Abstract
Spatial interpolation is employed to improve exposure estimates and to assess adverse health effects associated with environmental risk factors. Since various studies have reported that high ozone (O3) concentrations can give rise to adverse effects on respiratory symptoms and lung function, we investigated the association between O3 levels and lung function using a variety of spatial interpolation techniques and evaluated how different methods for estimating exposure may influence health results for a cohort from an industrial complex (Gwangyang Bay) in South Korea in 2009. To estimate daily concentrations of O3 in each subject, four different methods were used, which include simple averaging, nearest neighbor, inverse distance weighting, and kriging. Also, to compare the association between O3 levels and lung function by age-groups, we explored ozone’s impacts on three age-related groups: children (9–14 years), adults (15–64 years), and the elderly (≥65 years). The overall change of effect size on lung function in each age group tended to show similar patterns for lag and methods for estimating exposure. A significant negative association was only observed between O3 levels and FVC and FEV1 for most of the lag and methods in children. The largest effect of O3 levels was found at the average for the lung function test day and last 2 days (0–2 days). In conclusions, the spatial interpolation methods may benefit in providing individual-level exposure with appropriate temporal resolution from ambient monitors. However, time-activity patterns of residents, monitoring site locations, methodological choices, and other factors should be considered to minimize exposure misclassification.
Keywords: spatial interpolation, ozone, lung function, kriging
1. Introduction
Air pollution exposure assessment is a crucial component to investigate the relationship between air pollution and health effects in epidemiological studies. As the magnitude of the health effects of ambient air pollution often relies on exposure assessment methods, the method for estimating exposure is an important factor to determine the actual or potential exposure level of humans. The most desirable method for estimating personal exposure is to measure directly through personal monitoring [1]. However, since direct measurement of exposure at the individual level can be time consuming and expensive, in large-scale epidemiological studies it can be commonly estimated by fixed ambient air pollution monitoring data. Also, most epidemiology studies use data spatially aggregated at the area level because of data limitations.
The advantage of monitoring data includes the ability to use existing data and to cover a large spatial area [2]. Previous epidemiological studies for air pollution have assigned exposures using data from a few nearest air monitors and used the exposure as a surrogate for the actual personal exposure [3,4]. However, this approach may cause uncertainty such as exposure misclassification, and may underestimate or overestimate the health effects of air pollution because it does not reflect the spatial heterogeneity of individuals [5,6].
Recently, spatial interpolation is increasingly being used to assess adverse health effects associated with environmental risk factors. Advances in geostatistical methods and geospatial technologies based on geographic information systems (GIS) have made it possible to improve air pollution exposure assessments. Specifically, a number of studies have been conducted to estimate the relationship between air pollution and health outcomes using spatial methods such as proximity models [7,8], geostatistical methods [9,10] and land-use regression models [11,12]. In particular, to minimize exposure misclassification, advanced interpolation methods have been applied to better estimate a population’s or individual’s exposures to air pollution. Kriging is one of these interpolation methods, which is developed based on statistical techniques in geostatistics for optimal spatial prediction at unobserved locations [13,14].
The short-term exposure effects of ozone have been demonstrated to cause lung function impairment, lung inflammation and respiratory symptoms [15]. In addition, high O3 concentrations have been associated with adverse effects on respiration and lung function [16]. While many studies have focused on the characteristics of surface ozone concentration and distribution, relatively few have investigated how this pollutant affected health outcomes. Also, almost no investigations have utilized improved methods for estimating exposure for the association between ozone and health outcomes.
In this study, we investigated the association between ozone level at an industrial complex in South Korea and lung function with two objectives, one of which is estimating and comparing ozone exposures using four different methods including simple averaging across all monitors in the study area, spatial interpolation by the nearest monitoring station, inverse distance weighting and ordinary kriging and the other of which is evaluating how different methods for estimating exposure influence health outcomes.
2. Materials and Methods
2.1. Study Area and Subjects
The study area is a major national industrial complex (Gwangyang Bay) where petrochemical and steel-related industries such as steel mills and a thermal power plant are densely placed. This area is a geographically closed coastal area where air pollutants become stagnant and do not spread into the atmosphere. It is well established from the national environmental project [17] that surface ozone is a secondary air pollutant, which affects human health.
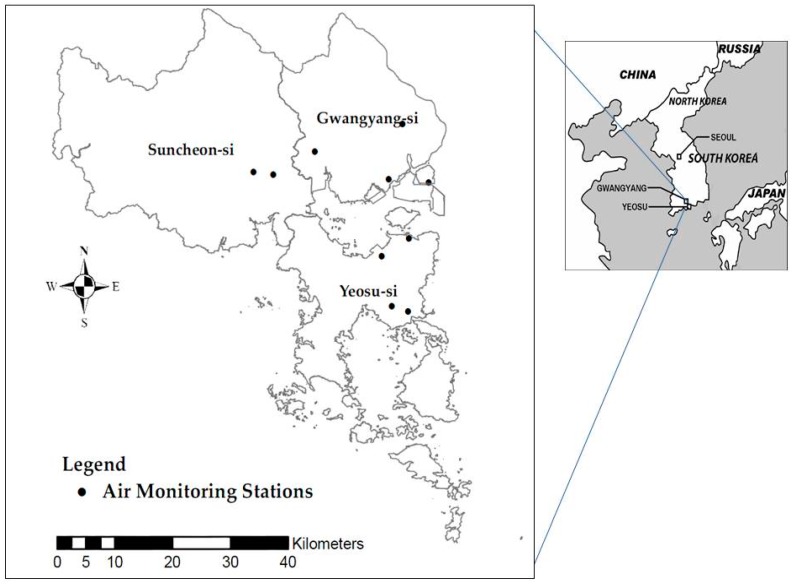
Information concerning demographic characteristics, and residential and medical histories was investigated. A total of 2283 participants who had registered accurate residential addresses and undergone a pulmonary function test were recruited in 2009. Figure 1 shows the study area and location of the fixed monitoring stations. This study was approved by the Institutional Review Board of Soonchunhyang University (IRB ID: 2007-15-02).
Figure 1.
The locations of the study area and air monitoring stations.
2.2. Ambient Ozone Exposure Estimation
Concentrations of O3 were measured for every hour of every day by an ultraviolet photometric analyzer using 10 ambient air monitoring stations located in Gwangyang Bay. We obtained hourly O3 data to analyze their association with the lung function of the study subjects. For statistical analysis, the maximum daily 8 h moving average of the ozone data was calculated.
To estimate daily concentrations of O3 in each subject, four different methods were used. Individual O3 exposure was assigned the values of the average of all monitors in the study area (Method 1), the predicted concentration observed at the nearest monitoring station (Method 2), inverse distance weighting (IDW) proportional to the distance from the estimated value (Method 3), and kriging were used to quantify the tendency of the differences between values at points to increase with distance (Method 4). Method 1 assigns exposure as the average value of all monitors in the study area. We calculated the average of the values from 10 monitors and assigned that value to the subjects as the same level of ozone exposure. Method 2 selects the value of the nearest monitor and does not consider the values of neighboring monitors at all. In this study, each subject was assigned the ozone concentration level of the single monitor nearest to the subject’s residence. Method 3 is the simplest interpolation method. The IDW interpolation method is based on the assumption that unknown values are influenced more by nearby points than far away points. The interpolation weights of each monitor’s values are calculated as a function of distance between the observed values of a sample site and the site at which the prediction is sought [18].
The observed values that are closer to the sample point of interest are more heavily weighted; however, a large search window can still preserve some of the local variations in pollutant levels [19]. To predict a value for location of subject’s residence, the data from all monitors within the Gwangyang Bay area were included in the IDW interpolation for O3. We utilized λi = 1/di as a weighting factor for monitor i, where di is the distance between monitor i and the point to be predicted [19]. Kriging is an interpolation technique that estimates values at unmonitored locations from observed values and semi-variograms. IDW uses a simple algorithm based on distance, but the kriging method (Method 4) was weighted based on spatial autocorrelation, which was determined from the variogram developed by the spatial structure of the data. Kriging requires the estimation of the empirical semi-variogram, based on a theoretical semi-variogram function such as spherical, gaussian and exponential models [20]. We used ordinary kriging with a spherical model to estimate the daily O3 concentration [21]. The spherical variogram is one of the most commonly chosen forms, and the ordinary kriging algorithm takes advantage of the assumptions made about an unknown constant mean value and is appropriate for our data.
2.3. Cross-Validation
In order to ensure the validity of the values predicted by the interpolation methods we performed cross-validation. Each air monitor’s ozone value was excluded, and then the remaining monitor values were used to estimate the ozone value at the excluded monitor location. To evaluate the quality of prediction by interpolation methods and similarity between the measured and predicted values, the root mean square error (RMSE) was calculated [22]. The consistency of the measured and predicted values was applied with the coefficient of divergence (COD). A COD of zero implies there are no differences between measured and predicted values, while COD values approaching one indicates maximum differences [23]. The RMSE and COD are defined as:
| (1) |
| (2) |
where Xij and Xik represent the average concentrations of the measured and predicted ozone for day i, and p is the number of observations.
2.4. Lung Function Tests
The lung function was measured with the forced vital capacity (FVC) and forced expiratory flow in 1 s (FEV1) methods during the morning hours in indoor buildings. These measurements were performed by experienced technicians in accordance with the American Thoracic Society criteria [24]. Each subject was asked repeated measurements no more than five times until the best result appeared. Pulmonary function was performed using a spirometer (Spirovit model SP1, Schiller, Ottobrunn, Germany).
2.5. Statistical Analysis
We estimated O3 concentrations at the study subjects’ locations for the lung function test day (0 day), 1 day before the lung function test (1 day), 2 days previous (2 days), average of the test day and previous day (0–1 day), average of the last 2 days (1–2 days), and average of the test day and last 2 days (0–2 days). In order to compare the associations between O3 levels and lung functions by age-group, the ozone’s impacts on three age-related groups were explored with children (9–14 years), adults (15–64 years), and the elderly (≥65 years). In this study, multivariable regression analysis was used to determine FVC and FEV1 against predicted values of ozone at each lag and to assess the association of ozone levels with pulmonary function (FVC and FEV1) by the four different methods for estimating exposure. A p-value < 0.05 was considered significant for all analyses. The data were analyzed using the R3.0.1 (R Foundation for Statistical Computing, Seoul, Korea) and SAS 9.2 (SAS Institute, Cary, NC, USA).
3. Results
A total of 2283 ozone exposures were estimated at the individual level from information based on the residential locations of the subjects. The summary descriptive statistics of O3 concentrations for each method are shown in Table 1.
Table 1.
Summary statistics comparing the result of the four different methods for daily O3 concentrations at the the Gwangyang Bay industrial complex (n = 2283).
| Model | Mean | SD | Min | 25th | 50th | 75th | Max |
|---|---|---|---|---|---|---|---|
| Method 1 | 42.2 | 15.5 | 10.4 | 30.1 | 39.4 | 54.0 | 88.6 |
| Method 2 | 40.9 | 16.6 | 3.7 | 28.9 | 38.9 | 51.8 | 113.8 |
| Method 3 | 41.4 | 15.6 | 3.8 | 29.7 | 39.0 | 52.2 | 113.1 |
| Method 4 | 41.7 | 15.9 | 5.9 | 29.6 | 39.2 | 52.6 | 113.5 |
Daily 8 h maximum moving average, Method 1: simple averaging, Method 2: nearest neighbor, Method 3: inverse distance weighting, Method 4: kriging.
These summary statistics are the average across the 2283 subjects. The mean O3 values were 42.2 ppb from Method 1, 40.9 from Method 2, 41.4 from Method 3 and 41.7 from Method 4. The average values of estimated O3 exposures were not significantly different in each method. The largest variation in spatial concentration among the four methods was nearest the monitors (Method 2). Table 2 shows the mean and range levels of daily max 8 h moving average of O3 concentrations on each day, and the lung function test was performed for 1 lag, 2 lag, 0–1 lag, 1–2 lag and 0–2 lag by the four different methods for estimating exposure.
Table 2.
Distributions of O3 concentrations measured on lung function test day, 1 day, 2 days, 0–1 day, 1–2 days and 0–2 days by the four different methods.
| Lag Days | Method 1 | Method 2 | Method 3 | Method 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| 0 day | 59.8 (12.0) | 31.5–85.9 | 56.0 (14.6) | 26.0–85.7 | 57.8 (12.6) | 30.2–93.9 | 58.1 (13.0) | 31.5–97.8 |
| 1 day | 58.1 (11.7) | 35.8–82.8 | 53.9 (13.6) | 26.0–93.9 | 55.4 (12.1) | 30.9–93.4 | 55.7 (12.2) | 30.0–98.2 |
| 2 days | 58.9 (12.2) | 28.3–85.0 | 56.2 (12.3) | 24.0–96.4 | 57.7 (12.5) | 24.0–90.5 | 57.9 (12.3) | 23.9–90.1 |
| 0–1 day | 58.9 (10.6) | 36.7–84.4 | 54.9 (12.7) | 30.0–95.4 | 56.6 (10.8) | 33.5–93.6 | 56.9 (11.0) | 36.3–91.6 |
| 1–2 days | 58.5 (10.7) | 32.0–81.8 | 55.0 (11.2) | 26.8–93.6 | 56.6 (10.9) | 27.9–89.6 | 56.8 (10.8) | 27.3–88.3 |
| 0–2 days | 58.9 (9.8) | 36.2–83.2 | 55.3 (10.7) | 34.1–90.7 | 57.0 (9.8) | 34.7–89.0 | 57.2 (9.9) | 34.7–87.2 |
Method 1: simple averaging, Method 2: nearest neighbor, Method 3: inverse distance weighting, Method 4: kriging, SD: standard deviation, 0 day: lung function test day, 1 day: 1 day before lung function test, 2 days: 2 days before lung function test, 0–1 day: average of the test day and previous day, 1–2 days: average of the last 2 days, 0–2 days: average of the test day and last 2 days.
Ranges of concentrations of ozone were 28.3–85.9 ppb (Method 1), 24.0–96.4 (Method 2), 24.0–93.9 (Method 3), 23.9–98.2 (Method 4). The concentrations of ozone on the day of the lung function test (0d) were the highest in all methods except for Method 2 (nearest monitor). We found that the estimated ozone exposure from kriging showed more excellent cross-validation results than those from the other interpolation methods from the cross-validation analysis. The RMSE indicated the difference between observed and predicted ozone concentration for kriging was the lowest. The COD value for kriging was less than those of nearest monitor and IDW (Table 3).
Table 3.
Results from cross-validation data in this study.
| Pollutant | Method | RMSE | COD |
|---|---|---|---|
| O3 | Nearest monitor | 10.5 | 0.139 |
| O3 | IDW | 8.75 | 0.113 |
| O3 | Kriging | 7.48 | 0.099 |
RMSE: root mean square error, COD: coefficient of divergence.
A summary statistic of the demographic characteristics and lung function measurements of the study populations is presented in Table 4. The mean age of subjects was 41.8 year, and 56.9% were female. The mean FVC and FEV1 of each age group were 2.26, 1.92 in children, 3.23, 2.69 in adults and 2.39, 1.82 in the elderly, respectively (Table 4).
Table 4.
Demographic characteristics of the study subjects.
| Variables | All | Age 9–14 | Age 15–64 | Age ≥ 65 | |
|---|---|---|---|---|---|
| n | 2283 | 200 | 1419 | 664 | |
| Age (years) | 41.8 ± 25.7 | 12.8 ± 1.23 | 30.7 ± 17.8 | 74.2 ± 5.60 | |
| Gender (%) | Male | 43.1 | 47.5 | 46.5 | 34.5 |
| Female | 56.9 | 52.5 | 53.5 | 65.5 | |
| Height (cm) | 158.7 ± 10.0 | 145.4 ± 8.50 | 162.3 ± 8.57 | 155.2 ± 8.52 | |
| Weight (kg) | 55.6 ± 12.3 | 37.8 ± 9.89 | 57.4 ± 11.6 | 57.1 ± 9.90 | |
| BMI (kg/m2) | 21.9 ± 3.89 | 17.6 ± 3.53 | 21.7 ± 3.70 | 23.6 ± 3.25 | |
| Lung function | FEV1 (L) | 2.37 ± 0.73 | 1.92 ± 0.35 | 2.69 ± 0.66 | 1.82 ± 0.55 |
| FVC (L) | 2.90 ± 0.84 | 2.26 ± 0.42 | 3.23 ± 0.76 | 2.39 ± 0.71 | |
| FEV1/FVC (%) | 81.6 ± 8.76 | 85.5 ± 6.57 | 83.4 ± 7.65 | 76.6 ± 9.48 |
BMI: body mass index, FVC: forced vital capacity, FEV1: forced expiratory flow in 1 s.
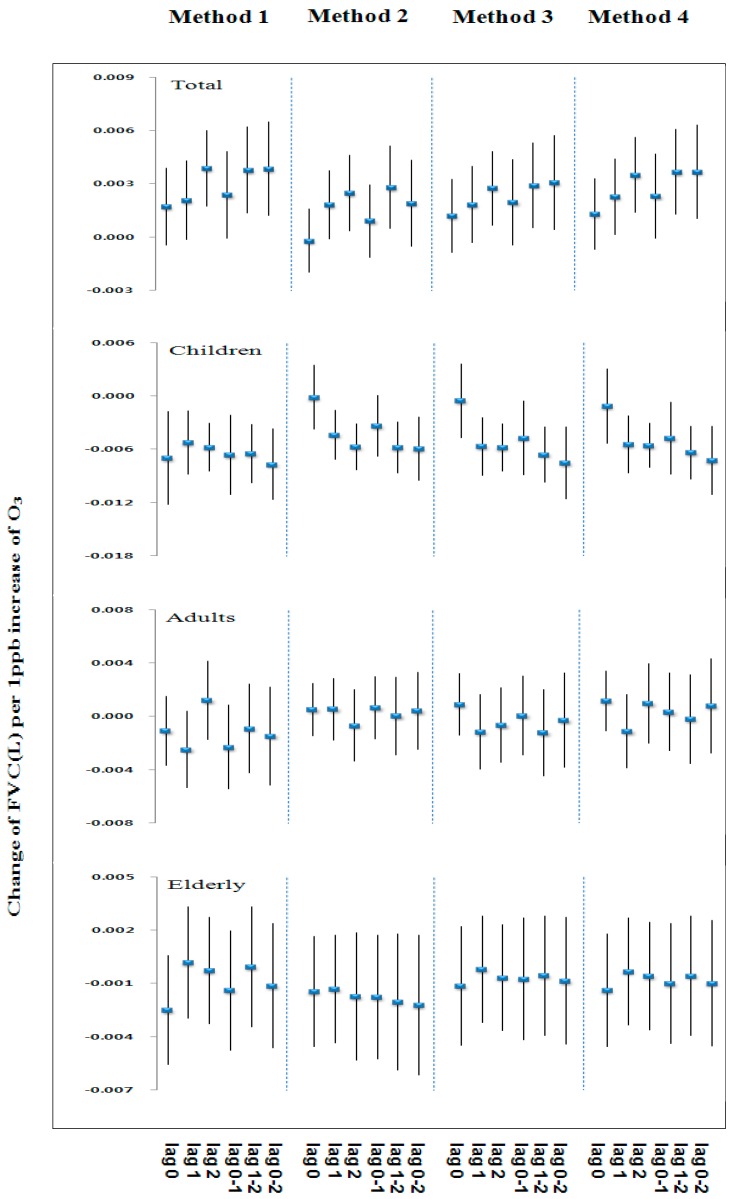
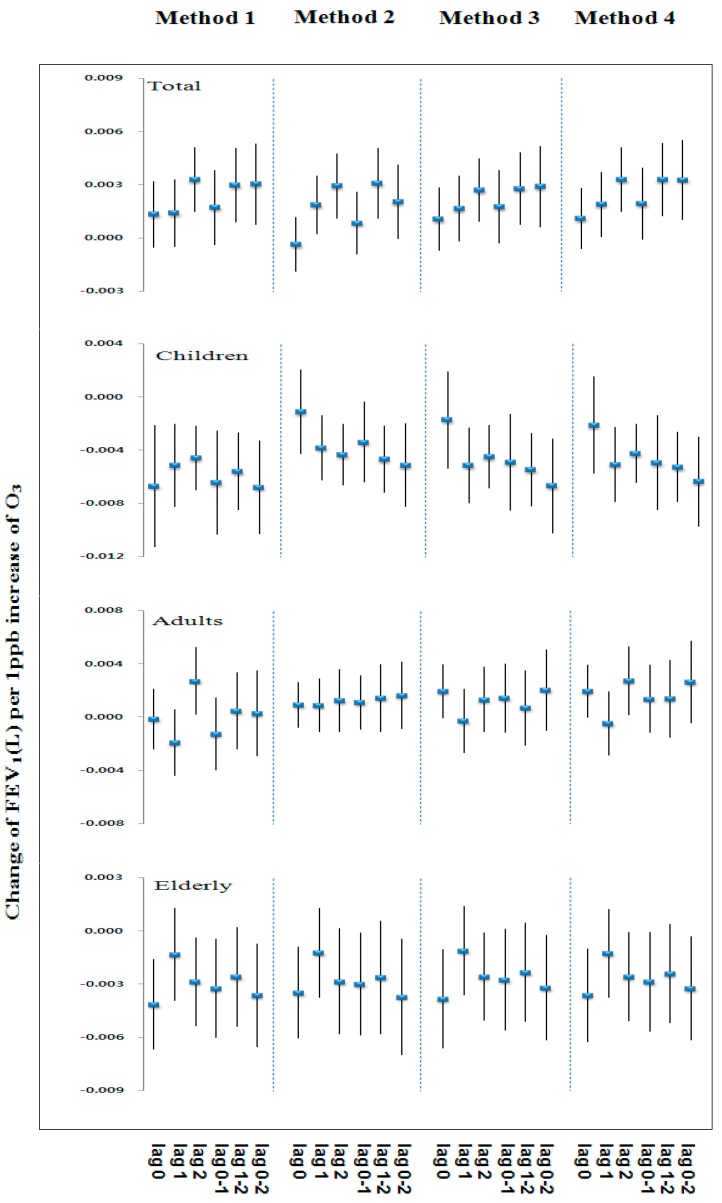
Figure 2 and Figure 3 show similar patterns of changes in effect size on FVC and FEV1 associated with exposure to 1 ppb increase of ozone concentrations by lag and the four methods for estimating exposure in each age group. The overall change of effect size on lung function in each age group tended to show similar patterns for lag and the methods for estimating exposure. Table 5 and Table 6 show the estimated changes in FVC and FEV1 for an increase to the inter-quartile range of O3 concentrations by the four methods and age groups. In children (age 9–14), the significant negative association were observed between O3 and FVC and FEV1 for most of the lag and the methods.
Figure 2.
Change of FVC (L) by O3 concentration for various lags and methods for estimating exposure.
Figure 3.
Change of FEV1 (L) by O3 concentration for various lags and methods for estimating exposure.
Table 5.
Age-specific effect of change in FVC (L) per increase IQR † of O3 concentration by various lags and four methods for estimating exposure.
| Lag Day | Age | FVC (L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | Method 4 | ||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | (95% CI) | ||
| 0 day | 9–14 | −0.12 | (−0.21, −0.03) * | −0.00 | (−0.07, 0.07) | −0.01 | (−0.07, 0.06) | −0.02 | (−0.10, 0.06) |
| 15–64 | −0.02 | (−0.06, 0.03) | 0.01 | (−0.03, 0.05) | 0.01 | (−0.02, 0.05) | 0.02 | (−0.02, 0.07) | |
| ≥65 | −0.04 | (−0.10, 0.01) | −0.03 | (−0.09, 0.03) | −0.02 | (−0.07, 0.03) | −0.03 | (−0.09, 0.04) | |
| All | 0.03 | (−0.01, 0.07) | −0.00 | (−0.04, 0.03) | 0.02 | (−0.01, 0.05) | 0.03 | (−0.01, 0.06) | |
| 1 day | 9–14 | −0.09 | (−0.15, −0.03) * | −0.08 | (−0.13, −0.03) * | −0.09 | (−0.14, −0.04) * | −0.11 | (−0.17, −0.04) * |
| 15–64 | −0.04 | (−0.09, 0.01) | 0.01 | (−0.03, 0.05) | −0.02 | (−0.06, 0.03) | −0.02 | (−0.08, 0.03) | |
| ≥65 | 0.00 | (−0.05, 0.06) | −0.03 | (−0.08, 0.03) | −0.00 | (−0.05, 0.04) | −0.01 | (−0.07, 0.05) | |
| All | 0.04 | (−0.00, 0.07) | 0.03 | (−0.00, 0.07) | 0.03 | (−0.01, 0.06) | 0.04 | (0.00, 0.09) | |
| 2 days | 9–14 | −0.10 | (−0.15, −0.05) ** | −0.11 | (−0.16, −0.06) ** | −0.09 | (−0.13, −0.05) ** | −0.11 | (−0.16, −0.06) ** |
| 15–64 | 0.02 | (−0.03, 0.07) | −0.01 | (−0.06, 0.04) | −0.01 | (−0.05, 0.03) | 0.02 | (−0.04, 0.08) | |
| ≥65 | −0.01 | (−0.06, 0.05) | −0.03 | (−0.10, 0.04) | −0.01 | (−0.06, 0.04) | −0.01 | (−0.07, 0.05) | |
| All | 0.07 | (0.03, 0.10) | 0.05 | (0.01, 0.09) | 0.04 | (0.01, 0.07) | 0.07 | (0.03, 0.12) | |
| 0–1 day | 9–14 | −0.11 | (−0.19, −0.04) * | −0.06 | (−0.13, 0.00) | −0.07 | (−0.14, −0.01) * | −0.09 | (−0.17, −0.01) * |
| 15–64 | −0.04 | (−0.09, 0.02) | 0.01 | (−0.03, 0.06) | 0.00 | (−0.04, 0.05) | 0.01 | (−0.05, 0.06) | |
| ≥65 | −0.02 | (−0.08, 0.03) | −0.03 | (−0.10, 0.03) | −0.01 | (−0.06, 0.04) | −0.02 | (−0.09, 0.05) | |
| All | 0.04 | (−0.00, 0.08) | 0.02 | (−0.02, 0.06) | 0.03 | (−0.01, 0.07) | 0.05 | (−0.00, 0.09) | |
| 1–2 days | 9–14 | −0.11 | (−0.17, −0.06) ** | −0.11 | (−0.16, −0.06) ** | −0.10 | (−0.15, −0.05) ** | −0.12 | (−0.18, −0.07) ** |
| 15–64 | −0.02 | (−0.07, 0.04) | 0.00 | (−0.05, 0.06) | −0.02 | (−0.07, 0.03) | −0.00 | (−0.07, 0.06) | |
| ≥65 | −0.00 | (−0.060, 0.057) | −0.04 | (−0.11, 0.03) | −0.01 | (−0.06, 0.04) | −0.01 | (−0.08, 0.06) | |
| All | 0.07 | (0.02, 0.11) | 0.05 | (0.01, 0.10) | 0.05 | (0.01, 0.08) | 0.07 | (0.02, 0.12) | |
| 0–2 days | 9–14 | −0.13 | (−0.20, −0.06) ** | −0.11 | (−0.18, −0.04) * | −0.12 | (−0.18, −0.05) ** | −0.14 | (−0.22, −0.07) ** |
| 15–64 | −0.03 | (−0.09, 0.04) | 0.01 | (−0.05, 0.06) | −0.00 | (−0.06, 0.05) | 0.02 | (−0.05, 0.08) | |
| ≥65 | −0.02 | (−0.08, 0.04) | −0.04 | (−0.12, 0.03) | −0.01 | (−0.07, 0.04) | −0.02 | (−0.09, 0.05) | |
| All | 0.07 | (0.02, 0.11) | 0.04 | (−0.01, 0.08) | 0.05 | (0.01, 0.09) | 0.07 | (0.02, 0.12) | |
†: IQR is 17.3 ppb for O3, FVC: forced vital capacity, Method 1: simple averaging, Method 2: nearest neighbor, Method 3: inverse distance weighting, Method 4: kriging, CI: confidence interval, 0 day: lung function test day, 1 day: 1 day before lung function test, 2 days: 2 days before lung function test, 0–1 day: average of the test day and previous day, 1–2 days: average of the last 2 days, 0–2 days: average of the test day and last 2 days. **: p < 0.001, *: p < 0.05.
Table 6.
Age-specific effect of change in FEV1 (L) per increase IQR † of O3 concentration by various lags and four methods for estimating exposure.
| Lag Day | Age | FEV1 (L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | Method 4 | ||||||
| β | (95% CI) | β | (95% CI) | β | (95% CI) | β | 95% CI | ||
| 0 day | 9–14 | −0.12 | (−0.20, −0.04) * | −0.02 | (−0.08, 0.04) | −0.03 | (−0.08, 0.03) | −0.04 | (−0.11, 0.03) |
| 15–64 | −0.00 | (−0.04, 0.04) | 0.02 | (−0.01, 0.05) | 0.03 | (−0.00, 0.06) | 0.04 | (0.00, 0.08) | |
| ≥65 | −0.07 | (−0.12, −0.03) * | −0.07 | (−0.11, −0.02) * | −0.06 | (−0.10, −0.02) * | −0.07 | (−0.12, −0.02) * | |
| All | 0.02 | (−0.01, 0.06) | −0.01 | (−0.04, 0.02) | 0.02 | (−0.01, 0.04) | 0.02 | (−0.01, 0.05) | |
| 1 day | 9–14 | −0.09 | (−0.14, −0.04) * | −0.07 | (−0.12, −0.03) * | −0.08 | (−0.12, −0.04) * | −0.10 | (−0.15, −0.04) * |
| 15–64 | −0.03 | (−0.08, 0.01) | 0.02 | (−0.02, 0.06) | −0.00 | (−0.04, 0.03) | −0.01 | (−0.06, 0.04) | |
| ≥65 | −0.02 | (−0.07, 0.02) | −0.02 | (−0.07, 0.03) | −0.02 | (−0.06, 0.02) | −0.02 | (−0.07, 0.02) | |
| All | 0.02 | (−0.01, 0.06) | 0.04 | (0.00, 0.07) | 0.03 | (−0.00, 0.05) | 0.04 | (0.00, 0.07) | |
| 2 days | 9–14 | −0.08 | (−0.12, −0.04) * | −0.08 | (−0.13, −0.04) * | −0.07 | (−0.11, −0.03) * | −0.08 | (−0.13, −0.04) * |
| 15–64 | 0.05 | (0.00, 0.09) | 0.02 | (−0.02, 0.07) | 0.02 | (−0.02, 0.06) | 0.05 | (0.00, 0.10) | |
| ≥65 | −0.05 | (−0.09, −0.01) * | −0.05 | (−0.11, 0.00) | −0.04 | (−0.08, −0.00) * | −0.05 | (−0.10, −0.00) * | |
| All | 0.06 | (0.03, 0.09) | 0.06 | (0.02, 0.09) | 0.04 | (0.01, 0.07) | 0.06 | (0.03, 0.10) | |
| 0–1 day | 9–14 | −0.11 | (−0.18, −0.04) * | −0.06 | (−0.12, −0.01) * | −0.08 | (−0.13, −0.02) * | −0.10 | (−0.16, −0.03) * |
| 15–64 | −0.02 | (−0.07, 0.03) | 0.02 | (−0.02, 0.06) | 0.02 | (−0.02, 0.06) | 0.03 | (−0.02, 0.08) | |
| ≥65 | −0.06 | (−0.10, −0.01) * | −0.06 | (−0.11, −0.00) * | −0.04 | (−0.09, 0.00) | −0.06 | (−0.11, −0.00) * | |
| All | 0.03 | (−0.01, 0.07) | 0.02 | (−0.02, 0.05) | 0.03 | (−0.01, 0.06) | 0.04 | (−0.00, 0.08) | |
| 1–2 days | 9–14 | −0.10 | (−0.15, −0.05) ** | −0.09 | (−0.14, −0.04) * | −0.08 | (−0.13, −0.04) ** | −0.10 | (−0.15, −0.05) ** |
| 15–64 | 0.01 | (−0.04, 0.06) | 0.03 | (−0.02, 0.08) | 0.01 | (−0.03, 0.05) | 0.03 | (−0.03, 0.08) | |
| ≥65 | −0.04 | (−0.09, 0.00) | −0.05 | (−0.11, 0.01) | −0.04 | (−0.08, 0.01) | −0.05 | (−0.10, 0.01) | |
| All | 0.05 | (0.02, 0.09) | 0.06 | (0.02, 0.10) | 0.04 | (0.01, 0.07) | 0.06 | (0.02, 0.10) | |
| 0–2 days | 9–14 | −0.12 | (−0.18, −0.06) ** | −0.10 | (−0.16, −0.04) * | −0.10 | (−0.16, −0.05) * | −0.12 | (−0.19, −0.06) ** |
| 15–64 | 0.01 | (−0.05, 0.06) | 0.03 | (−0.01, 0.08) | 0.03 | (−0.02, 0.08) | 0.05 | (−0.01, 0.11) | |
| ≥65 | −0.06 | (−0.11, −0.01) * | −0.07 | (−0.13, −0.01) * | −0.05 | (−0.09, −0.00) * | −0.06 | (−0.12, −0.01) * | |
| All | 0.05 | (0.01, 0.09) | 0.04 | (−0.00, 0.08) | 0.04 | (0.01, 0.08) | 0.06 | (0.02, 0.11) | |
†: IQR is 17.3 ppb for O3, FEV1: forced expiratory flow in 1 s, Method 1: simple averaging, Method 2: nearest neighbor, Method 3: inverse distance weighting, Method 4: kriging, 0 day: lung function test day, 1 day: 1 day before lung function test, 2 days: 2 days before lung function test, 0−1 day: average of the test day and previous day, 1−2 days: average of the last 2 days, 0−2 days: average of the test day and last 2 days. **: p < 0.001, *: p < 0.05.
The largest effect of O3 was found at the average of the lung function for the test day and last 2 days (0–2 days). In adult (age 15–64), the effect of O3 on reduction in FVC and FEV1 were not significant for any lag and the methods. In the elderly (age ≥ 65), the negative association between FEV1 and O3 was significant except on 0 day, 0–1 day, but FVCs were not significant for any lag and the methods.
4. Discussion
This study assessed the association between ozone exposure and lung function using four methods for estimating exposure. To minimize exposure misclassification, we tried to estimate more accurate exposure levels by using the spatial analysis technique in the exposure assessment of epidemiological studies. Although other researchers have employed a similar method to estimate individual levels of exposure to air pollution, they did not use such approaches to compare the lung function effects of ozone in different age groups [25,26,27]. In addition, no investigations have been done utilizing interpolation methods for estimating exposure to identify the association between ozone and health outcomes in Gwangyang Bay.
In this study, we developed better exposure estimates through the application of a kriging method among the four interpolation methods. Previous studies with the application of interpolation methods have suggested the similar associations. A study by Son et al. predicted individual levels of exposure to air pollution using the four different approaches (average across all monitors, nearest monitor, IDW and kriging) to identify the association between air pollution and lung function [28]. They found that the kriging method provided the most accurate estimates of exposures, and the magnitudes of health effect estimates were generally higher when using exposures based on averaging across all monitors or kriging. A simulation study by Kim et al. examined exposure prediction approaches such as the nearest monitor and kriging methods for PM2.5 affecting relative risk estimates for cardiovascular events in Los Angeles [29]. Their findings indicated that a kriging method provided more accurate exposure estimates because it had smaller average mean square prediction error. Liao et al. reported that daily kriging estimations of residential−level ambient PM concentrations were feasible on a national scale [21]. Jerrett et al. suggested that the health effects associated with exposure to PM2.5 were approximately three times greater using the kriging approach than using the average ambient concentration approach previously employed in the American Cancer Society (ACS) cohort. This can be explained by the fact that the exposure estimation method used in estimating air pollution exposure may affect the results of epidemiologic studies [25]. However, there have been debates on which methods are most suitable for estimating air pollution from ambient monitors.
The advantage of regulatory monitoring network data includes the ability to use existing data and to estimate individual level exposure. This monitoring data would allow studies on the relationship between air pollution and health outcomes for times, locations, and pollutants for which monitoring data are limited or unavailable [2]. The Ministry of Environment (MOE) in Korea has generated hourly ambient air concentration data from 257 urban air quality monitors, including O3, PM10, NO2, SO2 and CO [30]. This monitoring network data provide good temporal resolutions, with hourly monitor coverage of 0.003 monitor/km2 at a national scale. Air monitoring stations have typically been installed in urban areas where population density is high [2,31]. For instance, the most densely populated Seoul, the capital of Korea, had hourly monitor coverage of 0.04 monitor/km2. Although fewer monitors were available in our study area, monitor coverage had a high spatial resolution of 0.01 monitor/km2.
Declines in FVC and FEV1 were significantly associated with ozone concentration in children. In this study, we assessed the relationship between changes in lung function and ozone exposure as estimated by the interpolation method. The results of this study show that there were no significant associations between ozone exposure and lung function in the whole subject, but a decrease in lung function was observed in children for most of the lag and methods. More specifically, the declines in FVC and FEV1 were significantly associated with most of the lag and methods, and the largest effect was observed for 0–2 days. Previous researches of decrease in lung function on O3 exposure in children have demonstrated similar results [32,33,34,35]. According to Chang et al., 1 ppb increase in O3 was significantly associated with decreased FVC 2.29 mL among 2919 students who lived in five school districts in Taipei [36]. Significant lag effects were observed in FVC for 0 day, 1 day and 2 days before the spirometry test. Son et al. reported that a 11 ppb increase (IQR) in O3 was associated with a decrease of 6.1% (95% confidence interval, 5.0% to 7.3%) in FVC and 0.5% (95% confidence interval, 0.03% to 0.96%) in FEV1 in lag 0–2 day, based on kriging exposures [28]. Moreover, ozone exposure is well documented in many studies to cause adverse lung injury effects, including reduced lung capacity, chronic obstructive pulmonary disease, and severe asthma exacerbation [37,38]. In this study, ozone level was calculated as the maximum daily 8-h moving average. The maximum daily 8-h moving average was computed by selecting the highest value among 17 daily 8-h moving average. Since lung function tests were performed in the morning, estimated ozone level for the lung function test day (0 day) may have implications for interpreting the association between ozone level and lung function.
It is well established that ground-level ozone is generated from the photochemical oxidation of NOx emissions and volatile organic compounds (VOCs). The major sources of NOx and VOCs are emitted during fuel combustion from automobiles, fossil fuel power plants and industrial processing. Our study area was a major industrial hub in Korea, which may release a wide range of air pollutants into the atmosphere. These air pollutants may affect the respiratory health of communities living close to the industrial areas. In particular, children are potentially more vulnerable than adults to environmental risk factors like air pollution. Unlike adults, the children’s respiratory system is constantly growing and they breathe more air than adults do, in proportion to their weight. During the stages of rapid growth, immature lungs may be the most susceptible [33,39,40]. Several studies have demonstrated the association between respiratory health such as asthma and lung function in children and proximity to petrochemical sites [41,42,43]. According to the study of Rusconi et al. children living in a petrochemical polluted area (Sarroch) versus a reference area (Burcei) showed a decrease in lung function (variation in FEV1 = −10.3% (90% CI = −15.0 to −6.0%)) [43].
To improve the accuracy and better reflect the linkages between air pollution and health outcomes, multiple methods for estimating exposure to ambient ozone were used. Use of spatial interpolation methods may be useful in providing individual-level exposures for locations without monitors and alternative for epidemiological analyses at the individual-level on the health effects. However, these approaches can contribute to uncertainty in estimating exposure, including monitoring site location, distance between a monitor and population, time-activity patterns of residents, and methodological choices. Because people spend more time indoors than outdoors, exposure misclassification bias can occur. For example, the average time spent per day in residential indoors was over 50% in 24 hours a day [44,45,46]. Thus, information on time spent in each micro-environment should be used in assessing actual personal exposures.
5. Conclusions
This study presents the association between ozone and lung function using spatial interpolation methods to estimate ozone exposure, and investigate how different methods for estimating exposure may influence health outcome for a cohort living near an industrial complex (Gwangyang Bay) in South Korea in 2009. We found an association between ozone and lung function, and a significant negative association was only observed in children by each spatial interpolation method. Our study illustrates that the kriging method provided more accurate estimates of exposure, and the effect size on lung function was generally higher when using the kriging approach. These results can be explained by the fact that the exposure estimation method used in estimating ozone exposure may affect the health outcome of epidemiologic studies. Future studies will be required to consider methodological choice with monitoring site locations, and time-activity patterns with exposure modeling estimates.
Acknowledgments
This research was supported by National Institute of Environmental Research and the Soonchunhyang University Research Fund.
Author Contributions
Soon-Won Jung and Kyoungho Lee conducted the data analysis, provided statistical support, and wrote the manuscript. Seung-Do Yu, Geun-Bae Kim and Choonghee Park aided with the concept and design of the study, and edited the manuscript. Tack-Shin Kang and Ji-Hee Choi contributed to data collected to data collection and analysis. Wonho Yang and Yong-Sung Cho contributed to data analysis and provided statistical support. Bu-Soon Son aided with the concept and design of the study, contributed to data collection and analysis, provided statistical support, and edited the manuscript. All authors have the final manuscript, agreed that it is ready for submission, and accept responsibility for contents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.NRC . National Research Council, Exposure Science in the 21st Century: A Vision and A Strategy. National Academy Press; Washington, DC, USA: 2012. [PubMed] [Google Scholar]
- 2.Bell M.L. The use of ambient air quality modeling to estimate individual and population exposure for human health research: A case study of ozone in the Northern Georgia Region of the United States. Environ. Int. 2006;32:586–593. doi: 10.1016/j.envint.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Bell M.L., McDermott A., Zeger S.L., Samet J.M., Dominici F. Ozone and short-term mortality in 95 U.S. urban communities, 1987–2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope I.C., Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K., Thurston G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng R.D., Bell M.L. Spatial misalignment in time series studies of air pollution and health data. Biostatistics. 2010;11:720–740. doi: 10.1093/biostatistics/kxq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo M.A., Bell M.L. Spatial heterogeneity of PM10 and O3 in São Paulo, Brazil, and implications for human health studies. J. Air Waste Manag. Assoc. 2011;61:69–77. doi: 10.3155/1047-3289.61.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Dadvand P., Ostro B., Figueras F., Foraster M., Basagana X., Valentin A., Martinez D., Beelen R., Cirach M., Hoek G., et al. Residential proximity to major roads and term low birth weight: The roles of air pollution, heat, noise, and road-adjacent trees. Epidemiology. 2014;25:518–525. doi: 10.1097/EDE.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 8.Allen R.W., Criqui M.H., Roux A.V.D., Allison M., Shea S., Detrano R., Sheppard L., Wong N.D., Stukovsky K.H., Kaufman J.D. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20:254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinichka C., Bundhamcharoen K., Shibuya K. Diseases burden of chronic obstructive pulmonary disease (COPD) attributable to ground-level ozone in Thailand: Estimates based on surface monitoring measurements data. Environ. Health Perspect. Glob. J. Health Sci. 2016;8:1–13. doi: 10.5539/gjhs.v8n1p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan T.-C., Chen M.-L., Lin I.-F., Lee C.-H., Chiang P.-H., Wang D.-W., Chuang J.-H. Spatiotemporal analysis of air pollution and asthma patient visits in Taipei, Taiwan. Int. J. Health Geogr. 2009 doi: 10.1186/1476-072X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerckhoffs J., Wang M., Meliefste K., Malmqvist E., Fischer P., Jnassen N.A., Beelen R., Hoek G. A national fine spatial scale land-use regression model for ozone. Environ. Res. 2015;140:440–448. doi: 10.1016/j.envres.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Wang M., Gehring U., Hoek G., Keuken M., Jonkers S., Beelen R., Eeftens M., Postma D.S., Brunekreef B. Air pollution and lung function in Dutch children: A comparison of exposure estimates and associations based on land use regression and dispersion exposure modeling approaches. Environ. Health Perspect. 2015;123:847–851. doi: 10.1289/ehp.1408541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berke O. Exploratory disease mapping: Kriging the spatial risk function from regional count data. Int. J. Health Geogr. 2004;3:1–11. doi: 10.1186/1476-072X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brus D.J., Heuvelink G.B. Optimization of sample patterns for universal kriging of environmental variables. Geoderma. 2007;138:86–95. doi: 10.1016/j.geoderma.2006.10.016. [DOI] [Google Scholar]
- 15.Amann M. Health Risks of Ozone from Long-Range Transboundary Air Pollution. WHO Regional Office Europe; Copenhagen, Denmark: 2008. [Google Scholar]
- 16.Bell M.L., Goldberg R., Hogrefe C., Kinney P.L., Knowlton K., Lynn B., Rosenthal J., Rosenzweig C., Patz J.A. Climate change, ambient ozone, and health in 50 U.S. cities. Clim. Chang. 2007;82:61–76. doi: 10.1007/s10584-006-9166-7. [DOI] [Google Scholar]
- 17.Seo J., Youn D., Kim J., Lee H. Extensive spatio-temporal analyses of surface ozone and related meteorological variables in South Korea for 1999–2010. Atmos. Chem. Phys. Discuss. 2014;14:1191–1238. doi: 10.5194/acpd-14-1191-2014. [DOI] [Google Scholar]
- 18.Tomczak M. Spatial interpolation and its uncertainty using automated anisotropic inverse distance weighting (IDW)-cross-validation/jackknife approach. J. Geogr. Inf. Decis. Anal. 1998;2:18–30. [Google Scholar]
- 19.Wong D.W., Yuan L., Perlin S.A. Comparison of spatial interpolation methods for the estimation of air quality data. J. Expo. Sci. Environ. Epidemiol. 2004;14:404–415. doi: 10.1038/sj.jea.7500338. [DOI] [PubMed] [Google Scholar]
- 20.Haylock M., Hofstra N., Klein Tank A., Klok E., Jones P., New M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. 2008 doi: 10.1029/2008JD010201. [DOI] [Google Scholar]
- 21.Liao D., Peuquet D.J., Duan Y., Whitsel E.A., Dou J., Smith R.L., Lin H.-M., Chen J.-C., Heiss G. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ. Health Perspect. 2006;114:1374–1380. doi: 10.1289/ehp.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrero M., Grimalt J.O., Cantón L. Prediction of daily ozone concentration maxima in the urban atmosphere. Chemom. Intell. Lab. Syst. 2006;80:67–76. doi: 10.1016/j.chemolab.2005.07.003. [DOI] [Google Scholar]
- 23.Wilson J.G., Kingham S., Sturman A.P. Intraurban variatons of PM10 air pollution in Christchurch, New Zealand: Implications for epidemiological studies. Sci. Total Environ. 2005;16:727–736. doi: 10.1016/j.scitotenv.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society Standardization of spirometry, 1994 update. Am. J. Respir. Crit. Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 25.Jerrett M., Burnett R.T., Ma R., Pope III C.A., Krewski D., Newbold K.B., Thurston G., Shi Y., Finkelstein N., Calle E.E. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 26.Marshall J.D., Nethery E., Brauer M. Within-urban variability in ambient air pollution: Comparison of estimation methods. Atmos. Environ. 2008;42:1359–1369. doi: 10.1016/j.atmosenv.2007.08.012. [DOI] [Google Scholar]
- 27.Pikhart H., Bobak M., Gorynski P., Wojtyniak B., Danova J., Celko M.A., Kriz B., Briggs D., Elliott P. Outdoor sulphur dioxide and respiratory symptoms in Czech and Polish school children: A small-area study (SAVIAH) Int. Arch. Occup. Environ. Health. 2001;74:574–578. doi: 10.1007/s004200100266. [DOI] [PubMed] [Google Scholar]
- 28.Son J.-Y., Bell M.L., Lee J.-T. Individual exposure to air pollution and lung function in Korea: Spatial analysis using multiple exposure approaches. Environ. Res. 2010;110:739–749. doi: 10.1016/j.envres.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.-Y., Sheppard L., Kim H. Health effects of long-term air pollution: Influence of exposure prediction methods. Epidemiology. 2009;20:442–450. doi: 10.1097/EDE.0b013e31819e4331. [DOI] [PubMed] [Google Scholar]
- 30.KMOE . Ministry of Environment. KOREA; Sejong-City, Korea: 2013. Environmental review. [Google Scholar]
- 31.Beelen R., Hoek G., Pebesma E., Vienneau D., de Hoogh K., Briggs D.J. Mapping of background air pollution at a fine spatial scale across the European Union. Sci. Total Environ. 2009;407:1852–1867. doi: 10.1016/j.scitotenv.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Chen C.-H., Chan C.-C., Chen B.-Y., Cheng T.-J., Guo Y.-L. Effect of particulate air pollution and ozone on the lung function in non-asthmatic children. Environ. Res. 2015;137:40–48. doi: 10.1016/j.envres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Hwang B.-F., Chen Y.-H., Lin Y.-T., Wu X.-T., Lee Y.-L. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ. Res. 2015;137:382–390. doi: 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Amadeo B., Robert C., Rondeau V., Mounouchy M.A., Cordeau L., Birembaux X., Citadelle E., Gotin J., Gouranton M., Marcin G., et al. Impact of close-proximity air pollution on lung function in schoolchildren in the French West Indies. BMC Public Health. 2015 doi: 10.1186/s12889-015-1382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas-Martinez R., Perez-Padilla R., Olaiz-Fernandez G., Mendoza-Alvarado L., Moreno-Macias H., Fortoul T., McDonnell W., Loomis D., Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am. J. Respir. Crit. Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 36.Chang Y.-K., Wu C.-C., Lee L.-T., Lin R.S., Yu Y.-H., Chen Y.-C. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere. 2012;87:26–30. doi: 10.1016/j.chemosphere.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 37.Pinkerton K.E., Balmes J.R., Fanucchi M.V., Rom W.N. Ozone, a malady for all ages. Am. J. Respir. Crit. Care Med. 2007;176:107–108. doi: 10.1164/rccm.200704-607ED. [DOI] [PubMed] [Google Scholar]
- 38.Finn P.W., Gozal D., Moss M., Ewart G., Moore N., du Melle F. Commnets from the American Thoracic Society Presented by Mary B. Rice MD before the House Committee on Science, Space and Technology on EPA’s Proposed Ozone National Ambient Air Quality Standard 17 March 2015. [(accessed on 11 July 2016)]; Avaiable online: http://docs.house.gov/meetings/SY/SY00/20150317/103159/HHRG-114-SY00-Wstate-RiceM-20150317.pdf.
- 39.Selgrade M.J.K., Lemanske R.F., Jr., Gilmour M.I., Neas L.M., Ward M.D., Henneberger P.K., Weissman D.N., Hoppin J.A., Dietert R.R., Sly P.D. Induction of asthma and the environment: What we know and need to know. Environ. Health Perspect. 2006;114:615–619. doi: 10.1289/ehp.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S., Williams G., Jalaludin B., Baker P. Panel studies of air pollution on children’s lung function and respiratory symptoms: A literature review. J. Asthma. 2012;49:895–910. doi: 10.3109/02770903.2012.724129. [DOI] [PubMed] [Google Scholar]
- 41.White N., van der Walt A., Ravenscroft G., Roberts W., Ehrlich R. Meteorologically estimated exposure but not distance predicts asthma symptoms in schoolchildren in the environs of a petrochemical refinery: A cross-sectional study. Environ. Health. 2009 doi: 10.1186/1476-069X-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smargiassi A., Kosatsky T., Hicks J., Plante C., Armstrong B., Villeneuve P.J., Goudreau S. Risk of asthmatic episodes in children exposed to sulfur dioxide stack emissions from a refinery point source in Montreal, Canada. Environ. Health Perspect. 2009;117:653–659. doi: 10.1289/ehp.0800010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusconi F., Catelan D., Accetta G., Peluso M., Pistelli R., Barbone F., di Felice E., Munnia A., Murgia P., Paladini L. Asthma symptoms, lung function, and markers of oxidative stress and inflammation in children exposed to oil refinery pollution. J. Asthma. 2011;48:84–90. doi: 10.3109/02770903.2010.538106. [DOI] [PubMed] [Google Scholar]
- 44.Chau C., Tu E.Y., Chan D., Burnett J. Estimating the total exposure to air pollutants for different population age groups in Hong Kong. Environ. Int. 2002;27:617–630. doi: 10.1016/S0160-4120(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 45.Sexton K., Mongin S.J., Adgate J.L., Pratt G.C., Ramachandran G., Stock T.H., Morandi M.T. Estimating volatile organic compound concentrations in selected microenvironments using time-activity and personal exposure data. J. Toxicol. Environ. Health. 2007;70:465–476. doi: 10.1080/15287390600870858. [DOI] [PubMed] [Google Scholar]
- 46.Yang W., Lee K., Yoon C., Yu S., Park K., Choi W. Determinants of residential indoor and transportation activity times in Korea. J. Expo. Sci. Environ. Epidemiol. 2011;21:310–316. doi: 10.1038/jes.2010.23. [DOI] [PubMed] [Google Scholar]