Abstract
Objective:
Nitinol stenting could bring the better outcome in endovascular therapy for femoropopliteal disease. However, it might be expected that recent marked advances in both device technology and operator technique had led to improved efficacy of balloon angioplasty even in this segment. The aims of this study were to evaluate the clinical impact of balloon angioplasty for femoropopliteal disease and make risk stratification clear by propensity score matching analysis.
Methods:
Based on the multicenter retrospective data, 2758 patients (balloon angioplasty: 729 patients and nitinol stenting: 2029 patients), those who underwent endovascular therapy for femoropopliteal disease, were analyzed.
Results:
The propensity score matching procedure extracted a total of 572 cases per group, and the primary patency rate of balloon angioplasty and nitinol stenting groups after matching was significantly the same (77.2% vs 82.7% at 1 year; 62.2% vs 64.3% at 3 years; 47.8% vs 54.3% at 5 years). In multivariate Cox hazard regression analysis, significant predictors for primary patency were diabetes mellitus, regular dialysis, cilostazol use, chronic total occlusion, and intra-vascular ultra-sonography use. The strategy of balloon angioplasty was not evaluated as a significant predictor for the primary patency. After risk stratification using five items (diabetes mellitus, regular dialysis, no use of intra-vascular ultra-sonography, chronic total occlusion, and no use of cilostazol: the DDICC score), the estimated primary patency rates of each group (low, DDICC score 0–2; moderate, DDICC score 3; high risk, DDICC score 4–5) were 88.6%, 78.3%, and 63.5% at 1 year; 75.2%, 60.7%, and 39.8% at 3 years; and 66.0%, 47.1%, and 26.3% at 5 years (p < 0.0001). The primary patency rate of balloon angioplasty and nitinol stenting groups was significantly the same in each risk stratification.
Conclusion:
This study suggests that balloon angioplasty does not have inferiority to nitinol stenting but does have favorable efficacy in femoropopliteal segment by careful risk stratification with the recent advance of technique.
Keywords: Balloon angioplasty, femoropopliteal segment, endovascular therapy, propensity score matching analysis, multivariate analysis, risk stratification
Introduction
Nitinol stent could bring the better outcome in endovascular therapy (EVT) for femoropopliteal segment.1–6 But there remains high-risk cases and lesions for vessel patency after EVT, such as female gender, diabetes mellitus, end stage of renal disease, critical limb ischemia (CLI), long lesion, chronic total occluded lesion (CTO), and poor below-the-knee run-off lesions.7–9 For a solution of this issue, a development of new stent platforms has been ongoing, and the new strategy by use of the drug-coated balloon (so-called nothing left behind strategy) is also going to challenge it. However, improved efficacy of plain balloon angioplasty (BA) might be expected by recent marked advances in both device technology and operator technique. However, only a few studies estimated the current efficacy of BA for this segment in daily clinical settings.5,6,10
With this background, those might be important to reevaluate the impact of BA compared with nitinol stenting (NT) and make appropriate candidates for BA clear. It should be the best to build up the data by high-volume controlled prospected multicenter study, but it might be hard to make a protocol. Propensity score matching technique was first reported in the 1980s11 and has been widely used to minimize the baseline differences due to treatment selection bias in clinical observational studies. So, the aims of this study were to evaluate the clinical impact of plain BA for femoropopliteal lesions in NT era and make risk stratification clear by propensity score matching analysis, based on high-volume retrospective multicenter registry data.
Method
Patient
From January 2004 to December 2011, consecutive 5002 patients who underwent EVT for femoropopliteal lesions in 13 centers in Japan were enrolled in the Retrospective Multicenter Analysis for Femoropopliteal stenting (REAL-FP) registry. Of them, 2254 cases were excluded because of past history of revascularization (1651 cases), lost during follow-up (323 cases), procedure failure (152 cases), and acute limb ischemia (128 cases). Therefore, 2758 patients who underwent successful EVT (BA: 729 cases and NT: 2029 cases) for de novo femoropopliteal lesions were identified retrospectively and included in this study. Propensity score matching was performed for minimizing intergroup baseline differences due to an operator’s bias. The study protocol was designed in accordance with the Declaration of Helsinki, approved by the ethics committee of each participating hospital, and registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR; no. UMIN000010986). All patients gave written informed consent for both intervention and inclusion in this study prior to procedure.
Procedure and follow-up
The procedure and follow-up protocol were described in the former report based on this registry database.4 At each procedure, a kind of guide-wire, use of intra-vascular ultra-sonography (IVUS), size of balloon, necessity of adjunctive stenting, and choice of nitinol self-expandable stents (Luminexx (Bard, Murray Hill, NJ) or S.M.A.R.T. (Cordis J&J, Miami, FL)) were left to each operator’s discretion. After the procedure, all patients were prescribed lifelong aspirin (100 mg/day), and prolonged dual anti-platelet therapy (aspirin 100 mg/day + clopidogrel 75 mg/day or ticlopidine 200 mg/day or cilostazol 200 mg/day) was recommended. The classification of the Rutherford categories, physical findings, the resting ankle-brachial index, and duplex ultrasound scan for treated segment were monitored within 30 days and every 6 months thereafter. Repeated revascularization was performed based on clinical symptoms and findings on duplex sonography or angiography.
Definitions
Nonambulatory status was defined as wheelchair bound or bedridden. Lesion length (LL) referred to was the whole portion that was dilated by a balloon or treated with stenting. Reference diameter was visually estimated on angiography compared with an easy ruler put beside a foot in cases without IVUS use. In cases with IVUS use, the reference diameter was estimated by its findings. Calcification was defined as obvious densities noted within the apparent vascular wall on angiography. Poor run-off was defined as one vessel or none below-the-knee run-off assessed by the angiography before or after the procedure. Restenosis was defined as >2.4 of peak systolic velocity ratio by duplex scan or >50% stenosis by angiography. Primary patency was defined as a treated vessel without restenosis and any repeat revascularization. Limb salvage was defined as free from any amputation above the ankle.
Statistical analysis
Continuous variables with or without normal distributions were compared between groups using either the unpaired t-test or the Mann–Whitney U test, respectively. Variables with a normal distribution were expressed as mean values ± standard deviation (SD), while median and interquartile range was used for asymmetrically distributed data. Chi-square test was used to compare proportions between groups. Each outcome was estimated using the Kaplan–Meier method and compared using the log rank test. Statistical significance level was set at p < 0.05.
To adjust for baseline differences between groups, propensity score matching analysis11 was performed using the following variables: age, gender, ambulatory status, hypertension, dyslipidemia, diabetes mellitus, regular dialysis, current smoking, cilostazol use, statin use, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) use, CLI, Trans-Atlantic Inter-Society Consensus (TASC) II C/D lesions, LL, reference diameter, arterial calcification, chronic total occlusion (CTO), poor below-the-knee run-off, and IVUS use. According to Austin’s12 recommendation, BA group and NT group were matched 1:1 on the logit of the propensity score within the caliper of 0.2 standard deviation of the logit of the propensity score.
Independent outcome determinants were identified by the Cox proportional hazard ratio in multivariable analysis including all variables from univariable analysis with a p-value of <0.05.
Propensity score matching was performed with R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria), whereas other statistical analyses were performed using the Statistical Package for Social Sciences (SPSS; SPSS, Inc., Chicago, IL) software.
Results
For baseline characteristics before propensity score matching, there were significant differences between the BA group and the NT group, in ambulatory status, hypertension, regular dialysis, current smoking, administration of cilostazol, ACEI or ARB, TASC II C/D prevalence, LL, reference diameter, appearance of vessel calcification, CTO, poor below-the-knee run-off, and IVUS use (Table 1). Table 2 shows all perioperative complications which had no significant difference between the groups.
Table 1.
Baseline characteristics and procedure detail before and after propensity score matching.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| BA group | NT group | p-value | BA group | NT group | p-value | |
| Patient characteristics, n | 729 | 2029 | 572 | 572 | ||
| Age (years; mean ± SD) | 72 ± 9 | 73 ± 9 | 0.9341 | 72 ± 9 | 73 ± 10 | 0.7712 |
| Female, n (%) | 240 (33) | 596 (29) | 0.0738 | 163 (28) | 179 (31) | 0.3015 |
| Ambulatory, n (%) | 596 (82) | 1746 (86) | 0.0068 | 475 (83) | 487 (85) | 0.3320 |
| Hypertension, n (%) | 582 (80) | 1724 (85) | 0.0017 | 472 (83) | 473 (83) | 0.9378 |
| Dyslipidemia, n (%) | 340 (47) | 1006 (50) | 0.1827 | 287 (50) | 288 (50) | 0.9528 |
| Diabetes mellitus, n (%) | 436 (60) | 1215 (60) | 0.9968 | 349 (61) | 343 (60) | 0.7167 |
| Regular dialysis, n (%) | 237 (33) | 469 (23) | <0.0001 | 169 (30) | 159 (28) | 0.5133 |
| Current smoking, n (%) | 157 (22) | 523 (26) | 0.0238 | 144 (25) | 151 (26) | 0.6361 |
| Cilostazol, n (%) | 255 (35) | 1028 (51) | <0.0001 | 218 (38) | 225 (39) | 0.6709 |
| Statin, n (%) | 256 (35) | 768 (38) | 0.1981 | 216 (38) | 208 (36) | 0.6243 |
| ACEI/ARB, n (%) | 343 (47) | 1071 (53) | 0.0087 | 293 (51) | 286 (50) | 0.6789 |
| CLI, n (%) | 249 (34) | 630 (31) | 0.0971 | 174 (30) | 176 (31) | 0.8979 |
| Lesion characteristics, n | 950 | 2520 | 572 | 572 | ||
| TASC II C/D, n (%) | 113 (12) | 1255 (50) | <0.0001 | 93 (16) | 104 (18) | 0.3890 |
| LL (mm; mean ± SD) | 64 ± 60 | 141 ± 88 | <0.0001 | 78 ± 68 | 80 ± 59 | 0.5873 |
| Reference diameter (mm; mean ± SD) | 4.9 ± 1.1 | 5.3 ± 0.9 | <0.0001 | 5.2 ± 1.1 | 5.1 ± 0.8 | 0.2749 |
| Calcification, n (%) | 609 (64) | 1439 (57) | 0.0002 | 326 (57) | 346 (60) | 0.2297 |
| CTO, n (%) | 251 (26) | 1322 (52) | <0.0001 | 192 (34) | 201 (35) | 0.5753 |
| Poor run-off, n (%) | 382 (40) | 1058 (42) | 0.0179 | 242 (42) | 237 (41) | 0.7644 |
| Procedure detail, n | 950 | 2520 | 572 | 572 | ||
| IVUS use, n (%) | 115 (12) | 621 (25) | <0.0001 | 96 (17) | 96 (17) | 1.0000 |
SD: standard deviation; BA: balloon angioplasty; NT: nitinol stenting; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CLI: critical limb ischemia; TASC: Trans-Atlantic Inter-Society Consensus; LL: lesion length; CTO: chronic total occlusion; IVUS: intra-vascular ultra-sonography.
Table 2.
Perioperative complications before propensity score matching.
| BA group (n = 729) | NT group (n = 2029) | p-value | |
|---|---|---|---|
| All perioperative complications, n (%) | 43 (5.9) | 158 (7.8) | 0.1687 |
| Distal embolism, n (%) | 5 (0.7) | 21 (1.0) | 0.4044 |
| Bypass conversion, n (%) | 4 (0.5) | 20 (1.0) | 0.2770 |
| Blood transfusion, n (%) | 19 (2.6) | 63 (3.1) | 0.4999 |
| Temporary hemodialysis, n (%) | 1 (0.1) | 5 (0.2) | 0.5880 |
| Pseudo-aneurysm, n (%) | 2 (0.3) | 14 (0.7) | 0.2062 |
| Hematoma, n (%) | 12 (1.6) | 35 (1.7) | 0.8866 |
BA: balloon angioplasty; NT: nitinol stenting.
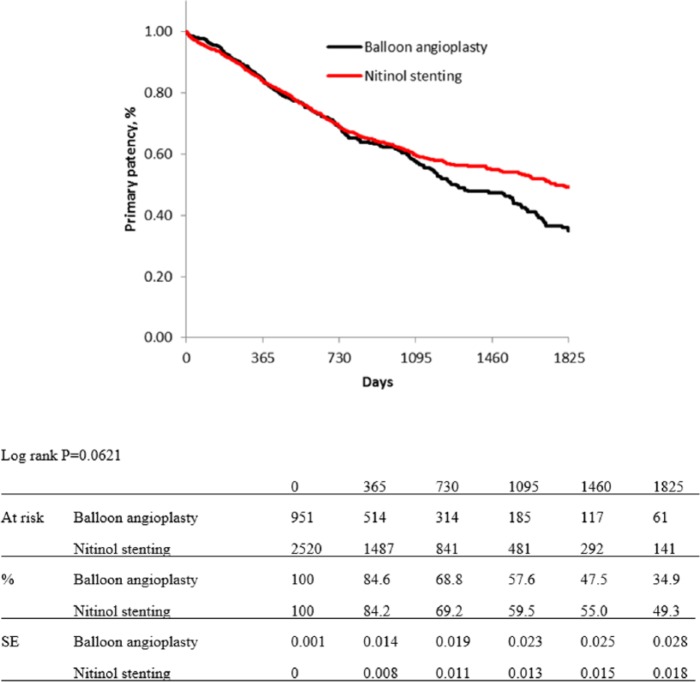
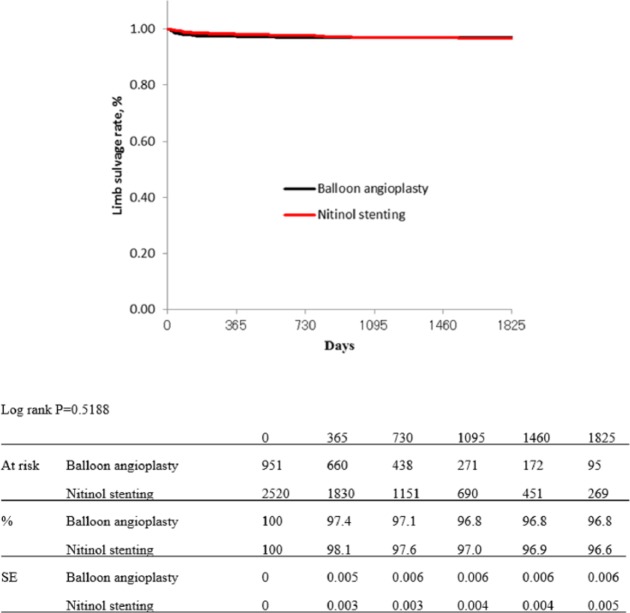
Figures 1 and 2 show Kaplan–Meier curves for primary patency and limb salvage rates of both groups before propensity score matching. The estimated primary patency rates were 84.6% in the BA group versus 84.2% in the NT group at 1 year, 68.8% versus 69.2% at 2 years, 57.6% versus 59.5% at 3 years, 47.5% versus 55.0% at 4 years, and 34.9% versus 49.3% at 5 years (p = 0.0621; Figure 1). The estimated limb salvage rates of both groups were kept over 96% for 5 years, and there were no significant differences between the groups (p = 0.5188; Figure 2).
Figure 1.
Kaplan–Meier curves show primary patency rate of balloon angioplasty and nitinol stenting groups before propensity score matching.
Figure 2.
Kaplan–Meier curves show limb salvage rate of balloon angioplasty and nitinol stenting groups before propensity score matching.
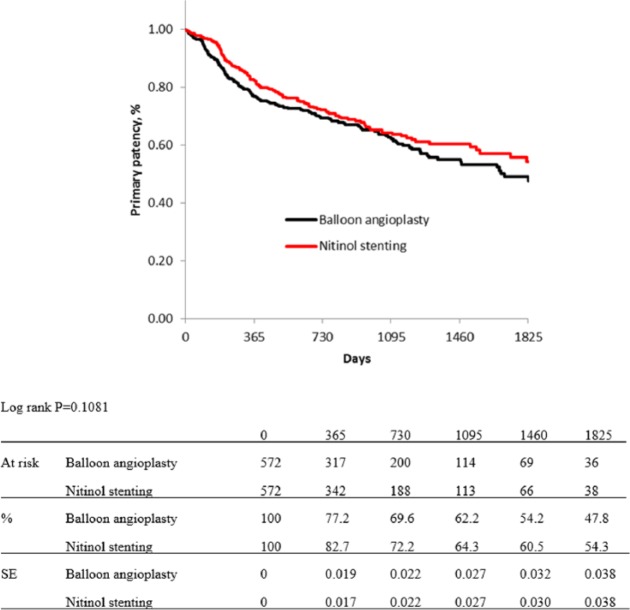
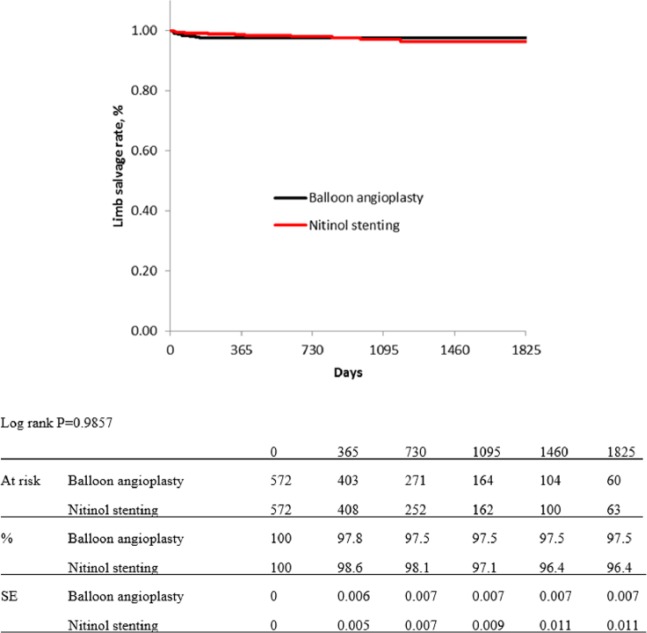
In the propensity score matching procedure, 255 cases with missing data on variables of interest were excluded, and a total 572 cases per group were extracted. Table 1 shows baseline characteristics of both groups, which were adjusted not to have significant differences. Figures 3 and 4 show Kaplan–Meier curves for primary patency and limb salvage rates of both groups after propensity score matching. The estimated primary patency rate after adjusting was 77.2% in BA group versus 82.7% in NT group at 1 year, 69.6% versus 72.2% at 2 years, 62.2% versus 64.3% at 3 years, 54.2% versus 60.5% at 4 years, and 47.8% versus 54.3% at 5 years (p = 0.1081; Figure 3). The estimated limb salvage rates of both groups were kept over 96% for 5 years, and there were no significant difference between the groups (p = 0.9857; Figure 4).
Figure 3.
Kaplan–Meier curves show primary patency rate of balloon angioplasty and nitinol stenting groups after propensity score matching.
Figure 4.
Kaplan–Meier curves show limb salvage rate of balloon angioplasty and nitinol stenting groups after propensity score matching.
Table 3 shows multivariate Cox hazard regression analysis for association of primary patency with baseline characteristics. In multivariate analysis, which included diabetes mellitus, regular dialysis, administration of cilostazol, administration of ACEI or ARB, CLI, LL ⩽ 100 mm, reference diameter ⩾ 6 mm, lesion calcification, CTO, and IVUS use, significant predictors for primary patency were diabetes mellitus, regular dialysis, administration of cilostazol, CTO, and IVUS use.
Table 3.
Association of primary patency with baseline characteristics after propensity score matching.
| Univariate analysis | Multivariate analysis | |
|---|---|---|
| Patient characteristics | ||
| Age | 1.0 (1.0–1.0) | – |
| Female gender | 1.2 (0.9–1.5) | – |
| Ambulatory | 0.8 (0.6–1.2) | – |
| Hypertension | 1.1 (0.8–1.4) | – |
| Dyslipidemia | 1.1 (0.9–1.3) | – |
| Diabetes mellitus | 1.4 (1.1–1.8)*** | 1.4 (1.1–1.7)** |
| Regular dialysis | 1.7 (1.4–2.2)****** | 1.4 (1.1–1.8)* |
| Current smoking | 1.0 (0.8–1.3) | – |
| Cilostazol | 0.6 (0.5–0.8)****** | 0.6 (0.5–0.8)***** |
| Statin | 1.0 (0.8–1.2) | – |
| ACEI or ARB | 0.8 (0.6–1.0)* | 0.9 (0.7–1.1) |
| CLI | 1.5 (1.2–1.9)**** | 1.2 (0.9–1.6) |
| Lesion characteristics | ||
| TASC II C/D | 1.6 (1.2–2.1)**** | – |
| Lesion length | 1.0 (1.0–1.0)***** | – |
| ⩽150 mm | 0.7 (0.5–1.0)* | – |
| ⩽100 mm | 0.7 (0.5–0.9)*** | 0.8 (0.6–1.1) |
| ⩽50 mm | 0.7 (0.6–0.9)*** | – |
| Reference diameter | 0.8 (0.7–0.9)***** | – |
| ⩾7 mm | 0.8 (0.5–1.3) | – |
| ⩾6 mm | 0.7 (0.6–0.9)* | 0.8 (0.6–1.0) |
| ⩾5 mm | 0.8 (0.6–1.0) | – |
| Calcification | 1.5 (1.2–1.9)**** | 1.1 (0.9–1.5) |
| CTO | 1.4 (1.1–1.7)*** | 1.3 (1.1–1.7)* |
| Poor run-off | 1.2 (1.0–1.5) | – |
| Procedure details | ||
| Balloon angioplasty | 1.2 (1.0–1.5) | – |
| IVUS use | 0.6 (0.4–0.8)**** | 0.5 (0.4–0.7)***** |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CLI: critical limb ischemia; TASC: Trans-Atlantic Inter-Society Consensus; CTO: chronic total occlusion; IVUS: intra-vascular ultra-sonography.
Data are hazard ratios and 95% confidence intervals.
p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001, *****p < 0.0005, ******p < 0.0001.
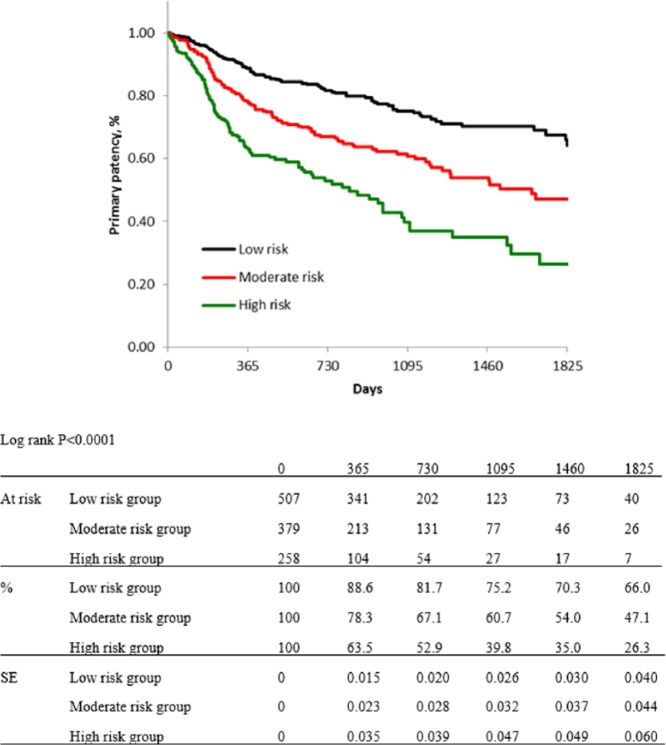
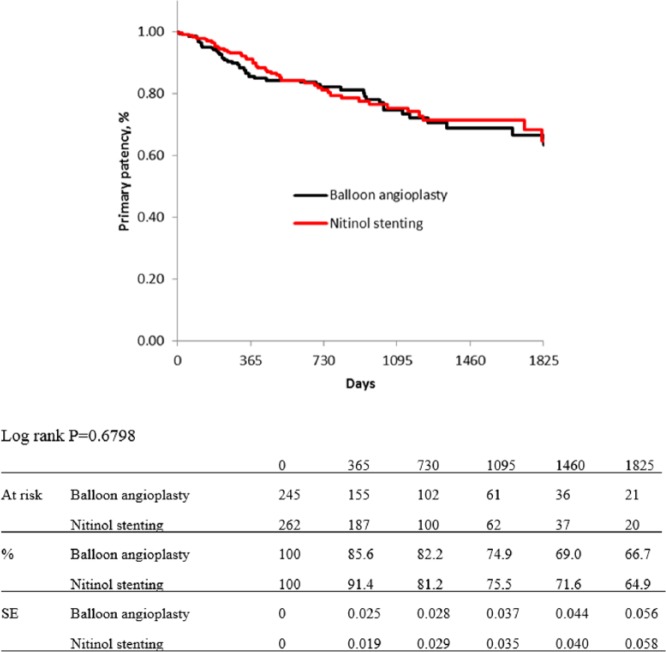
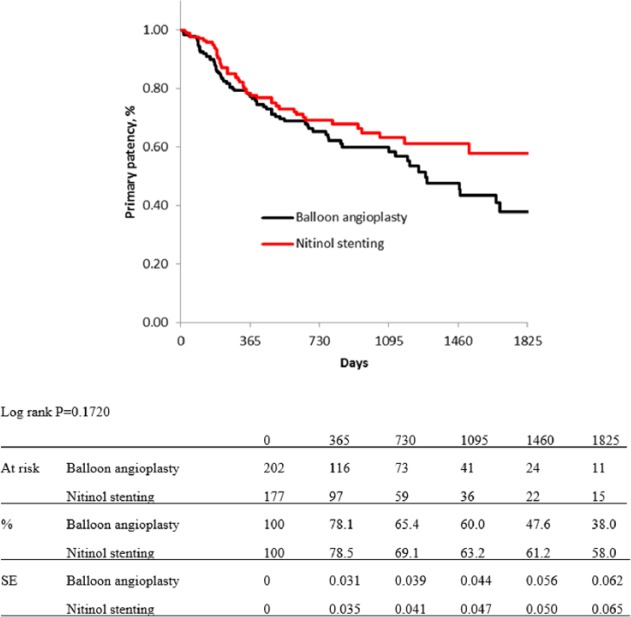
For risk stratification, all cases were classified into three groups, as low-, moderate-, and high-risk groups. Five items from independent predictors for primary patency were selected for this classification: diabetes mellitus, regular dialysis, no use of IVUS, CTO, and no administration of cilostazol: the DDICC score. These five items were each scored as 1 point. Therefore, the total score ranged from 0 to 5 points, and scores of 0–2, 3, and 4–5 points were used to indicate low-, moderate-, and high-risk patients, respectively. Kaplan–Meier curves for primary patency of each group based on the DDICC score are shown in Figure 5. The estimated primary patency rates of each group (low-, moderate-, and high-risk group) were 88.6%, 78.3%, and 63.5% at 1 year; 81.7%, 67.1%, and 52.9% at 2 years; 75.2%, 60.7%, and 39.8% at 3 years; 70.3%, 54.0%, and 35.0% at 4 years; and 66.0%, 47.1%, and 26.3% at 5 years (p < 0.0001). Figures 6–8 show no significant differences in primary patency rates between BA and NT groups in each risk stratification (low-risk group, p = 0.6798; moderate-risk group, p = 0.1720; high-risk group, p = 0.3048; log rank test).
Figure 5.
Kaplan–Meier curves show primary patency rate of each risk group after risk stratification using five items: diabetes mellitus, regular dialysis, no IVUS use, no cilostazol use, and CTO.
Figure 6.
Kaplan–Meier curves show primary patency rate of balloon angioplasty and nitinol stenting in low-risk group.
Figure 7.
Kaplan–Meier curves show primary patency rate of balloon angioplasty and nitinol stenting in moderate-risk group.
Figure 8.
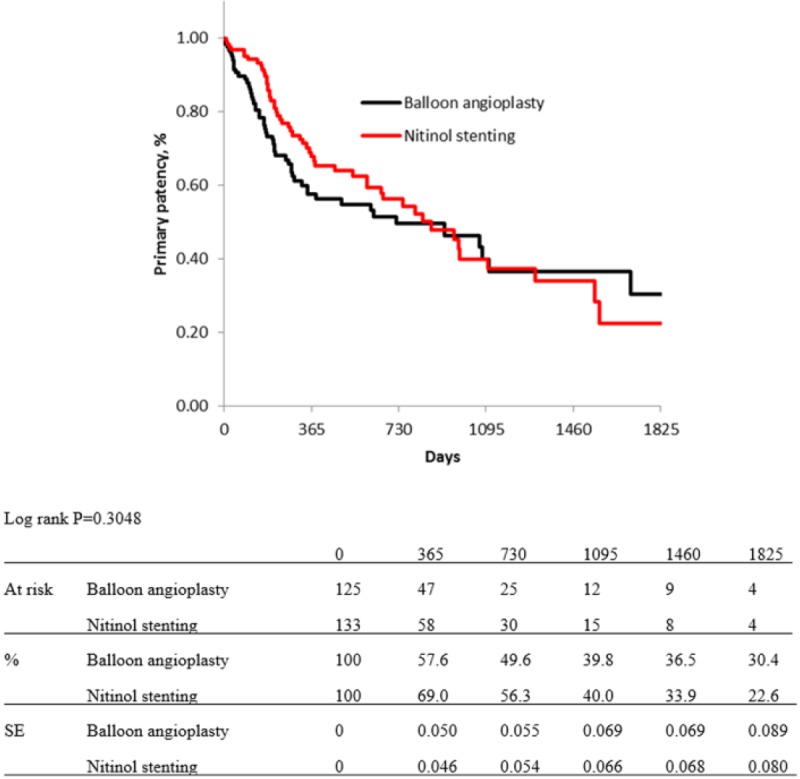
Kaplan–Meier curves show primary patency rate of balloon angioplasty and nitinol stenting in high-risk group.
In association of primary patency with BA in each subgroup, unfavorable impact was observed in patients over 80 years old, female gender, hypertension, no use of ACEI or ARB, or over 150 cm LL compared with nitinol stenting (Table 4).
Table 4.
Hazard ratios of balloon angioplasty for primary patency compared with nitinol stenting in each subgroup after propensity score matching.
| n (%) | Hazard ratio (95% CI) | |
|---|---|---|
| Patient characteristics | ||
| Age > 80 years | 220 (19) | 1.8 (1.1–3.0)* |
| Female gender | 342 (30) | 1.6 (1.1–2.4)* |
| Nonambulatory | 182 (16) | 0.9 (0.5–1.6) |
| Hypertension | 945 (83) | 1.4 (1.1–1.7)* |
| Dyslipidemia | 575 (50) | 1.1 (0.8–1.5) |
| Diabetes mellitus | 692 (60) | 1.0 (0.8–1.4) |
| Regular dialysis | 328 (29) | 1.3 (0.9–1.9) |
| Current smoking | 295 (26) | 1.1 (0.7–1.6) |
| No use of cilostazol | 701 (61) | 1.1 (0.9–1.5) |
| No use of statin | 720 (63) | 1.2 (0.9–1.5) |
| No use of ACEI or ARB | 565 (49) | 1.5 (1.1–2.0)* |
| CLI | 350 (29) | 1.3 (0.9–1.9) |
| Lesion characteristics | ||
| TASC II C/D | 197 (17) | 1.4 (0.9–2.2) |
| Lesion length | ||
| >150 mm | 138 (12) | 2.1 (1.1–3.9)* |
| >100 mm | 246 (22) | 1.1 (0.7–1.7) |
| Reference diameter | ||
| <6 mm | 805 (70) | 1.2 (0.9–1.5) |
| <5 mm | 337 (29) | 1.2 (0.8–1.8) |
| Calcification | 672 (59) | 1.3 (1.0–1.7) |
| CTO | 393 (34) | 1.3 (0.9–1.8) |
| Poor run-off | 479 (42) | 1.3 (1.0–1.9) |
CI: confidence interval; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CLI: critical limb ischemia; TASC: Trans-Atlantic Inter-Society Consensus; CTO: chronic total occlusion.
p < 0.05.
Discussion
Recent advances in both technology and operator technique could have been related to improved procedure success rate in EVT and expansion of the number of patients with peripheral artery diseases who are candidates for nonsurgical revascularization.13–15 Even in femoropopliteal segment, mid-term vessel patency had also improved through use of a nitinol stent1–5 in combination with pharmacotherapy,16–18 compared to conventional endovascular treatment before the use of nitinol stents. And to gain the efficacy of EVT in this segment, several studies with new technologies, such as drug-eluting stents,19,20 atherectomy,21 laser,22 and drug-coated balloon,23,24 have challenged. In most of these studies, inclusion criteria chose up to 10 cm in length, and their efficacy was evaluated in comparison with plain conventional BA. On this background, it might be important to reevaluate the impact of BA from a high-volume registry database. In our limited acknowledgement, this study was the highest volume study.
The mid-term efficacy of BA for de novo femoropopliteal segment showed statistically the same results with that of NT in this study despite both groups having quite different baseline characteristics. The estimated primary patency rates of BA at 1, 2, and 3 years (84.6%, 68.8%, and 57.6%) were quite acceptable compared with recent reports.3,5,6,10 Also, the limb salvage rate could have been kept quite high despite the fact that CLI cases were included in this study (30%). The appropriate selection bias left to each operator’s discretion might contribute to this result.
By the propensity score matching procedure, several differences in baseline characteristics between the groups were adjusted. Most of these extracted patients had TASC II A/B lesions and reference diameters which were equalized to over 5 mm in diameter. After propensity score matching, the primary patency of both groups was statistically the same, and the strategy of BA was not a significant predictor for the primary patency. The short- to mid-term estimated primary patency rates of BA after the propensity score matching were acceptable (77.2% at 1 year, 69.6% at 2 years, and 62.2% at 3 years), in comparison with outcomes of conventional BA arms in recent clinical trials23,24 those that evaluated the efficacy of drug-coated balloon in femoropopliteal segment. Mid- to long-term efficacy of drug-coated balloon might be expected to overcome the present results.
Even after the propensity score matching procedure, the limb salvage rate could have been kept high with no significant deference observed between the groups. This might be due to a relatively small prevalence of CLI cases. It needs a further study to examine whether BA for femoropopliteal lesions in CLI cases could be appropriate or not.
In multivariate analysis for association of primary patency with baseline characteristics, LL ⩽ 100 mm and reference diameter ⩾ 6 mm could not remain as significant predictors. This might be due to adjusting LL to around 80 mm and reference diameter to over 5 mm by the propensity score matching procedure. Diabetes mellitus, regular dialysis, and CTO lesions are well known as risk factors which affect the outcome of patients who underwent interventions. Clinical impact of cilostazol was already reported,16,17 and it was recognized that cilostazol administration could bring better outcome for the patients who underwent not only NT but also BA from this study. In previous reports25,26 that demonstrated the efficacy of IVUS use in coronary intervention, clinical impact of IVUS use in this segment might be thought due to recognition of guide-wire passage in true lumen, appropriate selection of device size, and/or recognition of concealed flow-limiting dissection which could not be detected on flash angiography.
Risk stratification of the patients who might be considered as the candidates for recanalization is important.27–29 This study tried risk stratification using five items, such as diabetes mellitus, regular dialysis, no use of IVUS, no use of cilostazol, and CTO: the DDICC score. In the condition of <10 cm LL and over 5 mm reference diameter, clinical efficacy of BA could be favorable in the patients with low risk (DDICC score, 0–2 points). It could also be acceptable in the patient with moderate risk (DDICC score, 3 points), but it seemed to keep falling even after 3 years compared with the NT group. It would be necessary for the patients with moderate risk to be paid careful follow-up over 3 years. Clinical efficacy of intervention, not only BA but also NT, was poor for the patients with high risk (DDICC score, 4–5 points). Therefore, other types of devise are promising and might be expected to bring the better outcomes even in such high-risk cases.
The overall estimation of clinical impact of BA showed no inferiority compared with NT; however, unfavorable impacts were still observed in extreme conditions, such as octogenarian and/or over 150-cm-long lesions.
It is clear that nitinol stent could bring the better outcome of EVT for femoropopliteal segment in this decade; however, this strategy is facing another issue, how to manage in-stent restenosis. In this point of view, easy metal implantation might be implemented and new drug-coated balloons would also be expected to bring better outcomes compared with plain BA. However, multiple nitinol stent implantation would still be required for immediate anatomical improvement in EVT against tough lesions, such as very long CTO lesions. Besides further development of stent platforms and drug technology, challenging effort to construct an appropriate strategy is necessary to improve the efficacy of EVT for high-risk cases.
Study limitations
This study was a retrospective and nonrandomized study despite the use of a prospectively maintained database with a large number of consecutive patients with femoropopliteal lesions. Patients considered unsuitable for revascularization, those who were failed in index intervention, or treated with bypass surgery were not managed in the study. In addition, a selection of strategy through all procedures was left to the physicians’ discretion, so that the failure rate of BA and prevalence of provisional stenting could not be evaluated. The available nitinol stents for femoropopliteal segments in this study period in Japan were only Luminexx (Bard) and S.M.A.R.T. (Cordis J&J) stents. The long-term efficacy of the next generations of nitinol stent is expected superior to those stents. Propensity score matching analysis was used for minimizing intergroup differences in characteristics. Cases with missing data on variables of interest were excluded during this procedure, and this exclusion could affect the results. Because of a lack of details about the indication of IVUS and/or its findings, it was uncertain how IVUS could affect the better outcome in this segment. A further prospective controlled study would be expected to estimate the relation between IVUS use and outcome.
Conclusion
In conclusion, this study based on high-volume multicenter registry data using propensity score matching analysis suggests that BA does not have inferiority to NT in femoropopliteal segment by close examination, not only anatomical condition but also patients’ backgrounds, effective pharmacotherapy, and the optional informative modality, such as IVUS.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the institutional review boards at all 13 participating cardiovascular and vascular institutions in Japan (UMIN000010986).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Retrospective Multicenter Analysis for Femoropopliteal stenting (REAL-FP) registry: the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR; UMIN000010986).
References
- 1. Mewissen MW. Self-expanding nitinol stents in the femoropopliteal segment: technique and mid-term results. Tech Vasc Interv Radiol 2004; 7: 2–5. [DOI] [PubMed] [Google Scholar]
- 2. Sabeti S, Schillinger M, Amighi J, et al. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score-adjusted analysis. Radiology 2004; 232: 516–521. [DOI] [PubMed] [Google Scholar]
- 3. Schillinger M, Sabeti S, Dick P, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007; 115: 2745–2749. [DOI] [PubMed] [Google Scholar]
- 4. Soga Y, Iida O, Hirano K, et al. Mid-term clinical outcome and predictors of vessel patency after femoropopliteal stenting with self-expandable nitinol stent. J Vasc Surg 2010; 52: 608–615. [DOI] [PubMed] [Google Scholar]
- 5. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther 2012; 19: 1–9. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong EJ, Saeed H, Alvandi B, et al. Nitinol self-expanding stents vs. balloon angioplasty for very long femoropopliteal lesions. J Endovasc Ther 2014; 21: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33(Suppl. 1): S1–S75. [DOI] [PubMed] [Google Scholar]
- 8. Gardner AW, Parker DE, Montgomery PS, et al. Gender differences in daily ambulatory activity patterns in patients with intermittent claudication. J Vasc Surg 2010; 52: 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soga Y, Iida O, Hirano K, et al. Utility of new classification based on clinical and lesional factors after self-expandable nitinol stenting in the superficial femoral artery. J Vasc Surg 2011; 54: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 10. Mwipatayi BP, Hockings A, Hofmann M, et al. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta-analysis. J Vasc Surg 2008; 47: 461–469. [DOI] [PubMed] [Google Scholar]
- 11. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 12. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45(Suppl. S): S5–S67. [DOI] [PubMed] [Google Scholar]
- 14. Vogel TR, Dombrovskiy VY, Carson JL, et al. Lower extremity angioplasty: impact of practitioner specialty and volume on practice patterns and healthcare resource utilization. J Vasc Surg 2009; 50: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 15. Andrew JK, Duane SP, Bruce HG, et al. SCAI expert consensus statement for femoral-popliteal arterial intervention appropriate use. Catheter Cardiovasc Interv 2014; 84: 529–538. [DOI] [PubMed] [Google Scholar]
- 16. Iida O, Nanto S, Uematsu M, et al. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. J Vasc Surg 2008; 48: 144–149. [DOI] [PubMed] [Google Scholar]
- 17. Soga Y, Yokoi H, Kawasaki T, et al. Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol 2009; 53: 48–53. [DOI] [PubMed] [Google Scholar]
- 18. Iida O, Yokoi H, Soga Y, et al. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the sufficient treatment of peripheral intervention by cilostazol study. Circulation 2013; 127: 2307–2315. [DOI] [PubMed] [Google Scholar]
- 19. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv 2011; 4: 495–504. [DOI] [PubMed] [Google Scholar]
- 20. Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol 2013; 61: 2417–2427. [DOI] [PubMed] [Google Scholar]
- 21. Zeller T, Rastan A, Sixt S, et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol 2006; 48: 1573–1578. [DOI] [PubMed] [Google Scholar]
- 22. Dave RM, Patlola R, Kollmeyer K, et al. Excimer laser recanalization of femoropopliteal lesions and 1-year patency: results of the CELLO registry. J Endovasc Ther 2009; 16: 665–675. [DOI] [PubMed] [Google Scholar]
- 23. Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008; 358: 689–699. [DOI] [PubMed] [Google Scholar]
- 24. Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008; 118: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 25. Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultra-sound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J 2008; 29: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 26. Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation 2000; 102: 523–530. [DOI] [PubMed] [Google Scholar]
- 27. Anderson PL, Gelijns A, Moskowitz A, et al. Understanding trends in inpatient surgical volume: vascular interventions, 1980–2000. J Vasc Surg 2004; 39: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 28. Rowe VL, Lee W, Weaver FA, et al. Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. J Vasc Surg 2009; 49: 910–917. [DOI] [PubMed] [Google Scholar]
- 29. Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009; 50: 54–60. [DOI] [PubMed] [Google Scholar]