Abstract
Quinazolinones are a group of fused heterocyclic compounds which have valuable biological properties including cytotoxic, antibacterial and antifungal activities. Thiazole group-containing compounds have been also reported to have a wide range of biological activities such as antitumor, anti-inflammatory, analgesic and antibacterial effects. Due to valuable cytotoxic effects of both thiazole groups and quinazoline derivatives, in this study a series of quinazolinone-thiazole hybrids were synthesized and evaluated for their cytotoxic effects on three cell lines including MCF-7, HT-29, and PC-3. Among tested compounds (quinazolinones and three intermediates), k5 and k6 showed highest cytotoxic activities against PC3 cell line. K6 and C were most active compounds against MCF7 and K6 showed best cytotoxicity on HT-29 cell line.
Keywords: Quinazolinone, Thiazole, Cytotoxicity
INTRODUCTION
Cancer is a major health problem in developing and undeveloped countries. Although major advances have been made in the treatment of this disease, due to the problems associated with drug resistance, the continued efforts to discover new anticancer agents is very important. To discover various chemical substances which may serve as leads to design new antitumor agents, we are mostly interested in this work with quinazoline derivatives, which are well known compounds as a new class of anticancer agents with significant therapeutic value against tumors (1). Quinazoline and their derivatives are building blocks for approximately 150 naturally occurring alkaloids isolated from a number of families of the plant kingdom, from microorganisms and animals (2). There are many reports about biological activities in synthetic and natural quinazolines including sedative (3), anticonvulsant(3,4,5), anti-inflammatory (3,6), antitumor (3,7), antibacterial (3,8,9,10), antifungal (4,5), antitubercular (4,6,8,11), antimalarial (9,12), antiviral (4,6), anti-HIV (3,8,9,10,13), and hypolipidemic activities (14,15). Some drugs have been synthesized with quinazoline structure such as cloroqualone (antitussive), diproqualone (analgesic) (16), gefitinib, lapatinib (anticancer) (1), piriqualone (anti-convulsant) (17), doxazocin (antihypertensive) (18), prazosin (antihypertensive) (19), trimetrexate (antibacterial), thymitaq (anticancer) (20) and raltitrexed (anticancer) (21). Thiazole group containing compounds have been also reported to have a wide range of biological activities including: antitumor, anti-inflammatory, analgesic, antibacterial, and antifungal effects(22,23,24,25). Thiazole, an important heterocyclic ring, is widely used in anticancer drug development. Several anticancer agents containing thiazole moiety have been discovered, like bleomycin and tiazofurin. Ritonavir (anti-HIV), meloxicam (anti-inflammatory), nizatidine (anti-peptic ulcer) and penicillin (antibiotic) are some other examples of thiazole bearing products with biological activities (22,26). Due to the valuable cytotoxic effects of both thiazole and quinazoline compounds, in this study, a series of quinazolinone-thiazole hybrids were synthesized. Furthermore, antiproliferative activity of derivatives was determined using tumor cells in culture against MCF-7, HT-29, and PC-3.
MATERIALS AND METHODS
Instrumentation
All starting materials, reagents and solvents were purchased from commercial suppliers like Merck (Germany) and Aldrich (USA) companies.
The purity of the prepared compounds was proved by thin layer chromatography (TLC) using various solvents of different polarities. Merck silica gel 60 F254 plates were applied for analytical TLC.
1HNMR spectra were recorded using a (Bruker 400 MHz, Germany) spectrometer, and chemical shifts are expressed as δ (ppm) with tetramethylsilane (TMS) as internal standard.
The IR spectra were obtained on a Shimadzu 470 spectrophotometer (potassium bromide disks). Melting points were determined using electrothermal melting point analyzer apparatus (IA 9000, UK) and are uncorrected. The mass spectra were run on a Finnigan TSQ-70 spectrometer (Finnigan, USA) at 70 eV. All cell lines were purchased from Pasteur Institute of Iran.
Preparation of compounds
To produce 6-nitroderivatives of thiazole-containing 4(3H) quinazolinones (K1-K6), the primary amine G was synthesized through a five-step procedure. In the first step, 4-phthalimido-2-butanone (compound B) (Fig. 1), was prepared through the addition of methyl vinyl ketone to phthalimide. In the second step, 1-bromo-4-N-phthalimido-2-butanone (compound C) (Fig. 1) was synthesized by bromination of the methyl group of compound B. Nucleophilic substitution of separately synthesized thiobenzamide (compound E) (Fig. 1) to the brominated intermediate compound C afforded 2-phenyl-4-[(2-N-phthalimido)ethyl]thiazole (compound F) (Fig. 1) which was reacted with hydrazine hydrate and deprotected to produce the 2-phenyl-4-(2-aminoethyl) thiazole (compound G) (Fig. 1). A group of benzoxazinones (J1-J6) with different substituent at position 2 were synthesized. The reaction of the primary amine (compound G) with these benzoxazinones yielded the final compounds as presented in Fig. 1.
Fig. 1.
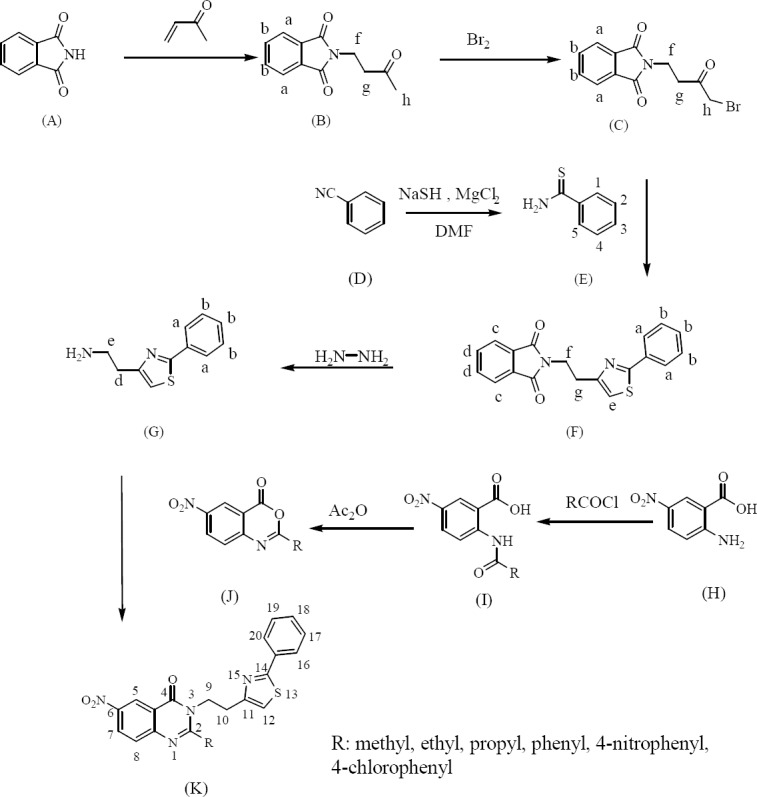
General reaction for preparation of the final compounds
Cell culture conditions
PC3 (prostate carcinoma), MCF-7 (breast cancer), and HT-29(colon carcinoma) cells were maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. PC3, MCF-7, and HT-29 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 5% v/v fetal bovine serum, 100 U/ml penicillin, and 100 mg/mL streptomycin. The medium was changed every two to three days and sub-cultured when the cell population density reached to 70–80% confluence. Cells were seeded at an appropriate density according to each experimental design (27).
Cytotoxicity assay
HT-29, MCF-7, and PC-3 cells were seeded in triplicate on 96-well tissue culture plates (15 × 103 cells/well) and incubated overnight. Cells were treated with different concentrations of the derivatives (0-275 μM) for 24 h. Then the medium was removed and the MTT substrate was prepared in a physiologically balanced solution (PBS), added to cells in culture, at a final concentration of 0.5 mg/ml, and incubated for 1 to 4 h. The formazan crystals were solubilized in dimethyl sulfoxide (DMSO) and the quantity of formazan (presumably directly proportional to the number of viable cells) was measured by recording changes in absorbance at 570 nm using a plate reading spectrophotometer. Cell viability was calculated using following formula:

IC50 values were calculated by plotting the cell viability against compound concentrations (27).
RESULTS
Details of preparation procedures and chemistry of synthesized compounds
4-Phthalimido-2-butanone (B)
To a well-stirred suspension of phthalimide (compound A) (Fig. 1) (36.75 g, 250 mmol) and 3-buten-2-one (17.5 g, 250 mmol) in 250 ml of ethyl acetate (EtOAc) was added a freshly prepared solution of sodium ethoxide (NaOEt) (0.67 g, 12 mmol) in 65 ml of anhydrous ethanol (EtOH) under an N2 atmosphere. After 2 h stirring at ambient temperature, the mixture was refluxed until an almost clear solution was obtained and refluxing was continued for an additional 2 h. After cooling, the solvent was removed in vacuo and the solid residue was crystallized from hot 96% EtOH to obtain compound B as a white powder (28). Yield:92%, m.p 112 °C, (Found: M217, C12H11NO3 requires 217), νmax = 3010, 2925, 1700 cm-1, 1HNMR (400 MHz, CDCl3) δ: 2.16 (3H, s, H-Ch), 2.85 (2H, t, J = 7.2 Hz, H-Cg), 3.93 (2H, t, J = 7.2Hz, H-Cf), 7.65-7.75 (2H, m, H-Cb Ar), 7.77-7.85 (2H, m, H-Ca Ar).
1-bromo-4-N-phthalimido-2-butanone (C)
4-Phthalimido-2-butanone (compound B) (14 g, 64 mmol) was dissolved in methylene chloride (105 ml) and methanol (85 ml). A solution of bromine (3.3 ml, 64 mmol) in methanol (20 ml) was added dropwise over a 2 h period. The reaction mixture was allowed to stir overnight, and was then treated with additional bromine (0.8 ml, 15.6 mmol); after 1 h, no starting material was visible by TLC (10:1, chloroform:EtOAc). The reaction mixture was concentrated in vacuo to leave a yellow solid, which was triturated with diethyl ether and dried with nitrogen flow to give compound C as white solid (29). Yield: 65%, m.p 105 °C, (Found: M296, C12H10BrNO3 requires 296), νmax = 3001, 2920, 1708 cm-1, 1HNMR (400 MHz, CDCl3) δ: 3.11 (2H, t, J = 7.2 Hz, H-Cg), 3.92 (2H, s, H-Ch), 4.0 (2H, t, J = 7.2 Hz, H-Cf), 7.70-7.77 (2H, m, H-Cb Ar), 7.80-7.90 (2H, m, H-Ca Ar).
Thiobenzamide (E)
To a solution of 7.9 g (99 mmol) of 70% sodium hydrosulfide hydrate and 10.1 g (50 mmol) of magnesium chloride hexahydrate in 100 ml of dimethylformamide (DMF) was added 5.15 g (50 mmol) of benzonitrile (compound D) (Fig. 1) in one portion, and the mixture was stirred at room temperature for 90 min. The resulting green solution was poured into 200 ml of water, and the product was extracted with diethyl ether. After evaporation of ether in vacuo, the crude product was suspended in 1N HCl and stirred for 20 min, then filtered and washed with water to give compound E (30). Yield: 82%, m.p. 187 °C, (Found: M137, C7H7NS requires 137), νmax = 3356, 3155, 1624 cm-1,1HNMR (400 MHz, CDCl3) δ: 7.34 (2H, t,J = 7.6 Hz, H-C2, H-C4 Ar), 7.45 (1H, t, J = 7.6 Hz, H-C3 Ar), 7.8 (2H, d, J = 7.6 Hz, H-C1, H-C5 Ar).
2- phenyl - 4 -[(2 - N - phthalimido) ethyl] thiazole (F)
1-Bromo-4-phthalimido-2-butanone (compound C) (5.92 g, 20 mmol) and thiobenzamide (compound E) (5.48 g, 40 mmol), were mixed with n-propanol (100 ml) and concentrated hydrochloric acid (10 ml) and the reaction mixture was heated to reflux for 2 h. After cooling, the precipitate was filtered and washed with n-propanol and then by diethyl ether. The hydrochloride product was obtained as a white solid. The free base, was obtained as follow: the product was mixed with saturated aqueous potassium carbonate solution overnight at room temperature. The solid was filtered, washed with water, diethyl ether and air-dried to leave compound F as a white solid (31). Yield: 55%, m.p 120 °C, (Found: M334, C19H14N2O2S requires 334), νmax = 3116, 2947, 1705 cm-1, 1HNMR (400 MHz, CDCl3) δ: 3.23 (2H, t, J = 6.6 Hz, H-Cg), 4.11 (2H, t, J = 6.6 Hz, H-Cf), 6.99 (1H, s, H-Ce), 7.32-7.38 (3H, m, H-CbAr), 7.68-7.72 (2H, m, H-CdAr), 7.76-7.80 (2H, m, H-CcAr), 7.81-7.85 (2H, m, H-CaAr).
2-phenyl-4-(2-aminoethyl) thiazole (G)
Compound F (3.67 g, 11 mmol) was added to a solution of hydrazine in methanol (200 ml, 4.0 M), and the reaction mixture was heated for 0.5 h until it became homogenous. The reaction mixture was then stirred at room temperature for further 2 h. Concentration in vacuo provided a white solid, which was purified by column chromatography (eluent: 89:10:1 chloroform:methanol: concentrated ammonium hydrochloride). The title product was obtained as sticky oil (31). Yield: 32%, (Found: M204, C11H12N2S requires 204), νmax = 3368, 1654 cm-1, 1HNMR (400 MHz, CDCl3) δ: 1.6-1.8 (2H, bs, NH2), 2.93 (2H, t, J = 6.0 Hz, H-Cd), 3.07-3.17 (2H, m, H-Ce), 6.93 (1H, m, H-Cc), 7.36-7.45 (3H, m, H-Cb), 7.88-7.96 (2H, m, H-Ca).
N-Acyl 5-nitroanthranilic acid (I)
Acyl chloride (0.37 mol) was added dropwise to a mixture of 5-nitroanthranilic acid (compound H) (0.25 mol) (Fig. 1) in dimethyl formamide (125 ml) at such rate that the temperature of the mixture did not rise above 40 °C. The mixture was stirred at room temperature for at least an additional 3 h. Completion of the reaction was determined by TLC and the mixture was poured into water (1 L) and stirred for 1 h. The precipitated product was collected by filtration, washed with cold water and dried under reduced pressure yielding (compound I) (Fig. 1) as a white powder. Yield: 55-70%.
2- substituted - 6 - nitro - 1,3 - benzoxazin - 4- one (J1-J6)
Compound I (0.125mol) was dissolved in acetic anhydride (90 ml) and slowly heated to 170-180 °C in a round-bottom flask equipped with a magnetic stirrer bar and a claisen- distillation head. Completion of the reaction was confirmed by TLC (20:1, chloroform:methanol), and the produced acetic acid was distilled under reduced pressure. The residue was then cooled and product was washed by n-hexane to give 3(2-methyl-6-nitro-1,3-benzoxazin-4-one) (compound J) (Fig. 1) as yellow crystals (32). Yield: 65-84%.
6-Nitroderivatives of thiazole containing 4(3H) quinazolinones (K1-K6)
To prepare 2-substituted-6-nitro-3-(2-(2-phenylthiazole-4-yl) ethyl) quinazolin-4(3H)-ones, 0.5 mmol of related benzoxazone was refluxed with 1 mmol of amine (compound G) in chloroform (10 ml) for 6-7 h. After completion of the reaction, chloroform was evaporated under reduced pressure and the residue was treated with ethylene glycol (5 ml) and NaOH pellets (0.003 g) in a flask equipped with a claisen-distilation head. The mixture was reheated to 130-140 °C for 5 h. After completion of the reaction, the clear solution was allowed to cool to room temperature and kept overnight to precipitate which was then crystallized from 2-propanol to obtain final products.
2 - ethy l- 6 - nitro- 3 - (2 - (2 - phenylthiazol - 4-yl)ethyl)quinazolin-4(3H)-one (K1)
Yield: 45%, m.p 203 °C, (Found: M406, C21H18N4O3S requires 406), νmax = 2997, 2912, 1658, 1535, 1311 cm-1, 1HNMR (400 MHz, CDCl3) δ:1.28 (3H, t, J = 8.0Hz, CH3:R), 2.77 (2H, q, J = 8.0 Hz, CH2:R), 3.22(2H, t, J = 8.0 Hz, H-C10), 4.74(2H, t, J = 8.0 Hz, H-C9), 6.89 (1H, s, H-C12), 7.28-7.40 (3H, m, H-C17, H-C18, H-C19), 7.67 (1H, d, J = 8.0 Hz, H-C8), 7.75-7.83 (2H, m, H-C16, H-C20),8.43 (1H, dd, J = 6.4, J = 2.8, H-C7), 9.07 (1H, d, J = 2.8Hz, H-C5)
6 - nitro - 2 - (4 - nitrophenyl) - 3 - (2 - (2 -phenylthiazol-4-yl)ethyl)quinazolin-4(3H)-one (K2)
Yield: 54%, m.p 218 °C (Found: M499, C25H17N5O5S requires 499), νmax = 3105, 2850,1678, 1516, 1334 cm-1, 1HNMR (400 MHz, CDCl3) δ: 3.23 (2H, t, J = 6.4 Hz, H-C10), 4.47 (2H, t, J = 6.8Hz, H-C9), 6.88 (1H, s, H-C12), 7.30-7.50(5H, m, H-C17, H-C18, H-C19, Ph-2H:R), 7.60-7.90 (5H, m, H-C16, H-C20, H-C8, Ph-2H:R), 8.42 (1H, dd, J = 6.4Hz, J = 2.8Hz, H-C7), 9.06(1H, d, J = 2.4 Hz, H-C5)
2 - methyl - 6 - nitro-3 - (2 - (2 - phenylthiazol-4-yl)ethyl)quinazolin-4(3H)-one (K3)
Yield: 52%, m.p 197 °C, (Found: M392, C20H16N4O3S requires 392), νmax= 3101, 2924, 1678, 1516, 1334 cm-1,1HNMR (400 MHz, CDCl3) δ:2.51 (3H, S, CH3:R), 3.23 (2H, t, J = 8.0 Hz, H -C10), 4.46 (2H, t, J = 8.0 Hz, H-C9), 6.88 (1H, s, H-C12), 7.30-7.42 (3H, m, H-C17, H-C18, H-C19), 7.62 (1H, d, J = 8.0 Hz, H-C8), 7.73-7.87 (2H, m, H-C16, H-C20), 8.42 (1H, dd, J = 8.0 Hz, J = 2.8 Hz, H-C7), 9.06 (1H, d, J = 2.4 Hz, H-C5)
6-nitro-3-(2-(2-phenylthiazole-4-yl)ethyl)-2-propylquinazolin-4(3H)-one (K4)
Yield: 75%, m.p 215 °C, (Found: M420, C22H20N4O3S requires 420), νmax = 3101, 2870, 1689, 1512, 1330 cm-1, 1HNMR (400 MHz, CDCl3) δ: 0.95 (3H, t, J = 8.0 Hz, CH3:R), 1.74 (2H, hex, J = 8.0 Hz, CH2:R), 2.68 (2H, t, J = 8.0Hz, CH2:R), 3.22 (2H, t, J = 8.0 Hz, H-C10), 4.74 (2H, t, J = 8.0 Hz, H-C9), 6.88 (1H, s, H-C12), 7.30-7.39 (3H, m, H-C17, H-C18, H-C19), 7.64 (1H, d, J = 8.0Hz, H-C8), 7.75-7.85 (2H, m, H-C16, H-C20), 8.42 (1H, dd, J = 8.0, J = 2.4, H-C7), 9.06 (1H, d, J = 2.4Hz, H-C5)
6 - nitro - 2 - phenyl - 3 - (2-(2-phenylthiazole-4-yl)ethyl)quinazolin-4(3H)-one (K5)
Yield: 49%, m.p 187 °C, (Found: M454, C25H18N4O3S requires 454), νmax = 3101, 1689,1504, 1315 cm-1,1HNMR (400 MHz, CDCl3) δ: 3.26 (2H, t, J = 6.0 Hz, H-C10), 4.44 (2H, t, J = 6.0Hz, H-C9), 6.84 (1H, s, H-C12), 7.30-7.45 (4H, m, H-C17, H-C18, H-C19, Ph-1H:R), 7.60-8.05 (7H, m, H-C16, H-C20, H-C8, Ph-4H:R), 8.43 (1H, dd, J = 6.4Hz, J = 2.8Hz, H-C7), 9.11(1H, d, J = 2.4 Hz, H-C5)
2 - (4 - chlorophenyl) – 6 – nitro – 3 - (2 - (2 -phenylthiazole-4-yl)ethyl)quinazolin-4(3H)-one (K6)
Yield: 61%, m.p 210 °C, (Found: M488, C25H17ClN4O3S requires 488), νmax = 3101, 2927, 1681, 1516, 1338 cm-1, 1HNMR (400 MHz, CDCl3) δ:3.03 (2H, t, J = 6.4 Hz, H-C10), 4.44 (2H, t, J = 6.8Hz, H-C9), 6.96 (1H, s, H-C12), 7.30 (2H, d, J = 8.4 Hz, H-C16, H-C20), 7.32-7.37 (4H, m, H-C17, H-C19, Ph-2H:R), 7.38-7.40 (2H, m, H-C18, H-C8), 7.70 (1H, dd, J = 6.4Hz, J = 2.8 Hz, H-C7), 7.84-7.87 (2H, m, Ph-2H:R),9.06 (1H, d, J = 2.4 Hz, H-C5).
Antiproliferative effects of the derivatives
MTT assay results for evaluation of cytotoxic effects of the compounds are shown in Figs. 2, 3, and 4. Their IC50 (μM) values are also listed in Table 1.
Fig. 2.
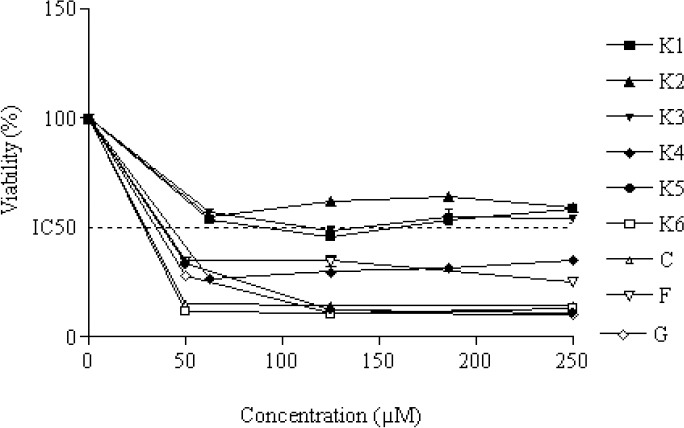
The percentage of cytotoxicity versus concentration by MTT exclusion on MCF-7 cancer cell line. IC50 value was obtained by plotting the percentage of proliferation values versus drug concentrations. Data are expressed as the mean ± SEM of three separate experiments.
Fig. 3.
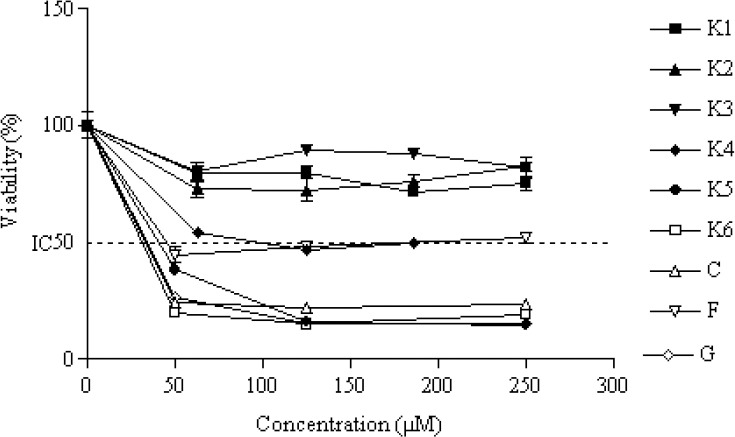
The percentage of cytotoxicity versus concentration by MTT exclusion on HT-29 cancer cell line. IC50 value was obtained by plotting the percentage of proliferation values versus drug concentrations. Data are expressed as the mean ± SEM of three separate experiments.
Fig. 4.
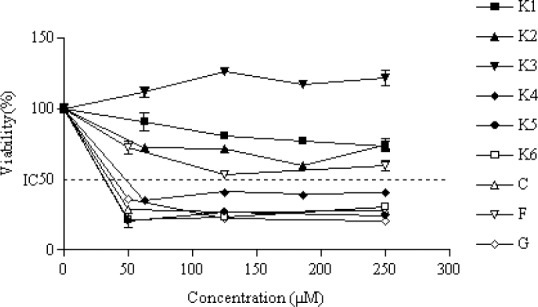
The percentage of cytotoxicity versus concentration by MTT exclusion on PC-3 cancer cell line. IC50 value was obtained by plotting the percentage of proliferation values versus drug concentrations. Data are expressed as the mean ± SEM of three separate experiments.
Table 1.
The IC50 (μM) of tested compounds against MCF-7, HT-29, and PC-3 cancer cell lines
DISCUSSION
The synthesized 4(3H) quinazolinones in this study contained 4-ethyl-2-phenylthiazole group on position 3 of quinazolinone structure. For preparation of these quinazolinones, a primary amine containing thiazole (compound G) (Fig. 1) was synthesized. The most practical method to prepare thiazoles is Hantzsch reaction which involves the condensation of α-haloketones and thiourea or thioamides in refluxing alcohol. Phthalimide as an NH2-synthon was used here for the preparation of the amine. Application of phthalimide in Gabriel synthesis for preparation of primary amines is well documented. After alkylation, the resulting alkyl phthalimide is reacted with hydrazine. The desired primary amine could be generated by reacting with hydrazine hydrate. Consequently phthalazine as a stable cyclic product is formed and precipitated (Fig. 5). The reaction of 2-phenyl-4-(2-aminoethyl) thiazole (compound G) with different benzoxazinones resulted in the production of new 4(3H)quinazolinones (Fig. 1).
Fig. 5.
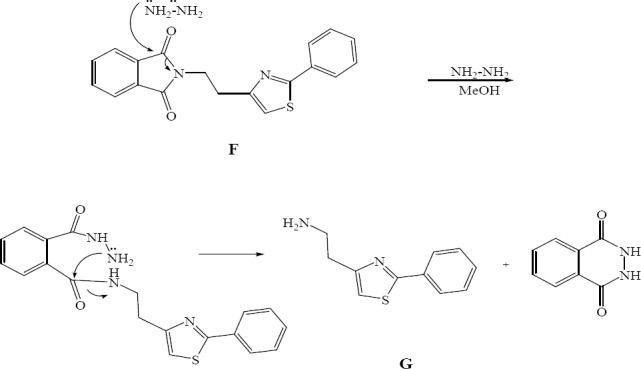
Amine generation mechanism.
CONCLUSION
In this study, quinazolinones as biologically active compounds were conjugated with another well-known moiety (thiazole ring) in a multi-step reaction procedure to produce interesting novel compounds. All synthesized compounds were tested for their cytotoxic effects on three cell lines including MCF-7, HT-29, and PC-3 of which quinazolinones K1-K6 and three intermediates, compound C, F, and G, k5 and k6 showed highest cytotoxic activities against PC3 cell line. K6 and compound C were most active against MCF-7 and K6 showed best cytotoxicity on HT-29 cell line.
ACKNOWLEDGMENT
Authors appreciate Research Council of Kermanshah University of Medical Sciences for financial support of this project.
REFERENCES
- 1.El-Azab A, Al-Omar M, Abdel-Aziz A, Abdel-Aziz N, El-Sayed M, Aleisa A, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur J Med Chem. 2010;45:4188–4198. doi: 10.1016/j.ejmech.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari A, Singh V, Bajpai A, Shukla G, Singh S, Mishra A. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur J Med Chem. 2007;42:1234–1238. doi: 10.1016/j.ejmech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Mhaske SB, Argade NP. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62:9787–9826. [Google Scholar]
- 4.Gursoy A, Unal B, Karali N, Otuk G. Synthesis, Characterization and Primary Antimicrobial Activity Evaluation of 3-Phenyl-6-methyl-4-(3H)-quinazolinone-2-yl-mercaptoacetic Acid Arylidenehydrazides. Turk J Chem. 2005;29:233–245. [Google Scholar]
- 5.Mourad AE, Aly AA, Farag HH, Beshr EA. Microwave assited synthesis of triazolo-quinazolinones and benzimidazoqinazolinones. Beilstein J Org Chem. 2007;3:1–5. doi: 10.1186/1860-5397-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Murphy DE, Sun Z, Gregor VE. Novel parallel synthesis of N-(4-oxo-2-substituted-4-H-quinazolin-3-yl)-substituted sulfonamides. Tetra-hedron Lett. 2004;45:8049–8051. [Google Scholar]
- 7.Dinakaran M, Selvam P, DeClercq E, Sridhar SK. Synthesis, antiviral and cytotoxic activity of 6-bromo-2, 3-disubstituted-4 (3H)-quinazolinones. Biol & Pharm Bull. 2003;26:1278–1282. doi: 10.1248/bpb.26.1278. [DOI] [PubMed] [Google Scholar]
- 8.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and antimicrobial activities of some novel substituted 2-Imidazolyl-N-(4-oxo-quinazolin-3 (4H)-yl)-acetamides. Chem Pharm Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 9.Laddha SS, Wadodkar SG, Meghal SK. Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1: Synthesis, characterization and anti-inflammatory-antimicrobial activity of 6, 8-disubstituted 2-phenyl-3-[substituted-benzothiazol-2-yl]-4 (3H)-quinazolinones. Arkivoc. 2006;11:1–20. [Google Scholar]
- 10.Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4 (3H)-one. Pharm Acta Helvet. 1999;74:11–17. doi: 10.1016/s0031-6865(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 11.Nanda AK, Ganguli S, Chakraborty R. Antibacterial activity of some 3-(Arylideneamino)-2-phenylquinazoline-4(3H)-ones: synthesis and preliminary QSAR studies. Molecules. 2007;12:2413–2426. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philipova I, Dobrikov G, Krumova K, Kaneti J. Convenient synthesis of some 2 substituted 4 (3H) quinazolinone derivatives. J Heterocycl Chem. 2006;43:1057–1063. [Google Scholar]
- 13.Gürsoy A, Karali N. Synthesis and primary cytotoxicity evaluation of 3-[[3-phenyl-4 (3H)-quinazolinone-2-yl) mercaptoacetyl] hydrazono]-1H-2-indolinones. Eur J Med Chem. 2003;38:633–643. doi: 10.1016/s0223-5234(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 14.Ghabrial SS, Gaber HM. Dipolar cycloaddition reactions with quinazolinones: a new route for the synthesis of several annelated pyrrolo and pyridazinoquinazoline derivatives. Molecules. 2003;8:401–410. [Google Scholar]
- 15.Refaie FM, Esmat AY, Gawad SMA, Ibrahim AM, Mohamed MA. The antihyperlipidemic activities of 4 (3H) quinazolinone and two halogenated derivatives in rats. Lipids Health Dis. 2005;4:22–33. doi: 10.1186/1476-511X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shcherbakova I, Balandrin MF, Fox J, Ghatak A, Heaton WL, Conklin RL. 3H-Quinazolin-4-ones as a new calcilytic template for the potential treatment of osteoporosis. Bioorg Med Chem Lett. 2005;15:1557–1560. doi: 10.1016/j.bmcl.2005.01.078. [DOI] [PubMed] [Google Scholar]
- 17.Liu JF, Lee J, Dalton AM, Bi G, Yu L, Baldino CM, et al. Microwave-assisted one-pot synthesis of 2, 3-disubstituted 3H-quinazolin-4-ones. Tetrahedron Lett. 2005;46:1241–1244. [Google Scholar]
- 18.Piller LB, Davis B, Cutler JA, Cushman WC, Wright JT, Williamson JD, et al. Validation of heart failure events in the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT) participants assigned to doxazosin and chlorthalidone. Curr Contr Trials Cardio Med. 2002;3:1–10. doi: 10.1186/1468-6708-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akazome M, Yamamoto J, Kondo T, Watanabe Y. Palladium complex-catalyzed intermolecular reductive N-heterocyclization: novel synthesis of quinazoline derivatives from 2-nitrobenzaldehyde or 2-nitrophenyl ketones with formamide. J Organomet Chem. 1995;494:229–233. [Google Scholar]
- 20.Al-Omary FAM, Abou-zeid LA, Nagi MN, Habib ESE, Abdel-Aziz AAM, El-Azab AS, et al. Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg Med Chem. 2010;18:2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett. 2005;15:1915–1917. doi: 10.1016/j.bmcl.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 22.Bharti S, Nath G, Tilak R, Singh S. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2, 4-disubstituted thiazole ring. Eur J Med Chem. 2010;45:651–660. doi: 10.1016/j.ejmech.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Khalil A, Berghot M, Gouda M. Synthesis and antibacterial activity of some new thiazole and thiophene derivatives. Eur J Med Chem. 2009;44:4434–4440. doi: 10.1016/j.ejmech.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Pandeya S, Sriram D, Nath G, DeClercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of schiff and mannich bases derived from isatin derivatives and N-[4-[4’-chlorophenyl) thiazol-2-yl] thiosemicarbazide. Eur J Pharm Sci. 1999;9:25–31. doi: 10.1016/s0928-0987(99)00038-x. [DOI] [PubMed] [Google Scholar]
- 25.Cukurovali A, Yilmaz I, Gur S, Kazaz C. Synthesis, antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring. Eur J Med Chem. 2006;41:201–207. doi: 10.1016/j.ejmech.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Xu Z, Tan M, Su W, Gong X. 3-(4-(Benzo[d]thiazol-2-yl)-1-phenyl-1H-pyrazol-3-yl) phenyl acetate induced Hep G2 cell apoptosis through a ROS-mediated pathway. Chemico Biol Interact. 2010;183:341–348. doi: 10.1016/j.cbi.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, Xiao F, Qian S, Lu W, Yang B. Synthesis and in vitro cytotoxic evaluation of some thiazolylbenzimidazole derivatives. Eur J Med Chem. 2011;46:417–422. doi: 10.1016/j.ejmech.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Eriks JC, Van der Goot H, Sterk GJ, Timmerman H. Histamine H2-receptor agonists. Synthesis, in vitro pharmacology, and qualitative structure-activity relationships of substituted 4-and 5-(2-aminoethyl) thiazoles. J Med Chem. 1992;35:3239–3246. doi: 10.1021/jm00095a021. [DOI] [PubMed] [Google Scholar]
- 29.Brighty KE, Lowe JA, Mcguirk PR. Heterocyclic-substituted quinoline-carboxylic acids. US patent. 1991 5037834. [Google Scholar]
- 30.Manaka A, Sato M. Synthesis of Aromatic Thioamide from Nitrile Without Handling of Gaseous Hydrogen Sulfide. Chem Inform. 2005;36:761–764. [Google Scholar]
- 31.Walczyski K, Timmerman H, Zuiderveld O, Zhang M, Glinka R. Histamine H1 receptor ligands: Part I. Novel thiazol-4-ylethanamine derivatives: synthesis and in vitro pharmacology. Il Farmaco. 1999;54:533–541. doi: 10.1016/s0014-827x(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 32.Al-Obaid AM, Abdel-Hamide SG, El-Kashef HA, Abdel-Aziz AA, El-Azab AS, Al-khamees HA, et al. Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur J Med Chem. 2009;44:2379–91. doi: 10.1016/j.ejmech.2008.09.015. [DOI] [PubMed] [Google Scholar]


