Abstract
Background
Sarcoptic mange, a parasitic disease caused by the mite Sarcoptes scabiei, is regularly reported on wolves Canis lupus in Scandinavia. We describe the distribution and transmission of this parasite within the small but recovering wolf population by analysing 269 necropsy reports and performing a serological survey on 198 serum samples collected from free-ranging wolves between 1998 and 2013.
Results
The serological survey among 145 individual captured Scandinavian wolves (53 recaptures) shows a consistent presence of antibodies against sarcoptic mange. Seropositivity among all captured wolves was 10.1 % (CI. 6.4 %–15.1 %). Sarcoptic mange-related mortality reported at necropsy was 5.6 % and due to secondary causes, predominantly starvation. In the southern range of the population, seroprevalence was higher, consistent with higher red fox densities. Female wolves had a lower probability of being seropositive than males, but for both sexes the probability increased with pack size. Recaptured individuals changing from seropositive to seronegative suggest recovery from sarcoptic mange. The lack of seropositive pups (8–10 months, N = 56) and the occurrence of seropositive and seronegative individuals in the same pack indicates interspecific transmission of S. scabiei into this wolf population.
Conclusions
We consider sarcoptic mange to have little effect on the recovery of the Scandinavian wolf population. Heterogenic infection patterns on the pack level in combination with the importance of individual-based factors (sex, pack size) and the north–south gradient for seroprevalence suggests low probability of wolf-to-wolf transmission of S. scabiei in Scandinavia.
Keywords: Canis lupus, Grey wolf, Sarcoptes scabiei, Sarcoptic mange, Ectoparasites, ELISA, Red fox, Vulpes vulpes, Wildlife disease
Background
Sarcoptic mange is an epizootic skin disease caused by the mite Sarcoptes scabiei worldwide infesting over 100 mammalian hosts including wild and domestic canids [1, 2]. The mite, burrowing through the stratum corneum, causes the host to mount a humoral immunological response [3–5]. Wolves Canis lupus infested by S. scabiei, develop alopecia due to intense scratching and biting triggered by a hypersensitive response and may become debilitated and emaciated due to secondary bacterial infections and difficulties in catching the natural prey [1, 2, 6]. S. scabiei infections can reduce pack size, annual pack growth rate and cause additive mortality [7, 8]. However, recovery from even severe sarcoptic mange on wolves is reported from northern Spain and Yellowstone National Park [7, 9].
S. scabiei actively seek olfactory and thermal stimuli and are able to survive, in suitable environments, for up to 19 days off the host. All life stages remain infective for at least one-half to two-thirds of their survival time [10]. Transmission normally occurs through close contact between hosts and is assumed to be host-density dependent [1] but also fomites in the host environment can be a source of transmission [10]. In Yellowstone National Park, the spatio-temporal patterns of S. scabiei infestation on wolves are related to distance to the next infested pack, indicating wolf-to-wolf transmission [7]. In Scandinavia interspecific transmission of S. scabiei var. vulpes from red fox Vulpes vulpes is the most likely origin for mange in wolves, domestic dog Canis lupus familiaris, arctic fox Alopex lagopus, lynx Lynx lynx and domestic cat Felis catus [11, 12]. In northern Spain, wolves are infested with S. scabiei originated from both red foxes and ungulates, emphasising the prey-to-predator transmission [13].
The wolf was regarded as functionally extinct in Scandinavia during the late 1960’s. In 1983, two immigrant wolves from the Finnish-Russian wolf population reproduced for the first time and became the founders of the present Scandinavian wolf population, [14–16]. During the following 30 years, the population increased from less than 10 individuals to an estimated size of approximately 400 wolves [17]. By 2013, only five Finnish-Russian founders had genetically contributed to this population, and severe inbreeding depression has been confirmed [15]. Although mortality of Scandinavian wolves is mainly human-caused [18], sarcoptic mange may be an important cause of natural mortality [11]. Sarcoptic mange arrived in Scandinavia in the mid-1970’s with devastating effects on the red fox population, [19, 20]. A previous study focusing on immunoglobulin E (IgE) levels found 14/57 Scandinavian wolves seropositive for sarcoptic mange [21]. Effects of sarcoptic mange on the demography of the Scandinavian wolf population remain unclear but have the potential to influence this small, inbred population. Here we analyse and present an overview of the distribution of sarcoptic mange in the Scandinavian wolf population.
Between 1998 and 2013 a total of 198 serum samples from live wolves were collected and analysed by two different enzyme-linked immunosorbent assays (ELISA) and complemented by Western Blot. In addition, we evaluated necropsy reports of 269 dead wolves collected in Sweden between 2003 and 2013.
The aim of our study is to describe the occurrence of sarcoptic mange in the Scandinavian wolf population and to identify demographic and environmental factors that relate to the probability of finding seropositive samples. Based on the literature and personal observations we expected sarcoptic mange to be a minor threat to the Scandinavian wolf population. We predicted the probability of sarcoptic mange occurrence to depend on population level factors including red fox and wolf territory density rather than on individual-based factors such as age and sex of wolves.
Results
Seropositivity on captured wolves
In total 178 of the 198 samples (89.9 %) (Tables 1 and 2) were tested seronegative and 20 samples (10.1 %, Wilson 95 % CI 6.4 %–15.1 %) were tested seropositive. Mean annual proportion of seropositive samples was 11.3 % (SE 2.5 %), ranging from 40.0 % in 1999 (N = 5) to zero in 2008 (N = 7) and 2010 (N = 15) (Fig. 1a). The annual proportion of the wolf population that was sampled decreased during the study period (χ1,13 = 18.82; p < 0.01) (Fig. 1b). The annual sample size did not allow for further temporal analysis.
Table 1.
Demographic distribution of the serum samples and observed lesions indicating sarcoptic mange among the captured individuals
| Total number of serum samples | Seropositive serum samples | ||
|---|---|---|---|
| Pups | Pups total | 56 | 0 |
| Females | 28 | 0 | |
| Males | 28 | 0 | |
| Single/Dispersing | 0 | 0 | |
| Pair | 0 | 0 | |
| Pack | 56 | 0 | |
| Unclear pack structure | 0 | 0 | |
| Alopecia reported | 0 | 0 | |
| Adults | Adults total | 142 | 20 |
| Females | 66 | 6 | |
| Males | 76 | 14 | |
| Single/Dispersing | 11 | 1 | |
| Pair | 54 | 6 | |
| Pack | 71 | 13 | |
| Unclear pack structure | 6 | 1 | |
| Alopecia reported | 9 | 7 |
Pups are < 1 year old, adults > 1 year old. Single/Dispersing wolves are outside the parental territory and have no territory established yet. Pairs are a male and a female in an established territory. Packs are one or two reproducing wolves with their < 2 year old offspring. Alopecia reported on the capture form
Table 2.
Results from ELISA and Western Blot analysis
| ID Wolf | Sampling date | Sex | Age class | OD MSA – 1 | OD Crude | Result ELISAs | Western Blots |
|---|---|---|---|---|---|---|---|
| 9802 | 15.12.1998 | Male | Adult | 0.71 | 0.83 | ++ | ++ |
| 9803 | 15.12.1998 | Female | Adult | 0.73 | 0.91 | ++ | + |
| 9808 | 27.01.2000 | Male | Adult | 0.11 | 0.32 | −+ | -- |
| 0001 | 28.01.2000 | Male | Adult | 1.54 | 2.18 | ++ | ++ |
| 0002 | 28.01.2000 | Female | Adult | 0.11 | 0.09 | -- | - |
| 0003 | 28.01.2000 | Female | Juvenile | 0.05 | 0.07 | -- | - |
| 0104 | 10.02.2001 | Female | Adult | 1.14 | 1.20 | ++ | ++ |
| 0105 | 10.02.2001 | Male | Adult | 1.66 | 1.96 | ++ | ++ |
| 0105 | 05.02.2003 | Male | Adult | 0.76 | 0.73 | ++ | + |
| 0109 | 12.02.2001 | Male | Adult | 0.63 | 0.11 | +− | - |
| 0109 | 08.12.2001 | Male | Adult | 0.34 | 0.13 | -- | - |
| 0106 | 12.02.2001 | Female | Juvenile | 0.11 | 0.14 | -- | - |
| 0111 | 21.12.2001 | Female | Adult | 0.20 | 0.30 | -? | + |
| 0203 | 13.01.2002 | Female | Adult | 0.17 | 0.24 | -? | - |
| 0208 | 26.01.2002 | Male | Adult | 0.41 | 0.15 | ?- | - |
| 0208 | 06.03.2003 | Male | Adult | 1.32 | 1.30 | ++ | + |
| 0215 | 01.02.2002 | Male | Adult | 0.20 | 0.25 | -? | - |
| 0214 | 05.03.2003 | Male | Adult | 0.70 | 0.92 | ++ | + |
| 0214 | 21.01.2004 | Male | Adult | 0.41 | 0.65 | ?+ | + |
| 0307 | 06.03.2003 | Female | Adult | 0.15 | 0.23 | -? | - |
| 0009 | 23.01.2004 | Male | Adult | 0.36 | 0.13 | ?- | - |
| 0504 | 14.03.2005 | Male | Adult | 0.40 | 0.42 | ?+ | + |
| 0506 | 16.03.2005 | Male | Adult | 0.56 | 0.19 | +− | ?+ |
| 0506 | 09.03.2007 | Male | Adult | 0.43 | 0.07 | ?- | - |
| 0507 | 16.03.2005 | Female | Juvenile | 0.26 | 0.15 | -- | - |
| 0507 | 08.02.2008 | Female | Adult | 0.65 | 0.13 | +− | - |
| 0601 | 27.01.2006 | Female | Adult | 0.44 | 0.37 | ?+ | - |
| 0606 | 01.02.2006 | Male | Adult | 0.22 | 0.36 | −+ | + |
| 0606 | 08.03.2007 | Male | Adult | 0.12 | 0.20 | -- | |
| 0611 | 13.02.2006 | Male | Adult | 0.22 | 0.25 | -? | + |
| 0611 | 11.03.2007 | Male | Adult | 0.43 | 0.21 | ?? | - |
| 0704 | 07.03.2007 | Female | Adult | 0.67 | 0.70 | ++ | + |
| 0913 | 30.01.2009 | Male | Juvenile | 0.10 | 0.35 | −+ | - |
| 0916 | 11.02.2009 | Male | Adult | 1.50 | 0.40 | ++ | + |
| 0918 | 12.02.2009 | Male | Adult | 1.01 | 0.80 | ++ | + |
| 0918 | 11.02.2010 | Male | Adult | 0.23 | 0.19 | -- | |
| 1004 | 10.02.2010 | Female | Juvenile | 0.18 | 0.22 | -? | - |
| 1114 | 21.03.2011 | Female | Adult | 0.73 | 0.39 | ++ | + |
| 1114 | 11.12.2011 | Female | Adult | 0.98 | 0.37 | ++ | - |
| 1114 | 06.02.2012 | Female | Adult | 0.81 | 0.35 | ++ | ?- |
| 1114 | 14.03.2013 | Female | Adult | 1.19 | 0.37 | ++ | + |
| 1202 | 16.12.2011 | Male | Adult | 1.54 | 0.98 | ++ | + |
OD MSA-1: Relative optical densities using the MSA-1 antigen in the ELISA, cut off are 0.35 (doubtful) and 0.499 (seropositive) respectively. OD Crude: Relative optical densities using the crude antigen in the ELISA, cut off 0.2 (doubtful) and 0.299 (seropositive). Result ELISAs are corresponding to the OD values and decide between: + (positive), − (negative) and ? (doubtful). Shown are all samples with either positive or doubtful results from one of the ELISA and five random selected with negative results
Fig. 1.
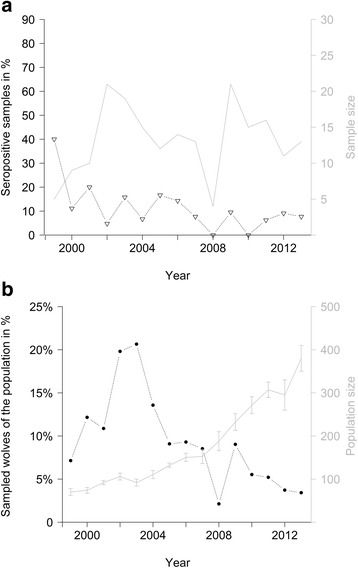
The proportion of seropositive samples (a) and the sample size as the percentage of the Scandinavian wolf population (b). Triangles (a) show the proportion of seropositive samples to the sampled number of wolves each year (grey line). Solid dots (b) show the percentage of sampled wolves. The grey line (b) represents the mean number of individual wolves reported in Scandinavia with error bars representing minimum and maximum estimations [17]
Among the 38 recaptured individuals, eight were tested seropositive at least once. Six were seropositive at first capture and four seronegative at recapture 1 year later. Two individuals were seropositive both at first capture and at recapture 2 years later (Table 2).
In 61 of 95 territory sampling events, multiple wolves were captured within the same territory. In 13 of these 61 sampling events, at least one individual was seropositive and there were always also seronegative wolves in the same sampling event. In two family groups, both the adult male and female tested seropositive but their captured pups (two each) were seronegative at time of capture. In eight territories, seropositive individuals were recaptured and six tested seronegative at recapture. In one territory, both the adult male and female were captured in two consecutive years, the male tested seropositive twice and the female seronegative twice.
Sarcoptic mange from wolves at necropsy
The average annual prevalence of sarcoptic mange among dead wolves collected in Sweden between 2003 and 2013 was 4.5 % (SE = 1.3 %, N = 269). In the same period, seropositivity rate of sarcoptic mange among captured wolves in Sweden was 7.6 % (SE = 2.5 %, N = 112). Of the total 21 wolves considered to have sarcoptic mange at necropsy, ten were euthanized due to mange-caused emaciated status (starvation and alopecia), two were illegally shot, two were shot during licence hunt, one was culled to prevent predation on livestock, one was euthanized after a traffic accident and five were found dead. Of the five wolves found dead, four died due to mange-related starvation and one after a traffic accident. In summary, 15 (5.6 %) of the known wolf mortalities reported by the Swedish National Veterinary Institute (SVA) in Sweden between 2003 and 2013 were either euthanized or died because of sarcoptic mange. A systematic serological survey was not carried out on the necropsied wolves, thus it was not possible to include the necropsy data in to the statistical analysis.
The age distribution of the 19 wolves with known age and lesions of mange at necropsy was nine pups (5 to 12 months old; 47.3 %), seven juveniles (1 and 2 years old 36.8 %), and three adults (2 to 6 years old 15.8 %). The majority (9 and 7 respectively) of these wolves came from two different wolf territories. In one territory the adult pair and two 9 months old pups were captured. At capture, the adults were seropositive, while the pups were seronegative. However, the pups were euthanized at the age of 15 and 19 months due to lesions from sarcoptic mange [22]. One of them was seropositive at necropsy, while the serum of the other was not tested.
Probability of seropositive samples
All 56 pups captured within their parental territories, were seronegative and did not show any lesions of sarcoptic mange. They were therefore excluded from the statistical analysis. Annual mean seroprevalence of the adult population was 19.9 % (SE 6.5 %).
The model combining the population-level predictor latitude and the individual-based interaction of sex and pack size was the most parsimonious (Table 3). According to the relative AICc weight ratio, it had 1.5 higher evidence than the next-best model, which included the wolf territory density. Both models passed model validation with rs > 0.8 (Table 3), but rs is not penalized for additional variables. By adding body condition or removing pack size, the models decreased in evidence but remained within the AICc cut off value (Table 3). Latitude correlated negatively with the likelihood of finding seropositive adult wolves for all combinations of sex and pack size except for females in pairs (Fig. 2a, Table 4). The probability of finding seropositive adult wolves was 6.1 to 8.2 times higher in the southern part (eg UTM 6 600 000, seroprevalence males 36.5 %, females 10.5 %) in packs with 6 wolves (mean size of reproducing packs), as compared to the northern edge of the wolf distribution. The probability of finding seropositive adult males increased linearly with pack size, from 6.6 % seroprevalence in pairs to 38.9 % seroprevalence in packs of eight wolves in the southern part of the study area. For adult females in the same area, the model predicted a seroprevalence of 37.1 % for packs of eight wolves. No adult female from a pair without pups was seropositive, the model predicts a seroprevalence < 1 %.
Table 3.
Model selection for predictors of seroprevalence of sarcoptic mange in adult wolves captured in Scandinavia
| Model | Parameters | K | AICc | ΔAICc | ωi | rs(SE) |
|---|---|---|---|---|---|---|
| Combined | Lat + Sex * Pack Size | 5 | 78.16 | 0 | 0.38 | 0.86(0.02) |
| + Density | Lat + Sex * Pack Size + Territory Density | 6 | 79.00 | 0.84 | 0.24 | 0.86(0.03) |
| + Body Condition | Lat + Sex * Pack Size + Body Condition | 6 | 79.04 | 0.88 | 0.24 | 0.59(0.03) |
| – Pack Size | Lat + Sex | 3 | 80.57 | 2.41 | 0.12 | 0.62(0.03) |
| Submodel intrinsic | Sex * Pack Size | 4 | 83.36 | 5.20 | 0.01 | - |
| Submodel density | Lat + Pack Size | 3 | 84.83 | 6.67 | 0.01 | - |
| Density | Lat + Long + Pack Size + Territory Density | 4 | 88.47 | 10.31 | 0 | - |
| Intrinsic | Sex * Pack Size + Body Condition + Age + Repro | 6 | 90.41 | 12.25 | 0 | - |
| Null model | 1 | 1 | 92.00 | 13.84 | 0 | - |
Top models are validated by k-fold cross validation (rs). Lat / Long: Latitude and longitude of the territory centre point, Repro: Reproducing or non-reproducing pair, Pack Size: Number of wolves within the territory, Territory Density: Mean Euclidian distance to the next three territory centre points, Body Condition: Individual residual distance to the linear regression line of log body weight and log body length. Presented are the two main models (Intrinsic, Density), the top models for each variable group, the model combining the top submodels, the combined model with variables ranking within the cut off in the variable group model selection and the null model
Fig. 2.
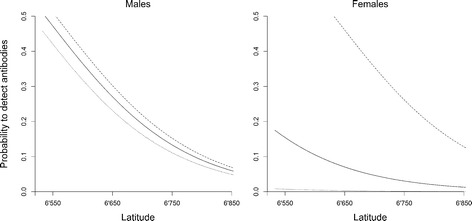
Probability of seropositive serum samples from captured wolves depending on sex, pack size and latitude. Dotted line for pairs, solid line for a pack of six wolves and dashed for a pack with eight wolves. Both figures show the back transformed and averaged predictions of the combined model with e(Combined/(1+Combined)
Table 4.
Top model estimates explaining seroprevalence of sarcoptic mange in adult wolves captured in Scandinavia
| Factors | Estimate | SE | Lower CI | Upper CI |
|---|---|---|---|---|
| Intercept | −57.16 | 26.3 | 5.56 | 108.76 |
| Lat | - 0.88 | 0.39 | - 1.64 | - 0.11 |
| Sex female | - 6.12 | 3.67 | - 13.31 | 1.07 |
| Pack Size | 0.05 | 0.15 | - 0.24 | 0.34 |
| Sex female * Pack Size | 0.76 | 0.46 | - 0.15 | 1.66 |
| Territory Density | 0.02 | 0.02 | - 0.01 | 0.05 |
| Body Condition | −4.09 | 3.53 | - 11.01 | 2.82 |
Estimates are averaged among the four top models. Lat: Latitude of the territory centre point, Pack Size: Number of wolves within the territory, Territory Density: Mean Euclidian distance to the next three territory centre points, Body Condition: Individual residual distance to the linear regression line of log body weight and log body length
Red fox harvest data was available for 49 Swedish territory centre points throughout the study period. There was no difference in red fox densities between territories with seropositive wolves (harvested red fox per 1000 ha ± SE = 1.6 ± 0.5) and territories with only seronegative wolves (harvested red fox per 1000 ha ± SE = 1.4 ± 0.2) (t = −0.31, df = 9.4; p = 0.77). However, the fox harvest decreased along the geographical gradient from the southern to the northern most territories (Fig. 3; r2 = − 0.59, df = 43, p < 0.001).
Fig. 3.
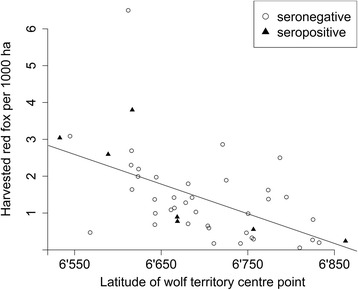
Harvested red fox per 1000 ha for each sampled Swedish wolf territory along the latitudinal gradient (N = 49). Circles indicate territories with only seronegative samples, and triangles indicate territories with at least one seropositive sample in the respective years
Discussion
The higher probability to find Scandinavian wolves seropositive to sarcoptic mange in their southern population range is consistent with a higher red fox density and higher habitat productivity resulting in smaller wolf territory size as described by J Mattisson, et al. [23] in these southern latitudes. The positive association between habitat productivity and frequency of mange on wolves is also reported from Yellowstone National Park [7]. In northern Spain reported seroprevalence (20 %, N = 17/88) is two times higher than the seropositivity rate in Scandinavia. This difference may be related to higher wildlife densities and more contact to livestock and domestic dogs in Spain [9, 24]. However, we could not find a direct relationship between red fox density and the occurrence of seropositive individual wolves. Reasons for this could be that red fox bag statistics do not represent the density of infectious red foxes especially after a regional mange outbreak [25], and the occurrence of infected wolves after a mange peak in the red fox might be delayed [9]. Bag statistics in general have been shown to be an appropriate index to estimate population densities [25, 26].
Consistent with previous reports [7, 9], we found wolves that had recovered from sarcoptic mange and also cases of coexistence of seropositive wolves, both with and without clinical symptoms, with seronegative and healthy wolves within the territory. But we also had other cases with high mortality on the pack scale. Co-infection with other diseases in this population such as canine distemper virus (CDV), causing immune depression, could lead to increased mortality [27, 28]. The risk for a host to get infected may relate to the initial mite load. Possibly a threshold needs to be reached to overwhelm the host’s immune system, resulting in a high total mite load and increased probability to spread the disease. In humans, S. scabiei transmission usually occurs in close body contact and patients carrying >100 adult female mites are much more likely to spread the disease than patients with lower mite rates [29]. Á Oleaga, et al. [9] reported mite rates of <100 isolated mites on wolves and a negative relation of isolated mites to the area of alopecic skin, suggesting a certain ability to control the mite development. The hypersensitive reaction leading to pruritus and alopecia might be present even if the mite rate is low [6]. In Yellowstone National Park, within-pack transmission occurs. Almberg et al. [30] reported a 61 % increased risk of individual infection with a 10 % increased prevalence within packs and that mortality hazards increased with the proportion of infected pack members or ambient temperatures above average but decreased with increasing prey availability.
The large proportion of young wolves among the infested individuals at necropsy demonstrates their sensitivity once infested with sarcoptic mange. In several North American wolf populations, sarcoptic mange decreased pup survival [8, 31, 32]. In Scandinavia, a large part of the mange-infested subadult wolves at necropsy came from only two territories and among the captured individuals, none of the pups were seropositive. We cannot rule out low detectability of infested pups due to high mortality before sampling during winter. In this case we would expect some seropositive survivors and, a negative relation between seroprevalence in adults and pack size. This is not supported by our findings. A seasonal cycle on wolves with lesions of mange has been observed in the Yellowstone National Park with peaks in November on population scale and in January on pack scale [30]. Our samples are collected between December and March.
During winter, Scandinavian wolf pups may be separated from their parents for a large part of the time even though they do not leave the territory until dispersal and do not generally contribute to the food acquirement of the family group [33]. For these wolf pups, exposure to live or recently-killed mange-infested red fox is likely to be very low. Experimentally measured seroconversion in domestic dogs and red foxes takes up to 1–5 weeks post-infection and 1–3 weeks from onset of clinical signs [3, 34] and persists for 1 to > 4.5 months after successful treatment in domestic dogs [35]. Infected pups, 7 to 10 months old at capture, would likely be detected by the ELISA. We suggest that wolf pups in Scandinavia are less exposed to S. scabiei than adults.
In contrast to other studies on sarcoptic mange and wolves [24, 30, 31], we found a lower probability of seroprevalence in adult females of small packs. Sex differences in the humoral response to sarcoptic mange are found in Iberian ibex Capra pyrenaica hispanica infested with S. scabiei and higher Immunoglobulin G (IgG) levels in females than males [36]. Higher IgG levels in females could lead to a longer duration of measurable antibody response potentially resulting, in contrast to our findings, in a higher detectability. Considering the observed recoveries, if higher antibody titres would lead to a faster clearance of the mite, detectability could be lower. IgG levels are currently not available for this data set and IgE or IgA levels measured on a subset of the wolf samples did not differ between males and females nor between mange seropositive and seronegative sera [21, 37] The observed positive correlation of mange prevalence in adult female wolves with pack size may be related to increased susceptibility to parasites during parturition and lactation [6].
Due to the high sociality and the monogamous mating system, sex differences in behaviour of breeding wolves are expected to be small, except during the early stage of nursing the pups [38]. Wolves display sexual dimorphism as male wolves are 20–30 % heavier than females [16, 39] and have a higher food intake [33, 40]. Exposure might be higher for males if they spend more time on carcasses shared with infested red foxes. The breeding wolf pairs in Scandinavia move together and use their territory equally during most of the year [23, 41]. However, we do not know if individual male and female wolves have different predation patterns on red fox or domestic dogs. Considering the assumption of restricted mite development on wolves and the heterogenic patterns on pack scale, individual variation regarding infection paths and immunological response might play an important role. Our small sample size, not allowing us to control for individual variation, might bias model based estimates on individual factors such as sex. Individuals to be captured where chosen according to the research- or management questions and sampling was opportunistic. However, to our knowledge this is the largest serological survey for sarcoptic mange on free-ranging wolves. This is a retrospective study, clinical evaluation of the captured animals was not standardized, did not classify mange lesions, or reports were incomplete. For seven of the 20 seropositive wolves, lesion consistent with sarcoptic mange (alopecia) was noted at capture. For five seropositive wolves normal fur was noted at capture and for eight seropositive wolves information was missing. Two wolves with seropositive test results had clinical signs (alopecia) at capture. No skin biopsies or skin scrapings have been conducted. We therefore were not able to define a nominal reference gold standard and do not report sensitivity and specificity of the ELISA. We confirmed the diagnosis using two ELISAs with different antigens in parallel and Western Blot in addition. Previous studies reported sensitivity and specificity of 92 and 96 % respectively, on domestic dogs with acute sarcoptic mange and 95 and 98 % in chronic cases applying the crude antigen [42]. For the same ELISA, sensitivity and specificity of 95 and 83 % respectively are reported on wild Norwegian red fox [43]. We assume similar values for the wolves, considering the close phylogenetic relation of the domestic dog and the red fox to the wolf. The ELISA using the MSA-1 along with the crude antigen is today used by SVA to diagnose sarcoptic mange on domestic dogs but not validated yet. We consider the three analyses to complement each other and assume sensitivity and specificity at least as high as in the validation studies.
Conclusions
Although continuously present, we do not consider sarcoptic mange as a significant factor of the sustainability and recovery of the Scandinavian wolf population. Wolf pups are less exposed to the parasite and mange dynamics in the wolf population are not related to the local density of wolf territories. Heterogenic seroprevalence distribution within the packs and repeated observed recovery suggest an effective host - parasite response and a restricted wolf-to-wolf transmission. This disease cannot be regarded as a potential factor controlling population growth at this stage of population development. However, devastating mortality may occur on individual pack scale as a result of this parasite. The higher probability of sarcoptic mange in the southern part of the wolf distribution is probably related to landscape factors and red fox population dynamics. Sarcoptic mange could potentially become more important along with a southern expansion of the Scandinavian wolf population. Future research should address the individual humoral response of wolves and other canids to S. scabiei. In Scandinavia, the scale of research should include the entire carnivore guild for a more complete view on the dynamics and effects of this important disease.
Methods
Study area
The Scandinavian wolf population is located in central Sweden and south-eastern Norway (between 59 and 62°N, and 10–15°E) (Fig. 4) [17]. The area is primarily covered by managed forest stands of Norway spruce Picea abies and Scots pine Pinus sylvestris and wet lands, agricultural areas and settlements covering minor areas, primarily in the southern part of the wolf range [23]. The human population density is less than 1/km2 in large areas of the main wolf range [16]. The climate is continental with average temperatures of 15 °C in June and −7 °C in January and the area is snow covered from December to March with an average depth of 30–60 cm [41]. The main prey species are moose Alces alces and roe deer Capreolus capreolus, the latter mainly in the southern range of the wolf area. Other prey species represent a minor proportion of the diet [33, 44].
Fig. 4.
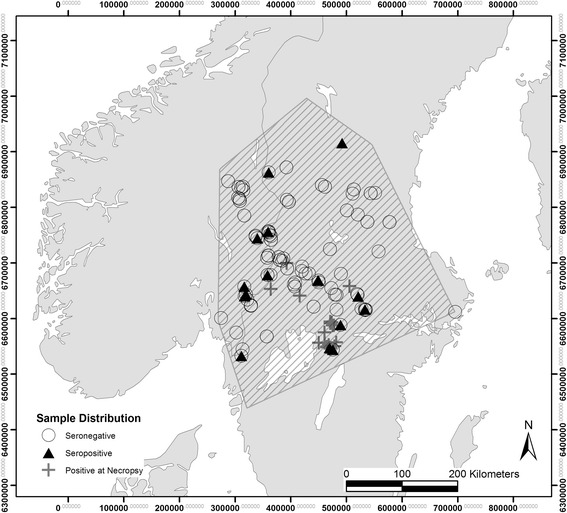
Study area. Distribution of the Scandinavian wolf population (hatched), pack centre points of captured territorial wolves or pups displayed as seropositive (N = 20) or negative (N = 178) according to the ELISA and the location of collected wolves with lesions of mange at necropsy (N = 21). Due to data collection of several animals at the same location, symbols might be overlapping
Serum collection and analysis
A total of 198 serum samples from 145 individual free-ranging wolves of 54 different packs in Sweden and Norway were analysed. All wolves were immobilized by darting from a helicopter during winter (December-March) between 1998 and 2013 as part of a long term joint Scandinavian research project or by national management, both following standard capture procedures [45] and in accordance with the NC3Rs ARRIVE guidelines for reporting animal research [46]. Captured animals were tagged with VHF or GPS collars and ear tags or microchips and the identity further confirmed by DNA- and pedigree analysis [15]. To change radio collars or for translocations 38 individuals were recaptured 1–3 times (Table 5). A sampling event per territory was defined as one or more wolves captured per winter within a pack, including recaptures. Sampled animals were sexed (104 males, 94 females), weighed and measured. As a proxy for body condition, we used the residual distance from each individual to the linear regression line of log body weight and log body length (nose to base of the tail) estimated from all individuals in the data set [47]. This residual index was no longer correlated to body length (r = −0.01, df = 104, p = 0.94). The animals were grouped into pups (<1 year old) and adults. The age was estimated from tooth wear in adults and pups were identified by the growth zone on the radius and ulna [48]. Based on movement data from GPS-collared wolves, pups were assumed to be born in early May [49]. The age of the sampled wolves ranged from 7 to 10 months for pups and over 10 years for adults. Age was adjusted if pedigree analysis could prove the year of birth. Clinical evaluation of the captured wolves was not standardized but anomalies, such as previous injuries, broken teeth or mange lesion, are mentioned on the capture form. Blood was collected from the cephalic vein using 8 ml sterile, evacuated serum-separator tubes with gel and clot activator (Venosafe™, Terumo Europe N.V, Leuven, Belgium). Serum was separated by centrifugation at 1500 rpm for 15 min the same day and kept frozen until shipment to the laboratory (Department of Virology, Immunobiology and Parasitology, SVA Uppsala, Sweden).
Table 5.
Age and recaptures among the captured individuals
| Recaptures | |||||
|---|---|---|---|---|---|
| Captured individuals | 1 | 2 | 3 | Total samples | |
| Pups | 56 | 56 | |||
| Pups, recaptured as adults | 13 | 5 | 1 | 19 | |
| Adults | 89 | 25 | 6 | 3 | 123 |
| Total | 145 | 38 | 11 | 4 | 198 |
Pups are < 1 year old, adults > 1 year old
Serum samples were analysed by running two parallel ELISAs using a crude (S. scabiei var. vulpes extract) antigen according to Bornstein et al. 1993 [34], modified by a change of the substrate from 5-amino-2-hydroxybenzoic to 3-3-5 tetramethylebenzidin (TBM) and a recombinant major S. scabiei var. vulpes antigen (MSA-1) respectively [50]. In each series, samples were tested in duplicates and positive and negative control samples from dogs were included. Optical density (OD) was measured at 450 nm with a multiscan EX (Thermo Labsystems, Vantaa, Finland). In order to get an OD value relative to the positive control, mean OD values for each duplicate were calculated and the mean OD of a blank (PBS-T instead of a sample) subtracted. For valid results, positive control OD values had to be between 0.8 and 1.6 for the crude antigen and between 0.7 and 2.0 for the MSA-1 antigen. OD values of the samples were divided by the OD value of the positive control. Positive results should reach > 0.3 for the crude antigen and > 0.5 for the MSA-1, relative to the positive control. Doubtful results were defined as 0.2–0.299 by the crude antigen and 0.35–0.499 by the MSA-1 antigen, OD values below 0.2 and 0.35 respectively were considered to be negative.
Western Blot as previously described [3], was used to confirm all seropositive samples, all doubtful samples and all samples with different results between the two ELISAs. In brief, the sarcoptes proteins in the crude antigen extract were separated by their atomic weight (kDa) on a nitrocellulose membrane. The samples were exposed to the proteins. When positive, the antibodies in the samples bound with the protein at 164 kDa or 147 kDa and 105 kDa. Samples with doubtful or positive ELISA values but confirmed positive by Western Blot were considered as seropositive.
The proportion of seropositive samples are presented with confidence intervals estimated by the Wilson score method [51].
Necropsy reports
After 2002, all dead wolves found in Sweden have been delivered for standard necropsy at SVA. Serology as described above (N = 15), microscopy (N = 4) or both (N = 2) were part of the standard necropsy if clinical signs of sarcoptic mange were detected. We compared observed seropositivity from captured Swedish wolves from 2003 to 2013 with the necropsy data.
Population data
The annual monitoring of the Scandinavian wolf population for the entire study area was based on snow tracking, DNA-analyses of scats and radio/GPS collar data [52]. This monitoring provided, for each winter, the total number and geographical distribution of established pairs and packs and estimates of pack- and population size. We expressed wolf density at two spatial scales. Within wolf territories, pack size represented the number of wolves per territory and ranged from one to ten wolves. The samples were classified as from single wolves, from pairs (two scent marking animals of opposite sex) or packs (one or two scent marking animals and pups or yearlings). Wolves older than two years within the parental territory and both parents present have until the end of the study period not been detected by the monitoring in Scandinavia [17]. At the landscape level, we used distance to neighbouring packs as a proxy for territory density. We estimated the territory densitiy for each wolf territory and for each winter as the average of the Euclidean distances between its centre point and the centre points of the three nearest neighbouring wolf territories using the spatstat package [53] in R 3.0.3 [54]. We estimated the centre point coordinates by averaging VHF or GPS collar locations of territorial adult wolves, and DNA collection sites for wolf territories without radio-collared individuals.
We used latitude as a habitat productivity proxy and checked for longitude effects. As a proxy of red fox density for each wolf territory, we used the yearly number of red fox harvested per 10 km2. These bag statistics were provided by the Swedish Association for Hunting and Wildlife Management on the hunting management unit level (mainly corresponding to a municipality) in Sweden. Mange lesions or other qualitative measures of the shot red fox were not reported. Using a two sample t-test, we tested if occurrence of seropositive wolves was related to high red fox densities. We used the red fox harvest record from those hunting management units that contained the centre points of the wolf territories. Accordingly, we tested if the latitude of the centre points was related to the red fox harvest record in the respective hunting management units.
Modelling seroprevalence
According to our hypothesis we formulated two full models à priori: 1) The individual-based model correlated the probability to find a seropositive serum sample to individual-based intrinsic factors; reproductive state, age, body condition, and the interaction of sex and pack size, assuming more physical contact of reproducing females with their pups, as well as a potential negative effect on pup survival with seropositive females. 2) The population-based model correlated the probability to find a positive serum sample to territory density, and to the projected, metric latitude and longitude of the wolf territory. Territory size of Scandinavian wolves increases with latitude, independently of available moose biomass but related to landscape structure and climate [23]. We did not include red fox density in this model because of missing values for the Norwegian territories. The response variable in both models was a binary term with 1 for seropositive and 0 for seronegative wolves, and models were statistically estimated using logistic regression. Due to a low number of events (seropositive) the risk of over-fitted models increases rapidly with every included variable [55]. We formulated sub models with fewer variables and included them in the selection. Within both variable groups sub-models performed better than the full models (Table 3).
All analyses were done using statistical extensions available in R 3.0.3 [54]. Despite the inclusion recaptured individuals we did not include nested random factors to the models. This decision was based on the lack of the among-individual variance to the binary response variable resulting in a negligible effect when included as a random factor [56]. A large proportion of the individuals (107 of 145, Table 5) were sampled only once. In addition, the time interval between capture events was long enough for individuals to change from seropositive and seronegative and vice versa [34, 36]. The variance inflation factor of any continuous variable was <1 indicating no multicollinearity [57] and models did not show outliers (Cook’s distance) [58].
We selected by parsimony for each full model the best sub-model (lowest AICc using the R-package AICcmodvag, no cut off). Then we used a cut-off point of delta AICc = 4 to find the best combination of predictors from both sub-models. Further, we used model-averaging to present the effect sizes of the predictors of the models within the cut-off [59]. Observations with lacking data were omitted before model selection (N = 36). Robustness of the selected models was verified by k-fold cross validation. The models were run 100 times on a training set including randomly selected 90 % of the data. Using the estimates of the training set, probabilities of being seropositive were predicted for the remaining 10 %. The predicted values were sorted and grouped into 10 equal-sized, ranked bins. For each bin, the frequency of seropositive individuals was correlated with the rank of the bin using a Spearman rank correlation (rs). This process was repeated 10 times and the mean values of rs are presented.
For the modelling process we omitted 36 out of the 198 samples either because data on body condition were missing (N = 21), the wolves were dispersing (N = 4) or were immigrants from the Finnish-Russian population resident but captured for translocation (N = 2). Four samples from one individual were excluded due to unclear results from both ELISA and Western Blot. On average, 9.3 % (SE 1.4 %) of the estimated mean Scandinavian wolf population was sampled each winter (Fig. 1a).
Acknowledgments
This paper is based on a Master thesis at Hedmark University College [60]. We are grateful to Per Ahlqvist, Thomas H. Strømseth and pilot Ulf Grinde, who captured and handled the wolves, together with Jon M. Arnemo. The SKANDULV project have been funded primarily by the Norwegian Research Council, Norwegian Environment Agency, Hedmark University of Applied Sciences, Norwegian Institute for Nature Research, County Governor of Hedmark, Swedish Research Council Formas, Swedish Environmental Protection Agency, Swedish University of Agricultural Sciences, Swedish Association for Hunting and Wildlife Management, and Worldwide Fund for Nature (Sweden). We thank A. Söderberg and K. Näslund for technical assistance and C. Milleret for R support. We also thank two anonymous reviewers for valuable comments on earlier versions of the manuscript.
Funding
This study was funded by Hedmark University College and the Scandinavian Wolf Research Network, SKANDULV.
Availability of data and materials
Wolf population data for Fennoscandia is published in annual monitoring reports eg [17]. Individual wolf data, serum samples and red fox harvest statistics are available upon request to the corresponding author.
Authors’ contributions
BF, BZ, JMA, ALE and PW designed the study. JMA, ALE, PW and HS collected samples and data from live wolves, EOÅ and OL collected the data from the dead wolves and JK collected and organized the red fox data. BF and ALE organized and combined the data. BF participated in the laboratory analysis. BF and BZ performed the statistical analysis. BF drafted the manuscript. BZ, PW, JM, SB, JMA, ALE, HS, EOÅ and OL critically and substantially revised the manuscript. All authors approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Capture, tagging, and sampling of blood were approved by the Ethical Committee on Animal Experiments, Uppsala, Sweden (C 407/12) and the National Animal Research Authority, Oslo, Norway (2015/7224).
Contributor Information
Boris Fuchs, Email: boris.fuchs@hihm.no.
Barbara Zimmermann, Email: barbara.zimmermann@hihm.no.
Petter Wabakken, Email: petter.wabakken@hihm.no.
Set Bornstein, Email: set.bornstein@sva.se.
Johan Månsson, Email: Johan.Mansson@slu.se.
Alina L. Evans, Email: alina.evans@hihm.no
Olof Liberg, Email: Olof.Liberg@slu.se.
Håkan Sand, Email: Hakan.Sand@slu.se.
Jonas Kindberg, Email: Jonas.Kindberg@slu.se.
Erik O. Ågren, Email: erik.agren@sva.se
Jon M. Arnemo, Email: jon.arnemo@hihm.no
References
- 1.Pence DB, Ueckermann E. Sarcoptic mange in wildlife. Rev Sci Tech. 2002;21(2):385–398. [PubMed] [Google Scholar]
- 2.Bornstein S, Mörner T, Samuel WM. Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. 2. Ames: Iowa State University Press; 2001. pp. 107–119. [Google Scholar]
- 3.Bornstein S, Zakrisson G, Thebo P. Clinical picture and antibody response to experimental Sarcoptes scabiei var. vulpes infection in red foxes (Vulpes vulpes) Acta Vet Scand. 1995;36(4):509–519. doi: 10.1186/BF03547665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlian LG, Morgan MS, Vyszenskimoher DL, Stemmer BL. Sarcoptes scabiei: The circulating antibody response and induced immunity to scabies. Exp Parasitol. 1994;78(1):37–50. doi: 10.1006/expr.1994.1004. [DOI] [PubMed] [Google Scholar]
- 5.Falk ES. Serum immunoglobulin values in patients with scabies. Br J Dermatol. 1980;102(1):57–61. doi: 10.1111/j.1365-2133.1980.tb05672.x. [DOI] [PubMed] [Google Scholar]
- 6.Wobeser GA. Essentials of disease in wild animals. 1. Ames: Blackwell Publishing Professional; 2006. [Google Scholar]
- 7.Almberg ES, Cross PC, Dobson AP, Smith DW, Hudson PJ. Parasite invasion following host reintroduction: A case study of Yellowstone’s wolves. Phil Trans R Soc B. 2012;367(1604):2840–2851. doi: 10.1098/rstb.2011.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez MD, Bangs EE, Sime C, Asher VJ. Sarcoptic mange found in wolves in the Rocky Mountains in western United States. J Wildl Dis. 2010;46(4):1120–1125. doi: 10.7589/0090-3558-46.4.1120. [DOI] [PubMed] [Google Scholar]
- 9.Oleaga Á, Casais R, Balseiro A, Espí A, Llaneza L, Hartasánchez A, Gortázar C. New techniques for an old disease: Sarcoptic mange in the Iberian wolf. Vet Parasitol. 2011;181(2–4):255–266. doi: 10.1016/j.vetpar.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Arlian LG. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu Rev Entomol. 1989;34(1):139–159. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- 11.Mörner T, Eriksson H, Bröjer C, Nilsson K, Uhlhorn H, Ågren E, Segerstad CH, Jansson DS, Gavier-Widén D. Diseases and mortality in free-ranging brown bear (Ursus arctos), gray wolf (Canis lupus), and wolverine (Gulo gulo) in Sweden. J Wildl Dis. 2005;41(2):298–303. doi: 10.7589/0090-3558-41.2.298. [DOI] [PubMed] [Google Scholar]
- 12.Ryser-Degiorgis MP, Hofmann-Lehmann R, Leutenegger CM, Segerstad CH, Mörner T, Mattsson R, Lutz H. Epizootiologic investigations of selected infectious disease agents in free-ranging eurasian lynx from Sweden. J Wildl Dis. 2005;41(1):58–66. doi: 10.7589/0090-3558-41.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Oleaga A, Casais R, Prieto JM, Gortázar C, Balseiro A. Comparative pathological and immunohistochemical features of sarcoptic mange in five sympatric wildlife species in Northern Spain. Eur J Wildl Res. 2012;58(6):997–1000. doi: 10.1007/s10344-012-0662-y. [DOI] [Google Scholar]
- 14.Vilà C, Sundqvist AK, Flagstad Ø, Seddon J, Björnerfeldt SB, Kojola I, Casulli A, Sand H, Wabakken P, Ellegren H. Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc R Soc Lond Ser B Biol Sci. 2003;270(1510):91–97. doi: 10.1098/rspb.2002.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberg O, Andrén H, Pedersen HC, Sand H, Sejberg D, Wabakken P, Åkesson M, Bensch S. Severe inbreeding depression in a wild wolf Canis lupus population. Biol Lett. 2005;1(1):17–20. doi: 10.1098/rsbl.2004.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wabakken P, Sand H, Liberg O, Bjärvall A. The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Can J Zool. 2001;79(4):710–725. doi: 10.1139/z01-029. [DOI] [Google Scholar]
- 17.Wabakken P, Svensson L, Kojola I, Maartmann E, Strømseth TH, Flagstad O, Akesson M, Zetterberg A. Ulv i Skandinavia og Finland: Sluttrapport for bestandsovervåking av ulv vinteren 2012–2013. Elverum: Høgskolen i Hedmark; 2013. [Google Scholar]
- 18.Liberg O, Chapron G, Wabakken P, Pedersen HC, Hobbs NT, Sand H. Shoot, shovel and shut up: Cryptic poaching slows restoration of a large carnivore in Europe. Proc R Soc Lond Ser B Biol Sci. 2012;279(1730):910–915. doi: 10.1098/rspb.2011.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mörner T. Sarcoptic mange in Swedish wildlife. Rev Sci Tech. 1992;11(4):1115–1121. [PubMed] [Google Scholar]
- 20.Lindström ER, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L, Lemnell PA, Martinsson B, Sköld K, Swenson JE. Disease reveals the predator: Sarcoptic mange, red fox predation, and prey populations. Ecology. 1994;75(4):1042–1049. doi: 10.2307/1939428. [DOI] [Google Scholar]
- 21.Ledin A, Arnemo JM, Liberg O, Hellman L. High plasma IgE levels within the Scandinavian wolf population, and its implications for mammalian IgE homeostasis. Mol Immunol. 2008;45(7):1976–1980. doi: 10.1016/j.molimm.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Wabakken P, Aronson A, Sand H, Steinset OK, Kojola I. Ulv i Skandinavia: Statusrapport for vinteren 2001–2002. Elverum: Høgskolen i Hedmark; 2002. [Google Scholar]
- 23.Mattisson J, Sand H, Wabakken P, Gervasi V, Liberg O, Linnell JDC, Rauset GR, Pedersen HC. Home range size variation in a recovering wolf population: Evaluating the effect of environmental, demographic, and social factors. Oecologia. 2013;173(3):813–825. doi: 10.1007/s00442-013-2668-x. [DOI] [PubMed] [Google Scholar]
- 24.Oleaga A, Vicente J, Ferroglio E, Pegoraro de Macedo MR, Casais R, del Cerro A, Espí A, García EJ, Gortázar C. Concomitance and interactions of pathogens in the Iberian wolf (Canis lupus) Res Vet Sci. 2015;101:22–27. doi: 10.1016/j.rvsc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Elmhagen B, Hellström P, Angerbjörn A, Kindberg J. Changes in vole and lemming fluctuations in northern Sweden 1960–2008 revealed by fox dynamics. Ann Zool Fenn. 2011;48(3):167–179. doi: 10.5735/086.048.0305. [DOI] [Google Scholar]
- 26.Ueno M, Solberg EJ, Iijima H, Rolandsen CM, Gangsei LE. Performance of hunting statistics as spatiotemporal density indices of moose (Alces alces) in Norway. Ecosphere. 2014;5(2):art13. doi: 10.1890/ES13-00083.1. [DOI] [Google Scholar]
- 27.Sykes JE. Immunodeficiencies caused by infectious diseases. Vet Clin N Am Small Anim Pract. 2010;40(3):409–423. doi: 10.1016/j.cvsm.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Åkerstedt J, Lillehaug A, Larsen IL, Eide NE, Arnemo JM, Handeland K. Serosurvey for canine distemper virus, canine adenovirus, Leptospira interrogans, and Toxoplasma gondii in free-ranging canids in Scandinavia and Svalbard. J Wildl Dis. 2010;46(2):474–480. doi: 10.7589/0090-3558-46.2.474. [DOI] [PubMed] [Google Scholar]
- 29.Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35(04):197–206. doi: 10.1017/S0031182000021612. [DOI] [Google Scholar]
- 30.Almberg ES, Cross PC, Dobson AP, Smith DW, Metz MC, Stahler DR, Hudson PJ. Social living mitigates the costs of a chronic illness in a cooperative carnivore. Ecol Lett. 2015;18(7):660–67. [DOI] [PMC free article] [PubMed]
- 31.Todd AW, Gunson JR, Samuel WM. Sarcoptic mange: An important disease of coyotes and wolves in Alberta, Canada. In: Worldwide Furbearer Conference Proceedings: 3–11 August 1980; Frostburg: MD. Ed. J.A. Chapman D. and Pursley; 1980: 706–729.
- 32.Kreeger TJ. The internal wolf: Physiology, pathology, and pharmacology. In: Mech LD, Boitani L, editors. Wolves: Behavior, Ecology and Conservation. Chicago: The University of Chicago Press; 2003. pp. 192–217. [Google Scholar]
- 33.Zimmermann B, Sand H, Wabakken P, Liberg O, Andreassen HP. Predator-dependent functional response in wolves: From food limitation to surplus killing. J Anim Ecol. 2015;84(1):102–112. doi: 10.1111/1365-2656.12280. [DOI] [PubMed] [Google Scholar]
- 34.Bornstein S, Zakrisson G. Humoral antibody response to experimental Sarcoptes scabiei var. vulpes infection in the dog. Vet Dermatol. 1993;4(3):107–110. doi: 10.1111/j.1365-3164.1993.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lower KS, Medleau LM, Hnilica K, Bigler B. Evaluation of an enzyme-linked immunosorbant assay (ELISA) for the serological diagnosis of sarcoptic mange in dogs. Vet Dermatol. 2001;12(6):315–320. doi: 10.1046/j.0959-4493.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 36.Sarasa M, Rambozzi L, Rossi L, Meneguz PG, Serrano E, Granados JE, González FJ, Fandos P, Soriguer RC, Gonzalez G, et al. Sarcoptes scabiei: Specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp Parasitol. 2010;124(3):265–271. doi: 10.1016/j.exppara.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Frankowiack M, Olsson M, Cluff HD, Evans AL, Hellman L, Månsson J, Arnemo JM, Hammarström L. IgA deficiency in wolves from Canada and Scandinavia. Dev Comp Immunol. 2014;1:26–28. doi: 10.1016/j.dci.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Packard JM. Wolf behavior: Reproductive, social, and intelligent. In: Mech LD, Boitani L, editors. Wolves: Behavior, ecology, and conservation. Chicago: University of Chicago Press; 2003. pp. 35–65. [Google Scholar]
- 39.Mech LD, Peterson RO. Wolf-prey relations. In: Mech LD, Boitani L, editors. Wolves: Behavior, ecology, and conservation. Chicago: University of Chicago Press; 2003. pp. 131–160. [Google Scholar]
- 40.Peterson RO, Ciucci P. The wolf as a carnivore. In: Mech LD, Boitani L, editors. Wolves: Behavior, ecology, and conservation. Chicago: University of Chicago Press; 2003. pp. 104–130. [Google Scholar]
- 41.Zimmermann B, Nelson L, Wabakken P, Sand H, Liberg O. Behavioral responses of wolves to roads: Scale-dependent ambivalence. Behav Ecol. 2014;25(6):1353–64. [DOI] [PMC free article] [PubMed]
- 42.Bornstein S, Thebo P, Zakrisson G. Evaluation of an enzyme-linked immunosorbent assay (ELISA) for the serological diagnosis of canine sarcoptic mange. Vet Dermatol. 1996;7(1):21–28. doi: 10.1111/j.1365-3164.1996.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 43.Davidson RK, Bornstein S, Handeland K. Long-term study of Sarcoptes scabiei infection in Norwegian red foxes (Vulpes vulpes) indicating host/parasite adaptation. Vet Parasitol. 2008;156(3–4):277–283. doi: 10.1016/j.vetpar.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Sand H, Wabakken P, Zimmermann B, Johansson Ö, Pedersen HC, Olof L. Summer kill rates and predation pattern in a wolf-moose system: Can we rely on winter estimates? Oecologia. 2008;156(1):53–64. doi: 10.1007/s00442-008-0969-2. [DOI] [PubMed] [Google Scholar]
- 45.Arnemo JM, Evans AL, Fahlman Å. Biomedical protocols for free-ranging brown bears, gray wolves, wolverines and lynx. Evenstad: Hedmark University College; 2012. [Google Scholar]
- 46.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakob EM, Marshall SD, Uetz GW. Estimating fitness: A comparison of body condition indices. Oikos. 1996;77(1):61–67. doi: 10.2307/3545585. [DOI] [Google Scholar]
- 48.Gipson PS, Ballard WB, Nowak RM, Mech LD. Accuracy and precision of estimating age of gray wolves by tooth wear. J Wildl Manage. 2000;64(3):752–758. doi: 10.2307/3802745. [DOI] [Google Scholar]
- 49.Alfredéen A-C. Department of Conservation Biology, Swedish University of Agricultural Sciences. 2006. Denning behaviour and movement pattern during summer of wolves Canis lupus on the Scandinavian Peninsula. [Google Scholar]
- 50.Ljunggren EL, Bergström K, Morrison DA, Mattsson JG. Characterization of an atypical antigen from Sarcoptes scabiei containing an MADF domain. Parasitology. 2006;132(01):117–126. doi: 10.1017/S0031182005008747. [DOI] [PubMed] [Google Scholar]
- 51.Harrell FE, Charles D. Hmisc: Harrell Miscellaneous. R-package version 3.15-0. 2015. [Google Scholar]
- 52.Liberg O, Aronson Å, Sand H, Wabakken P, Maartmann E, Svensson L, Åkesson M. Monitoring of wolves in Scandinavia. Hystrix. 2011;23(1):29–34.
- 53.Baddeley A, Turner R. {spatstat}: An {R} package for analyzing spatial point patterns. J stat soft. 2005;12:1–42. doi: 10.18637/jss.v012.i06. [DOI] [Google Scholar]
- 54.R Core Team . Warm Puppy. Vienna: R Foundation for Statistical Computing; 2014. R: A language and environment for statistical computing. [Google Scholar]
- 55.Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 56.Pasch B, Bolker BM, Phelps SM. Interspecific dominance via vocal interactions mediates altitudinal zonation in neotropical singing mice. Am Nat. 2013;182(5):E161–E173. doi: 10.1086/673263. [DOI] [PubMed] [Google Scholar]
- 57.Fox J, Weisberg S. An {R} companion to applied regression. 2. Thousend Oaks: Sage; 2011. [Google Scholar]
- 58.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. 1. New York: Springer; 2009. [Google Scholar]
- 59.Mazerolle MJ. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2014.
- 60.Fuchs B. Sarcoptic mange in the Scandinavian wolf population. Evenstad: Høgskolen i Hedmark; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Wolf population data for Fennoscandia is published in annual monitoring reports eg [17]. Individual wolf data, serum samples and red fox harvest statistics are available upon request to the corresponding author.


