Abstract
Purpose/Aim
Previous studies have indicated that the sulfated polysaccharide heparin has anti-inflammatory effects. However, the mechanistic basis for these effects has not been fully elucidated.
Materials and Methods
NCI-H292 (mucoepidermoid) and HBE-1 (normal) human bronchial epithelial cells were treated with LPS alone or in the presence of high-molecular-weight (HMW) fully-sulfated heparin or desulfated HMW heparin. Cells were harvested to examine the phosphorylation levels of ERK1/2, p38, and NF-κB p65 and COX-2 protein expression by Western blot and gene expression of both COX-2 and CXCL-8 by TaqMan qRT-PCR.
Results
Heparin is known to exert an influence on receptor-mediated signaling through its ability to both potentiate and inhibit the receptor-ligand interaction, depending upon its concentration. In H292 cells, fully-sulfated HMW heparin significantly reduced LPS-induced gene expression of both COX-2 and CXCL-8 for up to 48 hours, while desulfated heparin had little to no significant suppressive effect on signaling or on COX-2 gene or protein expression. Desulfated heparin, initially effective at preventing LPS-induced CXCL8 up-regulation, reduced CXCL8 transcription at 24 hours. In contrast, in normal HBE-1 cells, fully-sulfated heparin significantly suppressed only ERK signaling, COX-2 gene expression at 12 hours, and CXCL-8 gene expression at 6 and 12 hours, while desulfated heparin had no significant effects on LPS-stimulated signaling or on gene or protein expression. Sulfation determines heparin’s influence and may reflect the moderating role of GAG sulfation in lung injury and health.
Conclusions
Heparin’s anti-inflammatory effects result from its non-specific suppression of signaling and gene expression and are determined by its sulfation.
Keywords: Heparin, Sulfation, COX-2, LPS, lung
INTRODUCTION
The extracellular matrix (ECM) in the lung has various biological properties. It provides supporting structure, facilitates gas exchange by providing tensile strength, and functions in tissue repair (1, 2). In the ECM, various types of glycosaminoglycans (GAGs) fill the spaces between the interlocking mesh structures, mostly consisting of collagen. These GAGs can be separated into two groups: non-sulfated GAGs (hyaluronic acid) and sulfated GAGs (heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate). To clarify the roles of GAGs in the inflammatory processes in the lung, the interactions between GAGs and the neighboring pulmonary cells or residual immune cells have been investigated. It has been demonstrated that fragments of hyaluronan generated during inflammation can trigger the innate immune response by activation of toll-like receptor (TLR) 4 and can modulate inflammation by binding to CD44 in acute lung injury (3). CD44 appeared to prevent an exaggerated inflammatory response to LPS in alveolar macrophages through induction of negative regulators of TLR4 signaling, such as IL-1R-associated kinase-M, toll-interacting protein, and A20 (4). Regulation of inflammation mediated by GAGs, as seen in the response to hyaluronan, is essential to repair mechanisms following tissue injuries in the lung (5).
Heparin is a highly sulfated GAG, rich in N- and O-sulfate groups, and is known to have anti-inflammatory properties. Heparin binds leukocyte (L)-selectin and platelet (P)-selectin, both of which are associated with leukocyte migration (6). In an in-vivo model of acute peritoneal inflammation, heparin administration significantly decreased the neutrophil migration to the target tissue (6). Heparin appeared to be effective in inhibition of neutrophil migration by blocking the initial tethering and rolling of neutrophils along the vessels mediated by the L- and P-selectins (7). Sulfate residues on the repeating disaccharide units of heparin are considered to play a role in the inhibition of neutrophil migration, and among them 6-O-sulfated residues appeared to be the key factor (8). Additionally, heparin decreased mRNA expression of ICAM-1 (Intercellular Adhesion Molecule 1) in endothelial cells, which promotes leukocyte transmigration (9).
LPS, a polysaccharide component of bacterial cell wall and one of the important triggers of inflammation in the lung, is responsible for eliciting excessive inflammation due to neutrophil migration, which can result in sepsis. Sepsis is a major risk factor of acute lung injury (ALI) and sepsis-induced ALI is often associated with high mortality (10). LPS elicits the innate immune response through activation of TLR4, mediated by LPS-binding protein and CD14 (11). TLR4 signal transduction occurs through myeloid differentiation factor 88 (MyD88)-dependent and independent pathways. Both MyD88-dependent and independent signaling result in NF-κB activation, while MyD88-dependent signaling also activates MAPKs, including p38, ERK, and JNK (12). Phosphorylation of ERK, p38, and NF-κB leads to activation of transcription factors related to inflammation, such as JNK/Activator protein 1 (AP-1), resulting in production of various pro-inflammatory cytokines and cyclooxygenase-2 (COX-2).
It is well established that COX-2 plays a crucial role in inflammation, proliferation, apoptosis, and tumorigenesis (13). In the lung, up-regulation of COX-2 expression has been implicated in various inflammatory conditions such as ALI, asthma, and bacterial pneumonia (14, 15, 16). LPS challenge has been shown to increase COX-2 expression levels in rat pulmonary tissues (17). In COX-2 deficient mice, an improved survival rate and a significantly lower inflammatory response than in wild-type mice were found after LPS administration. Based on these findings, it has been suggested that COX-2 could be an important target to resolve the inflammation and decrease the mortality rate in sepsis associated with LPS-induced injury (18).
Heparin successfully decreases LPS-induced inflammation by well-understood mechanisms inhibiting neutrophil migration (6, 8, 9). However, the mechanism behind the protective anti-inflammatory effects of heparin on lung epithelium in LPS-induced injury has not been elucidated. Pulmonary epithelial cells are known to express TLR4 and induce both COX-2 expression and subsequent PGE2 production in response to LPS (19, 20, 21). The purpose of this study was to investigate whether heparin’s sulfation level affects LPS-induced COX-2 expression in-vitro, comparing mucoepidermoid and normal human bronchial cells. In the present study, we demonstrate that in the H292 cell line, fully-sulfated heparin both prevented activation of the ERK1/2, p38, and NF-κB p65 signaling pathways and significantly reduced LPS-induced COX-2 gene and protein expression while desulfated heparin not only did not suppress but actually enhanced LPS-induced COX-2 gene and protein expression with time. In the HBE-1 normal bronchial cell, only fully-sulfated heparin’s effects on ERK signaling were of significance and again, desulfated heparin had little effect upon LPS-induced ERK signaling. Neither significantly affected COX-2 protein or COX-2 and CXCL-8 gene expression. These findings suggest a plausible mechanism behind the epithelial response to heparin’s modulation of LPS-induced airway inflammation and support an anti-inflammatory role for fully-sulfated heparin in the lung.
MATERIALS AND METHODS
Cell Culture and treatment
NCI-H292 (CRL-1848™) airway epithelium-like cells, a human mucoepidermoid pulmonary carcinoma cell line, was obtained from ATCC and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 µg/mL streptomycin, 2.5 µg/mL amphotericin, and 50 µg/mL gentamicin. The cells were grown at 37°C with 7.5% CO2 in fully humidified air. H292 cells were cultured on 6-well plates. When cells were 60% confluent, media were removed and then cells were incubated in RPMI 1640 medium containing 2% FBS for 24 hrs. The cells were treated with 10 µg/mL of LPS (from E. coli 0111:B4; Sigma-Aldrich, St Louis, MO) with or without high-molecular-weight (HMW) heparin (sodium salt, from bovine lung [Western blot analysis] or porcine intestine [real-time PCR analysis], 13,500–15,000 MW; Calbiochem, La Jolla, CA) at 50 µg/mL or 500 µg/mL, which was given 10 mins prior to the LPS stimulation. The concentration of LPS used in these experiments (10 µg/mL) has been determined to be the optimal dose for induction of IL-8 (CXCL8) in H292 cells (22). Both LPS and heparins were first dissolved in fresh RPMI containing 2% FBS and added to the cultures to achieve the effective concentrations so that fresh medium made up 10% of the final total volume of culture medium. For controls, the cells were incubated in unchanged medium with an added 10% total volume of fresh RPMI containing 2% FBS for the same time periods.
The HBE-1 normal human bronchial cell line, immortalized with the HPV-18 E6 and E7 genes (23), was cultured in DMEM:Ham’s F-12 containing Clonetics BEGM supplements, cat. no. CC-4175 (insulin, transferrin, hEGF, hydrocortisone, retinoic acid, gentamicin, amphotericin B, triiodothyronine, epinephrine, and bovine pituitary extract) (Lonza, Walkersville, MD) and propagated to near-confluence on 12-well plates. An LPS concentration of 1 µg/mL was used for HBE-1 cells. LPS and heparins were dissolved in fresh DMEM:F12 and quiescent cells were treated as for H292 cells. Extended quiescence (16 to 24 hours) in DMEM:F12 without BEGM supplements was found to cause cell stress and detachment; therefore, a 6-hour quiescence period was used for HBE-1 signaling experiments. For treatment times longer than 30 minutes, HBE-1 cells were returned to complete medium containing LPS and heparins to avoid cell detachment.
The optimal time point for visualizing LPS effects on multiple signaling pathways was previously determined to be 30 mins after treatment; therefore, this time point was selected for harvesting cells in RIPA (Pierce Biotechnology, Rockford, IL) containing phosphatase inhibitors (PhosStop, Roche, Indianapolis, IN) for signaling analysis. Cells were harvested in RLT Plus (Qiagen, Valencia, CA) for total RNA isolation at 6, 12, and 24 hrs after treatment to evaluate gene expression levels or lysed in RIPA at 12, 24, and 48 hrs to evaluate protein expression levels.
Effects of the Sulfation Level of Heparin
To determine the effect of the sulfation level of heparin, cells were similarly pre-treated with 500 µg/mL HMW heparin, either fully sulfated or desulfated, and cultured for the same time periods as detailed above without further treatment or stimulated with 10 µg/mL (H292) or 1µg/mL (HBE-1) of LPS. Desulfated heparin was obtained by dissolving the pyridinium salt of HMW heparin (from bovine lung) in dimethyl sulfoxide (DMSO) with 10% dH2O and incubating the mixture at 80°C for 5 hours, followed by pH adjustment to 9.14 with 0.1 M NaOH, extensive dialysis against water, and lyophilization, resulting in 85% desulfation, as previously described (24, 25).
Western Blot Analysis
Cells were washed with phosphate buffered saline (PBS) and lysed on ice in RIPA buffer (Pierce Biotechnology). Cell lysates were sonicated and equal amounts of protein from each sample were subjected to electrophoresis on 4–12% Bis-Tris NuPAGE gels in MOPS running buffer (Invitrogen, Grand Island, NY), followed by transfer to nitrocellulose membranes. The membranes were blocked with 5% non-fat dry milk in TBST (20 mM Tris· HCl [pH 7.6], 150 mM NaCl, and 0.1% Tween-20) for 1 hour at room temperature and incubated overnight with primary antibodies in TBST/5% BSA at 4°C. Primary antibodies used for this study include those against the phosphorylated and total forms of p38, ERK1/2, and NF-κB p65 and against COX-2 (all from Cell Signaling Technology, Danvers, MA), and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). After washing with TBST, the membranes were incubated with secondary antibodies coupled to horseradish peroxidase (Cell Signaling). The signals were detected by chemiluminescence using SuperSignal West Pico (Pierce Biotechnology). The signals were analyzed using densitometry software (SigmaScan; Systat Software, Inc., San Jose, CA) and the values were calculated and expressed as mean ± SD of the ratios of COX-2 to GAPDH and of bands of phosphorylated proteins to those of their total forms.
RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated using the RNeasy Plus Mini kit (Qiagen) with QiaShredder homogenization, following manufacturer’s instructions. cDNA synthesis was performed from 2 µg RNA using a cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR (qRT-PCR) was performed for GAPDH, COX-2, and CXCL8 (IL-8) using TaqMan assays (Applied Biosystems) in a Bio-Rad MyiQ iCycler (Bio-Rad, Hercules, CA). Relative gene expression was analyzed using the 2T−ΔΔc method. Due to the discontinuation and unavailability of HMW heparin extracted from bovine lung, HMW heparin prepared from porcine intestinal mucosa (Calbiochem) was used in the cell treatment for qRT-PCR analysis. Both preparations of HMW heparin were effective in suppressing the gene expression of COX-2 in H292 cells at both 50 and 500 µg/mL concentrations.
Statistical Analysis
All experiments were repeated at least three times. The differences in the degrees of phosphorylation and expression of COX-2 and CXCL8 between experimental groups were analyzed by unpaired, two-tailed t-test. Comparisons and probabilities were determined using computerized statistical software (StatCrunch, Integrated Analytics LLC, Columbia, SC or the Microsoft Excel Analysis ToolPak Add-in software suite) with significance at P ≤ 0.05.
RESULTS
Effects of HMW Heparin on LPS-treated H292
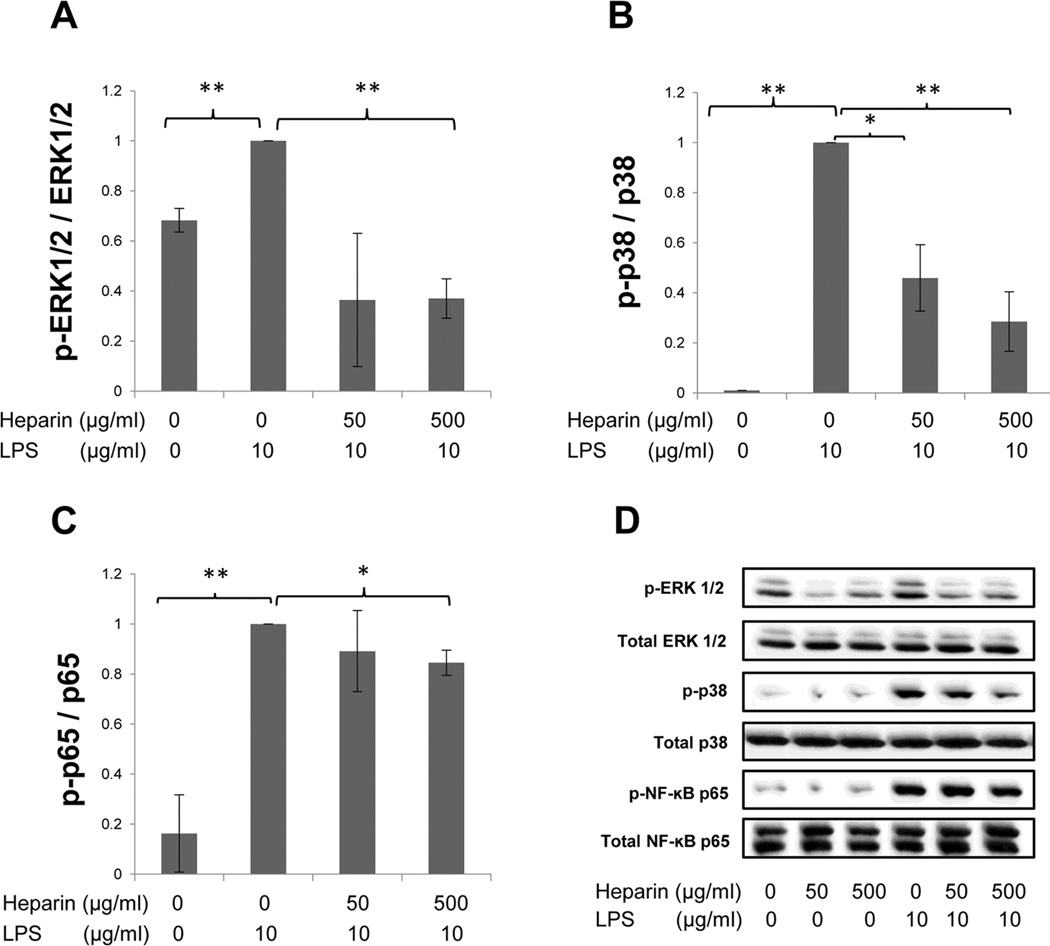
Signaling pathways, such as ERK1/2, p38, and NF-κB p65, have been reported to induce COX-2 in several cell types (26, 27). To investigate the effects of heparin on these signaling pathways in LPS-treated H292 cells, cells were treated with either 50 µg/mL or 500 µg/mL of HMW heparin, alone or in the presence of 10 µg/mL of LPS. Cells were harvested at 30 min after treatment to determine the phosphorylation levels of ERK1/2, p38, and NF-κB p65. As shown in Fig. 1, LPS treatment significantly increased phosphorylation of ERK1/2, p38, and NF-κB p65 compared with untreated samples. HMW heparin at 500 µg/mL significantly inhibited LPS-induced elevation of the phosphorylation levels of these signaling pathways (ERK1/2: P=0.0003; p38: P=0.0038; NF-κB p65: P=0.0361) (Fig. 1A–C). HMW heparin at 50 µg/mL significantly suppressed LPS-induced phosphorylation of p38 only (P=0.015) (Fig. 1B). Both 50 µg/mL and 500 µg/mL of HMW heparin significantly decreased phosphorylation of ERK1/2 compared with untreated samples (Fig. 1A). Fig 1D shows a representative signaling blot for each pathway examined.
Fig. 1.
The effect of HMW heparin on phosphorylation of ERK1/2 (A), p38 (B), and NF-κB p65 (C) in LPS-treated H292 cells. Levels of phosphorylation were determined 30 mins after treatment. HMW heparin at 500 µg/mL significantly prevented LPS-induced phosphorylation of these signaling pathways. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group. A representative Western blot for each signaling pathway is shown in (D).
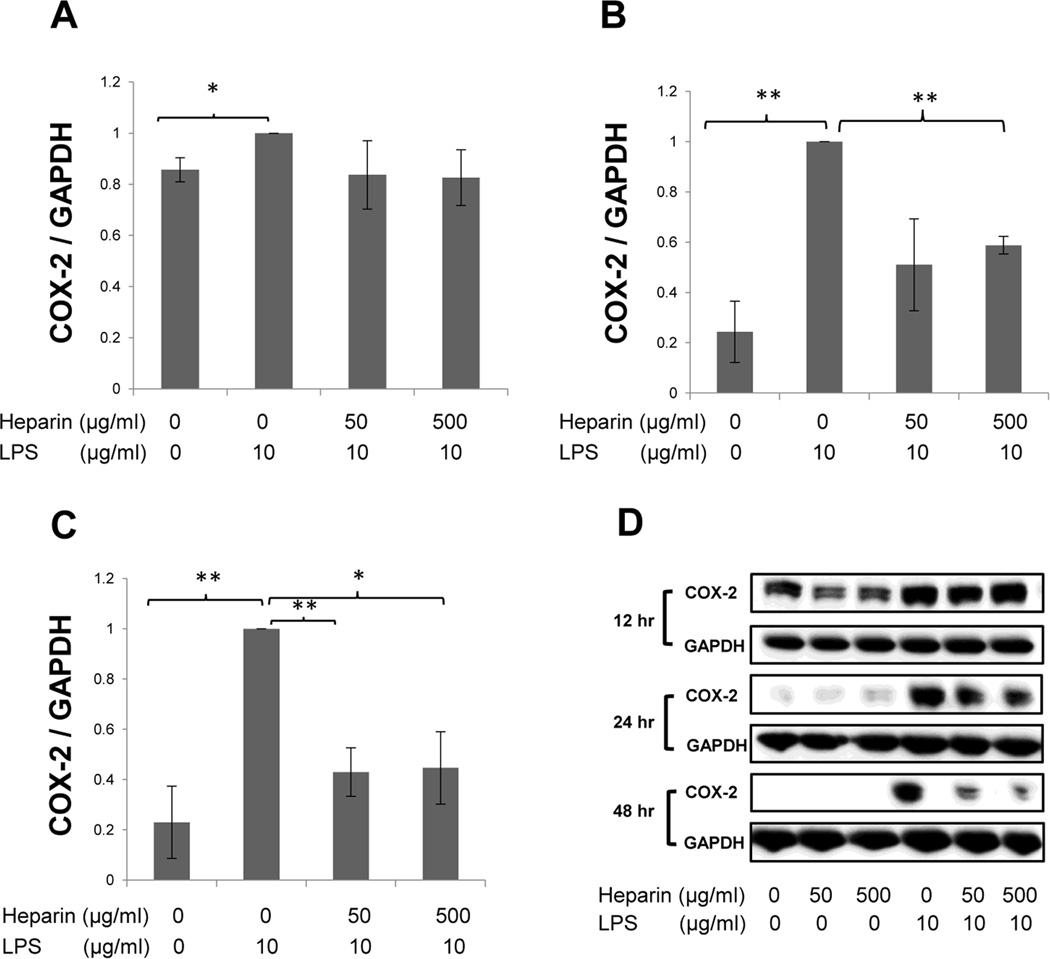
Next, the effect of heparin on COX-2 expression in LPS-treated H292 cells was determined. The protein and gene expression levels of COX-2 were evaluated by Western blotting and qRT-PCR, respectively. Cells were treated in the same fashion as described above and harvested at 12, 24, and 48 hrs after treatment to evaluate protein expression by Western blotting (Fig. 2) and at 6, 12, and 24 hrs for qRT- PCR (Fig. 3A).
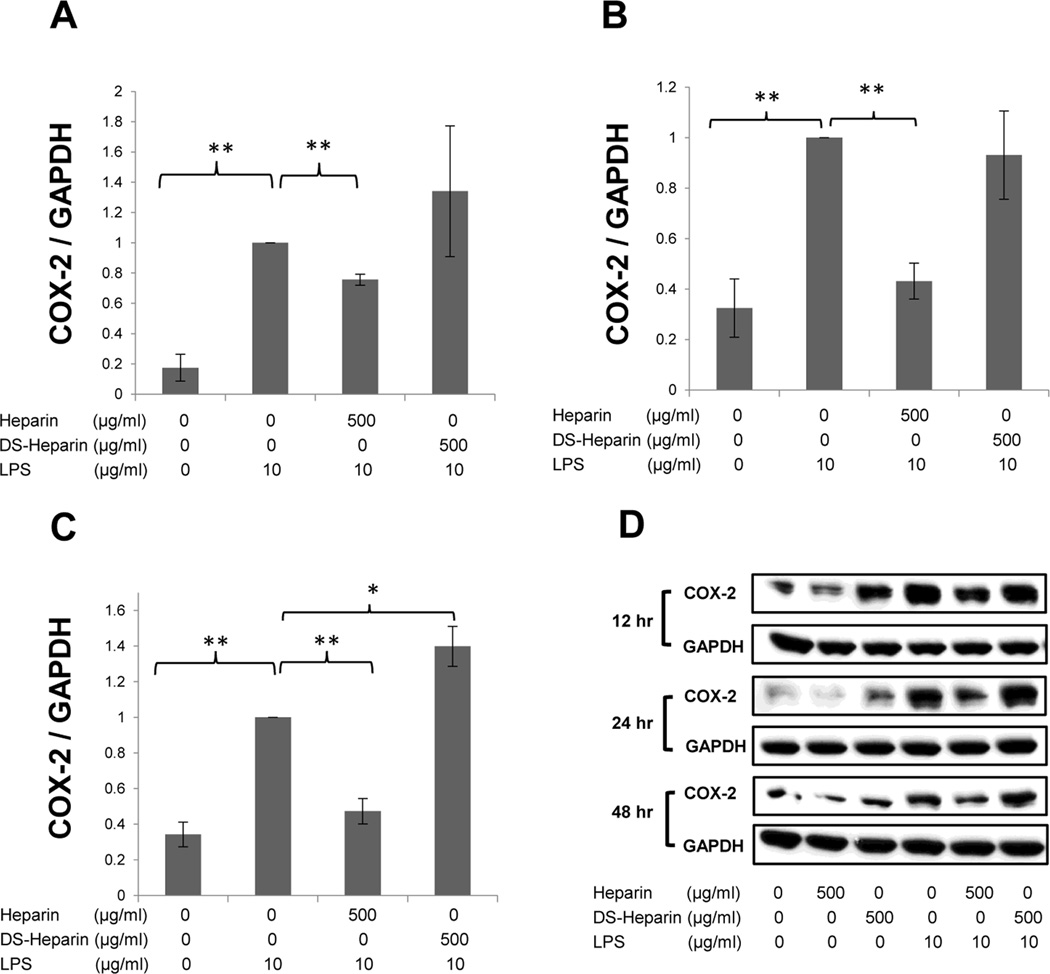
Fig. 2.
The effect of HMW heparin on COX-2 protein expression in LPS-treated H292 cells at 12 hrs (A), 24 hrs (B), and 48 hrs (C) after treatment. 500 µg/mL of HMW heparin prevented LPS-induced COX-2 expression at 24 and 48 hrs after treatment. (D) Representative Western blots for each time point are shown. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group.
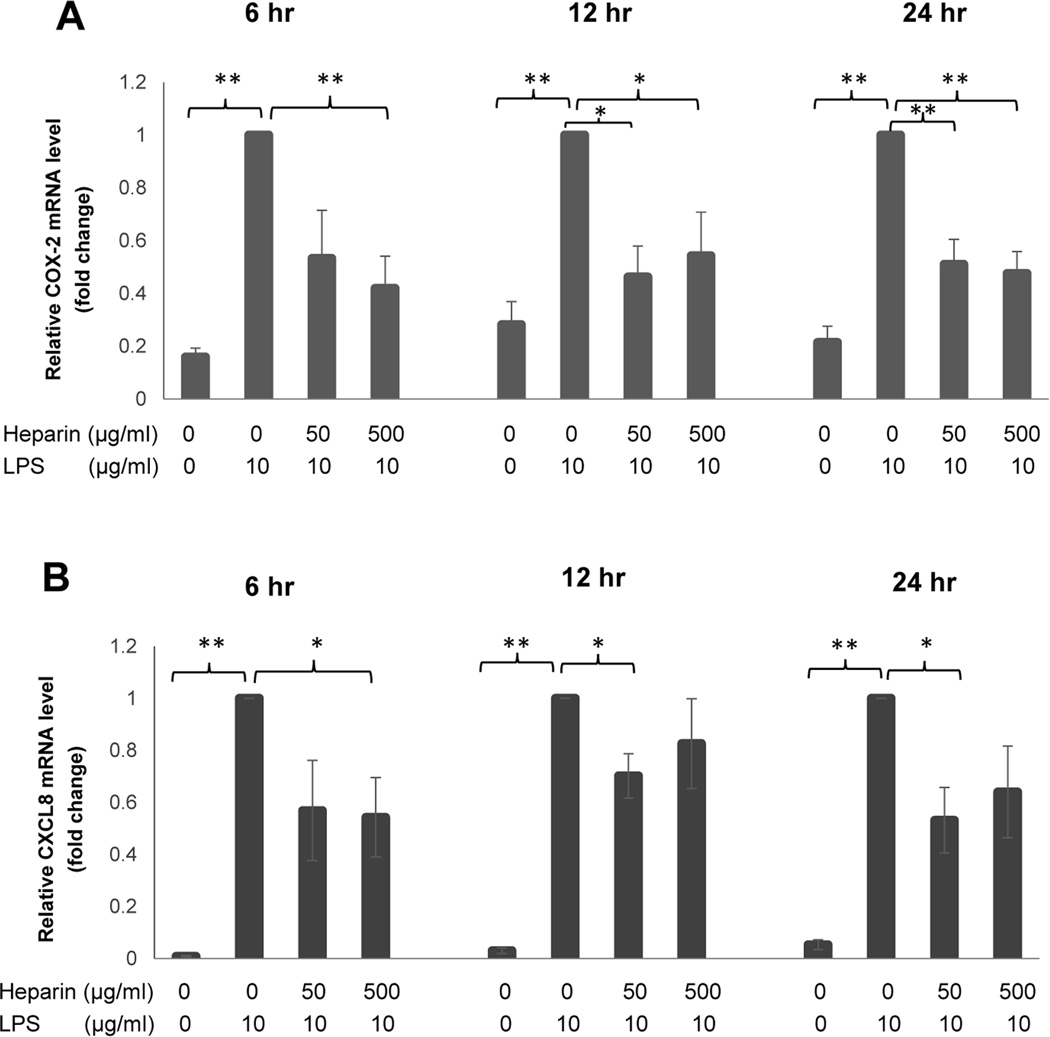
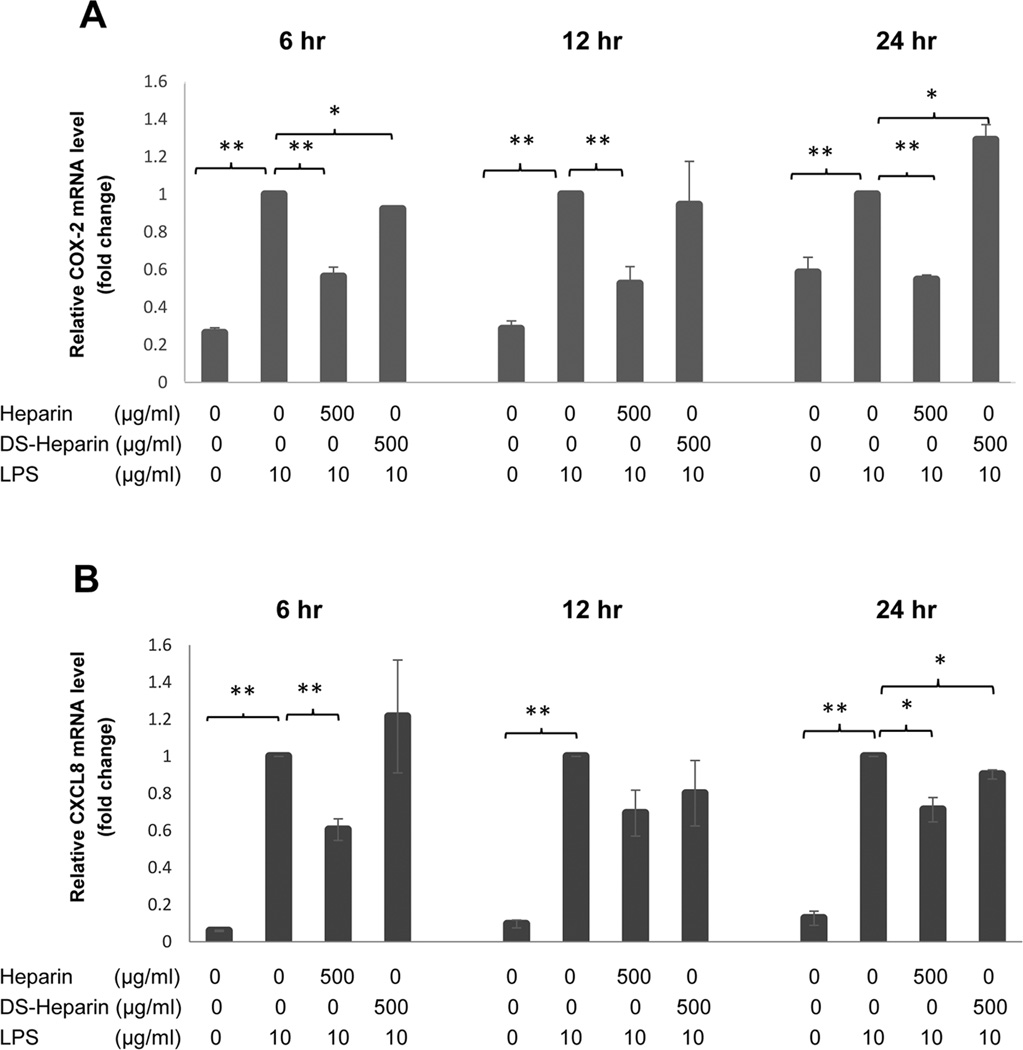
Fig. 3.
Fold change in gene expression in H292 cells at 6 hrs, 12 hrs, and 24 hrs following heparin treatment. Quantitative real-time PCR (TaqMan assay) was performed and GAPDH was used as the housekeeping gene. (A) 500 µg/mL of HMW heparin significantly inhibited mRNA expression of COX-2 in H292 stimulated with LPS at all time points. (B) HMW heparin at 50 µg/mL significantly prevented LPS-induced CXCL8 mRNA expression at 12 and 24 hrs, and 500 µg/mL of HMW heparin significantly prevented LPS-induced CXCL8 mRNA expression at 6 hrs after treatment. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group of that time point.
HMW heparin significantly inhibited the LPS-induced increase in COX-2 protein expression. LPS treatment significantly up-regulated COX-2 protein expression at 12 hrs (Fig. 3A), 24 hrs (Fig. 3B), and 48 hrs (Fig. 3C) after treatment. HMW heparin at 500 µg/mL significantly suppressed the elevation of COX-2 expression in LPS-treated cells at 24 hrs (P=0.0003) and 48 hrs (P=0.0183) after treatment (Fig. 2B–C), whereas 50 µg/mL of HMW heparin had a significant effect only at 48 hrs (P=0.0041) (Fig. 2C). Heparin treatment alone, at both 50 µg/mL and 500 µg/mL, resulted in a significant decrease in COX-2 expression only at 12 hrs after treatment, compared with untreated samples (Fig. 2D).
Similarly, mRNA expression of COX-2 was significantly elevated in LPS-treated cells compared with untreated samples, and 500 µg/mL of HMW heparin significantly prevented LPS-induced elevation of COX-2 mRNA expression at all time points (Fig. 3A). HMW heparin at 50 µg/mL had a significant inhibitory effect at 12 and 24 hrs (Fig. 3A). Collectively, these data demonstrate that heparin treatment significantly prevents LPS-induced up-regulation of COX-2 mRNA and protein expression in H292 cells.
CXCL8 is one of the important pro-inflammatory chemokines secreted by various types of cells during inflammation (28). When H292 cells were stimulated with LPS, there was a significant increase in mRNA expression of CXCL8 at 6, 12, and 24 hrs (P < 0.001) (Fig. 3B). HMW heparin at 50 µg/mL significantly suppressed LPS-induced CXCL8 mRNA expression at 12 hrs (P=0.0257) and 24 hrs (P=0.0194), and 500 µg/mL of HMW heparin significantly prevented LPS-induced CXCL8 mRNA expression at 6 hrs after treatment (P=0.0414) (Fig. 3B).
Effects of the Sulfation Level of Heparin in H292 cells
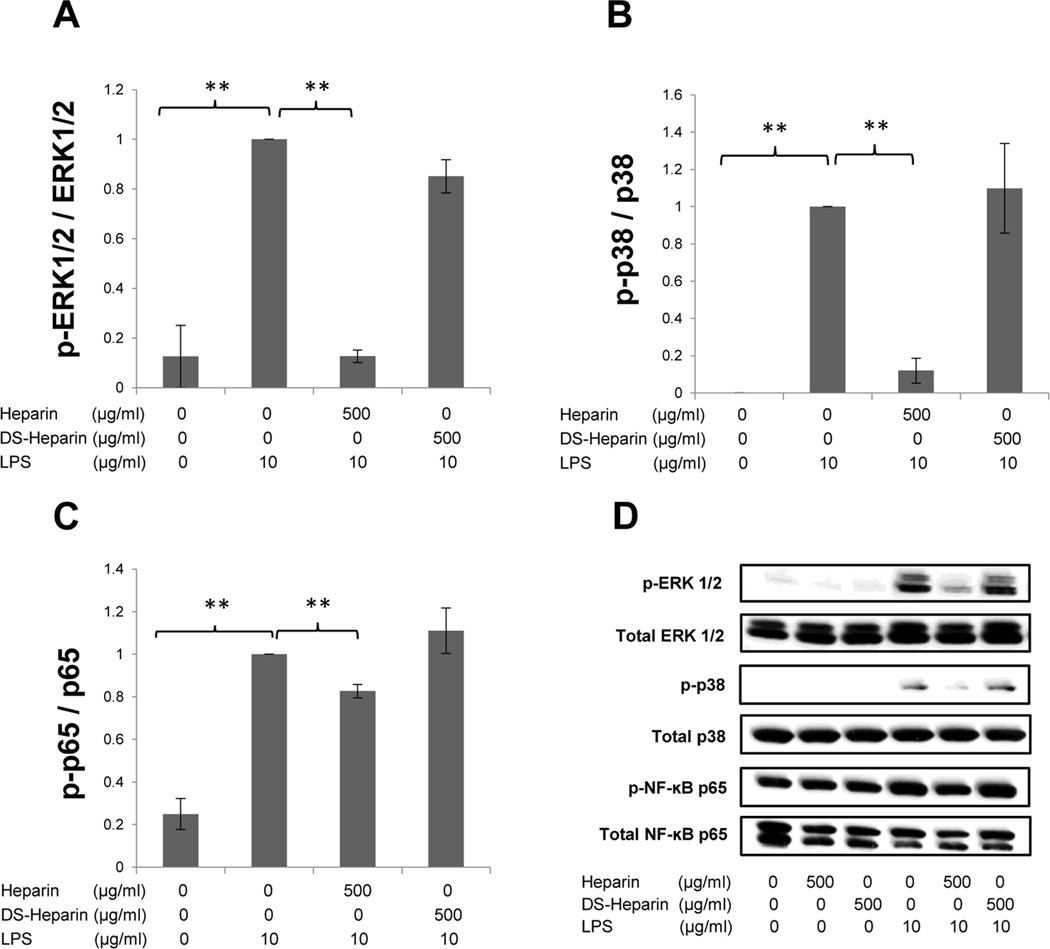
To investigate the role of sulfation in heparin’s effects on the signaling pathways and COX-2 expression levels, this study was repeated in H292 cells treated with either 500 µg/mL of HMW heparin or 500 µg/mL of desulfated HMW heparin, alone or in the presence of 10 µg/mL of LPS. The concentration of 500 µg/mL for desulfated heparin was chosen because 500 µg/mL of HMW heparin was effective in the suppression of LPS-induced phosphorylation in all signaling pathways tested. As before, there were significant LPS-induced increases in the phosphorylation of ERK1/2, p38, and NF-κB p65, and they were significantly prevented by 500 µg/mL of HMW heparin (ERK1/2: P < 0.0001; p38: P=0.0002; NF-κB p65: P=0.0052) (Fig. 4). However, there were no significant differences in any of the phosphorylation levels in the “LPS plus desulfated heparin” treatment group compared with LPS treatment alone.
Fig. 4.
The effect of sulfation levels of heparin on phosphorylation of ERK1/2 (A), p38 (B), and NF-κB p65 (C). H292 cells were treated with either HMW heparin (500 µg/mL) or desulfated HMW heparin (500 µg/mL) in the presence of 10 µg/mL of LPS for 30 mins. HMW heparin significantly prevented LPS-induced phosphorylation of these signaling pathways. However, desulfated HMW heparin (DS-Heparin) had no significant effect on phosphorylation levels increased by LPS. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group. (D) Representative Western blots for each signaling pathway are shown.
In further analysis of these experiments, H292 cells were harvested at 12, 24, and 48 hrs and evaluated for protein expression of COX-2 and harvested at 6, 12, and 24 hrs for gene expression analysis of both COX-2 and CXCL8 LPS treatment significantly increased protein expression of COX-2 and 500 µg/mL of HMW heparin significantly prevented LPS-induced COX-2 protein expression at all time points (Fig. 5A–C). Desulfated HMW heparin significantly intensified LPS-induced up-regulation of COX-2 protein at 48 hrs after treatment (P=0.0242) (Fig. 5C). Representative Western blots for each time point are shown in Fig. 5D.
Fig. 5.
The effect of the sulfation levels of heparin on COX-2 protein expression in LPS-treated H292 cells at 12hrs (A), 24 hrs (B), and 48 hrs (C) after treatment. HMW heparin significantly inhibited protein expression of COX-2 in H292 cells stimulated with LPS at all time points. Desulfated HMW heparin (DS-Heparin) significantly enhanced up-regulation of COX-2 protein at 48 hrs following treatment with LPS. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group. (D) Representative Western blots for each time point are shown.
Statistical analysis of all gene expression repeats combined revealed that LPS treatment significantly increased mRNA expression of COX-2 and 500 µg/mL of HMW heparin significantly prevented LPS-induced COX-2 mRNA elevation at all time points (Fig. 6A). Desulfated heparin significantly reduced LPS-induced COX-2 mRNA elevation at 6 hrs (P=0.0064) but significantly increased it at 24 hrs (P=0.023) after treatment (Fig. 6A).
Fig. 6.
Fold change in gene expression in H292 cells at 6 hrs, 12 hrs, and 24 hrs following treatment with either HMW heparin (500 µg/mL) or desulfated HMW heparin (DS-Heparin at 500 µg/mL) in the presence of LPS. (A) HMW heparin significantly inhibited mRNA expression of COX-2 in H292 cells stimulated with 10 µg/mL LPS at all time points. However, DS-Heparin significantly enhanced up-regulation of COX-2 mRNA expression by LPS at 24 hrs after treatment. (B) Both HMW heparin and DS-Heparin, at the same concentration of 500 µg/mL, significantly prevented LPS-induced CXCL8 mRNA expression at 24 hrs after treatment. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group of that time point.
Similarly, when H292 cells were stimulated with LPS there was a significant increase in mRNA expression of CXCL8 at 6, 12, and 24 hrs (P < 0.0001) (Fig. 6B). HMW heparin at 500 µg/mL significantly prevented LPS-induced CXCL8 mRNA expression at 6 hrs (P=0.0024) and 24 hrs (P=0.0118), and 500 µg/mL of desulfated HMW heparin significantly suppressed LPS-induced CXCL8 mRNA expression at 24 hrs after treatment (P=0.0184) (Fig 6B).
Effects of HMW Heparin on LPS-treated HBE-1 cells
Experiments in HBE-1 cells were conducted as were those in H292 cells. However, HBE-1 cells were found to have high basal ERK1/2 levels which obscured any additional signal from LPS stimulation. Nevertheless, the effects of fully-sulfated heparin on LPS-stimulated ERK1/2 signaling were marked and significant (P<0.001, n=7) while desulfated heparin had no significant effects (Fig. 7A). Neither p38 (Fig. 7B) nor NF-κB p65 (Fig. 7C) showed any significant effects from LPS stimulation nor any significant change in phosphorylation due to heparin addition of either sulfation type or concentration. Signaling experiments provided no significant differences, except for a consistent significant suppression of ERK1/2 signaling by fully-sulfated heparin.
Fig. 7.
The effect of HMW heparin on phosphorylation of ERK1/2 (A), p38 (B), and NF-κB p65 (C) in LPS-treated HBE-1 normal human bronchial epithelial cells. Levels of phosphorylation were determined 30 mins after treatment. HMW heparin at 50 and 500 µg/mL significantly suppressed phosphorylation of the ERK signaling pathway. ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group. (D) A representative Western blot for each signaling pathway is shown.
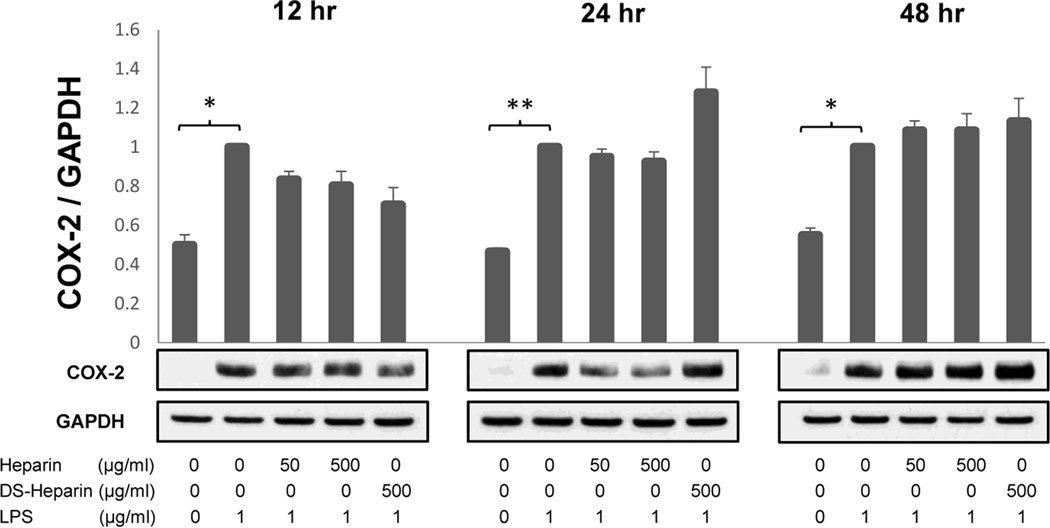
Despite the lack of a clear LPS effect on signaling via these three pathways, COX-2 protein was significantly upregulated by LPS (1 µg/mL) at all time points: 12 hr (P=0.0173), 24 hrs (P=0.0022), and 48 hrs (P=0.0105), n=3. However, again there was little suppressive effect of heparin on this upregulation (Fig. 8). A small increase in COX-2 in the “LPS + Desulfated Heparin” sample at 24 hours was not significant by the unpaired, two-tailed t-test (P=0.2276) (Fig. 8).
Fig. 8.
The effect of the sulfation levels of heparin on COX-2 protein expression in LPS-treated HBE-1 human bronchial epithelial cells at 12hrs (A), 24 hrs (B), and 48 hrs (C) after treatment. HMW heparin did not significantly inhibit protein expression of COX-2 in HBE-1 cells stimulated with LPS. Desulfated HMW heparin (DS-Heparin) apparent up-regulation of COX-2 protein at 24 hrs was not significant. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to the “LPS only” group. (D) Representative Western blots for each time point from the same experiment are shown.
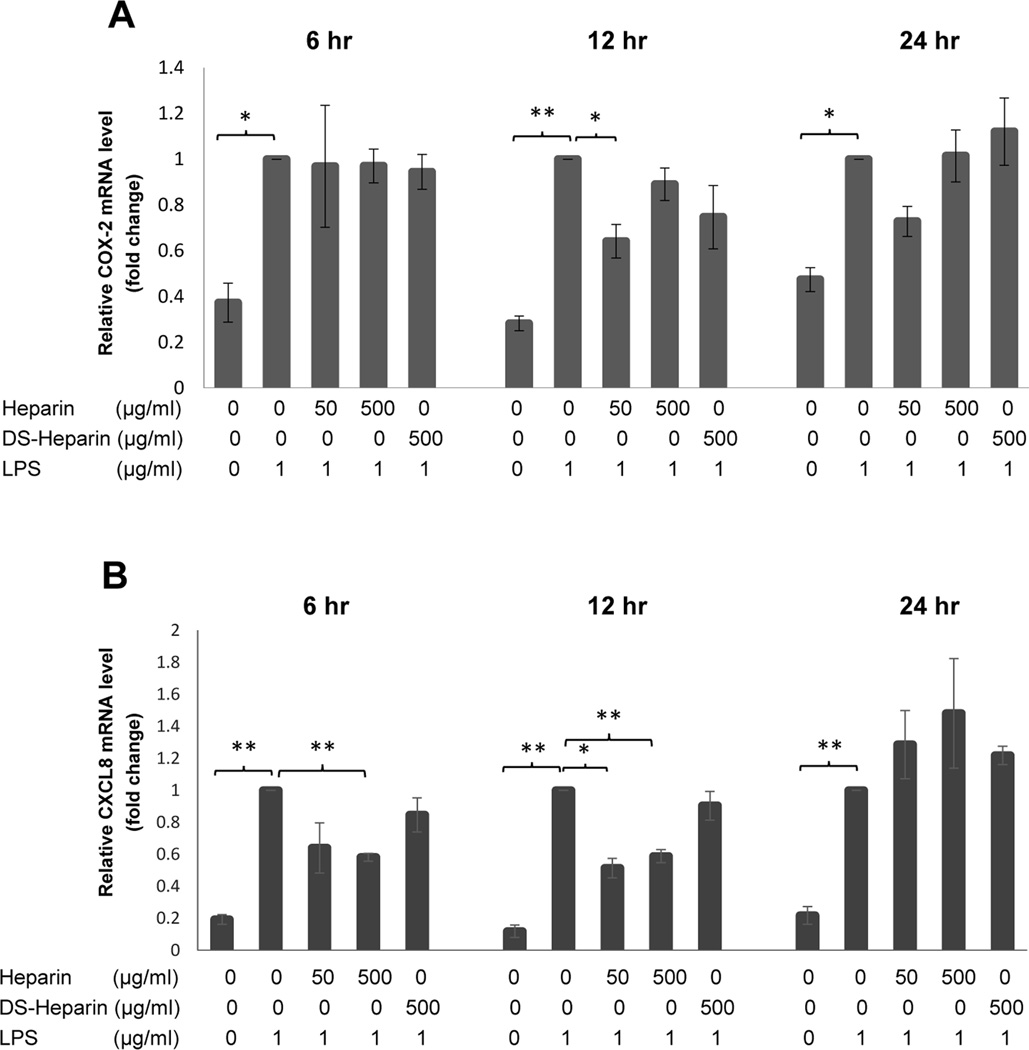
Similarly, gene expression analysis of COX-2 regulation by LPS (1 µg/mL) in these HBE-1 cells again showed a significant increase due to LPS at 6 hrs (P=0.0177), 12 hrs (P=0.0020), and 24 hrs (P=0.0098) (n=3), with non-significant heparin effects except for a decrease in the 12-hour “LPS + 50 µg/mL Heparin” sample (P=0.0394) (Fig. 9A). CXCL8 gene expression, however, showed strong and significant upregulation by LPS at 6 hrs (P=0.0032), 12 hrs (P=0.0020), and 24 hrs (P=0.0049) (n=3) and was also significantly downregulated by fully-sulfated 500 µg/mL heparin at both 6 hours (P=0.00325) and 12 hours (P=0.0033) and by 50 µg/mL heparin at 12 hours (P=0.0151) (Fig. 9B). By 24 hours, the suppressive effects diminished, possibly reflecting the depletion of heparin from the medium over time along with multiple rounds of signaling events.
Fig. 9.
Fold change in gene expression in HBE-1 cells at 6 hrs, 12 hrs, and 24 hrs following treatment with either HMW heparin (500 µg/mL) or desulfated HMW heparin (500 µg/mL) in the presence of LPS at 1 µg/mL. (A) HMW heparin significantly inhibited mRNA expression of COX-2 in HBE-1 cells stimulated with LPS only at 12 hours after treatment. DS-Heparin did not significantly affect regulation of COX-2 mRNA by LPS at any time point. (B) HMW heparin at 500 µg/mL significantly prevented LPS-induced CXCL8 mRNA expression at 12 and 24 hrs after treatment; 50 µg/mL HMW Heparin was effective only at 12 hrs. Desulfated HMW Heparin (DS-Heparin) had no significant effects on CXCL8 gene expression. * P < 0.05 and ** P < 0.01. Values represent mean ± SE for triplicate analyses, normalized to “LPS only” group of that time point.
DISCUSSION
When tissue injuries occur in the lung, the clearance of degraded ECM components and restoration of ECM is a critical step of tissue repair (5, 29). Degraded or selectively shed GAGs have been shown to influence re-epithelialization by interacting with growth factors. Therefore, various GAGs have been investigated to elucidate their effects on healing mechanisms following lung injury (29, 30, 31). Heparin is a highly sulfated GAG that has been used as a model of soluble sulfated GAGs which are shed after lung injury (31). In whole lung, the interplay of complex factors and responses of various cell types to the injuring agent affect the outcome of the injury. Isolated normal cells and cell lines treated in vitro may exhibit some of the same responses, but inherently lack the complexity of the whole lung. For example, important differences between lung cell lines and normal lung cells in their expression and sulfation of GAGs and their subsequent responses to challenges were observed when apoptosis was triggered in H292 cells by the forced over-expression of HSulf1, an endosulfatase that removes 6-O sulfate groups from the side chains of heparan sulfate. In conrast human alveolar Type II (hATII) cells were completely unaffected by HSulf1 over-expression (32). Cadmium, while toxic to hATII cells at all concentrations with or without HSulf1 over-expression, became toxic to H292 cells at low concentrations only when the sulfate groups necessary for ERK signaling were enzymatically-removed by HSulf1 over-expression (33). A survey of HSulf1 gene expression in various lung cells and cell lines revealed that five different normal lung cell types expressed HSulf1 at a significantly higher level than did five lung cell lines, including H292s (32). This difference may be crucial in the normal lung environment, where injury is prevented from becoming an overwhelming inflammatory response and where homeostasis must be regained after challenge. It is likely that the sulfation of GAGs plays a major role in this important function.
As in the previous study comparing normal and cancer lung cells, the differences in how H292 and HBE-1 cells in this study use GAG sulfation to respond to LPS challenge may give insight into how the normal human lung, as an integrated whole organ, recovers from inflammatory injury. Results of this study demonstrate that, in H292 epithelial cells, HMW heparin inhibits the ERK1/2, p38, and NF-κB p65 signaling pathways and LPS-induced COX-2 expression, while desulfated HMW heparin fails to suppress to the same extent as fully-sulfated heparin or even enhances LPS-induced COX-2 expression. These findings suggest that the amount of sulfation in heparin is the key factor in controlling the response to LPS-induced inflammation in this lung epithelial cell line. In contrast, normal HBE-1 cells’ response to LPS showed little effect of any heparin on COX2 expression, and an early suppression of CXCL8 gene expression by fully-sulfated heparin was lost within 24 hours. It is possible that normal cells depend more upon a whole organ response to challenge while cancer cell lines do not.
In H292 cells, culture medium containing 10% serum alone (without LPS) strongly up-regulates COX-2 expression (34). Here 2% FBS was used to reduce, but not eliminate, the background signaling for which serum components are co-factors and to demonstrate the suppression of LPS-stimulated signaling by heparin. Heparin treatment immediately suppressed LPS-induced signaling through the ERK, p-38, and NF-κB p65 pathways in this cell line. Heparin also reduced serum-induced background signaling in those cells not given LPS (Fig. 1D). These effects were sustained over 24 hours for gene expression and over 48 hours for protein expression, while both background and LPS-stimulated levels of expression decreased as serum factors became exhausted in the absence of medium changes. In contrast, desulfated heparin had little suppressive effects on either background or LPS-induced signaling or on COX-2 gene or protein expression.
In normal bronchial cells, LPS did not induce a significant response, which may be the result of their treatment as isolated cells rather than as part of the intact airway and the absence of macrophages, which may be necessary for LPS’ full inflammatory effect (35, 36, 37). In addition to the multiple differences in a cancer cell that sustain its fast growth and override checks on its continuous cell cycle, part of this difference could be due to the sensitivity of HBE-1 cells to medium lacking supplementation which includes insulin, retinoic acid, hydrocortisone, hEGF, and other growth stimulants. Basal levels of signaling may not be suppressed enough after 6 hours of quiescence to show a clear response to LPS in these cells, as ERK signaling could be sustained by growth factors adsorbed and sequestered in the extracellular matrix of these cells. Heparin’s immediate effects on signaling were limited to the ERK pathway. As a co-factor of FGF receptors, heparin acts to stimulate ERK signaling over a limited concentration range. We chose to use a high concentration of fully-sulfated heparin here to inhibit ERK signaling, as the same concentration of desulfated heparin was ineffective. The effects of heparin on LPS-stimulated COX-2 protein expression were not significant, which suggests that heparin’s observed anti-inflammatory effects on inflammation in vivo do not act primarily via ERK signaling in lung epithelium.
Fragments of hyaluronan, a non-sulfated GAG, were previously found to be responsible for eliciting inflammatory gene expression, such as CXCL2 and macrophage inflammatory protein (MIP)-1β, through TLR2 and TLR4 signaling pathways (5). Hyaluronan fragments also induced IL-8 (CXCL8) and IFN-γ-inducible protein 10 (IP-10) in airway epithelial cells, whereas heparin did not induce TLR4 dependent TNF-α secretion in murine dendritic cells (38, 39, 40). In contrast, HMW heparin, a highly sulfated GAG, has here driven an anti-inflammatory response in vitro. In this study, heparin suppressed LPS-induced COX-2 expression in the H292 mucoepidermoid cell line, and the removal of sulfate groups from heparin greatly reduced this effect. Although these results in this cell line may not reflect the in vivo normal lung, the outcomes of this study reinforce the premise that, after injuries to the lung, the sulfation level in shed GAGs may affect the extent and resolution of COX-2-induced inflammation.
While the non-sulfated GAG hyaluronan has been shown to induce CXCL8, soluble heparin has been reported to suppress the biological effect of CXCL8 by blocking its binding to the CXCR1 and CXCR2 receptors in endothelial cells (41). In this study, heparin decreased LPS-induced CXCL8 mRNA expression in H292 cells, implying that the blockage by heparin of CXCL8’s interactions with its receptors blocked the AP-1 signaling (28, 42) that initiates a positive feedback loop (43) and thereby decreased the gene transcription of CXCL8. In this way, sulfated heparin can serve as a moderating force on CXCL8 production as well as to directly block its biological effects. Desulfated heparin, in contrast, had no such suppressive effects on CXCL8 gene regulation at 6 hours, but at 12 and 24 hours some suppression was seen. As the desulfated heparin used here is typically depleted of only ~85% of its sulfates (24, 25), we propose that the delayed effect we observed is due to its internalization, with the intracellular concentration of sulfation increasing over time.
LPS has been shown to induce COX-2 expression through multiple pathways, including p38, ERK1/2, JNK, and NF-κB, in various epithelial cells and immune cells (29). COX-2 has been described to modulate cell proliferation, inflammation, and apoptosis, and up-regulation of COX-2 is associated with various diseases in the lung, such as ALI, asthma, and bacterial pneumonia (14, 15, 16). In this study, heparin inhibited the phosphorylation of p38, ERK, and NF-κB in H292 cells when stimulated by LPS. Inhibition of these signaling pathways by heparin is supported by previous results. High concentrations of heparin were found to inhibit multiple signaling pathway intermediates in rat alveolar Type II cells stimulated by FGFs, decreasing phosphorylation levels of Akt, ERK1/2, and p38 (31). Translocation of NF-κB into the nucleus, confirming TLR4 activation and signaling in LPS-stimulated human monocytes and endothelial cells, was inhibited by heparin treatment, which attenuated myocardial reperfusion injury (44, 45). Campo, et al., provided evidence that heparin sulfate from bovine kidney physically interacts with TLR4 in LPS-stimulated chondrocytes to prevent NF-κB subunits’ nuclear localization (46). A physical interaction between heparin and TLR4 has not previously been established in H292 cells.
Mucus hypersecretion is an important pathophysiologic feature of chronic inflammatory airway diseases, such as cystic fibrosis, chronic obstructive pulmonary disease, and asthma (47, 48). Excessive mucus production has been associated with up-regulation of COX-2 expression which correlated with up-regulation of MUC5AC expression, and the inhibition of the signaling pathways upstream of COX-2 (ERK1/2 and p38) blocked MUC2 and MUC5AC production in H292 cells (49). A recent study has shown that heparin suppresses LPS-induced mucus secretion in vivo. Intranasal instillation of either unfractionated or low molecular weight heparin significantly prevented LPS-induced mucus production in rats (50). Our study provides plausible dual mechanisms (receptor-mediated as well as sustained intracellular) for this heparin-driven decrease in LPS-induced in-vivo mucus production.
Our studies of normal bronchial cells give a more relevant picture of sulfated heparin’s potential uses in the injured human lung than do the H292 studies. During the course of our investigation, several different lung cell types were examined in vitro for the effects of LPS (including the bronchial cells used here as well as freshly-isolated normal hATII cells, normal lung fibroblasts, normal rat lung ATII cells, and the RLE-6TN rat epithelial cell line). ERK signaling was consistently difficult to quiet well enough to see clean effects of LPS treatment on signaling. This technical issue with HBE-1 cells may be one reason for the high basal level of ERK1/2 signaling seen in our untreated cells (Fig. 1D) and by others as well (36). Nevertheless, high concentrations of fully-sulfated heparin were consistently effective in reducing ERK signaling and suppressing proliferation in isolated normal lung cells while desulfated heparin was not (data not shown). These observations suggest that ERK signaling is resistant to LPS but sensitive to fully-sulfated heparin in normal lung cells. Heparin’s moderating effects on multiple signaling pathways and on gene and protein expression when internalized, while enhancing or inhibiting ERK signaling in a concentration dependent fashion, could allow for a balanced response in whole lung to inflammatory triggers. Such cross-talk and balance was hypothesized for ATI cells in cell-cell contact with macrophages (enhancing the production of cytokines) and ATII cells (producers of response-dampening pulmonary surfactant) in the LPS-challenged rat lung (37). Notably, analysis of pulmonary surfactant has shown it to be well-sulfated (51). The dampening effects on signaling and downstream events of sulfated heparin as a model for sulfated HSPGs and GAGs in the lung reflects the many ways GAG sulfation maintains homeostasis via multiple signaling pathways and cross-talk among other cell types in the complex in vivo environment.
Extensive research has emphasized the role of COX-2 in the pathogenesis of inflammatory lung diseases, such as acute lung injury, idiopathic pulmonary fibrosis (IPF), and asthma (52). Decreased production of PGE2 was found in the lungs of patients with IPF (53). Administration of PGE2 decreased apoptosis of isolated fibrotic lung epithelial cells and increased apoptosis of fibrotic lung fibroblasts, suggesting that COX-2 has a protective role in IPF (53, 54). However, in acute lung injury, PGE2 is considered an important mediator of pulmonary edema, and studies using animal models have shown that COX-2 is associated with development and progression of acute lung injury (14, 52, 55). The overall role of COX-2 in inflammatory lung disease remains unclear due to the complexity of the disease development (52). Further studies are needed to determine the effect(s) of heparin on the pathologic processes of these inflammatory lung diseases.
In conclusion, we have established that heparin suppresses the LPS-induced expression of the inflammatory intermediate COX-2 in human H292 pulmonary mucoepidermoid carcinoma cells via mechanisms related to charge density of sulfate groups. Removal of sulfate groups from heparin diminishes its suppressive effects on both LPS-induced signaling and LPS-induced COX-2 gene and protein expression, significantly increasing COX-2 over time. LPS-induced COX-2 expression has been implicated in the pathogenesis of various inflammatory respiratory diseases. Our data suggest that fully-sulfated heparin has a potential modulatory role in LPS-induced inflammation in the lung, and the sulfation level of HSPGs present in the ECM and/or GAGs shed into the intercellular spaces may help determine the extent of inflammation following lung injury. This study provides additional in vitro evidence for the in vivo physiological significance of heparan sulfate in the airway and suggests the potential utility of heparin in dampening the inflammatory response to exogenous (bacterial) stimuli.
Acknowledgments
FUNDING
This study was supported by National Institutes of Health grants HL44497 and HL95411.
The authors wish to thank Rheen Wu and Kenneth Adler for the HBE-1 cells.
Footnotes
DECLARATION OF INTERESTS
No conflicts of interest, financial or otherwise
AUTHOR CONTRIBUTIONS
NYY designed and performed experiments and developed the manuscript; DRN performed experiments, contributed to discussions, and edited the manuscript; HMJ and HYZ performed experiments and contributed to discussions; PLS designed experiments, contributed to discussions, and edited the manuscript.
REFERENCES
- 1.Smits NC, Shworak NW, Dekhuijzen PN, van Kuppevelt TH. Heparan sulfates in the lung: structure, diversity, and role in pulmonary emphysema. Anat Rec. 2010;293(6):955–967. doi: 10.1002/ar.20895. [DOI] [PubMed] [Google Scholar]
- 2.Souza-Fernandes AB, Pelosi P, Rocco PR. Bench-to-bedside review: the role of glycosaminoglycans in respiratory disease. Crit Care. 2006;10(6):237. doi: 10.1186/cc5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, et al. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178(4):2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3(5):401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82(11):3253–3258. [PubMed] [Google Scholar]
- 7.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52(3):349–374. [PubMed] [Google Scholar]
- 8.Wang L, Brown JR, Varki A, Esko JD. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110(1):127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SJ, Hoggat AM, Faulk WP. Heparin regulates ICAM-1 expression in human endothelial cells: an example of non-cytokine-mediated endothelial activation. Thromb Haemost. 1998;80(3):481–487. [PubMed] [Google Scholar]
- 10.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3(2):169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 12.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 13.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010 doi: 10.1155/2010/215158. Article ID 215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 15.N'Guessan PD, Hippenstiel S, Etouem MO, Zahlten J, Beermann W, Lindner D, et al. Streptococcus pneumoniae induced p38 MAPK- and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am. J Physiol Lung Cell Mol Physiol. 2006;290(6):L1131–L1138. doi: 10.1152/ajplung.00383.2005. [DOI] [PubMed] [Google Scholar]
- 16.Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, et al. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax. 1997;52(11):940–945. doi: 10.1136/thx.52.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermert L, Ermert M, Merkle M, Goppelt-Struebe M, Duncker HR, Grimminger F, et al. Rat pulmonary cyclooxygenase-2 expression in response to endotoxin challenge: differential regulation in the various types of cells in the lung. Am J Pathol. 2000;156(4):1275–1287. doi: 10.1016/S0002-9440(10)64998-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejima K, Layne MD, Carvajal IM, Kritek PA, Baron RM, Chen YH, et al. Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003;17(10):1325–1327. doi: 10.1096/fj.02-1078fje. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, et al. Expression of functional toll-like receptor-2 and-4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31(2):241–245. doi: 10.1165/rcmb.2004-0078OC. [DOI] [PubMed] [Google Scholar]
- 20.Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, et al. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31(3):330–336. doi: 10.1165/rcmb.2003-0438OC. [DOI] [PubMed] [Google Scholar]
- 21.Petrovic N, Knight DA, Bomalaski JS, Thompson PJ, Misso NL. Concomitant activation of extracellular signal-regulated kinase and induction of COX-2 stimulates maximum prostaglandin E2 synthesis in human airway epithelial cells. Prostaglandins Other Lipid Mediat. 2006;81(3–4):126–135. doi: 10.1016/j.prostaglandins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1289–L1296. doi: 10.1152/ajplung.00356.2006. [DOI] [PubMed] [Google Scholar]
- 23.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, et al. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;265(5 Pt 1):C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 24.Inoue Y, Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976;46(1):87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa K, Inoue Y, Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- 26.Looby E, Abdel-Latif MM, Athie-Morales V, Duggan S, Long A, Kelleher D. Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer. 2009;9:190. doi: 10.1186/1471-2407-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinton LJ, Mizgerd JP. NF-kappaB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res. 2011;343(1):153–165. doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 28.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L566–L577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 29.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 31.Newman DR, Li CM, Simmons R, Khosla J, Sannes PL. Heparin affects signaling pathways stimulated by fibroblast growth factor-1 and-2 in type II cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(1):L191–L200. doi: 10.1152/ajplung.00284.2003. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Newman DR, Sannes PL. HSULF-1 inhibits ERK and AKT signaling and decreases cell viability in vitro in human lung epithelial cells. Resp Res. 2012;13:69. doi: 10.1186/1465-9921-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Newman DR, Bonner JC, Sannes PL. Over-expression of human endosulfatase-1 exacerbates cadmium-induced injury to transformed human lung cells in vitro. Tox App Pharm. 2012;265:27–42. doi: 10.1016/j.taap.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung S, Park Y, Jo J-R, Jung N-K, Song D-K, Bae J, et al. Overexpression of cyclooxygenase-2 in NCI-H292 human alveolar epithelial carcinoma cells: Roles of p38 MAPK, ERK-1/2, and PI3K/PKB signaling proteins. J Cell Biochem. 2011;112:3015–3024. doi: 10.1002/jcb.23226. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Ling Y, Huang M, Yin T, Gou S-M, et al. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72:36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, et al. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar Type II epithelial cells and macrophages. J Immunology. 2007;178:463–473. doi: 10.4049/jimmunol.178.1.463. [DOI] [PubMed] [Google Scholar]
- 37.Wong MH, Johnson MD. Differential response of primary alveolar Type I and Type II cells to LPS stimulation. PLOS one. 2013;8(1):e55545. doi: 10.1371/journal.pone.0055545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boodoo S, Spannhake EW, Powell JD, Horton MR. Differential regulation of hyaluronan-induced IL-8 and IP-10 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L479–L486. doi: 10.1152/ajplung.00518.2005. [DOI] [PubMed] [Google Scholar]
- 39.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172(1):20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 40.Soejima K, Rollins BJ. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol. 2001;167(11):6576–6582. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- 41.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38(39):12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 42.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104(33):13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochart H, Jenkins PV, Smith OP, White B. Low-molecular weight and unfractionated heparins induce a downregulation of inflammation: decreased levels of proinflammatory cytokines and nuclear factor-kappaB in LPS-stimulated human monocytes. Br J Haematol. 2006;133(1):62–67. doi: 10.1111/j.1365-2141.2006.05959.x. [DOI] [PubMed] [Google Scholar]
- 45.Thourani VH, Brar SS, Kennedy TP, Thornton LR, Watts JA, Ronson RS, et al. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278(6):H2084–H2093. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- 46.Campo GM, Avenoso A, Campo S, Traina P, D’Ascola A, Calatroni A. Glycosaminoglycans reduced inflammatory response by modulating toll-like receptor-4 in LPS-stimulated chondrocytes. Arch Biochem Biophys. 2009;491(1–2):7–15. doi: 10.1016/j.abb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Rogers DF. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options. Pulm Pharmacol Ther. 2005;18(1):1–8. doi: 10.1016/j.pupt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4(3):241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Kim YD, Kwon EJ, Park DW, Song SY, Yoon SK, Baek SH. Interleukin-1beta induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol Pharmacol. 2002;62(5):1112–1118. doi: 10.1124/mol.62.5.1112. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa T, Shimizu S, Tojima I, Kouzaki H, Shimizu T. Heparin inhibits mucus hypersecretion in airway epithelial cells. Am J Rhinol Allergy. 2011;25(2):69–74. doi: 10.2500/ajra.2011.25.3562. [DOI] [PubMed] [Google Scholar]
- 51.Weaver TE, Kropp KL, Whitsett JA. In vitro sulfation of pulmonary surfactant-associated protein-35. Biochim Biophys Acta. 1987;914(2):205–211. doi: 10.1016/0167-4838(87)90065-3. [DOI] [PubMed] [Google Scholar]
- 52.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, et al. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144(5):1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 54.Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, et al. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182(1):73–82. doi: 10.1164/rccm.200905-0674OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuzzocrea S, Mazzon E, Sautebin L, Dugo L, Serraino I, De Sarro A, et al. Protective effects of Celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem Pharmacol. 2002;63(4):785–795. doi: 10.1016/s0006-2952(01)00908-x. [DOI] [PubMed] [Google Scholar]