Abstract
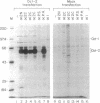
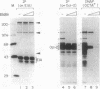
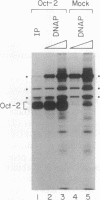
DNA-dependent and DNA-independent associations of DNA-binding proteins are important in transcriptional regulation. The analysis of DNA-independent associations frequently relies on assaying protein interaction in the absence of target DNA sequences. We have found that contaminating DNA in protein preparations can stabilize DNA-dependent associations that may appear DNA-independent. Three cellular proteins of 70, 85, and 110 kDa coimmunoprecipitated with the octamer motif-binding protein Oct-2 because of the presence of contaminating DNA in the cell extracts. In addition, heterodimer formation between Oct-1 (or Oct-2) and Pit-1 during protein-affinity chromatography was stabilized by the contaminating DNA. In both instances, these DNA-dependent protein associations were selectively inhibited by ethidium bromide in the precipitation reaction without any evident effect on DNA-independent protein associations. Thus, ethidium bromide may serve as a simple and general indicator of DNA-dependent and DNA-independent protein associations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurora R., Herr W. Segments of the POU domain influence one another's DNA-binding specificity. Mol Cell Biol. 1992 Feb;12(2):455–467. doi: 10.1128/mcb.12.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Cherney B. W., McBride O. W., Chen D. F., Alkhatib H., Bhatia K., Hensley P., Smulson M. E. cDNA sequence, protein structure, and chromosomal location of the human gene for poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8370–8374. doi: 10.1073/pnas.84.23.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc R. G., Corcoran L. M., LeBowitz J. H., Baltimore D., Sharp P. A. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev. 1988 Dec;2(12A):1570–1581. doi: 10.1101/gad.2.12a.1570. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. Footprinting at low temperatures: evidence that ethidium and other simple intercalators can discriminate between different nucleotide sequences. Nucleic Acids Res. 1987 Jan 26;15(2):491–507. doi: 10.1093/nar/15.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J. J., Hawk I. A., Levy C. C. Polyamine activation of staphylococcal nuclease. Biochim Biophys Acta. 1975 Apr 16;390(1):117–124. doi: 10.1016/0005-2787(75)90014-3. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. Roles of TFIID in transcriptional initiation by RNA polymerase II. Cell. 1991 Sep 20;66(6):1067–1070. doi: 10.1016/0092-8674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. J., Bautista C., Edwards G. M., Defeo-Jones D., Jones R. E., Harlow E. Antibodies specific for the human retinoblastoma protein identify a family of related polypeptides. Mol Cell Biol. 1991 Nov;11(11):5792–5799. doi: 10.1128/mcb.11.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., LeBowitz J. H., Sharp P. A. The octamer-binding proteins form multi-protein--DNA complexes with the HSV alpha TIF regulatory protein. EMBO J. 1989 Dec 20;8(13):4229–4238. doi: 10.1002/j.1460-2075.1989.tb08608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990 Dec;4(12B):2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Paillard S., Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- ROBERTS W. K., DEKKER C. A., RUSHIZKY G. W., KNIGHT C. A. Studies on the mechanism of action of micrococcal nuclease. 1. Degradation of thymus deoxyribonucleic acid. Biochim Biophys Acta. 1962 May 14;55:664–673. doi: 10.1016/0006-3002(62)90844-2. [DOI] [PubMed] [Google Scholar]
- Rustgi A. K., Dyson N., Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991 Aug 8;352(6335):541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- Schröter H., Maier G., Ponstingl H., Nordheim A. DNA intercalators induce specific release of HMG 14, HMG 17 and other DNA-binding proteins from chicken erythrocyte chromatin. EMBO J. 1985 Dec 30;4(13B):3867–3872. doi: 10.1002/j.1460-2075.1985.tb04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Stern S., Herr W. The herpes simplex virus trans-activator VP16 recognizes the Oct-1 homeo domain: evidence for a homeo domain recognition subdomain. Genes Dev. 1991 Dec;5(12B):2555–2566. doi: 10.1101/gad.5.12b.2555. [DOI] [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Herr W. The POU domain is a bipartite DNA-binding structure. Nature. 1988 Dec 8;336(6199):601–604. doi: 10.1038/336601a0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992 Feb 21;68(4):755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- Treacy M. N., He X., Rosenfeld M. G. I-POU: a POU-domain protein that inhibits neuron-specific gene activation. Nature. 1991 Apr 18;350(6319):577–584. doi: 10.1038/350577a0. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain mediates interactions between Oct-1 and other POU proteins. Mol Cell Biol. 1992 Feb;12(2):542–551. doi: 10.1128/mcb.12.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. W., Wilson L., Rosenfeld M. G. POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev. 1991 Jul;5(7):1309–1320. doi: 10.1101/gad.5.7.1309. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Xiao P., Capone J. P. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol Cell Biol. 1990 Sep;10(9):4974–4977. doi: 10.1128/mcb.10.9.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]