Abstract
Background
Tangshen Formula (TSF) is a traditional Chinese medicine for the treatment of diabetic kidney disease (DKD). Liver-type fatty acid binding protein (L-FABP) is expressed in various tissues, including the kidney, where it is known as urinary L-FABP. Other studies demonstrated that urinary L-FABP may be a useful biomarker for monitoring DKD. This post-hoc analysis and cross-sectional study evaluated the changes in urinary L-FABP in DKD patients treated with TSF and conventional medicine.
Methods
Post-hoc analysis was conducted on a multicenter, randomized, double-blind, placebo-controlled trial. A total of 180 participants with DKD including 98 with microalbuminuria and 82 with macroalbuminuria were enrolled in the original study. In addition to conventional treatment, 122 participants were randomly assigned to receive TSF and 58 to receive placebo. After 24-weeks of treatment, the intention-to-treat population in microalbuminuria stage was 56 in the TSF group and 25 in the placebo group, and in the macroalbuminuria stage 42 and 19, respectively. The primary outcome in the original trial was urinary protein level. In the current study, urinary and plasma L-FABP levels were measured in 30 microalbuminuria patients (15 in the TSF group and 15 in the placebo group) and 30 macroalbuminuria patients (15 in the TSF group and 15 in the placebo group). In addition, another 30 patients with normoalbuminuria (urinary albumin excretion rate (UAER) < 20 μg/min) were recruited for the cross-sectional study.
Results
(1) In microalbuminuria patients, UAER in the TSF group displayed a significant decrease after 24 weeks of treatment (P = 0.045). Levels of urinary L-FABP in the TSF group were markedly lower than in the placebo group after 12 and 24 weeks (P = 0.004 and P = 0.047, respectively). (2) In macroalbuminuria patients, 24-h urinary protein levels decreased significantly compared with baseline in the TSF group at week 12 (P = 0.042) and week 24 (P = 0.041). The TSF group showed a significant decrease in urinary L-FABP after 12 and 24 weeks (P = 0.036 and P = 0.046, respectively). (3) Levels of urinary L-FABP increased markedly, correlating with severity of DKD. L-FABP in patients with normoalbuminuria, microalbuminuria, and macroalbuminuria were 5.9 (5.2, 7.8) μg/ml, 11.4 (6.8, 13.4) μg/ml and 18.5 (10.9, 23.4) μg/ml, respectively (P = 0.000).
Conclusions
TSF combined with conventional therapy appeared to be effective in reducing urinary protein and urinary L-FABP. Urinary L-FABP levels appear to be associated with the severity of DKD.
Trial registration
Chinese Clinical Trial Registry ChiCTR-TRC-10000843. Registered 15 April 2010.
Keywords: Chinese herbal medicine, Tangshen formula, Liver-fatty acid binding protein, Diabetic kidney disease
Background
Diabetic kidney disease (DKD) is a critical problem, affecting approximately one third of people with diabetes mellitus (DM) [1]. As the number of persons with DM increases [2, 3], there has been a concomitant rise in DKD prevalence with associated cardiovascular mortality and end-stage renal disease (ESRD) [4]. DKD is also known as diabetic nephropathy. It is characterized by a pathologic series of structural abnormalities of all renal compartments, including glomerular and tubular basement membrane thickening, mesangial expansion, interstitial inflammation, glomerular and tubular hypertrophy, glomerulosclerosis, and tubulointerstitial fibrosis [5, 6], which eventually lead to renal dysfunction [7].
It is now widely accepted that the tubulointerstitium is closely involved in the pathogenesis of diabetic nephropathy and its degree of fibrosis corresponds with the rate of decline in kidney function [8, 9]. In the last decade, clinical studies have demonstrated that increased urinary liver-type fatty acid binding protein (L-FABP), which is expressed in the proximal tubules in the human kidney, is associated with the severity and clinical prognosis of DKD [10]. For example, Kamijo et al. [11] found that urinary L-FABP levels were progressively increased in patients with normo-, micro-, or macroalbuminuria and further increased in those with ESRD. After a 4-year follow-up, urinary L-FABP levels in patients who showed DKD progression were higher than those without progression. Thus, urinary L-FABP might be a suitable biomarker for predicting and monitoring deterioration of renal function in DKD. Furthermore, an accumulating number of interventional studies have reported that urinary L-FABP responds to renoprotective treatment [12–14].
Traditional Chinese Medicine (TCM) has been used for millennia in China to treat a variety of diseases. Records of using TCM to treat diabetes and its complications can be found in ancient TCM literature. Even in modern China, TCM is a primary or complementary therapy for kidney disease [15, 16]. The traditional Chinese herbal formula, Tangshen Formula (TSF), is composed of several herbs and formulated based on empirical evidence. In our previous studies, TSF showed a beneficial effect in attenuating development of DKD [17–21]. In light of those results, we conducted a multicenter randomized double-blind placebo-controlled trial to further demonstrate the efficacy and safety of TSF in treating DKD in persons with type 2 diabetes [22]. Since L-FABP is a novel biomarker for prognosis of DKD, we conducted this post-hoc analysis to evaluate the effect of TSF on urinary and plasma L-FABP in different DKD stages. In addition, we enrolled another 30 sex- and age-matched patients with normoalbuminuria to validate the clinical relevance of urinary L-FABP levels at various stages of DKD.
Methods
Study design
This post-hoc analysis was conducted using data from a previously published multicenter randomized double-blind placebo-controlled study that investigated the efficacy and safety of TSF in patients with type 2 diabetic kidney disease (Chinese Clinical Trial Registry ChiCTR-TRC-10000843) [22]. During that study, peripheral blood and urine samples of the participants were collected at baseline, 12 weeks, and 24 weeks and had been in frozen storage at −80 °C. We used these blood and urine samples to conduct this post-hoc analysis on L-FABP. Details of the the original trial design have been published previously [22]. The original trial was approved by the ethics committee of the China-Japan Friendship Hospital (No.2006–059). All participants in the trial signed written informed consent documents.
Diagnostic standards
Type 2 diabetes was diagnosed based on the American Diabetes Association guidelines (ADA; 2006) [23]. Diabetic kidney disease was diagnosed according to criteria of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI; 2007) [24]. Participants also underwent TCM diagnosis for their presenting syndrome (that is, TCM signs and symptoms). The predominant syndrome of qi-yin deficiency combined with blood stasis was based on criteria established in Clinical Research of New Investigational Drugs in Traditional Chinese Medicine [25].
Study population
The inclusion and exclusion criteria have been described previously [22] and are summarized here. Men and women between the ages of 25 and 75 with type 2 diabetes were eligible if they had urinary albumin excretion rate (UAER) between 20 and 200 μg/min, and/or 24-h urinary protein (24 h UP) between 0.5 and 2.0 g/d, and estimated glomerular filtration rate (eGFR) between 60 ml/min and 130 ml/min, fasting blood glucose (FBG) ≤ 7.8 mmol/L, and hemoglobin A1C ≤ 7.5 %; blood pressure < 140/90 mmHg; and TCM syndrome was deficiency of both qi and yin with blood stasis.
Patients were excluded if they had history of either kidney disease or other disease with elevated urinary protein, endocrine or metabolic disease, liver disease, cardiovascular disease within the past 3 months, or triglyceride (TG) > 10 mmol/L (>886 mg/dl). Patients taking glucocorticosteroids, thiazide diuretics, or niacin within the past 3 months were also excluded as were pregnant or breastfeeding women.
Randomization
A random allocation sequence was generated by computer based on blocks of 6. Patients were randomly assigned to either the TSF group (conventional treatments plus TSF) or the placebo group (conventional treatments plus placebo) in a 2:1 ratio and classified according to their different stages of DKD (microalbuminuria or macroalbuminuria). A total of 191 patients were screened, resulting in enrollment of 180 participants, including 98 with microalbuminuria and 82 with macroalbuminuria; 122 were randomly assigned to receive TSF and 58 to receive placebo. After 24-weeks’ clinical observation, the intention-to-treat population in microalbuminuria stage was comprised of 56 participants in the TSF group and 25 in the placebo group, while there were 42 and 19 participants in the TSF and placebo groups, respectively, in the macroalbuminuria stage. Blood and urine samples were collected at baseline, 12 and 24 weeks and stored at −80 °C for further analyses. Since only 15 macroalbuminuria participants in the placebo group permitted their blood and urine to be collected, 30 sex- and age-matched microalbuminuria participants (15 in the TSF group and 15 in the placebo group) and 30 sex- and age-matched macroalbuminuria participants (15 in the TSF group and 15 in the placebo group) were included to assay their urinary and plasma L-FABP levels. In addition, 30 sex- and age-matched participants patients with normoalbuminuria (UAER < 20 μg/min) were recruited from China to Japan Friendship Hospital for a cross-sectional study.
Treatment protocol
The treatment protocol has been described previously [22]. Based on ADA 2006 recommendations [23], antihypertensive treatment, glycemic control, and antilipemic agents were adopted as conventional treatment using open-label drugs (calcium channel blockers, insulin, statins). All participants received conventional treatment and participants with albuminuria received either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker agent. After a 2-week run-in period with diet control and programmed daily exercise, TSF and placebo were initiated for both groups with 8 g of granules dissolved in warm water, taken twice daily for 24 weeks.
The Chinese herbal formula TSF consists of seven natural herbs: astragalus root (Astragalus membranaceus (Fisch.) Bunge); rehmannia root (Rehmannia glutinosa (Gaertn.) DC.); notoginseng root (Panax notoginseng (Burk.) F.H. Chen); winged burning bush twig (Euonymus alatus (Thunb.) Sieb.); cornus fruit (Cornus officinalis Sieb. et Zucc.); rhubarb root and rhizome (Rheum palmatum L.); and bitter orange (Citrus aurantium L.). Main ingredients of the placebo were lactose, maltodextrin, and artificial food coloring. Preparation of TSF and placebo has been described previously [22]. TSF and placebo granules were identical in packaging, appearance, shape, size, and color.
Outcomes assessments
In the original trial, the primary outcome measure was urinary protein level, which was assessed by UAER for participants with microalbuminuria and 24 h UP for participants with macroalbuminuria. Secondary outcome measures were renal function, including eGFR, Scr, and BUN, and lipid profile, including TG, total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL). Safety indicators included alanine transaminase (ALT) and aspartate aminotransferase (AST). In addition, FBG and A1C were assessed. Blood and urine samples were collected from participants and frozen at −80 °C at baseline, 12 and 24 weeks. For this post-hoc analysis, both urinary and plasma L-FABP levels were measured using human L-FABP ELISA kits (RapidBio Lab, Calabasas, CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS 13.0 software (IBM, Armonk, NY, USA). Normally distributed variables were expressed as mean ± SD. Abnormally distributed variables were expressed as medians (interquartile range (IQR)). To compare 2 normally distributed variables in the same group, a paired t test was used. Student’s t test was used for analyzing the difference of variables between 2 groups with normal distribution. In the 3 DKD groups, normally distributed variables were compared using one-way ANOVA, and all comparisons between abnormally distributed parameters were performed with the Kruskal-Wallis test. In TSF treatment trial, normality was assessed using the Kolmogorov–Smirnov test, levels of abnormally distributed urinary and plasma L-FABP were exponentially- transformed (ln-transformed) before analysis, and if data were normally distributed after transformation, Student’s t test was used to compare differences between 2 groups. Differences with P < 0.05 were considered statistically significant.
Results
TSF treatment trial
Baseline characteristics
Baseline demographic, clinical, and laboratory characteristics of participants with microalbuminuria and macroalbuminuria in the TSF and placebo groups were similar (P > 0.05) (Table 1).
Table 1.
Baseline characterstics of participating diabetic kidney disease patients
| Microalbuminuria | Macroalbuminuria | |||||
|---|---|---|---|---|---|---|
| TSF (n = 15) | Placebo (n = 15) | P Value | TSF (n = 15) | Placebo (n = 15) | P Value | |
| Age (yr) | 56.1 ± 12.5 | 51.8 ± 8.5 | .382 | 55.5 ± 8.2 | 58.0 ± 10.8 | .522 |
| Male/Female | 9/6 | 7/8 | .384 | 6/9 | 5/10 | .400 |
| BMI (kg/m2) | 26.1 ± 3.7 | 26.0 ± 3.9 | .950 | 25.9 ± 3.0 | 27.3 ± 2.9 | .271 |
| Blood pressure | ||||||
| Systolic (mmHg) | 131.9 ± 7.8 | 128.3 ± 5.6 | .259 | 130.1 ± 8.3 | 131.1 ± 9.3 | .777 |
| Diastolic (mmHg) | 81.6 ± 6.4 | 76.2 ± 10.1 | .142 | 78.6 ± 7.4 | 82.0 ± 4.9 | .231 |
| Laboratory variables | ||||||
| FBG (mmol/L) | 6.7 ± 0.9 | 5.7 ± 1.0 | .097 | 6.9 ± 1.5 | 6.8 ± 0.7 | .752 |
| A1C (%) | 6.7 ± 0.6 | 6.5 ± 0.6 | .065 | 6.7 ± 1.0 | 6.5 ± 1.3 | .579 |
| TG (mmol/L) | 2.5 ± 1.6 | 2.2 ± 1.2 | .617 | 2.7 ± 1.8 | 2.2 ± 1.0 | .528 |
| TC (mmol/L) | 5.2 ± 0.9 | 4.9 ± 1.6 | .532 | 5.2 ± 0.9 | 5.6 ± 1.2 | .545 |
| LDL (mmol/L) | 3.0 ± 0.9 | 3.2 ± 1.4 | .729 | 3.0 ± 1.2 | 3.8 ± 1.6 | .293 |
| HDL (mmol/L) | 1.3 ± 0.6 | 1.0 ± 0.3 | .091 | 1.3 ± 0.4 | 1.5 ± 0.3 | .182 |
| ALT (U/L) | 26.2 ± 12.6 | 22.2 ± 9.1 | .417 | 22.2 ± 8.7 | 22.9 ± 7.8 | .835 |
| AST (U/L) | 22.4 ± 7.8 | 17.2 ± 5.5 | .102 | 20.3 ± 5.7 | 22.5 ± 8.5 | .443 |
Values expressed as mean ± SD
Abbreviations: ALT alanine aminotransferase, A1C glycosylated hemoglobin, AST aspartate aminotransferase, BMI body mass index, FBG fasting blood glucose, HDL high density lipoprotein, LDL low density lipoprotein, TC total cholesterol, TG triglyceride
Effects of TSF on renal function indices and urinary and plasma L-FABP levels in DKD patients with microalbuminuria
There was no statistical differences in levels of Scr, BUN, UAER, eGFR between the TSF group and the placebo group in participants with microalbuminuria at baseline (P = 0.136, P = 0.295, P = 0.975, P = 0.242, respectively), 12 weeks (P = 0.817, P = 0.080, P = 0.979, P = 0.713, respectively) and 24 weeks (P = 0.343, P = 0.484, P = 0.385, P = 0.697, respectively). However, after 12 and 24 weeks of TSF treatment, UAER decreased markedly when compared with that at baseline in the same group (P = 0.090 and P = 0.045), while in the placebo group, little or no change was exhibited (P = 0.199 and P = 0.259). Levels of Scr also declined and eGFR was increased over baseline in the TSF group although without significant difference (at 12 weeks, P = 0.222 and P = 0.354, respectively; at 24 weeks, P = 0.386 and P = 0.524, respectively). In the placebo group, Scr and eGFR were unchanged (at 12 weeks, P = 0.788, P = 0.860; at 24 weeks, P = 0.860, P = 0.713). Levels of plasma L-FABP were abnormally distributed in the TSF and placebo groups (P < 0.05), After ln-transformation, levels of ln plasma L-FABP in each group were normally distributed (P > 0.05). Compared with the placebo group, levels of ln plasma L-FABP in the TSF group showed no significant difference after 12 weeks or 24 weeks of treatment (P = 0.190 at baseline, P = 0.131 at 12 weeks, and P = 0.710 at 24 weeks, respectively) (Table 2). However, urinary L-FABP levels in the TSF group were significantly lower than levels in the placebo group after both12 weeks and 24 weeks of treatment (8.2 ± 10.1 μg/ml compared with 8.5 ± 4.4 μg/ml at baseline, P = 0.940, 6.8 ± 2.9 μg/ml compared with 11.1 ± 3.3 μg/ml at 12 weeks, P = 0.004 and 6.0 ± 3.0 μg/ml compared with 9.2 ± 4.9 μg/ml at 24 weeks, P = 0.047, respectively) (Fig. 1).
Table 2.
Effect of TSF and placebo on Scr, BUN, UAER, eGFR and ln plasma L-FABP levels in patients with microalbuminuria
| Parameters | Groups | Baseline | Week 12 | Week 24 |
|---|---|---|---|---|
| UAER (μg/min) | TSF | 157.6 ± 72.9 | 115.9 ± 50.7 | 104.0 ± 32.4a |
| Placebo | 158.6 ± 99.0 | 116.5 ± 69.7 | 121.8 ± 69.7 | |
| BUN (mmol/L) | TSF | 5.1 ± 1.2 | 5.6 ± 1.4 | 5.5 ± 1.4 |
| Placebo | 5.7 ± 1.6 | 6.6 ± 1.5 | 6.0 ± 2.3 | |
| Scr (μmol/L) | TSF | 73.5 ± 19.3 | 64.4 ± 19.7 | 67.6 ± 16.7 |
| Placebo | 60.8 ± 18.2 | 62.7 ± 19.8 | 61.9 ± 15.1 | |
| eGFR (ml/min/1.73 m2) | TSF | 102.7 ± 39.2 | 118.3 ± 49.4 | 113.9 ± 53.5 |
| Placebo | 128.3 ± 61.0 | 124.8 ± 45.2 | 121.0 ± 44.0 | |
| ln plasma L-FABP (μg/ml) | TSF | 1.0 ± 0.9 | 1.3 ± 0.8 | 1.2 ± 0.7 |
| Placebo | 1.4 ± 0.7 | 1.7 ± 0.6 | 1.1 ± 0.7 |
All values expressed as mean ± SD
a: P < 0.05 compared with baseline of the same group (Paired t test)
Abbreviations: BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, ln plasma L-FABP ln-transformed plasma liver-type fatty acid binding protein, Scr serum creatinine, UAER urinary albumin excretion rate
Fig. 1.
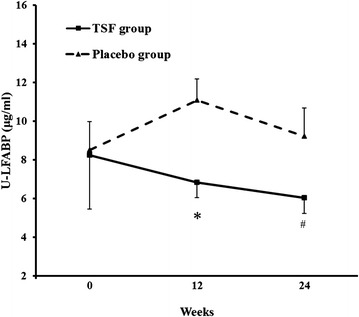
Effects of TSF on urinary L-FABP levels in microalbuminuria patients during the 24-week treatment period. Values presented as mean ± SE; *P = 0.004 and # P = 0.047 compared with the placebo group at the same time point. L-FABP = liver-type fatty acid binding protein
Effects of TSF on renal function indices and urinary and plasma L-FABP levels in DKD patients with macroalbuminuria
Levels of Scr, BUN, 24 h UP, eGFR were similar between the two groups in participants with macroalbuminuria at baseline (P = 0.594, P = 0.875, P = 0.996, P = 0.562, respectively), 12 weeks (P = 0.144, P = 0.158, P = 0.628, P = 0.376, respectively) and 24 weeks (P = 0.159, P = 0.090, P = 0.383, P = 0.646, respectively). In the TSF group, 24 h UP decreased significantly from 1.0 ± 0.7 g to 0.5 ± 0.5 g after 12 weeks (P = 0.042), remained at 0.5 ± 0.5 g after 24 weeks (P = 0.041). However, in the placebo group, little change was exhibited during the study period (P = 0.192 at 12 weeks and P = 0.415 at 24 weeks). In addition, Scr, BUN and eGFR remained unchanged compared to baseline in both the TSF group (P = 0.776, P = 0.979 and P = 0.911 at 12 weeks; P = 0.849, P = 0.998 and P = 0.538 at 24 weeks, respectively) and placebo group (P = 0.495, P = 0.119 and P = 0.664 at 12 weeks; P = 0.948, P = 0.113 and P = 0.914 at 24 weeks, respectively). Levels of plasma and urinary L-FABP were abnormally distributed in the TSF and placebo groups (P < 0.05). After ln-transformation, ln plasma and urinary L-FABP were normally distributed (P > 0.05). Ln plasma L-FABP showed little change in both the TSF and placebo groups (P = 0.643 at baseline, P = 0.328 at 12 weeks and P = 0.402 at 24 weeks, Table 3). However, ln urinary L-FABP decreased during 24 weeks of TSF treatment and significantly increased during 24 weeks of placebo treatment (baseline, TSF group 1.4 ± 0.6 μg/ml compared with placebo group 1.1 ± 1.0 μg/ml, P = 0.455; 12 weeks, TSF group 1.2 ± 0.3 μg/ml compared with placebo group 1.7 ± 0.3 μg/ml, P = 0.036; 24 weeks, TSF group 1.4 ± 0.5 μg/ml compared with placebo group 1.9 ± 0.5 μg/ml, P = 0.046) (Fig. 2).
Table 3.
Effect of TSF and placebo on Scr, BUN, UAER, eGFR and ln plasma L-FABP levels in patients with macroalbuminuria
| Parameters | Groups | Baseline | Week 12 | Week 24 |
|---|---|---|---|---|
| 24 h UP (g/24 h) | TSF | 1.0 ± 0.7 | 0.5 ± 0.5a | 0.5 ± 0.5a |
| Placebo | 0.9 ± 0.6 | 0.6 ± 0.6 | 0.7 ± 0.7 | |
| BUN (mmol/L) | TSF | 6.0 ± 1.7 | 6.0 ± 1.7 | 6.0 ± 1.9 |
| Placebo | 6.1 ± 1.1 | 6.9 ± 1.6 | 7.2 ± 1.7 | |
| Scr (μmol/L) | TSF | 74.9 ± 20.7 | 72.6 ± 22.6 | 73.5 ± 18.9 |
| Placebo | 79.4 ± 18.2 | 84.0 ± 17.4 | 83.1 ± 16.4 | |
| eGFR (ml/min/1.73 m2) | TSF | 104.1 ± 32.8 | 102.7 ± 37.7 | 97.3 ± 25.8 |
| Placebo | 96.2 ± 30.0 | 91.3 ± 30.1 | 92.3 ± 32.2 | |
| ln plasma L-FABP (μg/ml) | TSF | 1.7 ± 1.0 | 1.3 ± 1.3 | 1.5 ± 0.9 |
| Placebo | 1.6 ± 1.2 | 0.8 ± 1.4 | 1.2 ± 1.0 |
All values expressed as mean ± SD
a P < 0.05 compared with baseline of the same group (Paired t test)
Abbreviations: BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, ln-serum L-FABP ln-transformed serum liver-type fatty acid binding protein, Scr serum creatinine, UAER urinary albumin excretion rate
Fig. 2.
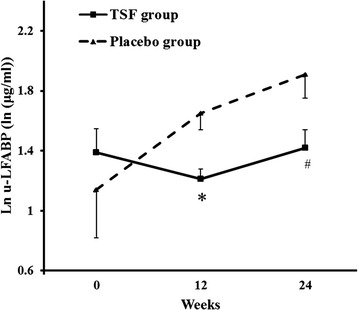
Effects of TSF on urinary L-FABP levels in macroalbuminuria patients during the 24-week treatment period. Values presented as mean ± SE; *P = .036 and # P = 0.046 compared with the placebo group at the same time point. ln urinary L-FABP = ln-transformed liver-type fatty acid binding protein
Cross-sectional study
Urinary L-FABP and Plasma L-FABP levels in DKD patients
Levels of urinary L-FABP in each DKD group differed significantly from levels in the other groups and increased markedly in accordance with the severity of DKD (P = 0.000 between three groups; DKD patients with microalbuminuria compared with DM patients with normoalbuminuria P = 0.002; DKD patients with macroalbuminuria compared with DM patients with normoalbuminuria P = 0.000; DKD patients with macroalbuminuria compared with DM patients with microalbuminuria P = 0.005, respectively) (Table 4). However, plasma L-FABP levels did not differ significantly between each DKD group (P = 0.888).
Table 4.
Urinary and plasma L-FABP levels in patients in different stages of DKD
| Groups | Urinary L-FABP (μg/ml) | Plasma L-FABP (μg/ml) |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| DM patients with normoalbuminuria (n = 30) | 5.9 (5.2, 7.8) | 4.4 (2.9,11.3) |
| DKD patients with microalbuminuria (n = 30) | 11.4 (6.7, 13.4)a | 6.7 (3.4,11.4) |
| DKD patients with macroalbuminuria (n = 30) | 18.8 (10.9, 23.4)a,b | 8.6 (2.5,12.4) |
a P < 0.05 compared with the DM patients with normoalbuminuria (Kruskal-Wallis test)
b P < 0.05 compared with the DKD patients with microalbuminuria (Kruskal-Wallis test)
Abbreviations: IQR interquartile range, serum L-FABP serum liver-type fatty acid binding protein, urinary L-FABP urinary liver-type fatty acid binding protein
Discussion
The renoprotective effects of the traditional Chinese herbal medicine TSF have been described in other reports [17, 21], including the original study [22] on which this post-hoc analysis and cross-sectional study were based. Results from the original study indicated that TSF as an adjunct to conventional diabetes treatments was more efficacious in lowering 24 h UP than conventional treatments alone in patients with macroalbuminuria [22]. Evidence from our previous studies have revealed that TSF attenuates development of diabetic nephropathy [17, 18, 22]. Specifically, TSF improves renal impairment by decreasing accumulation of extracellular matrix in the renal tissue by reducing expression of transforming growth factor-beta1 (TGF-β1) and increasing expression of matrix metallopeptidase 9 [19]. We have also demonstrated that TSF can reduce glomerulosclerotic and interstitial fibrotic indices and decrease serum TC and 24 h UP [17]. In this post-hoc analysis, we found that TSF decreased UP over the time course in macroalbuminuria patients, but did not differ from placebo. This phenomenon might be explained by the small sample size, since there were only 15 participants in each group.
The renal protective mechanisms of individual herbs in TSF have been discussed in numerous studies. Active components in astragalus, rehmannia, rhubarb, cornelian cherry, and winged burning bush twig have been found to reduce albuminuria, suppress activation of nuclear factor-kappaB, down-regulate TGF-β1 expression, and decrease extracellular matrix accumulation [26–30].
In this post-hoc analysis and cross-sectional study, we tested the hypothesis that TSF combined with conventional treatment would lower urinary and serum L-FABP both in micro- and macroalbuminuria patients. Our hypothesis originated from results of other clinical studies that showed urinary L-FABP was correlated with severity of tubulointerstitial injury, which suggested that urinary L-FABP is a predictor for the deterioration of renal function in DKD [10, 11]. L-FABP as an effective endogenous antioxidant is important for free fatty acid (FFA) metabolism [31, 32]. When experimental mice were exposed to albumin-bound FFAs, tubulointerstitial damage was more severe than mice exposed to albumin alone, as unbound albumin is reabsorbed in the proximal tubules [33]. Our previous study showed that TSF has a lipid-lowering effect, suggesting that TSF exerts a protective effect in the tubulointerstitium [17].
It is not clear whether urinary excretion of L-FABP is correlated with urinary protein. Results from a prospective observational follow-up study showed that although urinary L-FABP level was correlated with severity of albuminuria in all stages of DKD, this was not the case for a subgroup of patients with eGFR > 60 ml/min/1.73 m2 [11]. Therefore, in our study, the effect of TSF on urinary L-FABP might be independent of albuminuria.
To date, albuminuria and eGFR are widely used as markers of DKD progression. However, these tests are imprecise because long before the onset of microalbuminuria in diabetic patients, hemodynamic changes occur in the glomeruli [34]. For example, results from the Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes (DEMAND) study showed that 20.5 % of diabetic patients with decreased kidney function had normal albuminuria [35]. The National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM (NEFRON) study found that more than half (55 %) of type 2 diabetes patients with an eGFR < 60 ml/min per 1.73 m2 had normoalbuminuria [36]. In addition, estimated GFR is commonly used instead of direct GFR measurement, which compromises accuracy [37]. Thus, current tests to assess kidney function and damage are inadequate and new biomarkers that can monitor kidney function, injury and repair, and predict DKD risk are needed. Indeed, L-FABP has been the focus of intense study as a new biomarker for early diagnosis and prediction of DKD risk [10, 11, 38]. Moreover, the Ministry of Health, Labour and Welfare in Japan has approved urinary L-FABP as a tubular biomarker for monitoring DKD [39].
In our study, urinary L-FABP levels increased with the progression of DKD, similar to other reports such as by Chou and colleagues [40]. However, we did not test urinary creatinine level, and as such were unable to use it to calibrate urinary L-FABP levels, which might be affected by the concentration of the urine samples. In addition to the kidney, L-FABP is expressed in the liver. L-FABP derived from the liver is released into the circulation, filtered through glomeruli and taken up by proximal tubules [41]. But, in our study no difference in plasma L-FABP levels were observed among participants in different stages of DKD, suggesting that plasma L-FABP levels do not affect urinary L-FABP levels.
Our study revealed that TSF could reduce the urinary L-FABP level in DKD patients, and has the same trend as urinary protein. Furthermore, since urinary L-FABP levels have been found to be significantly correlated with urinary 8-OHdG levels [12], our finding suggests that the beneficial effect of TSF in DKD is partly mediated by reduction of oxidative stress.
Our study has limitations. Healthy participants were not included. As such, differences in urinary L-FABP levels between DKD patients and healthy individuals were not evaluated. In addition, as a tubular marker, urinary L-FABP does not predict a decline in GFR in overt DKD patients [42]. Since the progression of DKD is defined as an increase in urinary albumin concentration, it may be difficult to determine the potency of urinary L-FABP for diagnosis or prediction of progression of DKD. Further research is needed to validate the reference values of urinary L-FABP for distinguishing different stages of DKD, as well as the possible mechanism by which TSF affects urinary L-FABP.
Conclusions
In summary, results of this post-hoc analysis and cross-sectional study further extend observations that the traditional Chinese medicine Tangshen Formula appears to be a promising treatment for DKD. Moreover, urinary L-FABP may be a useful biomarker for predicting the progression of DKD. Further studies are needed to confirm its potential for clinical application.
Abbreviations
24 h UP, 24-h urinary protein; A1C, glycosylated hemoglobin; ADA, American Diabetes Association; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; DEMAND, Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes; DKD, diabetic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FBG, fasting blood glucose; FFA, free fatty acid; HDL, high density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; L-FABP, liver-type fatty acid binding protein; NEFRON, National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM; NKF-KDOQI, National Kidney Foundation Kidney Disease Outcomes Quality Initiative; Scr, serum creatinine; TC, total cholesterol; TCM, Traditional Chinese Medicine; TG, triglyceride; TGF-β1, transforming growth factor-beta1; TSF, Tangshen Formula; UAER, urinary albumin excretion rate
Acknowledgments
The authors thank Nissi S. Wang, MSc, for developmental editing of the manuscript.
Fundings
This work was supported by the International Science and Technology Cooperation Program of China (Grant no.2011DFA31860), the National Natural Science Foundation of China (Grant no.81130066) and the Beijing Municipal Science & Technology Commission (Grant no. Z151100003815015).
Availability of data and materials
Data are all contained within the paper.
Authors’ contributions
XY and BZ contributed equally to this work. XY, XL, MY and TZ collected the blood and urine samples. BZ and XL performed the statistical analysis. BZ drafted the manuscript. YW helped edit the manuscript. PL conceived of the study, and participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. There is no conflict of interest between the authors and the manufacturer of Tangshen Formula.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The original trial was approved by the ethics committee of the China-Japan Friendship Hospital (No.2006–059). All participants in the trial signed written informed consent documents.
Contributor Information
Xin Yang, Email: yangxinzs067@126.com.
Bingxuan Zhang, Email: zbx1118@126.com.
Xiaoguang Lu, Email: luxiaoguang878@163.com.
Meihua Yan, Email: meihua313@163.com.
Yumin Wen, Email: colline0725@163.com.
Tingting Zhao, Email: ttfrfr@163.com.
Ping Li, Email: lp8675@163.com.
References
- 1.Reutens AT, Atkins RC. Epidemiology of diabetic nephropathy. Contrib Nephrol. 2011;170:1–7. doi: 10.1159/000324934. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contrib Nephrol. 2011;170:36–47. doi: 10.1159/000324942. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 7.Sego S. Pathophysiology of diabetic nephropathy. Nephrol Nurs J. 2007;34:631–633. [PubMed] [Google Scholar]
- 8.Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20:551–555. doi: 10.1016/j.ejim.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17:247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Kamijo-Ikemori A, Sugaya T, Yamashita K, Yokoyama T, Koike J, et al. Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy. Nephron Clin Pract. 2009;112:c148–c156. doi: 10.1159/000214210. [DOI] [PubMed] [Google Scholar]
- 11.Kamijo-Ikemori A, Sugaya T, Yasuda T, Kawata T, Ota A, Tatsunami S, et al. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 2011;34:691–696. doi: 10.2337/dc10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care. 2005;28:2728–2732. doi: 10.2337/diacare.28.11.2728. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen SE, Sugaya T, Tarnow L, Lajer M, Schjoedt KJ, Astrup AS, et al. Tubular and glomerular injury in diabetes and the impact of ACE inhibition. Diabetes Care. 2009;32:1684–1688. doi: 10.2337/dc09-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb. 2011;18:1018–1028. doi: 10.5551/jat.9084. [DOI] [PubMed] [Google Scholar]
- 15.Wang YJ, He LQ, Sun W, Lu Y, Wang XQ, Zhang PQ, et al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol. 2012;139:757–764. doi: 10.1016/j.jep.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Li P, Xing CY, Zhao JY, He YN, Wang JQ, et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am J Kidney Dis. 2014;64:57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Li P, Burczynski FJ, Gong Y, Choy P, Sha H, Li J. Attenuation of diabetic nephropathy in Otsuka Long-Evans Tokushima fatty (OLETF) rats with a combination of Chinese herbs (Tangshen Formula) Evid Based Complement Alternat Med. 2011;2011:613737. doi: 10.1155/2011/613737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Li P, Zhao J, Zhao S, Luo Y, Sha H, et al. Renal protective effect of Tangshen Formula in rats with diabetic nephropathy. Journal of the Beijing University of Traditional Chinese Medicine. 2009;32:244–248. [Google Scholar]
- 19.Zhang H, Li P, Zhao J, Zhao S, Yan M, Luo Y, et al. Effect of Tangshen Formula on expression of TGF-β1 and MMP-9 of renal tissue in STZ-induced diabetic nephropathy rats. Chinese Journal of Integrated Traditional and Western Nephrololgy. 2009;10:290–294. [Google Scholar]
- 20.Yu H, Li L, Liang Q, Wang Y, Li P, Luo G. A metabonomic study on the treatment of diabetic nephropathy with traditional Chinese medicine tang-shen-fang. Chin J Chromatogr. 2011;29:320–324. doi: 10.3724/SP.J.1123.2011.00320. [DOI] [PubMed] [Google Scholar]
- 21.Jiang ZT, Liang LQ, Wang YM, Li P, Li J, Luo G. Effect of Tangshen Recipe on the homocysteine metabolism of patients with diabetic nephropathy. Chinese Journal of Integrated Traditional and Western Medicine. 2011;31:1057–1061. [PubMed] [Google Scholar]
- 22.Li P, Chen Y, Liu J, Hong J, Deng Y, Yang F, et al. Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: a multicenter double-blinded randomized placebo-controlled trial. PLoS One. 2015;10(5):e0126027. doi: 10.1371/journal.pone.0126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association Standards of medical care in diabetes--2006. Diabetes Care. 2006;29(Suppl 1):S4–S42. [PubMed] [Google Scholar]
- 24.KDOQI KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.XY Z. Clinical Research Guideline of New Investigational Drugs in Traditional Chinese Medicine. Beijing: China Medical Science Press; 2002. 163-168. Chinese.
- 26.Zhang J, Xie X, Li C, Fu P. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. J Ethnopharmacol. 2009;126:189–196. doi: 10.1016/j.jep.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 27.Baek GH, Jang YS, Jeong SI, Cha J, Joo M, Shin SW, et al. Rehmannia glutinosa suppresses inflammatory responses elicited by advanced glycation end products. Inflammation. 2012;35:1232–1241. doi: 10.1007/s10753-012-9433-x. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Qin WS, Jia ZH, Zheng JM, Zeng CH, Li LS, et al. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med. 2010;76:27–33. doi: 10.1055/s-0029-1185948. [DOI] [PubMed] [Google Scholar]
- 29.Yamabe N, Kang KS, Goto E, Tanaka T, Yokozawa T. Beneficial effect of Corni Fructus, a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediated renal injury in Streptozotocin-treated diabetic rats. Biol Pharm Bull. 2007;30:520–526. doi: 10.1248/bpb.30.520. [DOI] [PubMed] [Google Scholar]
- 30.Chang B, Jin C, Zhang W, Kong L, Yang JH, Lian FM, et al. Euonymus alatus in the treatment of diabetic nephropathy in rats. Am J Chin Med. 2012;40:1177–1187. doi: 10.1142/S0192415X12500875. [DOI] [PubMed] [Google Scholar]
- 31.Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein in renal disease. Clin Chim Acta. 2006;374(1-2):1–7. doi: 10.1016/j.cca.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, et al. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 33.Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- 34.Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int. 1991;40:792–807. doi: 10.1038/ki.1991.277. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND study. Cardiorenal Med. 2012;2:1–10. doi: 10.1159/000333249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, Atkins RC. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11) Diabetes Care. 2009;32:1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, et al. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36:1248–1253. doi: 10.2337/dc12-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamijo-Ikemori A, Ichikawa D, Matsui K, Yokoyama T, Sugaya T, Kimura K. Urinary L-type fatty acid binding protein (L-FABP) as a new urinary biomarker promulgated by the Ministry of Health, Labour and Welfare in Japan. The Japanese Journal of Clinical Pathology. 2013;61:635–640. [PubMed] [Google Scholar]
- 40.Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8(1):e54863. doi: 10.1371/journal.pone.0054863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyama Y, Takeda T, Hama H, Tanuma A, Iino N, Sato K, et al. Evidence for megalin-mediated proximal tubular uptake of L-FABP, a carrier of potentially nephrotoxic molecules. Lan Invest. 2005;85:522–531. doi: 10.1038/labinvest.3700240. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int. 2011;79:1113–1118. doi: 10.1038/ki.2010.554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are all contained within the paper.


