Abstract
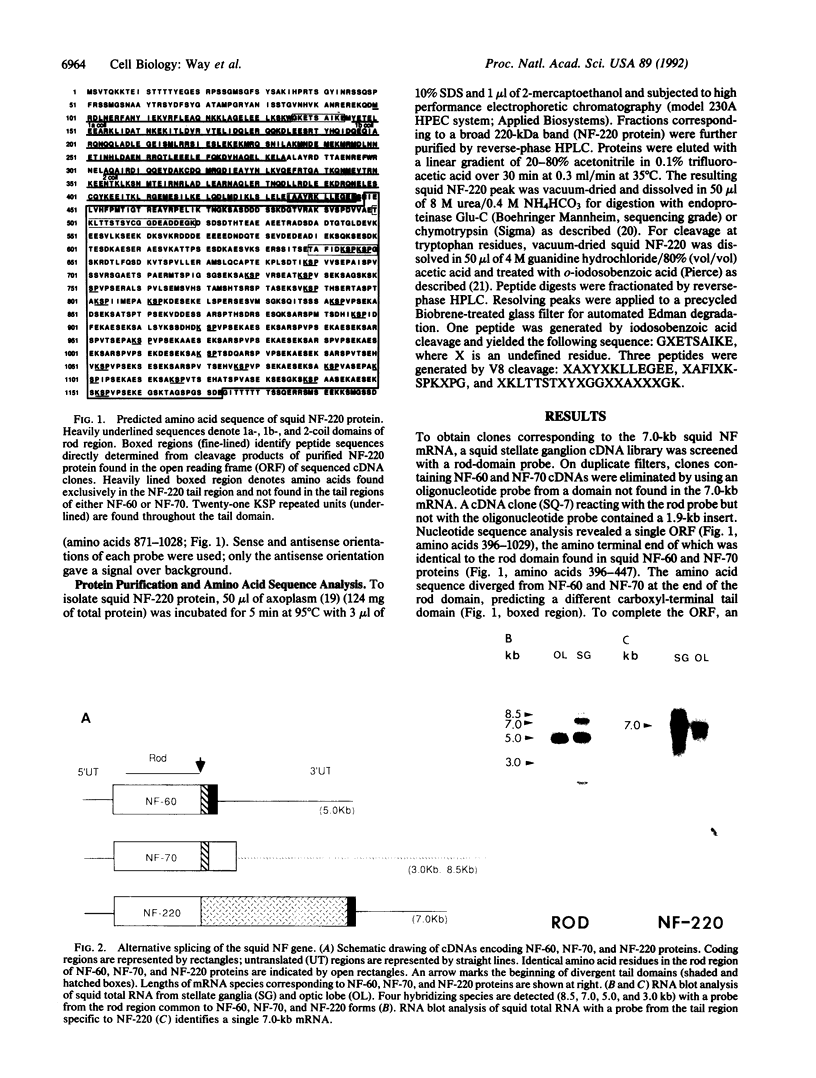
Previous studies have shown that two low molecular-weight neurofilament (NF) proteins (NF-60 and NF-70) from the squid Loligo pealei are translated from mRNAs that are splice variants of a single squid NF gene. In this study, we report the isolation and characterization of cDNA clones encoding a high-molecular-weight squid NF protein (NF-220), the mRNA of which derives from the same squid NF gene. All three proteins are identical in their amino-terminal and lamin-like rod domains but differ in their carboxyl-terminal tail regions. In contrast to the short tail domains of NF-60 and NF-70, the NF-220 protein has a longer tail domain containing an acidic cluster of amino acids immediately followed by repeated copies of the sequence motif Lys-Ser-Pro. The Lys-Ser-Pro domain is similar to that of mammalian medium NF (NF-M) and high NF (NF-H) proteins, where the serines are highly phosphorylated. Except for these Lys-Ser-Pro motifs, there is surprisingly little structural similarity between the squid NF-220 protein and mammalian NF-M and NF-H proteins. Furthermore, the location of introns in squid NF-220 protein shows that it is more closely related to nuclear lamins and type III intermediate-filament proteins than to vertebrate NF proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capano C. P., Giuditta A., Castigli E., Kaplan B. B. Occurrence and sequence complexity of polyadenylated RNA in squid axoplasm. J Neurochem. 1987 Sep;49(3):698–704. doi: 10.1111/j.1471-4159.1987.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Pant H. C., House S., Gainer H. Biochemical and immunocytochemical characterization and distribution of phosphorylated and nonphosphorylated subunits of neurofilaments in squid giant axon and stellate ganglion. J Neurosci. 1987 Jul;7(7):2056–2074. doi: 10.1523/JNEUROSCI.07-07-02056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodemont H., Riemer D., Weber K. Structure of an invertebrate gene encoding cytoplasmic intermediate filament (IF) proteins: implications for the origin and the diversification of IF proteins. EMBO J. 1990 Dec;9(12):4083–4094. doi: 10.1002/j.1460-2075.1990.tb07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring V., Stick R. Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J. 1990 Dec;9(12):4073–4081. doi: 10.1002/j.1460-2075.1990.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Vandekerckhove J., Weber K. Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 1987 Sep 14;221(2):403–407. doi: 10.1016/0014-5793(87)80964-x. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hisanaga S., Kusubata M., Okumura E., Kishimoto T. Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules. J Biol Chem. 1991 Nov 15;266(32):21798–21803. [PubMed] [Google Scholar]
- Julien J. P., Grosveld F., Yazdanbaksh K., Flavell D., Meijer D., Mushynski W. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophys Acta. 1987 Jun 6;909(1):10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Anomalous placement of introns in a member of the intermediate filament multigene family: an evolutionary conundrum. Mol Cell Biol. 1986 May;6(5):1529–1534. doi: 10.1128/mcb.6.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberburg I., Spinner N., Snyder S., Anderson J., Goldgaber D., Smulowitz M., Carroll Z., Emanuel B., Breitner J., Rubin L. Cloning of a cDNA encoding the rat high molecular weight neurofilament peptide (NF-H): developmental and tissue expression in the rat, and mapping of its human homologue to chromosomes 1 and 22. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2463–2467. doi: 10.1073/pnas.86.7.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant H. C., Gallant P. E., Gainer H. Characterization of a cyclic nucleotide- and calcium-independent neurofilament protein kinase activity in axoplasm from the squid giant axon. J Biol Chem. 1986 Feb 25;261(6):2968–2977. [PubMed] [Google Scholar]
- Roder H. M., Ingram V. M. Two novel kinases phosphorylate tau and the KSP site of heavy neurofilament subunits in high stoichiometric ratios. J Neurosci. 1991 Nov;11(11):3325–3343. doi: 10.1523/JNEUROSCI.11-11-03325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Szaro B. G., Pant H. C., Way J., Battey J. Squid low molecular weight neurofilament proteins are a novel class of neurofilament protein. A nuclear lamin-like core and multiple distinct proteins formed by alternative RNA processing. J Biol Chem. 1991 Aug 15;266(23):15035–15041. [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Dodemont H., Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J. 1988 Oct;7(10):2995–3001. doi: 10.1002/j.1460-2075.1988.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]