Abstract
Background:
Proximal humerus fractures are common problems plaguing the elderly population.
Purpose:
The purposes of this study were to determine the outcomes of fibular strut allografts in treatment of proximal humerus fractures with open reduction internal fixation (ORIF) and to present the authors’ preferred surgical technique. The hypothesis was that the use of fibular strut allografts in treating proximal humerus fractures with ORIF will provide low reoperation rates with acceptable outcomes.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review was registered with PROSPERO and performed with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting arthroscopic elbow outcomes with levels of evidence 1 through 4 were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents and countries. Statistics were calculated using Student t tests, 1-way analysis of variance, chi-square tests, and 2-proportion Z tests.
Results:
Four studies met the inclusion criteria. While there is great heterogeneity existing in the literature surrounding use of a fibular strut allograft as an adjunct to ORIF of proximal humerus fractures, current evidence shows a humeral head screw penetration rate of 3.7% with acceptable functional outcome scores, with a reoperation rate of 4.4% at a weighted mean 80.78 weeks (1.55 years) of postoperative follow-up.
Conclusion:
There is great heterogeneity that exists in the literature surrounding the use of a fibular strut allograft as an adjunct to ORIF of proximal humerus fractures. Current evidence shows a screw penetration rate of 3.7% with acceptable functional outcome scores, demonstrating fibular strut allograft is a viable option for treating proximal humerus fractures.
Keywords: proximal humerus fracture, fibular strut, open reduction internal fixation, surgical, shoulder
Proximal humerus fractures (OTA 11-B)13 are common in elderly individuals, reported as the third-most-common fracture in this population behind distal radius and hip fractures at 105 per 100,000 patients per year.2,10,18 In part, the reason for this is the decreasing bone quality from osteoporosis or osteopenia that presents with increasing age. The rate of proximal humerus fractures has been steadily increasing in the United States over the past 30 years at a rate of approximately 13% per year.7–9,18 Proximal humerus fractures are also common in the overall population, accounting for approximately 5% of all fractures; many of these fractures are managed nonoperatively by treating orthopaedic surgeons.3,15 Operative versus nonoperative treatment is typically dictated by the type of fracture in addition to other patient-oriented factors, including health and functional status.
The optimal treatment for proximal humerus fractures has yet to be elucidated. Gupta et al6 recently reviewed various types of surgical treatments for complex proximal humerus fractures and found significantly better clinical outcomes but a significantly higher reoperation rate in patients who underwent an open reduction internal fixation (ORIF) versus hemiarthroplasty and reverse total shoulder arthroplasty. To combat some of the issues with ORIF of proximal humerus fractures including implant failure, loss of reduction, fracture malunion or nonunion, osteonecrosis of the humeral head, or impingement syndrome, use of a fibular strut graft has been described to attempt to decrease postoperative screw penetration into the joint as well as varus collapse.14
The purposes of this study were to perform a systematic review of the literature to determine patient-reported clinical outcomes and complication rates with fibular strut augmentation of ORIF of proximal humerus fractures at short-term follow-up as well as to present the authors’ preferred surgical technique.
Methods
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.11 Systematic review registration was performed on February 1, 2015 using the PROSPERO International prospective register of systematic reviews (registration number CRD42015016777). Two reviewers independently conducted the search on February 16, 2014 using the following databases: MEDLINE, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: ((proximal humerus fracture) AND fibular strut). Level 1 through 4 evidence (2011 update by the Oxford Centre for Evidence-Based Medicine) clinical studies in English were eligible while medical conference abstracts were ineligible. All references within included studies were cross-referenced for inclusion if missed by the initial search. If a duplicate population was noticed, the study with the longer mean follow-up was included to avoid including the same patients twice. Studies that were excluded were basic science, biomechanical studies, level 5 evidence, open reduction of proximal humerus fractures without the use of a fibular strut graft, imaging, surgical technique, classification studies, and those studies that evaluated the treatment of nonunions, malunions, floating shoulders, proximal humerus fractures with humeral shaft extension, hemiarthroplasty, total shoulder arthroplasty, reverse shoulder arthroplasty, or revision procedures.
Figure 1 provides the PRISMA flow diagram used to determine the articles appropriate for inclusion in the final analysis. Patients of interest in this systematic review underwent ORIF of an acute proximal humerus fracture with the use of a fibular strut graft. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included publication year, level of evidence, dates of subject enrollment, country and continent of publication, presence or absence of study financial conflict of interest, number of subjects and shoulders, sex, age, bone mineral density, presence/absence of osteoporosis/osteopenia, smoking status, etiology of fracture, type of proximal humerus fracture (number of parts using Neer classification16), surgical technique, and postoperative rehabilitation protocol. Clinical outcome scores common to the included studies were recorded, and included Disability of the Arm, Shoulder, and Hand (DASH); Constant-Murley; and Short Form–36 (SF-36). Radiographic data were extracted when available. Postoperative range of motion was recorded. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).4
Figure 1.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flowchart.
Statistical Analysis: Best Evidence Synthesis
Given the heterogeneity of outcome measures reported by the 4 included studies, a best-evidence synthesis19 was used instead of a meta-analysis. Conceptually, the best-evidence synthesis combines the strengths of traditional reviews and meta-analyses by incorporating the detailed, unbiased analysis by the former and the quantification and systematic literature search methods of the latter. The utility of a best-evidence synthesis exists when there are many studies on a given topic, done by different investigators using different methods or patient populations to arrive at different conclusions, and no one of which clearly supersedes the others with findings accepted as conclusive. This requires reviewers to carefully consider the evidence available and put forth conclusions based on where the weight of the evidence lies. In the context of this study, the best-evidence synthesis begins with the aforementioned best-evidence criteria to justify and describe the study selection criteria employed. The literature synthesis then requires a presentation and discussion of the individual study characteristics, pooling of results when appropriate (as is performed in this study), and subsequently an intelligent, critical examination of the literature to answer questions related to the efficacy of the variable in question, here being the use of fibular strut graft augmentation for ORIF of proximal humerus fractures.19
The results of the quality assessments of the individual studies were used to classify the level of evidence.23 This qualitative analysis was performed with 5 levels of evidence based on the quality and results of the included studies: (1) strong evidence—provided by 2 or more high-quality studies and by generally consistent findings in all studies (75% of the studies reported consistent findings), (2) moderate evidence—provided by 1 high-quality study and/or 2 or more low-quality studies and by generally consistent findings in all studies (75% of the studies reported consistent findings), (3) limited evidence—provided by only 1 low-quality study, (4) conflicting evidence—inconsistent findings in multiple studies (<75% of the studies reported consistent findings), and (5) no evidence—when no studies could be found.
Authors’ Preferred Surgical Technique
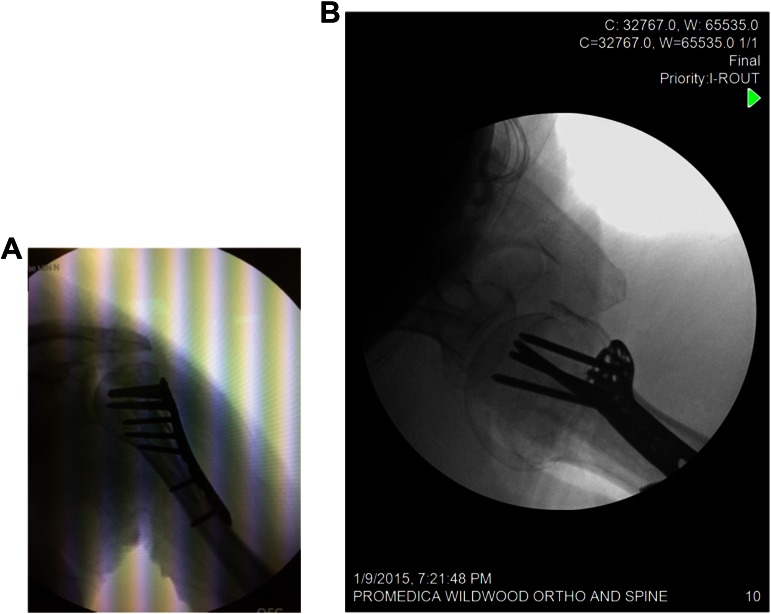
A standard operating table is used, with the head of the bed raised to 30°. A C-arm is brought in parallel to the table coming in from the head of the patient, and its ability to obtain proper anteroposterior (AP) and axillary radiographic views is confirmed (Figure 2, A and B). A standard deltopectoral approach is used. The cephalic vein is preferentially retracted medially, and the clavipectoral fascia and any fibrous tissue is resected. The subcoracoid space is developed and the axillary nerve is identified. A heavy suture is placed into the tendon of the subscapularis. In 3-part fractures, the head is internally rotated and the heavy stitch or fibertape allows the surgeon to derotate the head. The strategy required to reduce 3- and 4-part fractures requires that the fracture be deconstructed. To accomplish this, the joint must be entered. The bone of the bicipital groove is dense, and in complex fractures, the primary fracture line lies posterior to the groove. The joint is entered through this fracture line so that the head segment can be manipulated and the greater tuberosity mobilized. Heavy sutures are placed into the tendon of the infraspinatus, and these sutures can be used to manipulate the greater tuberosity. In complex fractures, the greater tuberosity is pulled posterior and superior by the deforming forces of the rotator cuff. In elderly and osteoporotic patients, the greater tuberosity is often friable, and no attempt should be made to place sutures through the bone. It is also critical that these heavy sutures are secured to the plate at the completion of the case to avoid tuberosity failure. Most fracture plates do not allow the surgeon to gain screw purchase in the greater tuberosity segment and the plate merely serves as a buttress and tuberosity security relies on suture augmentation. The head-shaft segment is then addressed and the head disimpacted from the shaft with an elevator (Figure 3). To avoid a cavitary defect or a bone void, the shaft may be impacted into the humeral head. In cases in which this is not possible, a structural graft can be used to support the head. An allograft fibular strut works well in this situation. The fibular strut is contoured with the distal end shaped with a small oscillating saw and a small round bur: the end with the thickest cortex should support the calcar region of the fracture site (Figure 4). The graft is placed into the humeral shaft and impacted into position with about 2 cm of bone left proud (Figure 5). If the humeral canal has a large diameter, the graft can be provisionally stabilized with a screw that transfixes it in place. It is also preferable to only use the minimum length necessary to support the head as in the revision setting an integrated strut can prove difficult to remove. The humeral head is set over the fibula with the strut allograft impacted into the humeral head. The humeral head and shaft are provisionally fixated to one another with temporary Kirschner wires (K-wires), as is the greater tuberosity to the humeral head. AP and axillary views are taken with the C-arm to confirm anatomic reduction. A locking plate is placed approximately 5 mm posterolateral to the bicipital groove. A bicortical screw is placed in the center of the oblong hole in the humeral shaft to provisionally hold the plate and allow for adjustment of the plate’s height. With the calcar guide, another K-wire is placed to ensure a proper trajectory of the calcar screw; this is drilled and the cannulated calcar locking screw is inserted. Thereafter, all plate screw holes with trajectories into the humeral head are filled with locking screws: Screw tips should be placed 2 to 4 mm from subchondral bone to allow for the possibility of varus collapse without consequential humeral head cut-out. Bicortical humeral shaft screws are then placed (Figure 6, A and B). The tuberosities are repaired to the plate with heavy suture through the islets in the plate. The soft tissue is then closed in layers. The patient’s affected upper extremity is placed in a sling for 4 weeks, with pendulums and table slides allowed at 2 weeks postoperative.
Figure 2.
(A) Anteroposterior and (B) axillary radiographic views obtained intraoperatively with use of a C-arm parallel to the operating table.
Figure 3.

The humeral head-shaft segment of the fracture line is addressed via disimpaction of the humeral head from the humeral shaft with use of an elevator.
Figure 4.
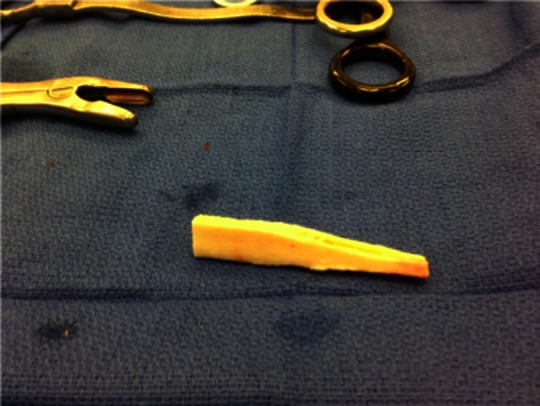
The fibular strut allograft is contoured with the distal end shaped using a small oscillating saw and a small round bur.
Figure 5.

The fibular strut allograft is placed into the humeral shaft and impacted into position with approximately 2 cm of bone left proud.
Figure 6.
(A) Anteroposterior and (B) axillary radiographic views obtained intraoperatively confirming adequate reduction with the final locking plate construct.
Results
Four studies with a total of 136 patients (mean age, 67.97 ± 6.56 years; 70% women) met the inclusion criteria.12,14,17,21 Table 1 provides a summary of the individual studies’ data. All patients sustained a displaced proximal humerus fracture (OTA 11-B) treated with an ORIF and fibular strut augmentation. No studies compared ORIF with fibular strut augmentation to ORIF alone for proximal humerus fractures. Only 127 patients were reported with the specific Neer classification of the proximal humerus fracture, which included 28 patients (22.1%) with 2-part fractures, 60 patients (47.2%) with 3-part fractures, and 39 patients (30.7%) with 4-part fractures. The final study by Tan et al21 notes that all patients had either 2-, 3-, or 4-part Neer classification fractures but did not specify the exact numbers of each category. All studies were level 4 evidence; 3 studies reported no conflict of interest (COI) while 1 study21 did not report the presence or absence of a COI. The mean MCMS was 37 ± 9.06 (poor). Of the patients included in this study, 94.12% were treated with a Philos locking plate (Synthes) while 5.88% were treated with the Peri-LOC locking plate (Smith & Nephew). In 3 studies12,14,17 out of 4, all procedures were performed by a single surgeon; 2 of the 4 studies performed the procedure in the beach chair14,21 position while 1 study17 used the semilateral position and 1 study did not specify.14
TABLE 1.
Summary of Included Individual Study Demographic, Surgical, and Outcome Dataa
| Study | No. of Patients (% Male) | Patient Age, y, Mean/Median (Range) | Overall Cohort Neer Classification of Fracture | Surgical Technique Details | Postoperative Rehabilitation Protocol | Mean Final Follow-up, mo | Subgroup Analyses Performed? |
|---|---|---|---|---|---|---|---|
| Little et al12 | 72 (0.278) | 63 (26-90) | 2-part: 29.2% 3-part: 41.7% 4-part: 29.2% | Single surgeon; deltoid-splitting approach; additional sutures used to stabilize tuberosities |
• Rehab: CPM machine for FF until postop day 1 • Passive FF, ABD, and IR/ER with a licensed OT • Sling at rest, but expected to perform daily home ROM |
Radiographic, 13.1; functional outcomes, 19 | Preoperative varus fractures, preoperative “varus displacement,” preoperative “varus impaction,” preoperative valgus fractures, comparison of varus to valgus subgroups (preoperative data provided: patient age, sex, smoking status, diabetes status, calcar comminution, Neer classification) |
| Matassi et al14 | 17 (0.412) | 62 (54-73) | 2-part: 0% 3-part: 64.7% 4-part: 35.3% | Single surgeon; beach chair positioning; deltopectoral approach (41.2%) and deltoid-splitting approach (58.8%); additional sutures used to stabilize tuberosities |
• Immobilization with sling • Pendulum movements started postop day 1 and shoulder mobilized with passive-assisted exercises • Active exercises at 3 wk |
28 | NR |
| Neviaser et al17 | 38 (NR) | 65.5 (44.1-82.7) | 2-part: 18.4% 3-part: 50.0% 4-part: 31.6% | Single surgeon; sloppy lateral decubitus position; deltoid-splitting approach |
• Active-assisted and passive shoulder ROM under the direction of OT beginning postop day 1 • Therapy 2 times/d during hospital stay • CPM machine for FF used for 4-6 h/d in hospital • Daily therapy at home with FF and ER stretching exercises 3 times/wk • Strengthening after radiographic evidence of healing |
17.2 | Neer types 2, 3, and 4; comparison of Neer fracture type 2 vs 3 vs 4 |
| Tan et al21 | 9 (22.2) | 75.4 (62-86) | Neer 2-, 3-, and 4-part (breakdown not reported) | Beach chair positioning; deltopectoral approach and deltoid-splitting approach; additional sutures used to stabilize tuberosities |
• Immobilize with sling • Passive ROM exercises started 2 d postop • Controlled active mobilization with ABD and FF beyond 90° started 3 wk postop |
3 | NR |
| Overall Cohort Information | |||||||
| Study | Interval Follow-up ROM, deg | Final Follow-up ROM, deg | Final Radiographic Measurements | Complications | Patient-Reported Outcomes | ||
| Little et al12 | NR | NR (active/passive FF, ER provided for all subgroups) | Mean time to radiographic union: 5 mo (range, 2-12); % patients with varus displacement, neck-shaft angle, change in humeral height, GT displacement provided for all subgroups | NR (% AVN, superficial wound epidermolysis, deep infection, screw perforation, revision surgery provided for all subgroups) | NR (DASH, SF-36, UCLA, Constant-Murley provided for all subgroups) | ||
| Matassi et al14 | NR | Medians: FF, 149°; extension, 47°; IR, 40°; ER, 65°; ABD, 135° | Anatomic alignment, 94.1%; slight varus alignment, 5.9%; humeral head collapse, 0%; screw penetration, 0%; complete fracture healing, 100%; mean change in humeral head height, 0.3 cm; restoration of medial cortical continuity, 100% | Superficial infections, 5.8%; major complications, 0% |
Medians: Constant-Murley, 79; VAS pain, 1; DASH, 33; SF-36, 83 • Return to previous activities: 88.2% • Experienced restrictions to activities: 11.8% |
||
| Neviaser et al17 | NR | Means: FF, 147.9°; IR, 0.8° (difference in No. of vertebral levels); ER, 60.7° | Collapse of humeral head, 2.6%; complete AVN, 0%; partial AVN, 2.6%; screw penetration, 0%; complete fracture healing, 97.3%; loss of reduction, 2.6%; restoration of medial cortical continuity, 97.4% | Superficial infections, 2.6%; reoperation for superficial infections, 0%; HO formation, 5.3% |
• Means: Constant-Murley, 87; DASH, 15 • Mean SF-36 scores: overall, 80; physical health, 79; mental health, 79.9; physical function, 83.1; pain, 78.6; general health, 86.7; vitality, 71.1 (Constant-Murley and SF-36 total, physical health, mental health, physical function, pain, general health, and vitality scores provided for all subgroups) |
||
| Tan et al21 | Mean 6 wk: FF, 87°; ABD, 85°; ER, 31°; IR, 40° | Means: FF, 109°; ABD, 107°; ER, 41°; IR, 55° |
• Immediate mean head-shaft angle, 139.2° • At 6 wk: mean head-shaft angle, 137.4°; screw cut-out, 0%; evidence of callus formation, 0% • At 12 wk: evidence of callus formation, 100%; maintenance of head-shaft angle, 100%; mean head-shaft angle, 136.6° • Screw penetration: 0% |
Superficial infections, 0%; deep infections, 0%; unplanned readmissions, 0%; major complications, 0%; axillary nerve deficits, 0% | NR | ||
aABD, abduction; AVN, avascular necrosis; CPM, continuous passive motion; DASH, Disabilities of the Arm, Shoulder, and Hand; ER, external rotation; FF, forward flexion; GT, greater trochanter; HO, heterotopic ossification; IR, internal rotation; NR, not reported; OT, occupational therapist; POD, postoperative day; PROs, patient-reported outcomes; ROM, range of motion; SF-36 = Short-Form–36; UCLA, University of California, Los Angeles; VAS, visual analog scale.
With regard to surgical technique, those described by the included articles are similar overall to our preferred method but with some variation. The articles mention both semilateral position17 and beach chair position14 and both deltoid-splitting12,14,17 and deltopectoral14 approaches (in comparison with our preferred method with beach chair position and a standard deltopectoral approach). Two publications14,17 similar to our technique placed sutures into the rotator cuff for control and fixation of the tuberosities; K-wires were used by several authors to joystick the fragments and achieve graft position.14,17 Some of the articles describe use of the fibular allograft as a reduction tool itself,12,14,17 and Matassi et al14 describes the placement of a single screw through the locking plate to push the fibula medially until it apposes the medial cortex of the humerus (to indirectly reduce the medial column) before subsequently placing several shaft screws through it. Finally, 1 publication14 used a postoperative drain, which our method does not call for, and the other 3 included publications do not report use of a drain.
With regard to outcome scores, the Constant-Murley score averaged 85.11 at weighted final follow-up of 89.52 weeks (1.72 years; N = 55). The DASH score averaged 19.45 at weighted final follow-up of 89.52 weeks (1.72 years; N = 55). In studies that looked at complete rates of fracture healing at weighted mean follow-up of 89.52 weeks (1.72 years; N = 55), 54 (98.2%) patients showed complete fracture healing.14,17
Five patients (3.7%) had screw penetration into the articular surface of the humeral head at some time postoperatively (exact timing not recorded by included studies); all were subsequently removed arthroscopically. Four patients were found to have a superficial infection (2.9%) defined as “wound breakdown/epidermolysis” treated with local wound care (1.45%) or 2 weeks of oral antibiotics (1.45%) with full resolution, 3 patients sustained deep infections (2.2%) necessitating implant removal after fracture union, and no patient had an unplanned readmission to the hospital. Thus, 8 patients (5.9%) in total required reoperation by the time of final follow-up, 5 of which were for the aforementioned screw penetration into the glenohumeral joint and the remaining 3 for implant removal related to deep infection. No patient had complete osteonecrosis but 1 patient (0.75%) had radiographic evidence of partial osteonecrosis of the humeral head at final follow-up (timing not reported) and did not require reoperation as this was not symptomatic; additionally, 1 patient (0.75%) showed a loss of reduction with varus collapse (timing not reported) and had no further surgery given adequate clinical outcomes. Two patients (1.5%) had asymptomatic heterotopic ossification (grade or classification not reported).
Discussion
Proximal humerus fractures are a common and often times complex problem for treating orthopaedic surgeons given their relative complexity. The use of a fibular strut allograft to aid in fracture reduction and fixation as an adjunct to standard locking plates has been recently described with promising clinical results.12,14,17,21 The use of fibular strut grafts to augment ORIF in the treatment of proximal humerus fractures has been shown to increase construct stiffness and maximal failure load.1 Its purpose is to provide medial support to prevent collapse as well as screw penetration, as the rate of screw penetration into the articular surface of the humeral head has been reported as high as 29%.5
Solberg et al20 reported a complication rate of 79% in 24 patients treated by locked plating alone who presented with a proximal humerus fracture in varus as compared with valgus (19% complication rate) due to a significantly higher rate of varus malreduction (71%), screw perforation into the joint, and varus subsidence. Hardeman et al7 reported on 307 patients treated with locked plating alone and found a failure rate of 47% in varus-aligned fracture with a reoperation rate of 52.9% due to avascular necrosis (AVN), screw cut-out, loss of reduction, or malunion. Thanasas et al22 systematically reviewed the literature on locking plate use in proximal humerus fractures and reported an incidence of 7.9% for AVN, 11.6% for screw cut-out, and a 13.7% reoperation rate but with an overall mean Constant score of 74.3 for the 791 patients included; the authors attributed the high incidence of these findings to the rigidity of the implant and the concomitant medial inadequate support with underlying severely osteoporotic bone.
The purpose of this study was to evaluate the outcomes of fibular strut allografts in treatment of proximal humerus fractures with ORIF. The authors’ hypotheses were confirmed in that patients with proximal humerus fractures treated with ORIF with fibular strut augmentation showed excellent clinical outcomes, including a low reoperation rate at 4.4% with acceptable functional outcome scores. This compares favorably to the aforementioned complication rates from the available literature on the fixation rates of proximal humerus fractures without strut augmentation.
It is important to review the indications for fibular strut grafting in each of the 4 included studies within this review to better understand the clinical scenario in which this technique can and should be employed. Little et al12 utilized the technique for patients with varus or valgus coronal plane fracture angulation who had 2-part Neer classification fractures with angulation greater than 45° and displacement greater than 1 cm, or 3- or 4-part Neer classification fractures. These authors in total had 21 (29.2%) Neer 2-part fractures, 30 (41.7%) Neer 3-part fractures, and 21 (29.2%) Neer 4-part fractures. Tan et al21 utilized this technique in any patient who underwent surgery for a displaced 2-part, 3-part, or 4-part Neer classification fracture pattern; they did not report the exact numbers of each of these categories that their patients fell into. Matassi et al14 indicated this technique for patients with “unstable” fracture patterns, designated as those with displacement, a disrupted medial hinge, and significant metaphyseal comminution. Eleven fractures (64.7%) were 3-part Neer classification fracture and 6 (35.3%) were 4-part fractures. Finally, Neviaser et al17 utilized fibular strut graft augmentation on those patients with displaced fractures and cortical comminution in the region of the surgical neck. They included 7 (18.4%) 2-part fractures, 19 (50.0%) 3-part fractures, and 12 (31.6%) 4-part fractures.
Overall, proximal humerus fractures with preoperative displacement, varus coronal malalignment, and/or medial cortical comminution are indicated for fibular strut allograft augmentation use due to the aforementioned increased risk of screw pull-out, varus malreduction, and humeral head subsidence with poor resultant functional outcomes when these proximal humerus fractures exist in this orientation due to their likelihood for further varus subsidence and screw cut-out.7,12,20,22 Patients with overall poor bone quality can be considered for augmentation use as well. Those patients with fracture patterns that maintain relative alignment outside of varus (neutral or valgus, per the suggestion of some authors) and without comminution of the medial calcar bone may not require the use of this augmentation option.
When comparing the 4 included articles independently, their findings were overall very similar with overwhelmingly favorable clinical and radiographic results for the fibular allograft strut augmentation in each, in addition to only a small number of complications. Neviaser et al17 evaluated 38 patients at a minimum follow-up of 49 weeks and reported no intra-articular screw penetration, cut-out, or complete AVN, with just 1 case of partial AVN and 1 loss of reduction. DASH (mean, 15) and Constant-Murley scores (mean, 87) were favorable as well. Matassi et al14 additionally reported no major complications, humeral head collapse, AVN, or screw cut-out with 100% radiographic healing at a mean 13 months postoperatively in their 17 patients. The mean Constant score (79) was similar as well, but with a higher DASH score (median, 33) than the study by Neviaser et al.17 Range of motion measurements were as follows: mean active forward flexion, 149°; extension, 47°; internal rotation, 40°; external rotation, 65°; and abduction, 135°. Tan et al21 reported no loss of reduction or screw cut-out in their 9 patients that were treated. At 12 weeks postoperatively, all patients had radiographic callus formation with maintenance of head-shaft angles, but range of motion measurements were generally lower than that reported by Matassi et al14: mean forward flexion, 109°; abduction, 107°; external rotation, 41°; and internal rotation, 55°. Little et al12 compared 32 patients with varus fractures to 40 valgus fractures. They reported no significant difference in the initial or final postoperative neck-shaft angles or change in humeral height, with no differences in what were successful functional outcome scores similar to those in the study by Neviaser et al17 (Constant, 85.2 vs 88.7; DASH, 21.4 vs 13.9). Complications were similarly rare, with only 3 total deep infections, 1 case of AVN, and 5 asymptomatic humeral head screw penetrations.
The primary limitation to this study is its relatively small overall cohort size, which is an inherent limitation to any systematic review with low numbers of published studies. None of the included studies were better than level 4 evidence, being entirely retrospective case series–based. In addition, none of the included studies compared the cohort of proximal humerus ORIF cases with fibular strut graft augment to a cohort of patients without fibular strut graft augmentation or a cohort of patients treated nonoperatively; thus, a comparison of this technique to other generally accepted strategies is not possible. Finally, given that there was appreciable heterogeneity in terms of patient fracture classification type (2-, 3- or 4-part fracture patterns) in those studies that reported it—and possibly in the other studies that did not provide this information—the results of this report must be viewed cautiously with regard to generalizability of its efficacy for all proximal humerus fracture patterns. Future higher level research is needed on the topic, with a randomized controlled trial comparing proximal humerus fracture fixation with and without fibular strut graft augmentation being the gold standard.
Conclusion
There is great heterogeneity that exists in the literature surrounding the use of a fibular strut allograft as an adjunct to ORIF of proximal humerus fractures. Current evidence shows a humeral head screw penetration rate of 3.7% with acceptable functional outcome scores, with a reoperation rate of 4.4% at a weighted mean 80.78 weeks (1.55 years) of postoperative follow-up, demonstrating fibular strut allograft is a viable option for treating proximal humerus fractures.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.M.S. receives royalties from Nova Science Publishers and the Postgraduate Institute for Medicine. J.D.H. receives research support from DePuy and Smith & Nephew and royalties from SLACK Inc. M.M. is a paid consultant for DJ Orthopaedics and Stryker and receives research support from DJ Orthopeadics. A.A.R. receives royalties from Arthrex, Saunders//Mosby-Elsevier, and SLACK Inc; is a paid consultant for Arthrex; and receives research support from DJO Surgical, Ossur, and Smith & Nephew.
References
- 1. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93:937–941. [DOI] [PubMed] [Google Scholar]
- 2. Baron JA, Barrett JA, Karagas MR. The epidemiology of peripheral fractures. Bone. 1996;18(3 suppl):209S–213S. [DOI] [PubMed] [Google Scholar]
- 3. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52:243–249. [DOI] [PubMed] [Google Scholar]
- 4. Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89:1693–1699. [DOI] [PubMed] [Google Scholar]
- 5. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21:185–191. [DOI] [PubMed] [Google Scholar]
- 6. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures -a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2015;29:54–59. [DOI] [PubMed] [Google Scholar]
- 7. Hardeman F, Bollars P, Donnelly M, Bellemans J, Nijs S. Predictive factors for functional outcome and failure in angular stable osteosynthesis of the proximal humerus. Injury. 2012;43:153–158. [DOI] [PubMed] [Google Scholar]
- 8. Kim SH, Szabo RM, Marder RA. Epidemiology of humerus fractures in the United States: nationwide emergency department sample, 2008. Arthritis Care Res (Hoboken). 2012;64:407–414. [DOI] [PubMed] [Google Scholar]
- 9. Launonen AP, Lepola V, Flinkkilä T, et al. Conservative treatment, plate fixation, or prosthesis for proximal humeral fracture. A prospective randomized study. BMC Musculoskelet Disord. 2012;13:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3:127–132. [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 12. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28:338–347. [DOI] [PubMed] [Google Scholar]
- 13. Marsh JL, Slongo TF, Agel J, et al. Fracture and Dislocation Classification Compendium–2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(suppl 10):S1–S133. [DOI] [PubMed] [Google Scholar]
- 14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43:1939–1942. [DOI] [PubMed] [Google Scholar]
- 15. Neer CS., 2nd Displaced proximal humeral fractures: part I. Classification and evaluation. 1970. Clin Orthop Relat Res. 2006;442:77–82. [DOI] [PubMed] [Google Scholar]
- 16. Neer CS., 2nd Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077–1089. [PubMed] [Google Scholar]
- 17. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469:3300–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop Relat Res. 2006;442:87–92. [DOI] [PubMed] [Google Scholar]
- 19. Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol. 1995;48:9–18. [DOI] [PubMed] [Google Scholar]
- 20. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23:113–119. [DOI] [PubMed] [Google Scholar]
- 21. Tan E, Lie D, Wong MK. Early outcomes of proximal humerus fracture fixation with locking plate and intramedullary fibular strut graft. Orthopedics. 2014;37:e822–e827. [DOI] [PubMed] [Google Scholar]
- 22. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18:837–844. [DOI] [PubMed] [Google Scholar]
- 23. van Tulder M, Furlan A, Bombardier C, Bouter L; Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2003;28:1290–1299. [DOI] [PubMed] [Google Scholar]