Abstract
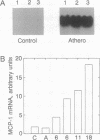
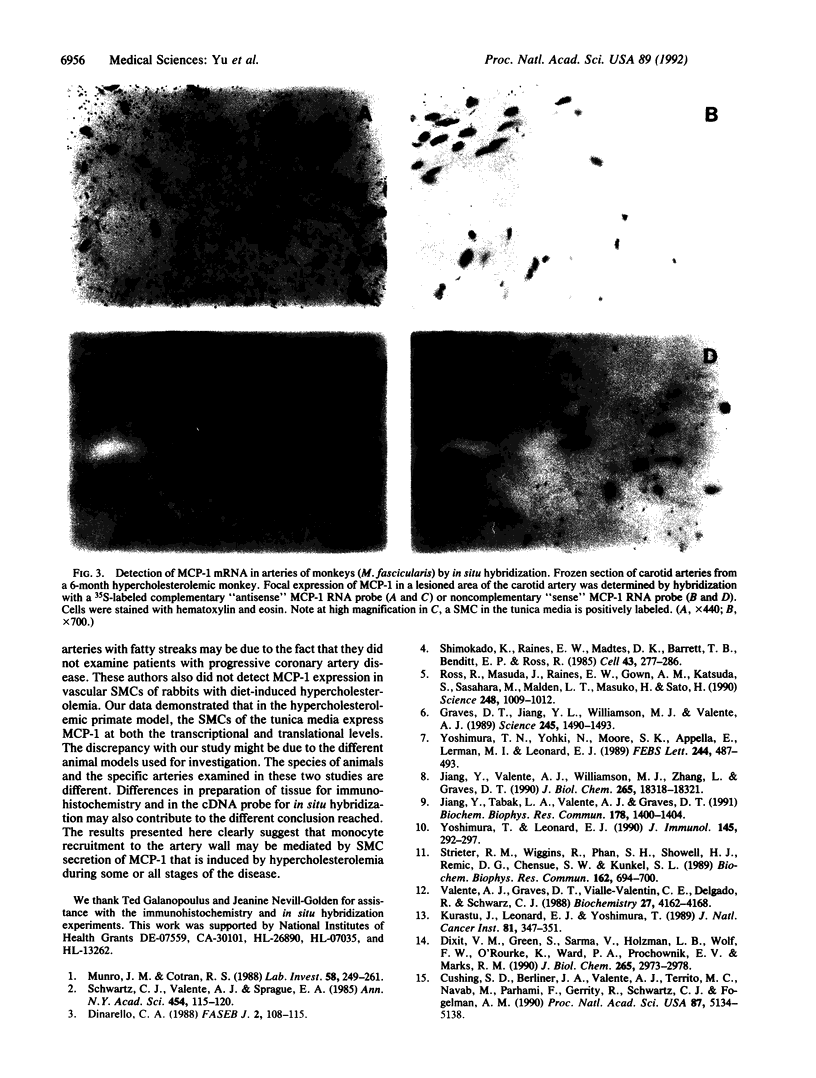
Atherosclerosis is marked by an overt inflammatory infiltrate, with enhanced recruitment of monocytes/macrophages observed in both human and experimental atherosclerosis. We previously determined that monocyte chemoattractant protein 1 (MCP-1) accounts for virtually all of the chemotactic activity produced by vascular (aortic) smooth muscle cells in culture. We now report that arteries from a primate model of atherosclerosis with dietary-induced hypercholesterolemia exhibit increased levels of MCP-1 mRNA expression in vivo, whereas their normal counterparts demonstrate minimal MCP-1 expression. Furthermore, immunohistochemistry and in situ hybridization clearly indicate that the expression of MCP-1 protein and mRNA is in the smooth muscle cells of the medial layer of the artery and in monocyte-like and smooth muscle-like cells found in the overlying intimal lesion. These studies indicate that one of the responses to dietary hypercholesterolemia is the expression of MCP-1 by vascular smooth muscle cells. This expression, when augmented with other cellular and molecular factors, could significantly contribute to the recruitment of monocytes/macrophages to the vessel wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbolini G., Scilabra G. A., Botticelli A., Botticelli S. On the origin of foam cells in cholesterol-induced atherosclerosis of the rabbit. Virchows Arch B Cell Pathol. 1969;3(1):24–32. doi: 10.1007/BF02901924. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., RITCHIE A. C. The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol; together with a description of the anatomy of the intima of the rabbit's aorta and the spontaneous lesions which occur in it. Am J Pathol. 1957 Sep-Oct;33(5):845–873. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dixit V. M., Green S., Sarma V., Holzman L. B., Wolf F. W., O'Rourke K., Ward P. A., Prochownik E. V., Marks R. M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990 Feb 15;265(5):2973–2978. [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Hollander W., Kirkpatrick B., Paddock J., Colombo M., Nagraj S., Prusty S. Studies on the progression and regression of coronary and peripheral atherosclerosis in the cynomolgus monkey. I. Effects of dipyridamole and aspirin. Exp Mol Pathol. 1979 Feb;30(1):55–73. doi: 10.1016/0014-4800(79)90081-9. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Tabak L. A., Valente A. J., Graves D. T. Initial characterization of the carbohydrate structure of MCP-1. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1400–1404. doi: 10.1016/0006-291x(91)91049-i. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Valente A. J., Williamson M. J., Zhang L., Graves D. T. Post-translational modification of a monocyte-specific chemoattractant synthesized by glioma, osteosarcoma, and vascular smooth muscle cells. J Biol Chem. 1990 Oct 25;265(30):18318–18321. [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kuratsu J., Leonard E. J., Yoshimura T. Production and characterization of human glioma cell-derived monocyte chemotactic factor. J Natl Cancer Inst. 1989 Mar 1;81(5):347–351. doi: 10.1093/jnci/81.5.347. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Sprague E. A., Kelley J. L., Valente A. J., Suenram C. A. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):175–191. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Sprague E. A., Kelley J. L., Suenram C. A., Graves D. T., Rozek M. M., Edwards E. H., Delgado R. Monocyte-macrophage participation in atherogenesis: inflammatory components of pathogenesis. Semin Thromb Hemost. 1986 Apr;12(2):79–86. doi: 10.1055/s-2007-1003539. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Sprague E. A., Kelley J. L., Suenram C. A., Rozek M. M. Atherosclerosis as an inflammatory process. The roles of the monocyte-macrophage. Ann N Y Acad Sci. 1985;454:115–120. doi: 10.1111/j.1749-6632.1985.tb11849.x. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Kindy M. S., Franzblau C., Sonenshein G. E. Complex regulation of collagen gene expression in cultured bovine aortic smooth muscle cells. J Biol Chem. 1986 May 15;261(14):6542–6547. [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Identification of high affinity receptors for human monocyte chemoattractant protein-1 on human monocytes. J Immunol. 1990 Jul 1;145(1):292–297. [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]