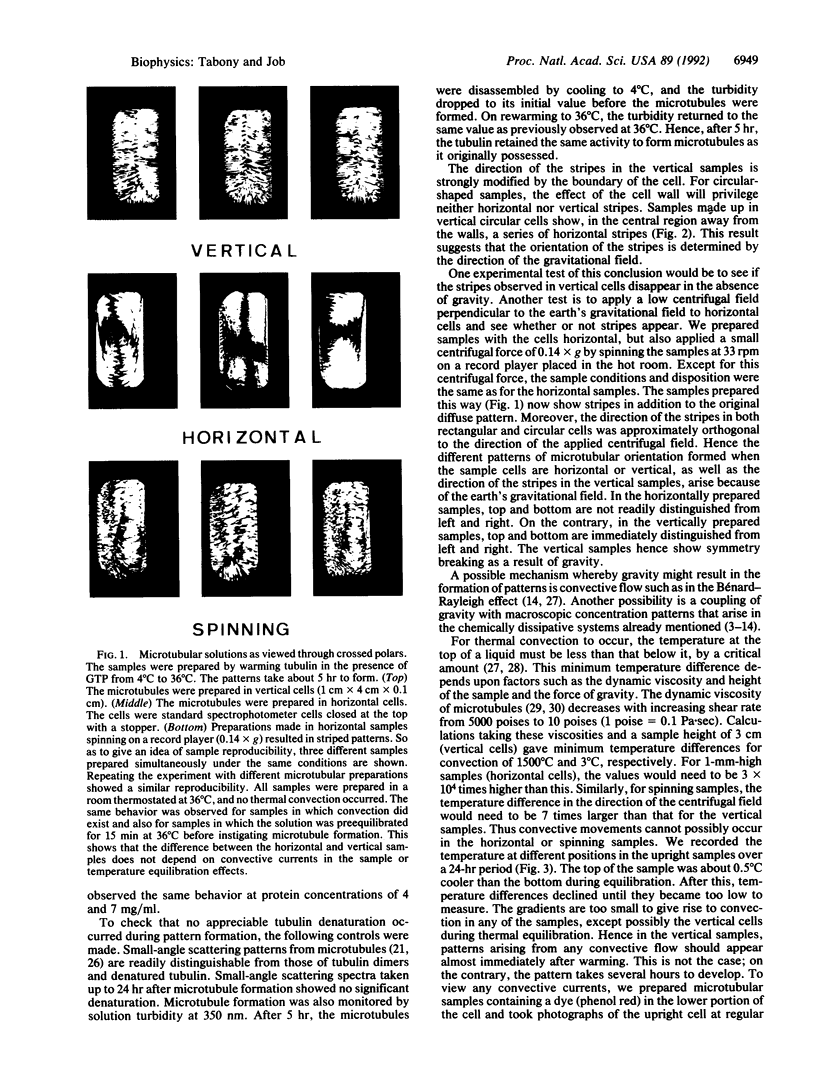
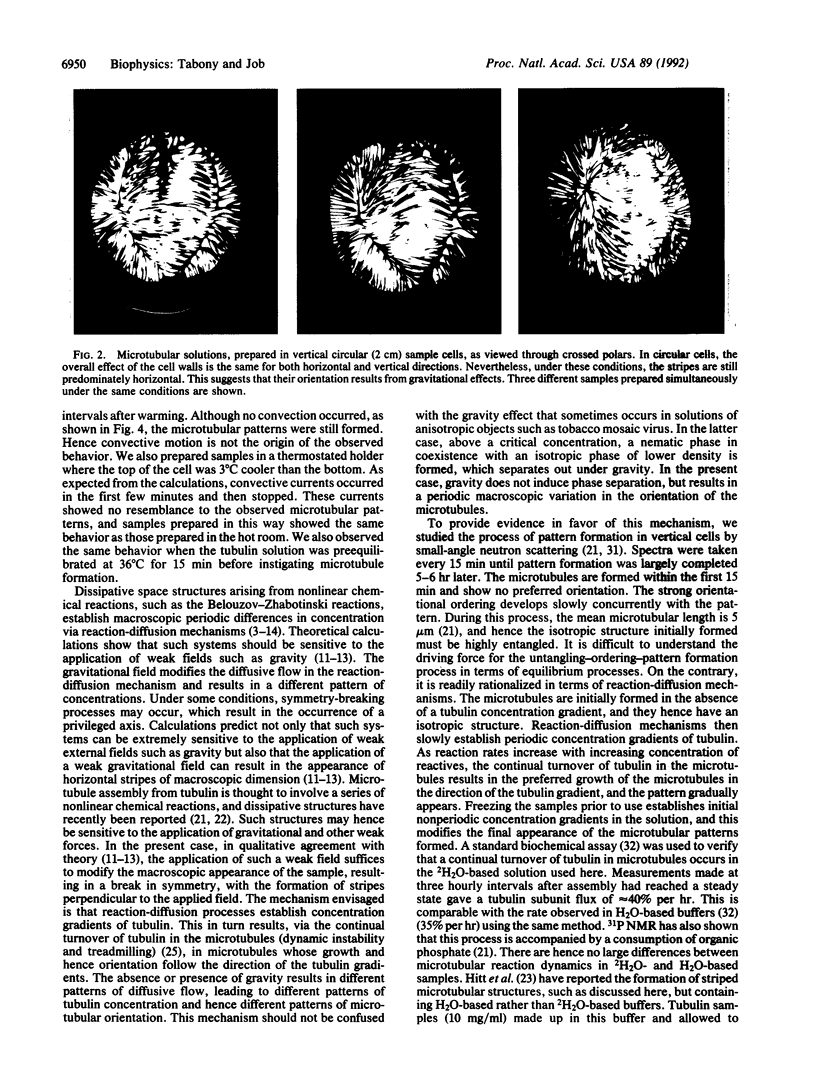

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordas J., Mandelkow E. M., Mandelkow E. Stages of tubulin assembly and disassembly studied by time-resolved synchrotron X-ray scattering. J Mol Biol. 1983 Feb 15;164(1):89–135. doi: 10.1016/0022-2836(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Buxbaum R. E., Dennerll T., Weiss S., Heidemann S. R. F-actin and microtubule suspensions as indeterminate fluids. Science. 1987 Mar 20;235(4795):1511–1514. doi: 10.1126/science.2881354. [DOI] [PubMed] [Google Scholar]
- Caspar T., Pickard B. G. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Elinson R. P., Rowning B. A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev Biol. 1988 Jul;128(1):185–197. doi: 10.1016/0012-1606(88)90281-3. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Keller R. Region-specific cell activities in amphibian gastrulation. Annu Rev Cell Biol. 1986;2:201–229. doi: 10.1146/annurev.cb.02.110186.001221. [DOI] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt A. L., Cross A. R., Williams R. C., Jr Microtubule solutions display nematic liquid crystalline structure. J Biol Chem. 1990 Jan 25;265(3):1639–1647. [PubMed] [Google Scholar]
- Job D., Pabion M., Margolis R. L. Generation of microtubule stability subclasses by microtubule-associated proteins: implications for the microtubule "dynamic instability" model. J Cell Biol. 1985 Nov;101(5 Pt 1):1680–1689. doi: 10.1083/jcb.101.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacinski G. M., Neff A. W. The amphibian egg as a model system for analyzing gravity effects. Adv Space Res. 1989;9(11):169–176. doi: 10.1016/0273-1177(89)90071-9. [DOI] [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M., Hotani H., Hess B., Müller S. C. Spatial patterns from oscillating microtubules. Science. 1989 Dec 8;246(4935):1291–1293. doi: 10.1126/science.2588005. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Regulation of the microtubule steady state in vitro by ATP. Cell. 1979 Nov;18(3):673–679. doi: 10.1016/0092-8674(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Sato M., Schwartz W. H., Selden S. C., Pollard T. D. Mechanical properties of brain tubulin and microtubules. J Cell Biol. 1988 Apr;106(4):1205–1211. doi: 10.1083/jcb.106.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwuchow J., Sack F. D., Hartmann E. Microtubule distribution in gravitropic protonemata of the moss Ceratodon. Protoplasma. 1990;159:60–69. doi: 10.1007/BF01326635. [DOI] [PubMed] [Google Scholar]
- Tabony J., Job D. Spatial structures in microtubular solutions requiring a sustained energy source. Nature. 1990 Aug 2;346(6283):448–451. doi: 10.1038/346448a0. [DOI] [PubMed] [Google Scholar]