Abstract
Background
Adrenocortical carcinoma (ACC) is a rare malignancy. The aim of this study was to determine the incidence and patterns of recurrence after curative-intent surgery for ACC.
Methods
Patients who underwent curative-intent resection for ACC between 1993 and 2014 were identified from 13 academic institutions participating in the United States ACC study group. Patients with metastasis or an R2 margin were excluded. Patterns and rates of recurrence were determined and classified as locoregional and distant recurrence.
Results
A total of 180 patients with a median age of 52 years (interquartile range 43–61) were identified. Most patients underwent open surgery (n = 111, 64.5 %) and had an R0 resection margin (n = 117, 75.0 %). At last followup, 116 patients (64.4 %) had experienced recurrence (locoregional only, n = 41, 36.3 %; distant only, n = 51, 45.1 %; locoregional and distant, n = 21, 18.6 %). Median time to recurrence was 18.8 months. Several factors were associated with locoregional recurrence, including left-sided ACC location (odds ratio [OR] 2.71, 95 % confidence interval [CI] 1.06–6.89) and T3/T4 disease (reference T1/T2, OR 3.04, 95 % CI 1.19–7.80) (both p < 0.05). Distant recurrence was associated with larger tumor size (OR 1.11, 95 % CI 1.01–1.24) and T3/T4 disease (reference T1/T2, OR 5.23, 95 % CI 1.70–16.10) (both p < 0.05). Patients with combined locoregional and distant recurrence had worse survival (3- and 5-year survival: 39.5, 19.7 %) versus patients with distant-only (3- and 5-year survival 55.1, 43.3 %) or locoregional-only recurrence (3- and 5-year survival 81.4, 64.1 %) (p = 0.01).
Conclusions
Nearly two-thirds of patients experienced disease recurrence after resection of ACC. Although a subset of patients experienced recurrence with locoregional disease only, many patients experienced recurrence with distant disease as a component of recurrence and had a poor prognosis.
Adrenocortical carcinoma (ACC) is a rare malignancy, with an annual incidence of 0.7–2.0 cases per million in the United States.1,2 Although the increase in the use of imaging studies has led to some ACC tumors being detected at an early stage, most patients with ACC are diagnosed with advanced disease.3,4 For patients with local or locally advanced disease, surgical resection is the only chance of cure.5–7 Despite attempts at curative resection, ACC is a lethal malignancy, with a 5-year survival of only approximately 35–50 %.6,8–10 This is largely due to the high incidence of recurrence after curative resection of ACC tumors, which can range from 37 to 80 %.11–13 However, only limited data on recurrence patterns and risk factors of recurrence after surgery for ACC have been published.14–16 In particular, most studies on ACC have focused on overall survival (OS) rather than recurrence.14–16 Furthermore, most previous studies that examined patterns of recurrence after curative resection of ACC were derived from single-institution case series.12,17,18 Although several reports on outcomes of ACC patients after surgery have been published from the United States, few studies have focused on recurrence.14,15,19
A handful of studies derived from patient data in Europe and Australia have suggested that certain factors such as margin resection, tumor size, depth of invasion, and functional status of tumor may be associated with recurrence.11,20,21 However, these data have not been validated in a US cohort. Furthermore, specific patterns of recurrence remain poorly elucidated. Data on recurrence may be important to provide patients and providers information on long-term prognosis, help tailor possible adjuvant therapy, and inform surveillance strategies.
Given the relative paucity of data, the objective of the current study was to define the rate and patterns of recurrence among patients undergoing curative resection of ACC tumors. Specifically, using a large multi-institutional cohort of patients, we sought to define factors associated with recurrence-free survival (RFS), as well as specific patterns of recurrence.
METHODS
Study Design
Between 1993 and 2014, patients who underwent curative intent resection for ACC at one of 13 academic institutions participating in the United States Adrenocortical Carcinoma Group were identified (Johns Hopkins Hospital, Stanford University, Vanderbilt University, Emory University, Wake Forest University, Washington University, The Ohio State University, University of California San Diego, University of California San Francisco, Medical College of Wisconsin, University of Texas Southwestern Medical Center, University of Wisconsin, New York University). Patients who had known metastasis at the time of operation or who had macroscopically positive margins (R2) were excluded (Supplemental Fig. 1). In addition, only patients who underwent their initial surgery at one of the index centers were included; no patient who had initial surgery elsewhere and was then referred to one of the centers for reoperation was included in the study cohort. The institutional review boards at each institution approved the study.
Standard demographic and clinicopathologic data were collected, including age, sex, race, tumor-related symptoms, functional status, laterality of tumor, depth of invasion, lymph node status, capsular invasion, and tumor size. The surgical approach was classified as open abdominal or posterior, thoracoabdominal, or minimally invasive. Histopathologic resection margin was classified as microscopically negative (R0) or positive (R1). Tumors were classified according to the 7th edition of the American Joint Committee on Cancer (AJCC) manual.22 Data on preoperative and adjuvant therapy, when available, were also collected. Information on vital status, date of last follow-up, and recurrence were collected. Recurrence was defined as biopsy-proven ACC or imaging highly suspicious for tumor recurrence. RFS was calculated from the date of surgery to the development of recurrent disease. Patterns of recurrence were classified as locoregional and distant metastasis; for the purposes of analyses, peritoneal recurrence was classified as distant metastasis.
Statistical Analysis
Categorical variables were provided as totals and frequencies. Continuous variables were reported as median with interquartile range (IQR). Comparisons between patient groups were assessed by χ2 or Wilcoxon rank-sum test. The Kaplan–Meier method was used to calculate RFS and OS with potential differences in survival examined using the log-rank test. The association of clinicopathologic factors and RFS were analyzed by the Cox regression model. Variables significant on univariable analysis were included in the multivariable logistic regression. Results were reported as odds ratio (OR) or hazard ratio (HR) as appropriate, with 95 % confidence intervals (CIs). A p value of less than 0.05 was considered to be statistically significant. Analyses were performed by Stata 12 (Stata-Corp, College Station, TX, USA).
RESULTS
Demographic and Clinicopathologic Characteristics
A total of 180 patients who underwent curative resection for ACC and met the inclusion criteria were identified. Median patient age was 52 years (IQR 43–61); the majority of patients were female (n = 115, 63.9 %) and white (n = 144, 82.3 %) (Table 1). Approximately one-third of patients had functional tumors (n = 64, 38.5 %), and the most common location was the left adrenal gland (n = 95, 53.7 %); median tumor size was 11 cm (IQR 7.5–14.3). At the time of surgery, most patients underwent an open abdominal or posterior resection (n = 111, 64.5 %), while a smaller subset (n = 23, 13.4 %) had a thoracoabdominal approach; the remaining 38 patients (22.1 %) underwent minimally invasive surgery. Multiorgan resection was performed in 67 patients (39.6 %). An R0 margin status was achieved in 117 patients (75.0 %) and an R1 margin in 39 patients (25.0 %). Roughly one-half of patients had T1/T2 (n = 85, 52.5 %) tumors. Lymph node status was not assessed in most patients (n = 131, 72.8 %); in the 49 patients who did have at least one lymph node sampled, 16 (32.7 %) had lymph node metastasis (N1). A small subset of patients received preoperative chemotherapy (n = 2, 1.2 %); in the postoperative setting, 73 patients (40.6 %) received some type of adjuvant therapy (mitotane, n = 54). Only 14 patients (9.5 %) received adjuvant radiotherapy.
TABLE 1.
Clinicopathologic characteristic of patients who underwent curative resection
| Characteristic | Total (n = 180) | No recurrence (n = 64) | Recurrence (n = 116) | p value |
|---|---|---|---|---|
| Age, years, median (IQR) | 52 (43–61) | 51 (41.5–61.5) | 53.5 (43–61) | 0.76 |
| Female, n (%) | 115 (63.9) | 42 (65.6) | 73 (62.9) | 0.72 |
| White race, (n = 175) | 144 (82.3) | 54 (85.7) | 90 (80.4) | 0.52 |
| Incidental finding, (n = 170) | 80 (47.1) | 34 (53.1) | 45 (43.4) | 0.21 |
| Hormone functional, (n = 166) | ||||
| Nonsecreting | 102 (61.5) | 43 (68.2) | 59 (57.3) | 0.54 |
| Cortisol | 35 (21.1) | 11 (17.5) | 24 (23.3) | |
| Mineralocorticoid | 8 (4.8) | 2 (3.2) | 6 (5.8) | |
| Sex hormone | 21 (12.6) | 7 (11.1) | 14 (13.6) | |
| Laterality (n = 177) | ||||
| Right | 82 (46.3) | 35 (55.6) | 47 (41.2) | 0.07 |
| Left | 95 (53.7) | 28 (44.4) | 67 (58.8) | |
| T stage (n = 162) | ||||
| T1 | 11 (6.8) | 7 (11.7) | 4 (4.0) | 0.005 |
| T2 | 74 (45.7) | 35 (58.3) | 39 (38.2) | |
| T3 | 62 (38.3) | 14 (23.3) | 48 (47.0) | |
| T4 | 15 (9.2) | 4 (6.7) | 11 (10.8) | |
| N stage | ||||
| N0 | 33 (18.3) | 12 (18.8) | 21 (18.1) | 0.2 |
| N1 | 16 (8.9) | 3 (4.7) | 13 (11.2) | |
| Nx | 131 (72.8) | 49 (76.5) | 82 (70.7) | |
| Tumor size, cm, median (IQR) | 11 (7.5–14.3) | 9.5 (6.5–13) | 12 (8–15.4) | 0.005 |
| Capsular invasion, (n = 120) | 71 (59.2) | 20 (44.4) | 51 (68.0) | 0.01 |
| Time between diagnosis and surgery, months, median (IQR) | 1.12 (0.56–2.34) | 1.07 (0.6–2.8) | 1.18 (0.4–2.2) | 0.59 |
| Operation type, (n = 172) | ||||
| Open abdominal or posterior | 111 (64.5) | 39 (61.9) | 72 (66.1) | 0.34 |
| Minimally invasive surgery | 38 (22.1) | 18 (28.6) | 20(18.3) | |
| Thoracoabdominal surgery | 23 (13.4) | 6 (9.5) | 17 (15.6) | |
| Margin (n = 156) | ||||
| R0 | 117 (75.0) | 45 (75.0) | 72 (75.0) | 1.0 |
| R1 | 39 (25.0) | 15 (25.0) | 24 (25.0) | |
| Multiorgan resection | 67 (39.6) | 22 (34.4) | 45 (42.9) | 0.27 |
| Neoadjuvant chemotherapy (n = 169) | 2 (1.2) | 1 (1.6) | 1 (1.0) | 0.74 |
| Adjuvant chemotherapy (n = 172)* | 61 (35.5) | 19 (30.2) | 42 (38.5) | 0.27 |
| Adjuvant radiotherapy (n = 147)* | 14 (9.5) | 5 (8.7) | 9 (10.1) | 0.81 |
| Adjuvant mitotane (n = 150) | 54 (36.0) | 17 (32.1) | 37 (38.1) | 0.46 |
IQR interquartile range
2 patients received both adjuvant chemotherapy and radiotherapy
Patterns of Recurrence and Risk Factors Associated with Recurrence
With a median follow-up of 26.4 months (IQR 8.3–59.9), 116 patients (64.4 %) developed recurrence. Among the 113 patients (97.4 %) with available data on recurrence pattern, 82 (72.6 %) developed recurrence only at one site (locoregional only: n = 41, 36.3 % vs. distant metastasis only: n = 51, 45.1 %). The remaining 21 patients (18.6 %) had multiple sites of recurrence. Among patients who had distant disease as a component of their recurrence, the most common site of distant recurrence included lung (n = 35, 48.6 %), liver (n = 30, 41.7 %), peritoneum (n = 8, 11.1 %), and spine (n = 8, 11.1 %). Of note, several factors were associated with specific patterns of recurrence. Left-sided ACC location (OR 2.71, 95 % CI 1.06–6.89; p = 0.04) and T stage (reference T1/T2, T3/T4 OR 3.04, 95 % CI 1.19–7.80; p = 0.02) were associated with locoregional recurrence (Supplemental Table 1). Factors such as tumor size (OR 1.11, 95 % CI 1.01–1.24; p = 0.04) and T stage (reference T1/T2: T3/T4 OR 5.23, 95 % CI 1.70–16.10; p = 0.004) were associated with increased risk of distant metastasis (Supplemental Table 2). Of note, while only a small subset of patients had lymph node sampling, the risk of recurrence increased in each T stage according to nodal status (Table 2).
TABLE 2.
Recurrence, timing, patterns and sites according to tumor stage and lymph node status
| Tumor invasion | Nodal status | No. of patients | Recurrence, n (%) |
Median recurrence free-survival (months) | Recurrence pattern |
|---|---|---|---|---|---|
| T1 | N0 | 3 | 0 | – | – |
| NX | 8 | 4 (50) | 51.2 | 1 LR, 3 distant (1 liver, 1 lung, 1 peritoneum) | |
| T2 | N0 | 10 | 5 (50) | 40.4 | 2 LR, 3 distant (2 lung, 1 other sitea) |
| N1 | 6 | 4 (66.7) | 9.0 | 1 LR, 3 bothb (1 liver, 1 lung, 1 liver–lung) | |
| NX | 58 | 30 (51.7) | 24.7 | 11 LR, 11 distant, 6 both (6 liver, 3 lung, 2 liver–lung, 1 bone, 3 other site) | |
| T3 | N0 | 17 | 14 (82.4) | 17.2 | 4 LR, 9 distant, 1 both (3 liver, 3 lung, 1 liver–lung, 1 bone, 2 other site) |
| N1 | 7 | 7 (100) | 2.8 | 4 LR, 1 distant, 1 both (1 liver, 1 bone) | |
| NX | 38 | 27 (71.1) | 12.5 | 8 LR, 15 distant, 4 both (7 lung, 3 liver, 2 liver–lung, 1 peritoneum, 1 bone, 5 other) | |
| T4 | N0 | 2 | 1 (50) | 7.5 | 1 LR |
| N1 | 3 | 2 (66.7) | 10.5 | 2 both (1 liver–lung, 1 spine) | |
| NX | 10 | 8 (80.0) | 8.1 | 1 LR, 7 distant (2 lung, 2 liver–lung, 1 liver, 1 spine, 1 other) |
LR locoregional
Other site includes brain, diaphragm, pancreas, spleen
Both indicates both LR and distance metastasis
Several factors were also associated with RFS after curative resection of ACC. On univariable analysis, tumor size, hormone functional status, T3/T4 stage, capsular invasion, lymph node metastasis, adjuvant mitotane therapy, and R1 margin resection were each associated with recurrence and RFS (all p < 0.05) (Table 3). On multivariable analysis, after controlling for competing risk factors, tumor size (HR 1.07, 95 % CI 1.01–1.13; p = 0.03) and capsular invasion (HR 2.36, 95 % CI 1.21–4.64; p = 0.01) remained associated with recurrence.
TABLE 3.
Univariable and multivariable analysis of recurrence-free survival of adrenocortical carcinoma who underwent curative resection
| Characteristic | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Age | 0.99 (0.98–1.01) | 0.32 | – | |
| Male sex | 1.14 (0.78–1.67) | 0.50 | – | |
| Tumor size | 1.04 (1.01–1.07) | 0.02 | 1.07 (1.01–1.13) | 0.03 |
| Hormone functional | 1.64 (1.10–2.44) | 0.02 | 1.39 (0.78–2.50) | 0.26 |
| Laterality | ||||
| Left | Ref | – | ||
| Right | 1.05 (0.72–1.52) | 0.82 | ||
| T stage | ||||
| T1/T2 | Ref | Ref | ||
| T3/T4 | 1.91 (1.28–2.85) | 0.002 | 1.71 (0.84–3.49) | 0.14 |
| Capsular invasion | 2.14 (1.32–3.49) | 0.002 | 2.36 (1.21–4.64) | 0.01 |
| N stage | ||||
| N0 | Ref | Ref | ||
| N1 | 3.15 (1.52–6.52) | 0.002 | 1.93 (0.44–8.40) | 0.38 |
| Nx | 1.14 (0.71–1.85) | 0.59 | 1.28 (0.59–2.78) | 0.53 |
| Operation type | ||||
| Open abdominal or posterior | Ref | – | ||
| Minimally invasive Surgery | 0.72 (0.44–1.18) | 0.19 | ||
| Thoracoabdominal surgery | 1.08 (0.64–1.85) | 0.76 | ||
| Multiorgan resection | 1.20 (0.82–1.77) | 0.36 | – | |
| Tumor rupture during surgery | 1.42 (0.76–2.68) | 0.27 | – | |
| Margin | ||||
| R0 | Ref | Ref | ||
| R1 | 1.27 (1.01–1.60) | 0.04 | 0.85 (0.39–1.83) | 0.68 |
| Neoadjuvant chemotherapy | 0.68 (0.09–4.86) | 0.70 | – | |
| Adjuvants radiotherapy | 1.48 (0.74–2.96) | 0.27 | – | |
| Adjuvant chemotherapy | 1.53 (0.90–2.61) | 0.12 | – | |
| Adjuvant mitotane | 1.82 (1.16–2.80) | 0.006 | 1.43 (0.73–2.80) | 0.29 |
HR hazard ratio, CI confidence interval
Timing of Recurrence and Long-Term Outcomes
The median RFS among all patients who underwent curative resection of ACC was 18.8 months (95 % CI 12.5–24.7). Among patients who experienced a recurrence, the median RFS was 10.0 months (95 % CI 7.9–13.41) (Fig. 1). Of note, median RFS among patients who experienced a locoregional recurrence only was 16.1 months (95 % CI 8.9–23.3), versus only 9.8 months (95 % CI 5.2–13.0) for patients who only had distant metastasis; RFS was 7.9 months (95 % CI 4.0–10.5) for patients who had both locoregional and distant metastasis (Fig. 2a). Among those patients with distant metastasis, the median RFS was 8.1 months for patients who had liver-only metastasis, 6.0 months for lung-only metastasis, and 10.5 months for both liver and lung metastasis (p = 0.22).
FIG. 1.
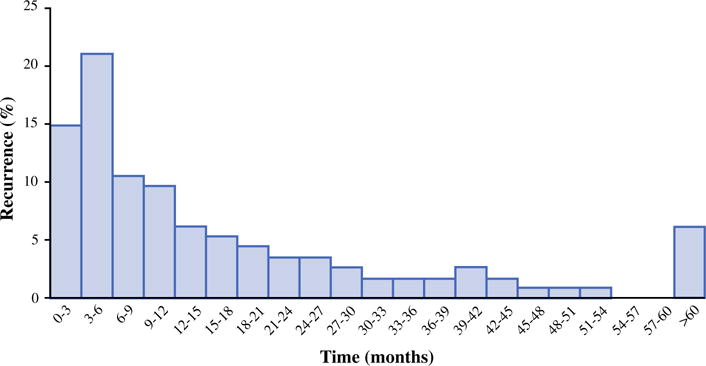
Incidence of recurrence over time
FIG. 2.
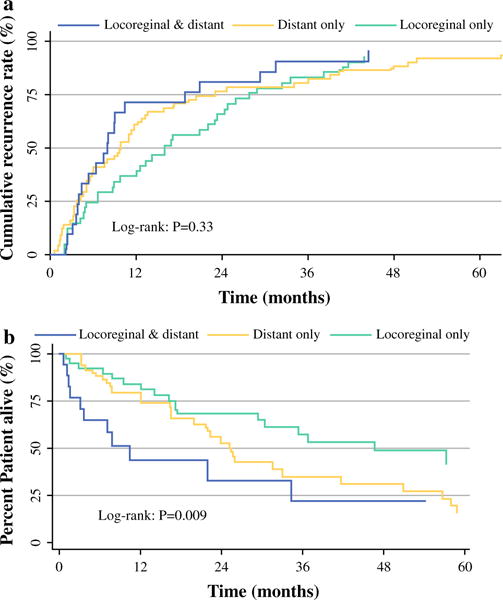
a Cumulative recurrence rate in patients with locoregional, distant, and both locoregional and distant metastasis. b Kaplan–Meier curve for survival after recurrence in patients with locoregional, distant, and both locoregional and distant metastasis
Median OS was 68.0 months (95 % CI 50.4–96.3), while 3- and 5-year OS was 68.7 and 54.4 %, respectively. Median OS after recurrence was 30.5 months (locoregional recurrence only, 46.7 months vs. distant metastasis only, 25.2 months vs. both locoregional and distant metastasis, 10.5 months; p = 0.009) (Fig. 2b). Similarly, 3- and 5-year survival was worse among patients with locoregional and distant metastasis (39.5 and 19.7 %, respectively) compared to patients who had locoregional recurrence only (81.4 and 64.1 %, respectively) or distant metastasis only (55.1 and 43.3 %, respectively) (p = 0.01).
DISCUSSION
ACC is a rare tumor for which surgical resection provides the best chance at cure. Most previous studies reporting on outcomes after surgical resection of ACC have focused largely on OS.14–16 In fact, data on recurrence and pattern of recurrence after curative resection of ACC are relatively scarce, and most reports are from small single-center case series or from centers outside of the United States.6,18,20,23 The current study is important because it represents the experience treating ACC from multiple large tertiary-care centers across the United States. Of note, almost two-thirds (64 %) of patients developed recurrence within 2 years of undergoing curative-intent surgery for ACC. Although a subset experienced recurrence with local disease only, the most common pattern of recurrence was distant metastasis to lung and/or liver. Factors associated with recurrence were largely tumor related, such as tumor size, hormone functional status, lymph node metastasis, and capsular invasion. Although the surgical approach and multiorgan resection were not associated with overall risk of recurrence, achieving an R0 margin at the time of surgery did decrease the likelihood of recurrence.
As with other malignancies, recurrence after surgery for ACC can be classified into three distinct categories: locoregional, distant, and both distant and locoregional.11,12,24 Data from several previous small case series have noted a heterogeneous recurrence pattern for ACC.11,12,20,23 For example, in a single-institution study from the Mayo Clinic, locoregional recurrence was noted to be the main pattern of recurrence.12 Similarly, Bellantone et al. reported an Italian series and noted locoregional recurrence to be the most common pattern of recurrence.11 In contrast, in a different study from Germany, Erdogan and colleagues reported that the most common pattern of recurrence was distant metastasis, followed by distant metastasis combined with locoregional recurrence.20 We similarly found that distant metastasis was the overwhelmingly predominant recurrence pattern: nearly 65 % of patients who underwent resection for ACC had distant metastasis as a component of their recurrence pattern. In addition, among those patients who experienced recurrence at a distant site, lung and liver were the most common sites of recurrent disease. Similarly, others have noted lung and liver to be the most common sites of distant recurrence.11,12,20 Taken together, these data demonstrate that recurrence is extremely common within the first 2 years after ACC resection. As such, providers need to be vigilant with postoperative surveillance within this time period, with a particular emphasis on detecting distant metastasis in the lung and liver.
Several factors were associated with recurrence. Specifically, in this study, factors associated with RFS included tumor size, hormone function status, depth of invasion, lymph node metastasis, capsular invasion, adjuvant mitotane therapy, and margin status (Table 3). These data are consistent with several smaller studies that noted recurrence to be affected by similar factors after resection of ACC.17,18,25 We also sought to examine whether certain clinicopathologic factors were associated with the pattern of recurrence. We noted that tumor laterality and depth of invasion were associated with locoregional recurrence, while T stage, capsular invasion, and tumor size were the most important risk factors associated with distant metastasis. A small number of previous series have sought to determine specific factors associated with various patterns of recurrence.18,26 For example, Ayala-Ramirez et al. noted that tumor margin and European Network for the Study of Adrenal Tumors (ENSAT) stage III/IV disease were associated with a higher risk of locoregional recurrence.18 Other studies have questioned whether laparoscopic versus open resection of ACC was associated with an increased risk of local recurrence.27–29 In the current study, we did not find any association of the surgical approach with the risk of overall or locoregional recurrence.
Highlighting the grave prognosis associated with ACC, we noted that RFS in our cohort was less than 2 years (18.8 months). Unlike many other studies, we also defined RFS according to the different patterns of recurrence. Of note, patients with locoregional recurrence had a survival twice as long compared to patients who developed distant metastasis and almost four times longer than patients who experienced both locoregional and distant metastasis (p = 0.009) (Fig. 2a). Kendrick et al. and Reibetanz et al. reported similar overall RFS of 17 months and 14 months, respectively.12,30 The high incidence of recurrence combined with the short RFS after curative-intent resection of ACC emphasizes the need for more effective adjuvant therapy. In the current study, 73 patients (40.6 %) received any type of adjuvant therapy. Among those who did receive systemic postoperative chemotherapy, the majority received mitotane therapy. Although the role of mitotane in the adjuvant setting remains controversial and debated, some retrospective data have suggested a reduced risk of recurrence with mitotane use.17,31
In the current study, use of mitotane appeared to be associated with an increased, rather than a decreased, risk of recurrence (HR 1.82, p = 0.006). The reason for this finding probably relates to selection bias in that the use of mitotane was more common in higher-risk patients. Similarly, the use of adjuvant radiotherapy remains ill defined. In the current study, only a small subset (n = 14) of patients received postoperative radiotherapy. Of note, 9 out of these 14 patients experienced a recurrence; 2 patients had a locoregional recurrence, and receipt of radiotherapy was not associated with risk of locoregional failure. Sabolch et al. had reported on 20 patients with localized disease who received postoperative adjuvant radiotherapy matched to 20 patients who did not receive radiotherapy.32 Although OS and RFS were no different in the two groups, local recurrence was lower among those who received adjuvant radiotherapy. The reason for these disparate results may be multifactorial but are likely due to the small sample size and the variations in how patients were selected and analyzed. Future studies are needed to better define the role of adjuvant therapy, especially given the high incidence of recurrent ACC disease.
The present study had several limitations. Because of the retrospective nature of study, as well as the inclusion of patients treated at many different institutions, treatment approaches may have been affected by selection and center bias. For example, only approximately 30 % of patients had lymph node evaluation, consistent with previous reports that reported lymph node sampling of 17–30 %.6,19 For this reason, we were unable to use the tumor, node, metastasis (TNM) or ENSAT staging systems in this study.22,33 To overcome this limitation, we defined recurrence pattern by AJCC T category stratified by lymph node status (Table 2). Despite amassing the experience of 13 major health care centers in the United States, our sample size was still relatively small. In turn, this limited some analyses and increased the risk of a Type I statistical error.
In conclusion, nearly two-thirds of patients who underwent curative resection for ACC tumor developed recurrence. Distant metastasis in lung and liver was the most common pattern of recurrence, with these patients having a particularly poor prognosis. Most recurrences occurred within the first 2 years after surgery. Data from the current study can, we hope, help inform physicians and patients with regard to the chance of recurrence after curative resection of ACC, as well as direct follow-up surveillance.
Supplementary Material
Footnotes
CONFLICT OF INTEREST None.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4810-y) contains supplementary material, which is available to authorized users.
References
- 1.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–78. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 2.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–78. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fassnacht M, Johanssen S, Fenske W, et al. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J Clin Endocrinol Metab. 2010;95:4925–32. doi: 10.1210/jc.2010-0803. [DOI] [PubMed] [Google Scholar]
- 4.Dackiw AP, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World J Surg. 2001;25:914–26. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 5.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–70. [PubMed] [Google Scholar]
- 6.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–7. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 7.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best practice and research. Clin Endocrinol Metab. 2009;23:273–89. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–26. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 9.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–5. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 10.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92:1113–21. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–8. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 12.Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136:543–9. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 2005;138:1078–86. doi: 10.1016/j.surg.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol. 2014;21:3509–14. doi: 10.1245/s10434-014-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton BL, Novitsky YW, Zerey M, et al. Outcomes of adrenal cortical carcinoma in the United States. Surgery. 2006;140:914–20. doi: 10.1016/j.surg.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Tritos NA, Cushing GW, Heatley G, Libertino JA. Clinical features and prognostic factors associated with adrenocortical carcinoma: Lahey Clinic Medical Center experience. Am Surg. 2000;66:73–9. [PubMed] [Google Scholar]
- 17.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–70. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 18.Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169:891–9. doi: 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States. Cancer. 2008;113:3130–6. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 20.Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:181–91. doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 21.Ip JC, Pang TC, Glover AR, et al. Improving outcomes in adrenocortical cancer: an Australian perspective. Ann Surg Oncol. 2015;22:2309–16. doi: 10.1245/s10434-014-4133-4. [DOI] [PubMed] [Google Scholar]
- 22.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. New York: Wiley; 2011. [Google Scholar]
- 23.Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbeck Arch Surg. 2012;397:201–7. doi: 10.1007/s00423-011-0866-8. [DOI] [PubMed] [Google Scholar]
- 24.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20:941–50. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 25.Freire DS, Siqueira SA, Zerbini MC, et al. Development and internal validation of an adrenal cortical carcinoma prognostic score for predicting the risk of metastasis and local recurrence. Clin Endocrinol. 2013;79:468–75. doi: 10.1111/cen.12174. [DOI] [PubMed] [Google Scholar]
- 26.Leboulleux S, Deandreis D, Al Ghuzlan A, et al. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol. 2010;162:1147–53. doi: 10.1530/EJE-09-1096. [DOI] [PubMed] [Google Scholar]
- 27.Mihai R. Diagnosis, treatment and outcome of adrenocortical cancer. Br J Surg. 2015;102:291–306. doi: 10.1002/bjs.9743. [DOI] [PubMed] [Google Scholar]
- 28.Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol. 2010;58:609–15. doi: 10.1016/j.eururo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013;27:4026–32. doi: 10.1007/s00464-013-3034-0. [DOI] [PubMed] [Google Scholar]
- 30.Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg. 2012;255:363–9. doi: 10.1097/SLA.0b013e3182367ac3. [DOI] [PubMed] [Google Scholar]
- 31.Terzolo M, Baudin AE, Ardito A, et al. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169:263–70. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 32.Sabolch A, Else T, Griffith KA, et al. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:252–9. doi: 10.1016/j.ijrobp.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115:243–50. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


