Abstract
Sea algae are widely consumed in the world. There are several seaweeds including brown algae which are authorized for human consumption. These plants contain important phytochemical constituents and have various potential biological activities. The present study investigated the presence of phytochemical constituents and total phenolic quantity of the seaweeds Sargassum angustifolium, Sargassum oligocystum and Sargassum boveanum. Cytotoxicity of seaweeds was tested against HT-29, HeLa and MCF-7 cell lines. Antioxidant potential of these 3 Sargassum species was also analyzed. Cytotoxicity was characterized by IC50 of human cancer cell lines using sulforhodamine assay. Antioxidant activities were evaluated using 2,2-diphenyl-1- picrylhydrazil. The analysis revealed that tannins, saponins, sterols and triterpenes were the most abundant compounds in these Sargassum species while cyanogenic and cardiac glycosides were the least ones. Sargassum angustifolium had the highest content of total phenolics (0.061 mg/g) and showed the highest antioxidant activity (IC50 = 0.231). Cytotoxic results showed that all species could inhibit cell growth effectively, especially MCF-7 cell line (IC50 = 67.3, 56.9, 60.4 for S. oligocystum, S. angustifolium and S. boveanum respectively). Considerable phytochemicals and moderate cytotoxic activity of S. angustifolium, S. oligocystum and S. boveanum make them appropriate candidate for further studies and identification of their bioactive principles.
Keywords: Antioxidant, Cytotoxic, Sargassun angustifolium, Sargassum oligocystum, Sargassum boveanum, Seaweed
INTRODUCTION
Seaweeds are fresh sources of bioactive compounds with immense medicinal potential which have attracted the attention of pharmaceutical industries (1,2). They usually grow in all seas except in the Polar Regions and produce biologically active compounds. They are used as human food especially by coastal populations (3). There are many reports on the antibacterial (4), antifungal (5), antiviral (6), anti-inflammatory (7), antidiabetic (8), antioxidant (9) and cytotoxic activities of seaweeds (10).
Genus of Sargassum is widely distributed in the temperate and tropical oceans of the world. There are numerous reports on their secondary metabolites and biological activities (11). They usually contain terpenoids that exhibits biological activities such as cell toxicity, antioxidant activity, vasodilatory effects, induction of larval settlement of hydrozoan and inhibition of acetylcholine-esterase (12,13).
Different kinds of radicals are generated in the normal metabolic activities and sometimes the antioxidant capacity of the body is inadequate to cope with them. Therefore, there is a growing interest on the discovery of natural antioxidants because they reduce the risk of developing chronic disease such as cancer and also phytochemicals are generally safer than synthetic chemicals (14).
Iran has coastal lines about 1260 km along the Persian Gulf and the Oman Sea. More than 250 species of different algae have been identified in this area (15). Despite the existence of a great extent of marine algae in this region, there are only a few studies on the phytochemical analysis and biological activities of these seaweeds. In the current study in addition to phytochemical screening of three Sargassum extracts, their antioxidant activity and cytotoxic potential were investigated.
MATERIAL AND METHODS
Authentication of plant material
The seaweeds were collected in 2012 from the Persian Gulf coasts of Iran close to Bushehr Province. They were identified by Agricultural and Natural Resources Research Center of Bushehr and their voucher specimens coded as 2662 for S. angustifolium, 2663 for S. oligocystum, and 2664 for S. boveanum were deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences (Isfahan, Iran).
Preparation of the extracts
The plant samples were cut into small pieces, completely air-dried, and stored in glass containers until extraction. About 100 g of the dried plant material was macerated for five consecutive days with methanol. The extracts were filtered through 2 layers of cotton fabric and evaporated at room temperature under reduced pressure. Dried residues were stored in clean vials until phytochemical and cytotoxic screening (16).
Phytochemical screening
Tests for phytochemical constituents including alkaloids, steroid and triterpenes, anthraquinones, flavonoids, saponins, cyanogenic glycosides, cardiac glycosides and tannins followed the methods described previously (17).
Determination of alkaloids
Powdered specimen of the plants (200 mg) was boiled with 10 ml water and 10 ml of hydrochloric acid on a water bath. Finally it was filtered and its pH was adjusted to about 6-7 with ammonia. One ml of the filtrate was treated with a few drops of Mayer’s reagent (potassium mercuric iodide solution). In addition, 1 ml portion was treated similarly with Wagner’s reagent (solution of iodine in potassium iodide). Turbidity or colored precipitation with either of these reagents was taken as evidence for the presence of alkaloids (17).
Test for cardiac glycosides
A few drops of the Baljet’s reagent (picric acid, ethanol and sodium hydroxide) were added to 2-3 mg of sample. A positive reaction was indicated by orange to deep red color (17).
Test for tannins
Sample (1 g) was boiled with 20 ml distilled water for 5 min in a water bath and filtered while it was hot. Then 1 ml of cool filtrate was diluted to 5 ml with distilled water and a few drops (2,3) of 10% ferric chloride were added and observed for formation of precipitates and any color change. A bluish-black or brownish-green precipitate indicated the presence of tannins (17).
Test for flavonoids
Powdered sample (1 g) was boiled with 10 ml of distilled water for 5 min and filtered while it was hot. A few drops of 20% sodium hydroxide solution were added to 1 ml of the cooled filtrate. A change to yellow color which on addition of acid changed to colorless solution depicted the presence of flavonoids (17).
Test for saponins
The extract solution (1 ml) was diluted with distilled water to 20 ml and shaken for 15 min in a graduated cylinder. Development of stable foam suggests the presence of saponins (17).
Test for combined anthraquinones
Powdered sample (1 g) was boiled with 2 ml of 10% hydrochloric acid for 5 min. The mixture was filtered while it was hot and the filtrate was allowed to cool. The cooled filtrate was partitioned against equal volume of chloroform and the chloroform layer was transferred into a clean dry test tube using a clean pipette. Equal volume of 10% ammonia solution was added into the chloroform layer, shaken and allowed to separate. The separated aqueous layer was observed for any color change. Rose pink color indicated the presence of anthraquinones (17).
Test for sterols and triterpenes
Three grams of the powdered leaves was placed in a test tube and 10 ml of 50% alcohol was added, the tube was then placed on a water bath and heated for 3 min. It was then allowed to cool to room temperature and filtered. The filtrate was then evaporated in an evaporating dish to dryness and 5 ml of petroleum ether was added to the dish and stirred for 5 min, the petroleum ether portion was then decanted and discarded. 10 ml of chloroform was then added and stirred for about 5 min, it was then transferred into test tube and 0.5 mg of anhydrous sodium sulphate was added and shaken gently and filtered, the filtrate was then divided into two test tubes and used for the following tests.
Liebermann-Burchard’s reaction: To test tube I, equal volume of acetic anhydride was added and gently mixed. Then 1 ml of concentrated sulfuric acid was added down the side of the tube. The appearance of a brownish-red ring at the contact zone of the two liquids and a greenish color in the separation layer indicates the presence of sterols and triterpenes.
Salwoski’s test: To test tube II, 2 to 3 drops of concentrated sulphuric acid was added to form a lower layer. Reddish-brown color at the inter phase indicates the presence of steroidal ring (17).
Determination of total phenolics
The powdered plant material (20 g of each sample) were weighed in to 50 ml flask, sonicated with 30 ml of ethanol 40% for about 30 min and shaken for about 10 min. The extracts were allowed to cool down to room temperature and the flasks were made to volume with extracting solvent.
Preparation of standard
Twenty mg of gallic acid and 30 ml 40% ethanol were added into 50 ml volumetric flask and sonicated until no solid was present in the flask. After cooling down the solution to room temperature, the flask was filled with extracting solvent. The standard solution was diluted several times.
One ml of standard solution transferred to 100 ml volumetric flask with 60-70 high-performance liquid chromatography (HPLC) grade water. The contents swirled to mix. Five ml of Folin-Ciocalteu’s phenol reagent was added and mixed again. After 1 min and before 8 min, 15 ml of sodium carbonate solution was added and the time recorded as time zero. The volume was made up to 100 ml exactly with HPLC grade water. The flask stoppered and mixed thoroughly by inverting it several times. After 2 h, the sample was scanned at 550 to 850 nm and maximum absorbance of about 760 nm was recorded. Same solution without the extraction solution was used as the blank solution (18).
Gallic acid was used as a standard compound and the total phenols were expressed as mg/g gallic acid equivalent using the standard curve y = 1.1771 x – 0.0252, R2 = 0.9958 where, y is absorbance at 760 nm and x is total phenolic content in the extracts of different alga expressed in mg/L.
In vitro cytotoxicity assay
The extracts were tested using MCF-7 (human breast adenocarcinoma), HeLa (cervical carcinoma), and HT-29 (human colon adenocarcinoma) cells. The cancer cell lines were bought from Cell Line Service (Germany) and grown in Dulbeccos Modified Eagle Medium (DMEM) (from Invitrogen, USA) supplemented with 10% fetal bovine serum (from Seromed, India). Cells were seeded in 96-well at 3500 cancerous cells/well and 5000 normal cells/well and allowed to adhere for 24 h at 37 °C with 5% CO2 in a fully humidified incubator. Then 100 μl serially diluted concentrations of samples in medium were dispensed in to the wells of the cell plates and incubated further for 72 h. After removal of the sample medium, the cells were topped up with 200 μl DMEM medium and incubated for 72 h. Afterward cells were fixed with cold 40% trichloroacetic acid at 4 °C for 1 h and washed with tap water. Cell viability was determined by Sulforhodamine assay. The absorbances were measured at 492 nm using a microplate reader (BioTeck, Germany). Percent cell death was calculated relative to the control. The concentration of the extract that inhibited 50% cells growth (IC50) was determined from the graph percent inhibition against different plant extract concentrations. The cytotoxic activities of all extracts against breast cancer cell lines were labeled according to the National Cancer Institute (NCI, USA) criteria (highly inhibiting activity means IC50 ≤20 μg/ml) (19).
DPPH free radical scavenging assay
The free radical scavenging activity was measured using the 2,2-diphenyl-1- picrylhydrazil (DPPH) assay. Sample stock solutions (1.0 mg/ml) of the extracts were diluted to final concentrations of 243, 81, 27, 9, 3 and 1 μg/ml, in ethanol. One ml of a 50 μg/ml DPPH ethanolic solution was added to 2.5 ml of sample solutions of different concentrations, and allowed to react at room temperature. After 30 min the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using the following equation:
AA% = ((absorbance of the control – absorbance of the sample)/absorbance of the control) × 100
Ethanol (1.0 ml) plus plant extract solutions (2.5 ml) were used as the blank.
DPPH solution (1.0 ml) plus ethanol (2.5 ml) was used as a negative control. The positive controls were ascorbic acid, butylated hydroxyanisole and butylated hydroxytoluene. Assays were carried out in triplicate (20).
Statistical analysis
One-way analysis of variance (ANOVA) followed by Scheffe post hoc test were used for data analysis. All results were expressed as mean ± SD and P<0.05 was considered as statistically significant.
RESULTS
Phytochemical constituents
Phytochemical data (Table 1) shows distinct patterns of chemical compositions in constituents of the extracts. The patterns of composition differed considerably in their quantitaties. The results of phytochemical evaluation are shown in Table 1.
Table 1.
Phytochemical constituents of Sargassum species.
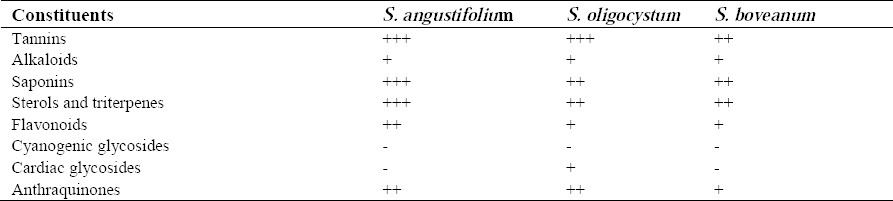
This analysis showed the most abundant compounds in S. angustifolium were tannins, saponins, sterols and triterpenes fallowed by flavonoids. Cyanogenic and cardiac glycosides were absent in this seaweed. In S. oligocystum tannins were the most abundant compounds fallowed by saponins, alkaloids, sterols and triterpenes. Cyanogenic glycosides were not present. Tannins, sterols, triterpenes and saponins were major constituents in S. boveanum too.
The amount of total phenol was determined with the Folin-Ciocalteu reagent. Phenolic compounds are a class of antioxidants which act as free radical terminators. Table 2 shows the contents of total phenols that were measured by Folin-Ciocalteu reagent in terms of gallic acid equivalent. The total phenol in selected seaweeds was 0.061, 0.019 and 0.035 mg in 1 g of dry extract in S. angustifolium, S. oligocystum and S. boveanum respectively.
Table 2.
Total phenolic content of seaweeds.

Cytotoxic assay
The criteria used to categorize the activity of extracts against MCF-7, HeLa, HT-29 cancer cell lines based on IC50 values, were modified from those of NCI and Geran and coworkers (19) as follows: IC50 ≤20 μg/ml; highly active, IC50 21-200 μg/ml; moderately active, IC50 201 - 500 μg/ml; weakly active and IC50 >501 μg/ml; inactive.
The cytotoxicity data for the extracts against MCF-7, HeLa and HT-29 cells are displayed in Table 3. Cytotoxic results showed that all species could inhibit cell growth effectively, especially MCF-7 cell line (IC50 were 67.3, 56.9, 60.4 for S. oligocystum, S. angustifolium and S. boveanum, respectively).
Table 3.
Cytotoxic activities (IC50, μg/ml) of the studied seaweeds on different cell lines.

Antioxidant activity
The antioxidant activities of the seaweed extracts were measured based on scavenging activity of the stable DPPH free radical. The value of IC50 is inversely related to the antioxidant activity. The extracts of three algae were found to have different levels of antioxidant activity. Table 4 shows that S. angustifolium indicated the highest antioxidant activity (IC50 of 0.231) while S. oligocystum showed the lowest one (IC50, 0.610).
Table 4.
Antioxidant activity (IC50) of the seaweeds.

DISCUSSION
Sea algae are widely consumed in Japan and there are several seaweeds including brown algae authorized in France for human consumption (21). Moreover, certain algae are used as capsule dosage form in different countries as diet integrator (22). These facts support safety of utilization of the algae extract as natural antioxidant. Based on the results obtained from studies on seaweeds, it has been shown that several algae species can prevent oxidative damage by scavenging free radical and hence able to prevent cancer cell formation (23). Certain algae have long been used in traditional Chinese herbal medicine in the treatment of cancer (24). Many studies have been performed in order to determine the bioactive compounds produced in marine algae. In this field, brown algae of the genus Sargassum, (Sargassaceae, Fucales) are known to contain structurally unique secondary metabolites such as plastoquinones (25), chromanols (26) and polysaccharides (27).
In the present research an effort was made to study the similarity and differences amongst three species based on phytochemical analysis and bioactivity. Phytochemical tests revealed many similarities. The only difference in terms of phytochemical analysis of the three plants was the cardiac glycosides which was present only in S. oligocystum. The amount of tannins, sterols and triterpenes, saponins and flavonoids were also different. With regard to cytotoxic activities, all species showed higher toxicity against MCF-7 cell lines compared to HT-29 and HeLa cell lines and the results were very similar. Phytochemical and biological results of the current work revealed that these edible seaweeds could be potential candidates in the field of drug development.
The result of the present study showed that the extract of S. angustifolium, which contained highest amount of phenolic compounds could exhibit a greater antioxidant activity. S. angustifolium is an important species from family Sargasseae. Different algae of this family have shown antibacterial, cytotoxic, antivirus, antioxidant and antitoxin activities. They seem to have hepatoprotective activity and in pharmacologic studies have reduced blood sugar (28).
The high scavenging property of S. angustifolium is related to hydroxyl groups existing in the phenolic compounds. In addition phenolics are strong antibacterial compounds and antibacterial properties of several plants are related to their phenolic contents. So the assessment of antibacterial effect of S. angustifolium in future studies is proposed.
In a study by Sadati and colleagues, S. swartzii from Asaloye-Niband marine protected area of the Persian Gulf was analyzed for its antioxidant and phenolic contents. The extract was partitioned in different solvents and methanol partition showed the highest phenolic content (0.12 mg/g) and antioxidant activity (73.92 ± 12.3 mmol FeII per 100 g dried plant) that is comparable with our study (29).
Tropical conditions affect phytochemical constituents of the plants especially phenolic content, particularly in marine organisms. In a study by Targete and coworker, a comparison between the total phenolic compounds of algae from different regions of tropical area was demonstrated (30). The results showed that there was significant difference in phenolics of algae related to the climate conditions. Therefore, it is suggested that phenolic content of algae from other regions of Persian Gulf be evaluated and results are compared.
It is the first time that these three Sargassum species of Persian Gulf are analyzed for their phytochemical and biological pattern. Further work is necessary to isolate the active principles and elucidate the structure and mode of action of these compounds.
CONCLUSION
The extracts of three Sargassum seaweeds from Persian Gulf, Iran were screened for their antioxidant, cytotoxic and phytochemical analysis. Phytochemical and cytotoxic results were similar with few differences in all species. Sargassum angustifolium showed the highest antioxidant activity. These seaweed extracts and their active components could emerge as natural and alternative antioxidants or serve as starting points for synthesizing more effective cytotoxic drugs.
ACKNOWLEDGMENT
This research project numbered 290161 was fully sponsored by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran. Also the authors would like to thank Dr. Zandi and Mr. Sartavi for their help in collection of the samples.
REFERENCES
- 1.Dhargalkar VK, Neelam P. Seaweed: Promising plant of the Millennium. Sci Cult. 2005;71:60–66. [Google Scholar]
- 2.Pietra F. Secondary metabolites from marine microorganisms; bacteria, protozoa, algae and fungi:achievements and perspective. Nat Pro Rep. 1997;14:453–464. doi: 10.1039/np9971400453. [DOI] [PubMed] [Google Scholar]
- 3.Hemminga MA, Duarte CM. Seagrass ecology. New York: Cambridge University Press; 2000. pp. 1–26. [Google Scholar]
- 4.Ragupathi Raja Kannan R, Arumugam R, Anantharaman P. Antibacterial potential of three seagrasses against human pathogens. Asian Pac J Trop Med. 2010;3:890–893. [Google Scholar]
- 5.Arumugam R, Ragupathi Raja Kannan R, Arivuselvan N, Anantharaman P. Antimicrobial potential of some seagrasses against phytopathogens. Seaweed Res Util. 2010;32:177–183. [Google Scholar]
- 6.Rowley DC, Hansen MST, Rhodes D, Sotriffer CA, Ni H, McCammon JA, et al. Thalassiolins A–C: New marine – Derived inhibitors of HIV cDNA integrase. Bioorg Med Chem. 2002;10:3619–3625. doi: 10.1016/s0968-0896(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 7.Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, et al. Study on the anti-inflammatory activity of methanol extract from seagrass Zostera japonica. J Agric Food Chem. 2004;54:306–311. doi: 10.1021/jf0509658. [DOI] [PubMed] [Google Scholar]
- 8.Gokce G, Haznedaroglu MZ. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol. 2008;115:122–130. doi: 10.1016/j.jep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Athiperumalsamy T, Devi Rajeswari V, Hastha Poorna S, Kumar V, Louis Jesudass L. Antioxidant activity of seagrasses and seaweeds. Bot Mar. 2010;53:251–257. [Google Scholar]
- 10.Numata A, Kanbara S, Takahashi C, Fujiki R, Yoneda M, Fujita E, et al. Cytotoxic activity of marine algae and a cytotoxic principle of the brown alga Sargassum tortile. Chem Pharm Bull (Tokyo) 1991;39:2129–2131. doi: 10.1248/cpb.39.2129. [DOI] [PubMed] [Google Scholar]
- 11.Itoh H, Noda H, Amano H, Zhuaug C, Mizuno T, Ito H. Antitumor activity and immunological properties of marine algal polysaccharides, especially fucoidan, prepared from Sargassum thunbergii of Phaeophyceae. Anticancer Res. 1993;13:2045–2052. [PubMed] [Google Scholar]
- 12.Cho SH, Kang SE, Cho JY, Kim AR, Park SM, Hong YK, et al. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J Med Food. 2010;10:479–485. doi: 10.1089/jmf.2006.099. [DOI] [PubMed] [Google Scholar]
- 13.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res Pharm Sci. 2013;8:113–118. [PMC free article] [PubMed] [Google Scholar]
- 14.Vinod BS, Maliekal TT, Anto RJ. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Signal. 2013;18:1307–1348. doi: 10.1089/ars.2012.4573. [DOI] [PubMed] [Google Scholar]
- 15.Sohrabipour J, Rabiei R. The checklist of green algae of the Iranian coastal lines of the Persian Gulf and Gulf of Oman. Iran Journ Bot. 2007;13:146–149. [Google Scholar]
- 16.Yegdaneh A, Putchakan S, Yueyongsawad S, Ghannadi A, Plubrukarn A. 3-Oxoabolene and 1- oxocurcuphenol, Aromatic Bisabolanes from the Sponge Myrmekioderma sp. Nat Pro Com. 2013;8:1355–1357. [PubMed] [Google Scholar]
- 17.Kamba AS, Hassan LG. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr J Pharm Pharmacol. 2010;4:645–652. [Google Scholar]
- 18.Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotech. 2006;5:1142–1145. [Google Scholar]
- 19.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemoth Rep. 1972;3:1–103. [Google Scholar]
- 20.Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Pro. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 21.Burtin P. Nutritional value of seaweeds. Electron J Environ Agric Food Chem. 2003;2:498–503. [Google Scholar]
- 22.Campanella L, Martini E, Tomassetti M. Antioxidant capacity of the algae using a biosensor method. Talanta. 2005;66:902–911. doi: 10.1016/j.talanta.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 23.Athukorala Y, Kim K, Jeon Y. Antiproliteractive and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006;44:1065–1074. doi: 10.1016/j.fct.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto I, Takahashi M, Tamura E, Maruyama H, Mori H. Antitumor activity of edible marine algae: Effect of crude fucoidan fractions prepared from edible brown seaweeds against L-1210 leukemia. Hydrobiologia. 1984;116:145–148. [Google Scholar]
- 25.Kim JA, Karadeniz F, Ahn BN, Kwon MS, Mun OJ, Bae MJ, et al. Bioactive quinone derivatives from the marine brown alga Sargassum thunbergii induce anti-adipogenic and pro-osteoblastogenic activities. J Sci Food Agric. 2015;10:48–54. doi: 10.1002/jsfa.7148. [DOI] [PubMed] [Google Scholar]
- 26.Lee JI, Seo Y. Chromanols from Sargassum siliquastrum and their antioxidant activity in HT 1080 cells. Chem Pharm Bull (Tokyo) 2011;59:757–761. doi: 10.1248/cpb.59.757. [DOI] [PubMed] [Google Scholar]
- 27.Costa LS, Fidelis GP, Cordeiro SL, Oliveira RM, Sabry DA, Câmara RB, Nobre LT, Costa MS, et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother. 2010;64:21–28. doi: 10.1016/j.biopha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Besednova NN, Zaporozhets TS, Kuznetsova TA, Kryzhanovski SP, Kovalev NN, Zviagintseva TN. Hepatoprotective effects of extracts and polysaccharides from seaweed. Antibiot Khimioter. 2014;59:30–37. [PubMed] [Google Scholar]
- 29.Sadati N, Khanavi M, Mahrokh A, Nabavi SMB, Sohrabipour J, Hadjiakhoondi A. Comparison of antioxidant activity and total phenolic contents of some Persian Gulf Marine Algae. J Med Plants. 2011;10:72–79. [Google Scholar]
- 30.Targete NM, Coen LD, Boettcher AA, Tanner ChE. Biogeographic comparisons of marine algal polyphenolics: evidence against a latitudinal trend. Oceologia. 1992;89:464–470. doi: 10.1007/BF00317150. [DOI] [PubMed] [Google Scholar]


