Abstract
Childhood stress and trauma are associated with substance use disorders in adulthood, but the neurological changes that confer increased vulnerability are largely unknown. In this study, maternal separation [MS] stress, restricted to the pre-weaning period, was used as a model to study mechanisms of protracted effects of childhood stress/traumatic experiences on binge drinking and impulsivity. Using an operant self-administration model of binge drinking and a delay discounting assay to measure impulsive-like behavior, we report that early life stress due to MS facilitated acquisition of binge drinking and impulsivity during adulthood in rats. Previous studies have shown heightened levels of corticotropin releasing factor (CRF) after MS, and here, we add that MS increased expression levels of GABAA α2 subunit in central stress circuits. To investigate the precise role of these circuits in regulating impulsivity and binge drinking, the CRF1 receptor antagonist antalarmin and the novel GABAA α2 subunit ligand 3-PBC were infused into the central amygdala [CeA] and medial prefrontal cortex [mPFC]. Antalarmin and 3-PBC at each site markedly reduced impulsivity and produced profound reductions on binge-motivated alcohol drinking, without altering responding for sucrose. Furthermore, whole-cell patch-clamp studies showed that low concentrations of 3-PBC directly reversed the effect of relatively high concentrations of ethanol on α2β3γ2 GABAA receptors, by a benzodiazepine site-independent mechanism. Together, our data provide strong evidence that maternal separation, i.e., early life stress, is a risk factor for binge drinking, and is linked to impulsivity, another key risk factor for excessive alcohol drinking. We further show that pharmacological manipulation of CRF and GABA receptor signaling is effective to reverse binge drinking and impulsive-like behavior in MS rats. These results provide novel insights into the role of the brain stress systems in the development of impulsivity and excessive alcohol consumption.
Keywords: GABA, alpha-2 receptor, Corticotropin Releasing Factor (CRF), benzodiazepine, central amygdala, medial prefrontal cortex (mPFC), antalarmin, 3-PBC, limbic, neuropsychiatric
Introduction
Individuals differ in the risk for developing drug addiction such that even after chronic usage, only a fraction of individuals develop drug dependence (Everitt et al., 2008). The rationale for this discrepancy is poorly understood; however, stress during the perinatal period is correlated to behavioral phenotypes linked to mood disorders and increased addiction risk during adulthood (Deminiere et al., 1992, Marinelli and Piazza, 2002). The experience of stress during infancy causes long lasting modulation of neurons in the limbic system, as well as hyperactivity of the hypothalamus-pituitary-adrenal (HPA) axis, which leads to elevated circulating levels of corticosterone, other gluococorticoids, and their metabolites (Koe et al., 2014) with widespread biochemical consequences.
Even less is known about the neuronal mechanisms that render the stressed offspring vulnerable to initiate binge drinking and to exhibit abnormal impulsivity. Binge drinking as defined by the National Institutes of Health is alcohol intake which increases blood alcohol level to > 80 mg% within a 2 hour period (Crabbe et al., 2011, NIH-NIAAA, 2004); a definition used by researchers and clinicians alike to investigate the brain circuits involved in this type of excessive alcohol intake (Gilpin et al., 2012, Vargas et al., 2014). Cognitive impulsivity is a core deficit present in many psychiatric conditions including drug addiction (Robinson et al., 2009). While there are increasingly more categorizations of impulsivity related to risky behavior and decision-making with various underlying neurochemical and neuroanatomical bases, it is generally accepted that impulsivity is the tendency to respond prematurely without foresight or regard for the consequences (Dalley et al., 2011). Although the behavioral task in this study focuses on impulsive choice where animals exhibit the temporal discounting of reward, response disinhibition involving the regulation of GABA signaling system in the cortico-limbic system is an important factor in impulsivity (Dick et al., 2013), and thus GABA dysregulation and modulation in MS is important.
Non-human primates (Huggins et al., 2012) and rodents exposed to MS, will self-administer ethanol during adolescence and adulthood (Garcia-Gutierrez et al., 2015, Cruz et al., 2008, Moffett et al., 2007, Romano-Lopez et al., 2012). Although the mechanism for stress-induced binge drinking is unknown, studies show that MS permanently alters expression of various GABAA receptor subunits [e.g., α1, α2, γ2] and their mRNA in the amygdala and hippocampus (Caldji et al., 2000, Edenberg et al., 2004, Hsu et al., 2003). The GABA receptors, especially the GABAA α1 receptor, has been extensively studied in relation to alcohol biochemistry, but recent human linkage studies also implicate the GABRA2 gene, encoding the GABAA α2 receptor in regulating excessive drinking and impulsivity, and reduced GABA levels in human frontal lobes are associated with significant levels of impulsivity in adolescents (Dick et al., 2013, Dick et al., 2006, Edenberg et al., 2004, Enoch et al., 2010). The approach in the present study focuses on the role of GABAA alpha2 subunit in modulating excessive drinking and impulsivity in adults exposed to MS.
Studies in MS models reveal elevated CRF in stress loci (Nemeroff, 2004a, Nemeroff, 2004b, O’Malley et al., 2011). This effect of MS can result in structural changes in neurons of the PFC and significantly affect development of neurons in reward and emotional memory circuits including nucleus accumbens and hippocampus (Yang et al., 2014, Monroy et al., 2010, Wang and Gondre-Lewis, 2013). In addition, pharmacological and genetic studies support the hypothesis that excessive alcohol consumption and binge drinking is mediated by elevated CRF, via activation of the CRF1 receptor [CRF1R] (Heilig et al., 2011, Koob, 2008, Phillips et al., 2015, Koob, 2014). Blockade of CRF1R in rodents, attenuates alcohol intake in dependent rodents (Funk et al., 2007, Gehlert et al., 2007, Koob, 2008, Lowery-Gionta et al., 2012). The literature supports a model where CRF signaling in the central amygdala [CeA] functions as a key regulator of binge drinking (Lowery-Gionta et al., 2012), recruited during excessive alcohol intake prior to the development of dependence, with CRF as a mediator of the transition to dependence.
A genetic polymorphism in the CRF1R gene was significantly linked to binge drinking in humans (Treutlein et al., 2006). Following exposure to stressful stimuli, adolescents expressing this polymorphism displayed a predisposition to excessive drinking leading to dependence in adulthood (Blomeyer et al., 2008). Moreover, early life adversity interacted with CRF to increase alcohol intake in primates (Barr et al., 2009). Indeed, addiction-related changes in prefrontal cortex CRF systems and their association with executive (George et al., 2012) or drinking phenotypes (Glaser et al., 2014) were reported; however, research to support a mechanism for the CRF system in impulsivity is lacking. Given that the experience of MS results in elevated CRF (Nemeroff, 2004b, O’Malley et al., 2011) and permanent alterations in GABA levels in stress circuits during adulthood (Caldji et al., 2000, Hsu et al., 2003), combined with the finding that MS results in long-term increases in alcohol in rodents (Cruz et al., 2008, Moffett et al., 2007), we hypothesized that the CeA and the mPFC, two loci of the stress circuits and cognitive impulsivity could influence vulnerability to binge drinking or impulsivity, following MS. Thus, the aim of this study was first to investigate the extent of binge drinking and impulsive-like behavior in our MS model, and second to determine if the action of pharmacological agents acting at CRF or GABA receptors in the CeA or mPFC could revert these behaviors to control levels.
METHODS
Animals
Pregnant Sprague Dawley dams were obtained from Harlan Laboratories [Frederick, MD, USA] and offspring used in this study were born onsite at the veterinary facility. They were subjected to the MS paradigm as described below, and were tested for drinking and impulsivity behaviors as adults. Equivalent numbers of males and females were used in the binge drinking and impulsivity studies. Subjects were housed in groups of 2-3 per plastic cage until drinking studies began. The vivarium was maintained at an ambient temperature of 21°C and was on a reverse 12 h light/dark cycle. All rats were provided ad libitum access to food and water. All training and experimental sessions for all subjects took place between 8:30 am and 5:30 pm. The treatment of all subjects was approved by the IACUC of the Howard University College of Medicine and all procedures were conducted in strict adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
MS Regimen
The maternal separation [MS] paradigm was performed as previously published (Roceri et al., 2002, Wang and Gondre-Lewis, 2013), and was meant to emulate recurrent stressful experiences during the neonatal period. The number of pups in each litter ranged from 10-14 pups. To prevent litter effects, pups were sexed, culled to n=10 with equal numbers of males and females, and redistributed to nursing dams at P1. Beginning at P2 until weaning at P21, the separation comprised of removal of pups from their nursing mothers. They were brought to a designated room, separate from the mother, where the temperature was monitored and maintained at 29°C. Each pup was placed in a cage located on a warmed pad, and visual access to other pups was blocked with cardboard. These conditions were maintained for 3 hours per day from 11AM to 2PM. After the 3 hr separation time, they were returned to their home cage and rooms. Non-MS (CTL) pups were not separated from their mothers and were treated according to standard animal facility regulations.
Use of Animals
40 adult rats from 21 litters were used; 12 for western blotting and 28 for the behavioral studies, used over several months. Although these studies are not aimed at examining sex differences, both males and females were always represented. Therefore, this is a mixed-sex study. In any behavioral experiment, to control for litter effects, the maximum number of pups used from a single mother was one male and one female. Therefore, for an n=10 as an example, the minimum number of dams was 5 for each condition. For the operant binge drinking paradigm in Figure 2, there were n=10 controls (5F, 5M), and n=10 MS (5M, 5F); 75% of these same animals were re-used and added to other animals for the delay discounting experiments; n=9 controls (5F, 4M) and n=11 for MS (8F, 3M). For Western blotting analysis, a different cohort of animals was used with the same principle of heterogeneity to reduce litter effects; n=5-6 controls (2-3F, 3M) and n=6 for MS (3F and 3M). For drug dosage studies, some animals used in figure 2 were combined with other rats of the same age that had undergone similar sustained operant training to have a sufficient number for surgical implantation of the cannulae and subsequent behavioral testing, n=5 for CeA drug infusion (3F, 2M) and n=4 for mPFC studies.
Figure 2. Baseline operant responding for alcohol, blood alcohol concentration and delay discounting (impulsivity) of MS vs. CTL rats.
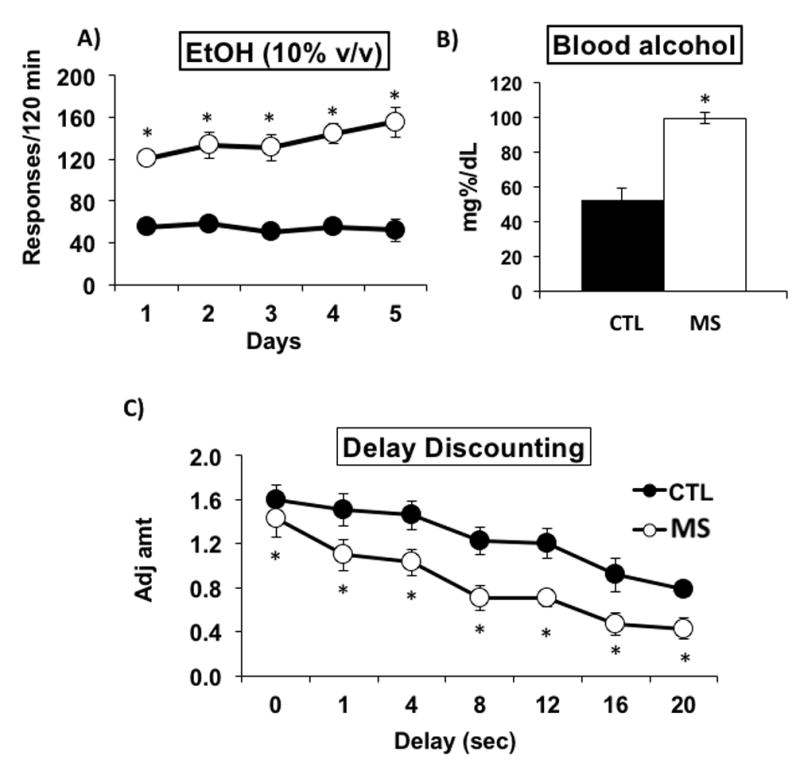
(A) Responding for alcohol is increased in maternally separated [MS] rats [N=10] compared to control [CTL] rats [N=10]. (B) BACs of MS rats [N=4] were elevated above those of CTL rats [N=4] and were >80 mg%/dL following 2 h of drinking. (C) Adjusted amount is decreased [impulsivity is elevated] in MS rats [N=11] compared to CTL SD rats [N=9]. * P ≤ 0.05 by ANOVA followed by post hoc tests.
Tissue Preparation and Immunoblotting
Naïve, randomly-selected adult rats at P70 were sacrificed and neural tissue was harvested for immunoblotting to semi-quantitatively evaluate baseline levels of GABAA α2 and CRF proteins in the CeA and mPFC of MS [N=4-6] and CTL [N=5-6] rats. The brain was removed from each animal and frozen, then sliced on a microtome in 300 μm sections. CeA and mPFC tissue sections were collected by 1.0 mm micropunch [Ted Pella, Redding, CA] from the right and left hemispheres and pooled. Tissue micropunches were lysed with CelLytic MT (dialyzable mild detergent, bicine, and 150 mM NaCl; Sigma-Aldrich) according to manufacturer’s instructions. Total protein was determined by the bicinchoninic assay (BCA) [Pierce, Rockford, IL]. Proteins were resolved by SDS- polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Blots were exposed to primary antibody overnight at 4°C followed by horseradish peroxidase (HRP)-labeled goat anti-mouse or anti-rabbit secondary antibody for 1 h at room temperature (RT) (Cell Signaling). Detection was with the ECL kit reagents (Amersham Life Science) followed by exposure to high-performance chemiluminescence film (Hyperfilm ECL; Amersham), and quantitation was by densitometric scanning with a Bio-Rad GS-700 imaging densitometer (Liu et al., 2011). Each lane represents an individual animal. The optical density of protein bands on each digital image was normalized to the O.D. of the loading control, and the animals for a given condition were averaged and expressed as densitometric units +/- SE. Normalized values across 3 blots were used for graph and analyzed with a student’s t-test.
Antibodies and reagents
The generation and specificity of the rabbit-derived GABAA α2 antibody was previously described (Pirker et al., 2000, Liu et al., 2011). The GABAA α2 antibody was the kind gift of Dr. W. Sieghart (Department of Biochemistry and Molecular Biology, Center for Brain Research, Medical University Vienna, A-1090 Vienna, Austria). It was raised in rabbits against peptides corresponding to amino acids 322-357 of the α2 protein coupled to keyhole-limpet hemocyanin, affinity purified and extensively characterized by various methods, including immunoprecipitation, western blotting and immunocytochemistry (Pirker et al., 2000). The Mouse anti-GAPDH (0411, Cat# sc-47724) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA) and is well characterized and used in numerous studies including our own previous publications (Liu et al., 2011).
Stereotaxic Implantation of Cannulae for Microinfusions
Adult MS rats were anesthetized via isofluorane/oxygen gas inhalation and placed in a stereotaxic apparatus to allow for bilateral implantation of 22-gauge guide cannulae into the CeA or mPFC. The cannulae were anchored to the skull by four stainless steel screws and dental acrylic. A stylet was inserted into each cannula to maintain its viability and was only removed during infusion times. The coordinates were based on the rat brain atlas of Paxinos and Watson as follows: CeA: AP, -2.0 mm; ML, ±3.6 mm; DV, -8.5 mm from bregma; mPFC: AP, +2.7 mm; ML, ±1.45 mm; DV, -2.5 mm from bregma at a 16° angle to the midline. Each cannula was placed 1.0 mm above the intended target. This allowed the injector tip to extend below the cannula tip. The animals were given a 3-day recovery period before re-stabilization on the delay discounting or operant drinking paradigms. After behavioral experiments, cannula placement was confirmed visually by examination of cryostat-generated 300μm brain slices post sacrifice.
Drugs and Microinfusion Procedure
3-propoxy-9H-pyrido[3,4-b]indole hydrochloride, commonly known as 3-propoxy-β-carboline hydrochloride (3-PBC), acting at the GABAA1/2 receptor, was obtained from Dr. James Cook at the University of Wisconsin-Milwaukee [Milwaukee, WI] (Namjoshi et al., 2011). Antalarmin hydrochloride, a CRF antagonist, was obtained from R&D Systems Inc. [Minneapolis, MN]. The drugs were mixed into 1 mL of sterile PBS with Tween-20 added dropwise until dissolved, and then bilaterally infused into the CeA or mPFC at a rate of 0.1 μL/min for 5 min using a Harvard infusion pump. The overall design of experiments was such that doses of vehicle, 2, and 4 μg of antalarmin, or 20 or 40 μg of 3-PBC were injected immediately prior to animals being placed in the operant or delay discounting chambers. Animals rested 1-3 days between doses. The antalarmin infusion studies occurred before the 3-PBC infusion studies, and were at least 2 weeks apart for any given animal. Different animals were used for the CeA and mPFC infusions. Antalarmin and 3-PBC were administered to MS rats to test their effects on the heightened operant responding and impulsivity profile of MS, whereas CTL rats do not consume significant levels of alcohol at baseline, nor do their impulsivity profile differ significantly at 8 sec compared to 0 sec delay.
Delay Discounting [Impulsivity]
The impulsivity paradigm was executed as described by Oberlin and Grahame (Oberlin and Grahame, 2009). Impulsivity is operationally defined as choosing a smaller, immediate reward to the exclusion of a larger delayed reward (Rachlin and Green, 1972), and was quantified using the adjusted amount delay discounting [DD] assay (Wilhelm and Mitchell, 2008). Operant boxes consisted of a nosepoke light, two levers, a cue light above each lever, a house light, and a 10 mL descending sipper tube for saccharin reinforcement [0.03% w/v]. Control of the operant boxes and data collection was with the MedPC IV software (MedAssociates, St. Albans, VT). Prior to actual testing, rats underwent 4 stages of behavioral shaping: Stage 1 is run for 1 session, and all center nose pokes are reinforced on a fixed ratio 1 (FR1) schedule with 20 second sipper access, where 1 lever press is required for sipper access. At stage 2, center nose pokes are reinforced on a FR1 schedule with 10 second sipper access, and the animal must complete 20 trials to move on to next stage. Stage 3 also requires 20 trials, but all trials are cued with a center light illuminated for 20 seconds. There is a 10 second intertrial interval. At stage 4, a nosepoke and lever press is required for the 10-second sipper access, and both right and left levers are reinforced equally, 20 trials with a 10 second intertrial interval in 60 minutes is required. (Oberlin and Grahame, 2009),
After shaping, side bias was assessed by averaging the last 3 days’ choices on each side. The large reinforcer was then assigned to their non-preferred side, to counter any initial side bias. After shaping was completed, rats were assessed at 0s delay. This time point is used as a task to assess discrimination of reinforcer (saccharin) magnitude discrimination prior to introduction of any delay to the larger reward. Immediate reward amount started at 1 s of saccharin access, and was adjusted upwards and downwards by 0.1 s based on the rat’s choices, i.e., an immediate choice resulted in down-adjustment of the sipper access time by 0.1s on the next trial, whereas a delay choice resulted in up-adjustment of the sipper access time by 0.1s in the next trial. The total adjustments in access were restricted to a minimum of 0s and a maximum of 2s. Average adjusted amounts of the reward over the last 20 trials of the session served as the measure of adjusted amount. All rats received 2-hour water access in their home cages at the end of daily testing (Oberlin and Grahame, 2009).
Phase 1
Following behavioral shaping rats were tested in the delay discounting paradigm at 0, 1, 4, 8, 12, 16 and 20 s delays. Each delay was tested for 2 consecutive sessions and the two-day data for each delay was averaged.
Phase 2
Following completion of Phase 1, rats were randomly separated into treatment groups and bilaterally implanted with cannulae in the mPFC or CeA. After re-stabilization on the DD paradigm at a delay of 8 s, rats were infused with 3-PBC [20 or 40 μg] or antalarmin [2 or 4 μg] as described above and run in the impulsivity paradigm with an 8 s delay.
Operant Drinking Apparatus
Animals were tested in 11 standard operant chambers [Coulbourn Instruments, Inc., Lehigh Valley, PA] enclosed in an isolated chamber as previously described ((Liu et al., 2011). The operant apparatus contained two levers, two dipper manipulanda, triple cue lights over each lever, and a house light. The dipper cup size which contained the 10% (v/v) alcohol or 3% (w/v) sucrose reinforcers was 0.1 mL. The Coulbourn Graphic State “3” operant software was used.
Drinking in the Dark Multiple Scheduled Access [DIDMSA] Paradigm
To initiate excessive “binge” alcohol drinking, we employed a modification of the drinking-in-the-dark-multiple-scheduled-access [DIDMSA] protocol (Liu et al., 2011, Bell et al., 2014). First, the procedure entailed adapting the rats to a reverse 12h/12h light/dark cycle which began at 7:00 pm [lights on] and lasted to 7:00 am [lights off]. Rats were trained to orally self-administer EtOH daily for two 45 min sessions with 30 min rest in between under an FR1 schedule employing the sucrose fading technique (Harvey et al., 2002). After a period of stabilization on the FR1 schedule, the response requirement was then increased to an FR4 schedule, where 4 lever presses are required for access to the reinforcer. For each schedule, responding was considered stable when responses were within ± 20% of the average responses for five consecutive days. Stabilization on the FR4 schedule took approximately 8 days. During the stabilization procedures, the animals were never deprived of food or fluid. These procedures are well established in our laboratory (June and Eiler, 2007, Liu et al., 2011). Other cohorts of rats were given a 3% [w/v] concentration of sucrose and trained in an identical manner under the FR1, then FR4, schedule. Following stabilization on the FR4 schedule for EtOH/sucrose, the DIDMSA protocol began using an FR4 schedule where the rats were given access to 10% alcohol, or 3% sucrose on both the left and right levers. To initiate the DIDMSA protocol during the dark phase, rats were given a 45 min operant session. After the session had elapsed, rats were then placed in the home cage with food and water ad libitum for 30 min. Rats were then given a second 45 min operant session and subsequently returned to their home cage. Rats engaged in the alcohol drinking for 21 consecutive days. Using this protocol, the MS rats in our laboratory produced consistent BACs of 99 ± 3 mg%. Sucrose control rats were trained in a similar manner; but lever pressed for a 3% sucrose solution instead of ethanol. The sucrose control rats allowed for evaluation of reinforcer specificity following MS and drug treatments.
Following 21 days of alcohol or sucrose drinking, rats were surgically implanted with bilateral cannulae into the CeA or mPFC. Rats (N=5/6) were then infused with 3-PBC [20 or 40 μg] or antalarmin [2 or 4 μg] as described above and were immediately placed in the operant chambers to respond for alcohol or sucrose. A 2 hour session consisted of two 45 min (90min) access and 30 minutes of rest. Figure 1 shows the timeline of experiments.
Figure 1. Map of behavioral experiments and locations of cannula implantation for animal studies.
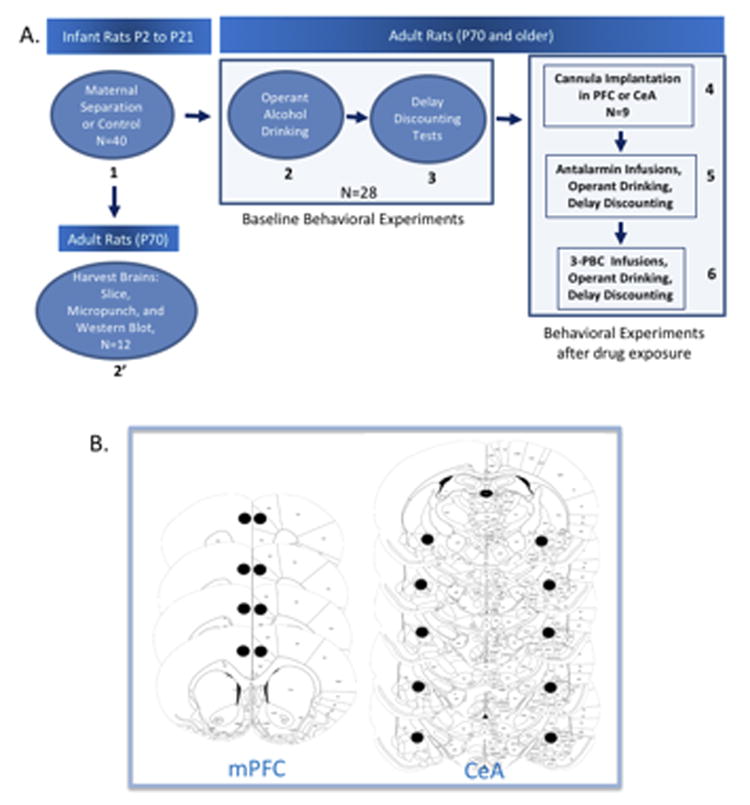
Panel A shows order of experiments demarcated by the numbers in bold and the direction of arrows. Panel B shows the location in the mPFC and the CeA where cannula were implanted. Each slice represents a different animal.
Blood Alcohol Concentration [BAC] Measurement
To ensure animals were consuming pharmacologically relevant amounts of EtOH to model human binge drinking (Bell et al., 2006, Naimi et al., 2003, NIH-NIAAA, 2004), approximately 100 μl of whole blood was collected from the tail vein of MS and CTL rats [N = 4/treatment group] into a heparin-coated tube. After collection, the whole blood was immediately centrifuged for 5 min at 1100 rpm. Plasma samples of 5 μl were analyzed in a GL-5 Analyzer (Analox Instruments, Luxenburg, MA). Microanalysis consisted of measuring the oxygen consumption in the reaction between the sample of alcohol and alcohol oxidase using a Clark-type amperometric oxygen electrode. Alcohol reagent buffer solutions (pH 7.4) and alcohol oxidase enzymes were used in all samples tested. BACs were determined in duplicates after 90 min of drinking.
Cell culturing and cell transfection
HEK 293 cells plated on 15-cm plates in 15 ml of Minimum Essential Medium [MEM, Gibco, Karlsruhe, Germany] supplemented with 158 mg/L sodium bicarbonate, 2 mM glutamine [Gibco, Karlsruhe, Germany], 100 U/mL penicillin-streptomycin [Gibco, Karlsruhe, Germany], and 10% fetal calf serum [Gibco, Karlsruhe, Germany]. Cultures were maintained at 37 °C in a humidified 95% O2/5% CO2 atmosphere for two days. Transfection with recombinant rat GABAA receptors were carried out as described in detail (Korpi and Luddens, 1993). Briefly, HEK 293 cells were transfected using the phosphate precipitation method with rat GABAA receptor cDNAs in eukaryotic expression vectors [pRK5] for α2. For optimal receptor expression, final concentrations [μg vector DNA per 15mm tissue culture plate] were: α2, 12.5 µg.
Electrophysiology
Two days after transfection, single coverslips containing HEK 293 cells were placed in a recording chamber mounted on the movable stage of a fluorescence microscope (Olympus IX70) and perfused at room temperature with a defined saline solution containing (in mM): 130 NaCl, 5.4 KCl, 2 CaCl2, 2 MgSO4, 10 glucose, 5 sucrose, and 10 HEPES (free acid), pH adjusted to 7.35, with about 35 mM NaOH. Transfected cells were identified by the fluorescence of the co-expressed Enhanced Green Fluorescent Protein (EGFP). The amplitudes of peak currents were measured from recorded traces. The GABA concentration response curve was analyzed with a sigmoidal non-linear regression fit, using the formula I = (Imax[L]nH)/(EC50nH+[L]nH), where Imax is the maximal induced current, L is the concentration of the agonist, and nH the Hill coefficient. Ligand-mediated membrane currents of these cells were studied in the whole-cell configuration (Hamill et al., 1981). Patch clamp pipettes were pulled from hard borosilicate capillary glass (0.5 mm ID, 1.5 mm OD, Vitrex, Science Products GmbH, Hofheim, Germany) using a horizontal puller (model P-97, Sutter Instruments, CA) in a multi-stage process. Using a fast Y-tube application system, the recombinant receptors were tested for EtOH mediated effects on the receptor current response with the approximate receptor subtype specific GABA EC10, and GABA EC10 plus 30 mM or 100 mM EtOH. Furthermore, both EtOH concentrations were tested together with the GABA EC10 and 1 nM and 30 nM 3-PBC. The responses of the cells were recorded by a patch clamp amplifier (EPC-8, HEKA-Electronic, Lambrecht, Germany) and the pClamp 8.1 software package (Axon Instruments, Foster City, CA). The standard holding potential for the cells was −40 mV. Whole cell currents were low pass-filtered by an eight-pole Bessel filter at 5 or 3 kHz before being digitized by a Digidata 1322A interface (Axon Instruments) and recorded by the computer at a sampling rate of at least 1 kHz.
Statistical Analyses
Data obtained using antalarmin and 3-PBC were analyzed by separate univariate ANOVAs for binge alcohol or sucrose drinking followed by Newman-Keuls post hoc tests. A two-tailed t-test was used to analyze the HEK cell data. A student’s t-test was used for western blotting analysis. All analyses were performed using the Sigma Plot 11.2 software program [Systat Software Inc., San Jose, CA].
RESULTS
MS facilitates acquisition of binge drinking and impulsivity during adulthood
We tested if the experience of chronic 3-hr daily postnatal MS, as a model of early life stress and childhood trauma, could have protracted effects on binge drinking and impulsivity. After stabilization on the FR4 schedule for 8 days, responding for alcohol within a two-hour period was recorded as presented in figure 2A. MS rats showed significantly elevated levels of responding for alcohol compared to CTL rats (Fig. 2A) with a significant main effect of Group [F[9,90] = 78.169, p < 0.001]. Post-hoc analyses confirmed the elevated responding for alcohol by MS rats for all 5 days tested [p ≤ 0.05]. BACs measured after the two 45 min drinking sessions were 99.3 ± 3.2 mg%/dL for MS animals and 52.9 ± 6.2 mg%/dL in CTL rats [Fig. 2B]. A significant main effect of Group [F[1,6] = 46.547, p < 0.001] was confirmed with post-hoc analysis [p ≤ 0.05].
While “impulsivity” is a complex behavioral phenotype (Dick et al., 2010), in the present study it was defined as choosing a smaller, immediate reward to the exclusion of a larger delayed reward (Rachlin and Green, 1972), and was quantified using the adjusted amount delay discounting [DD] assay (Wilhelm and Mitchell, 2008). The smaller the amount of the reward, the greater the impulsive inference. The MS rats showed significantly increased levels of impulsivity [lower adjusted amounts] compared with CTL rats [Fig. 2C], with significant main effects of Group [F[1,108] = 31.134, p < 0.001] and Delay [F[6,108] = 14.764, p < 0.001]. Post-hoc analyses confirmed the increased impulsivity of MS rats compared to CTL rats for 1, 4, 8, 12, 16 and 20 s delays [p ≤ 0.05]. These data are consistent with other findings that genetically bred high alcohol drinking (HAD) rats discounted delayed and probabilistic rewards more steeply than LAD rats (Wilhelm and Mitchell, 2008).
Antalarmin decreases impulsivity and binge alcohol drinking in MS rats
Because of the elevated levels of CRF in the CeA and mPFC of MS rats, we directly microinjected antalarmin, a CRF antagonist, into the CeA or mPFC of animals previously subjected to ethanol drinking or the delay discounting assay, to determine its effects on impulsivity and alcohol binge drinking, as well as the role CRF may play in regulating these two behaviors. When directly infused into the CeA of MS rats, antalarmin significantly reduced operant responding for alcohol [Fig. 3A; F[2,15] = 31.082; p < 0.001] and impulsivity [Fig. 3B; F[2,15] = 6.667; p = 0.008] compared to vehicle-treated MS rats. Post hoc analyses confirmed the reduction of impulsivity and operant responding by both 2 μg and 4 μg intracranial doses of antalarmin [p ≤ 0.05].
Figure 3. Effects of antalarmin injected into the CeA on delay discounting, operant binge drinking, sucrose drinking.
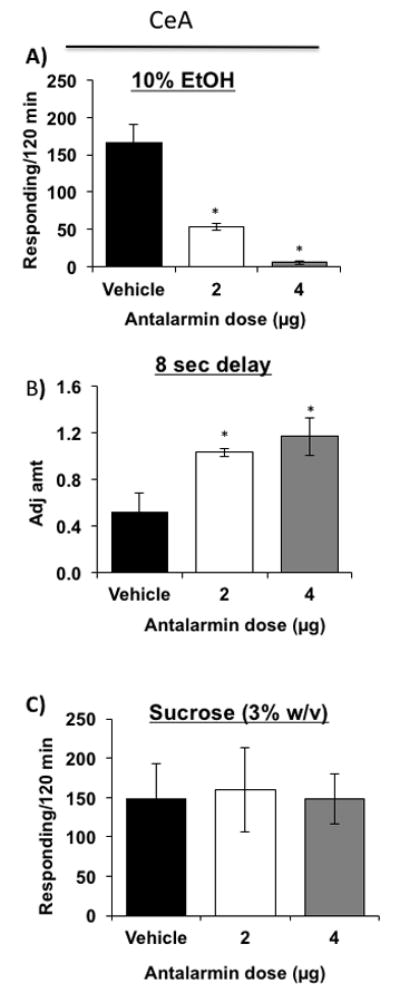
(A) Both doses of antalarmin [N=6/dosage group] reduced operant responding for alcohol of MS rats compared to vehicle-treated MS rats [N=6]. (B) Both 2 and 4 μg doses of antalarmin [N=6/dosage group] microinjected into the CeA of MS rats elevated adjusted amount [decreased impulsivity] compared to vehicle treatment in MS rats [N=6]. (C) Both doses of antalarmin in the CeA [N=5/dosage group] did not alter the responding of MS rats for sucrose compared to vehicle [N=5]. * P ≤ 0.05 by ANOVA.
To confirm that the antalarmin-induced reduction in operant responding for alcohol was not due to an overall reduction in the consumption of fluid or the drug’s potential sedative effects, we evaluated the effect of antalarmin on operant responding for sucrose in MS rats. Antalarmin did not reduce sucrose responding [Fig. 3C; F[2,12] = 0.0222; p = 0.978] compared to vehicle-treated MS rats.
Similar reductions of impulsivity and alcohol binge drinking were observed when antalarmin was microinjected into the mPFC. Antalarmin significantly reduced operant responding for alcohol [Fig. 4A; F[2,9] = 8.974; p = 0.007] and impulsivity [Fig. 4B; F[2,30] = 30.464; p < 0.001] compared to vehicle-treated MS rats. Post hoc analyses confirmed the reduction of impulsivity and operant responding by both 2 μg and 4 μg intracranial doses of antalarmin [p ≤ 0.05]. Antalarmin injected into mPFC did not reduce responding for sucrose [Fig. 4C; F[2,12] = 0.0843; p = 0.920] compared to vehicle-treated MS rats.
Figure 4. Effects of antalarmin injected into the mPFC on delay discounting, operant binge drinking and sucrose drinking.
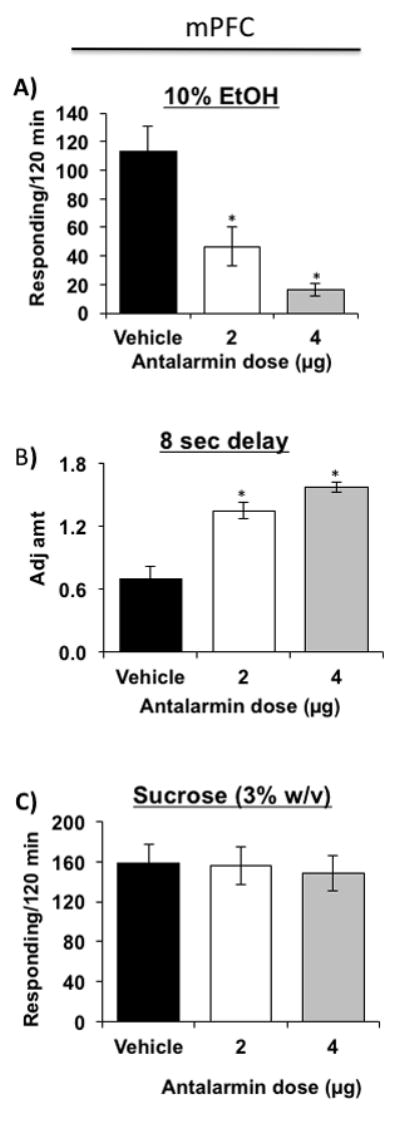
(A) Both 2 and 4 μg doses of antalarmin microinjected into the mPFC decreased impulsivity [elevated adjusted amount] in MS rats [N=6/dosage group] compared to vehicle treatment [N=6]. (B) Both doses of antalarmin also reduced responding of MS rats [N=4/dosage group] for alcohol compared to vehicle [N=4]. (C) Both doses of antalarmin in the mPFC [N=5/dosage group] did not alter the responding of MS rats for sucrose compared to vehicle [N=5]. * P ≤ 0.05 by ANOVA.
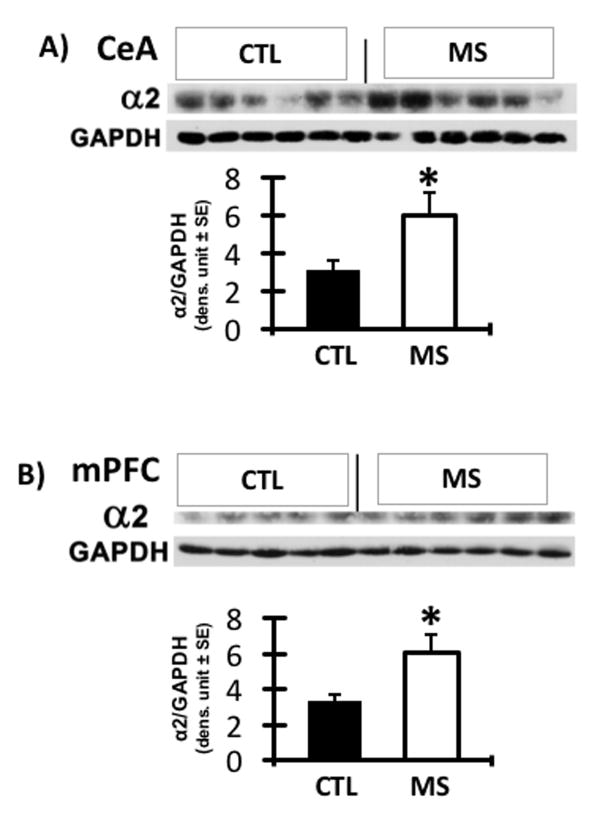
GABAA α2 is elevated in the CeA and mPFC of naïve MS rats
GABAA α2 receptors has been implicated in the mechanisms associated with excessive alcohol drinking behavior in genetically alcohol-preferring rats. To determine if MS rats share biochemical features of P rats, naïve MS rats, never exposed to any behavioral or alcohol drinking tests were examined for expression of GABAA α2 subunit because elevations of this receptor subunit is associated with excessive drinking. Compared with CTL rats, MS rats showed significantly elevated levels of GABAA α2 in the CeA and mPFC [Fig. 5A and 5B; p ≤ 0.05].
Figure 5. GABAA α2 protein concentration in CeA and mPFC of MS vs. CTL rats.
The levels of GABAA α2 expression were significantly higher in the CeA (A) and mPFC (B) of MS rats [N=6] compared to CTL rats [N=5 for mPFC, n=6 for CeA]. * P ≤ 0.05 by ANOVA.
3-PBC decreases impulsivity and binge alcohol drinking in MS rats
Because of the importance of GABAA receptors in modulating the effects of stress and alcohol and the importance of the CeA and mPFC in stress, impulsivity and addiction processes, we microinjected in vivo, 3-PBC, a GABA receptor modulator, directly into the CeA or mPFC of MS rats that were previously subjected to alcohol drinking or impulsivity. 3-PBC significantly reduced impulsivity [Fig. 6A; F[2,12] = 7.013; p = 0.010] and operant responding for alcohol [Fig. 6B; F[2,12] = 31.399; p < 0.001] compared to vehicle-treated MS rats. Post hoc analyses confirmed the reduction of impulsivity and operant responding by both 20 μg and 40 μg intracranial doses of 3-PBC [p ≤ 0.05]. 3-PBC in the CeA did not reduce sucrose responding [Fig. 6C; F[2,12] = 0.0537; p = 0.948] compared to vehicle-treated MS rats.
Figure 6. Effects of 3-PBC in the CeA and mPFC on delay discounting, operant binge drinking, and sucrose drinking.
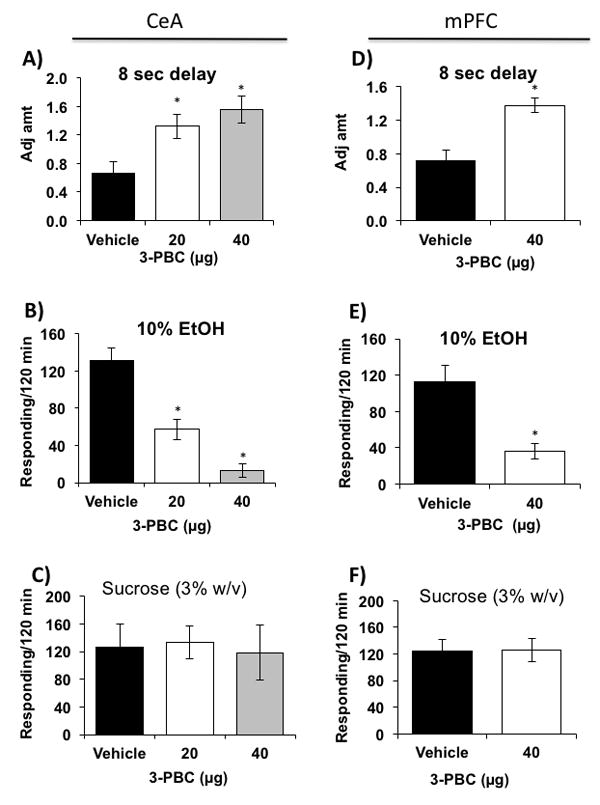
(A) Both 20 and 40 μg doses of 3-PBC microinjected into the CeA, elevated adjusted amounts [decreased impulsivity] in MS rats [N=5/dosage group] compared to vehicle treatment [N=5]. (B) Both doses of 3-PBC also reduced operant responding of MS rats [N=5/dosage group] for alcohol compared to vehicle [N=5]. (C) Neither dose of 3-PBC in the CeA [N=5/dosage group] altered the responding of MS rats for sucrose compared to vehicle [N=5]. (D) The 40 μg dose of 3-PBC microinjected into the mPFC, elevated adjusted amount [decreased impulsivity] in MS rats [N=4] compared to vehicle treatment [N=4]. (E) 40 μg of 3-PBC also reduced operant responding of MS rats [N=4] for alcohol compared to vehicle [N=4]. (F) 3-PBC in the mPFC [N=5] did not alter the responding of MS rats for sucrose compared to vehicle [N=5]. * P ≤ 0.05 by ANOVA.
Similar reductions of impulsivity and alcohol binge drinking were observed when 3-PBC was microinjected into the mPFC. Because 40μg of 3-PBC was shown to completely reverse excessive drinking and impulsive choice in the CeA, this single dose was used in the mPFC. It significantly reduced impulsivity [Fig. 6D; F[1,20] = 22.135; p < 0.001] and operant responding for alcohol [Fig. 6E; F[1,6] = 15.474; p = 0.008] compared to vehicle-treated MS rats. Post hoc analyses confirmed the reduction of impulsivity and operant responding by the 40μg intracranial dose of 3-PBC [p ≤ 0.05]. 3-PBC in the mPFC also did not reduce responding for sucrose [Fig. 6F; F[2,12] = 0.0600; p = 0.942] compared to vehicle-treated MS rats.
3-PBC modulates alcohol action in vitro at a non-benzodiazepine binding site
Given the consistent finding that 3-PBC is an antagonist of alcohol motivated behaviors as shown here and in the literature (Harvey et al., 2002, Kaminski et al., 2013), we evaluated the capacity of this ligand to block alcohol’s action at the GABAA α2-containing receptor subtype using electrophysiological whole cell recordings in HEK cells. Recent work has implicated the GABAA α2- receptor subtype as a direct substrate for the effects of alcohol. Figure 7A shows that low doses of 3-PBC, at 30 nM, reduced the low and high dose [30 and 100 mM] alcohol enhancement of currents at GABAA α2β3γ2 receptors [p ≤ 0.05] in HEK293 cells. To determine if 3-PBC binds at the benzodiazepine-specific binding site of GABA receptors, α2β3γ2 and α5β3γ2 expressing HEK cells were treated with either Diazepam or 3-PBC at varying doses. As expected, diazepam greatly potentiated the effects of GABA on whole cell currents beginning at 0.1µM, an effect which was effectively blocked by Ro15-1788 (flumanezil), a specific antagonist for the BDZ site (Figures 7B and 7C). By contrast, the potentiating effects of 1 to 100µM 3-PBC on α2β3γ2 but not α5β3γ2 were resistant to Ro15-1788 (Figures 6D and 6E). These findings suggest that the action site on α2β3γ2 at which 3-PBC blocks alcohol’s effects is distinct from the BDZ site.
Figure 7. Effects of 3-PBC in vitro on attenuating alcohol-mediated actions via a benzodiazepine-independent manner.
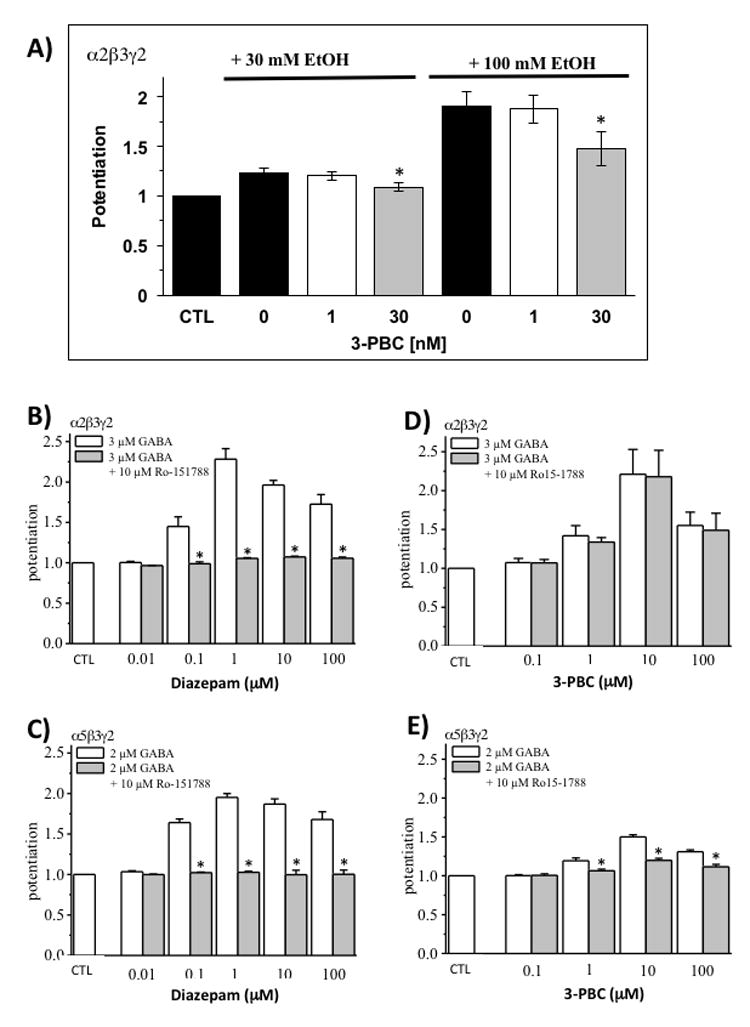
A) Whole-cell recordings of HEK 293 cells expressing recombinant rat α2β3γ2 GABAA receptors were performed. Currents were normalized to the GABA concentration specific for the receptor subtype EC10 under in vitro conditions. Two concentrations of EtOH [30 mM and 100 mM] in the absence or presence of 1 nM and 30 nM 3-PBC, respectively, were co-applied with 1.5 μM GABA. Asterisks [*] denote P ≤ 0.05 in a two-sided t-test, compared to 0 or 1nM PBC. (B-E) Increasing concentrations of diazepam (B, C) or 3-PBC (D,E) in the absence (white) or presence (gray) of 10 μM Ro15-1788 were co-applied with the receptor specific GABA concentrations at about the EC20. Asterisks (*) denote p < 0.05 in a two-sided t-test comparing diazepam and 3-PBC plus Ro15-1788 to the test compounds alone. Error bars indicate the standard error of the mean (± SEM) for at least four cells.
DISCUSSION
Despite the pervasive human clinical literature linking impulsivity and binge drinking during adolescence and young adulthood (Dick et al., 2013, Dick et al., 2006), little direct behavioral or neurobiological evidence exists to support this hypothesis. In the present study, MS, restricted to the early postnatal pre-weaning period, directly led to increased addiction risk illustrated by enhanced acquisition and maintenance of binge drinking during adulthood in rodents, with BACs ≥ 95 mg%/dL. While MS was previously reported to cause long-term increases in alcohol self-administration in adult animals (Cruz et al., 2008, Moffett et al., 2007), subjects did not approximate the binge alcohol levels that have been reported in human alcoholics (Liu et al., 2011, Yang et al., 2011). Thus, this study emulates human binge drinking due to protracted effects of childhood stress on adult alcohol-drinking behavior. MS also facilitated acquisition of cognitive impulsivity during adulthood.
Impulsivity is a behavioral phenotype associated with vulnerability to alcohol use initiation, onset of binge drinking behaviors, early-stage alcohol problems, and end-stage diagnoses of alcohol dependence and abuse (Dick et al., 2010, Lejuez et al., 2010, Rubio et al., 2008). Therefore, this behavior may be an important target of therapeutic intervention. As illustrated in Fig. 2C, MS produced a remarkable impulsivity phenotype across each of the 8 delay intervals tested, with the 16 s interval reaching the maximal level of impulsivity detectable. However, it is not known if binge drinking facilitated acquisition of the impulsivity phenotype, or vice versa. Nevertheless, our findings suggest, MS is a powerful factor in the initiation of both binge drinking and impulsivity, and may influence vulnerability to their co-morbidity.
The MS model employed here is well established (Wang and Gondre-Lewis, 2013, Hulshof et al., 2011, Monroy et al., 2010, Wang et al., 2015), and uses repeated 3-hour separation of newborns from the dams and their littermates over a 20-21 day period, with controls for temperature (room kept at 29C) and conditions that diminish potential auditory stressors. We prefer this MS model to others that use 6 hrs of MS (MS360) or a single 24hr maternal separation at P9 (Nylander and Roman, 2013, Penasco et al., 2015) because these prolonged means of inducing maternal deprivation disrupt the infant’s key metabolic needs for feeding, hydration, and warmth, necessary for survival. Additionally, in many reports, bodily contact (if any) between siblings is not specified, and this factor could introduce inconsistent outcomes across studies. These and other MS paradigms using 2-4 bottle free choice show results that range from no statistical difference in alcohol consumption compared to control animal facility reared animals whether MS was for 15 minutes or 360 minutes (Daoura et al., 2011, Jaworski et al., 2005, Gustafsson et al., 2005), to a reduction in alcohol intake, depending on the rat background and sex (Roman et al., 2003). However, many recent free-choice studies report a clear preference of MS animals for ethanol with MS180 (Huot et al., 2001) or a 24hr deprivation at P9 (Penasco et al., 2015). Therefore, although different MS paradigms or alcohol exposure regimen may influence the findings for MS-induced alcohol preference (Huot et al., 2001, Penasco et al., 2015, Roman et al., 2003), in the current study in which the animals must press the lever 4 times (i.e., work) for the 10% ethanol reinforcement, we clearly demonstrate that MS exposure enhances the propensity for alcohol self-administration. Further, using this same MS model for assessment of delay-discounting, we show that MS causes impulsive-like behavior compared to controls.
Persistent elevations of adult CRF levels were reported in brain regions that modulate stress both after maternal separation, (O’Malley et al., 2011) and in non-stressed high alcohol drinking rodents (Sommer et al., 2008, Zorrilla et al., 2013). Elevations in CRF are purported to regulate binge drinking in rodents (Lowery-Gionta et al., 2012) and humans (Treutlein et al., 2006), and were suggested to play a salient role in the transition to dependence (Koob, 2008). However, the role of CRF in regulating binge drinking and cognitive impulsivity in rodents triggered by stress/negative affective states has not been investigated. To test the hypothesis, the CRF1 receptor antagonist antalarmin was infused in the CeA and mPFC. Antalarmin in each locus produced profound and selective reductions on binge drinking and markedly reduced impulsivity-like responding. These findings provide strong evidence that CRF is a major neuronal regulator of binge drinking and cognitive impulsivity induced by MS. Hence, as with alcohol-dependent subjects (Heilig et al., 2011, Koob, 2008, Lowery and Thiele, 2010) CRF1R antagonists may represent an important therapeutic intervention for psychiatric disorders due to sustained uncontrollable stressors such as childhood trauma.
When applied directly into the CeA and mPFC, two brain regions that exhibit a high density of the GABAA α2 subunit protein (Fritschy and Mohler, 1995, Kaufmann et al., 2003), 3-PBC markedly reduced alcohol drinking in MS rats (Fig. 6), but sucrose drinking was undiminished between groups, indicative of a reinforcement specific behavior of MS rats, and also that 3-PBC was not acting as a sedative (Harvey et al., 2002). Consistent with naïve alcohol-preferring rats, which exhibited elevated GABAA α2 protein compared to non-preferring rats (Liu et al., 2011), the current study reveals increased baseline levels of GABAA α2 protein in MS relative to controls (Fig. 3). This could indicate similar modes of induction of binge drinking in both P and neonatally stressed rat models; We show that modulation of the alpha-2 receptor is sufficient to attenuate binge drinking in MS as was shown in alcohol preferring rats (Harvey et al., 2002, Liu et al., 2011). In addition to the GABAA receptor functions, reductions in overall cortical GABA levels in human adolescents and young adults are highly associated with “cognitive impulsivity” and response inhibition (Silveri et al., 2013). In the current study on the stressed rat model, we do not directly measure the levels of the GABA neurotransmitter, but this could be an important next step in further characterizing the MS model for alcohol, impulsivity and other neuropsychiatric presentations. Indeed, in the current study, the pups were isolated from each other as well as from their mother during the 3-hour separation, thus the possibility exists that infant peer isolation could interact with maternal separation to elicit the effects reported.
We tested the effectiveness of alcohol alone to modulate the GABAA α2β3γ2 receptors, and 3-PBC to modulate alcohol’s action at this GABAA receptor in HEK cells in vitro. The magnitude of the 100mM concentration suggests a response sensitivity of the GABAA α2β3γ2 receptors to moderate and high doses of alcohol. These data provide compelling evidence that 3-PBC was highly effective in attenuating alcohol’s agonistic effects on whole cell currents, particularly at the 30nM concentration. Because benzodiazepine action at the GABA site is well known for its sedative and anti-psychotic effects, the term binds at the ‘benzodiazepine receptor’ or ‘benzodiazepine site’ is promiscuously employed when there is an effect on the GABA receptor. However, our data in HEK cells show that 3-PBC does not seem to act via the classical benzodiazepine receptor because the potentiation of GABA at its EC50 by 3-PBC was not blocked by the universal GABAA receptor null modulator Ro 15-1788, also known as flumazenil (Fig. 7). It is increasingly evident that the GABAA receptor demonstrates specific sensitivity to many molecules aside from GABA, including ethanol (Borghese et al., 2014), dopamine(Hoerbelt et al., 2015) and BDZ (Sieghart, 2015), among others. Thus, although our data demonstrates a potent reduction of alcohol’s effects on α2β3γ by 3-PBC, and the association of GABAA α2 with impulsive behavior in alcoholics (Villafuerte et al., 2012), indicating that 3-PBC can specifically act at the α2 site, in a BDZ-independent manner, we cannot rule out the possibility that 3-PBC might interact with more than one binding site at the GABAA receptor. Additional studies are needed to further characterize the actions of 3-PBC in MS.
However, 3-PBC was shown to be a safe ligand devoid of untoward effects when given orally and did not work additively/synergistically with alcohol, or other benzodiazepine agonists (Harvey et al., 2002, June and Eiler, 2007). Hence, therapeutically, 3-PBC may represent a safe ligand to evaluate for stress-induced binge drinking and cognitive impulsivity induced by stressful life events such as childhood trauma. Although these results are compelling, there are sex-based differences in behavior, brain function and even alcohol clearance that cannot be resolved in the mixed-sex design of the current study. Future studies aimed at addressing sex differences in response to stress are necessary to enlighten a potential heterogeneity in ensuing psychoaffective behaviors following the experience of maternal separation or other early life stress. Further, other more common agonists and antagonists to GABA receptors as well as triple uptake inhibitors should be tested to expand our understanding of neurophysiological (dys) function after undergoing early life stress.
Conclusions
In summary, our data provide strong evidence that MS is a major risk factor for excessive drinking and impulsivity. These behaviors are greatly attenuated by the GABAA ligand, 3-PBC, and the CRF antagonist, antalarmin. These results provide novel insights into the role of the brain stress systems, especially CRF, in the development of impulsivity and concomitant excessive drinking. Therapeutically, these drugs provide two putative therapeutic agents demonstrated here to be effective in attenuating both binge drinking and cognitive impulsivity induced by stressful life events.
Acknowledgments
This research was supported in part by NIH grant NS076517 to JC and AA021262 to LA and MGL.
Footnotes
Declaration of Interest: The authors have no conflict of interest to disclose.
References
- BARR CS, DVOSKIN RL, GUPTE M, SOMMER W, SUN H, SCHWANDT ML, LINDELL SG, KASCKOW JW, SUOMI SJ, GOLDMAN D, HIGLEY JD, HEILIG M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–8. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL RL, RODD ZA, ENGLEMAN EA, TOALSTON JE, MCBRIDE WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–34. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL RL, RODD ZA, LUMENG L, MURPHY JM, MCBRIDE WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- BLOMEYER D, TREUTLEIN J, ESSER G, SCHMIDT MH, SCHUMANN G, LAUCHT M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–51. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- BORGHESE CM, HICKS JA, LAPID DJ, TRUDELL JR, HARRIS RA. GABA(A) receptor transmembrane amino acids are critical for alcohol action: disulfide cross-linking and alkyl methanethiosulfonate labeling reveal relative location of binding sites. J Neurochem. 2014;128:363–75. doi: 10.1111/jnc.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDJI C, FRANCIS D, SHARMA S, PLOTSKY PM, MEANEY MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- CRABBE JC, HARRIS RA, KOOB GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ FC, QUADROS IM, PLANETA CDA S, MICZEK KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–68. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLEY JW, EVERITT BJ, ROBBINS TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- DAOURA L, HAAKER J, NYLANDER I. Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin Exp Res. 2011;35:506–15. doi: 10.1111/j.1530-0277.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- DEMINIERE JM, PIAZZA PV, GUEGAN G, ABROUS N, MACCARI S, LE MOAL M, SIMON H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–9. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- DICK DM, ALIEV F, LATENDRESSE S, PORJESZ B, SCHUCKIT M, RANGASWAMY M, HESSELBROCK V, EDENBERG H, NURNBERGER J, AGRAWAL A, BIERUT L, WANG J, BUCHOLZ K, KUPERMAN S, KRAMER J. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Res Hum Genet. 2013;16:661–9. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK DM, BIERUT L, HINRICHS A, FOX L, BUCHOLZ KK, KRAMER J, KUPERMAN S, HESSELBROCK V, SCHUCKIT M, ALMASY L, TISCHFIELD J, PORJESZ B, BEGLEITER H, NURNBERGER J, JR, XUE X, EDENBER HJ, FOROUD T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- DICK DM, SMITH G, OLAUSSON P, MITCHELL SH, LEEMAN RF, O’MALLEY SS, SHER K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDENBERG HJ, DICK DM, XUEI X, TIAN H, ALMASY L, BAUER LO, CROWE RR, GOATE A, HESSELBROCK V, JONES K, KWON J, LI TK, NURNBERGER JI, JR, O’CONNOR SJ, REICH T, RICE J, SCHUCKIT MA, PORJESZ B, FOROUD T, BEGLEITER H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOCH MA, HODGKINSON CA, YUAN Q, SHEN PH, GOLDMAN D, ROY A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–7. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERITT BJ, BELIN D, ECONOMIDOU D, PELLOUX Y, DALLEY JW, ROBBINS TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITSCHY JM, MOHLER H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–94. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- FUNK CK, ZORRILLA EP, LEE MJ, RICE KC, KOOB GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-GUTIERREZ MS, NAVARRETE F, ARACIL A, BARTOLL A, MARTINEZ-GRAS I, LANCIEGO JL, RUBIO G, MANZANARES J. Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addict Biol. 2015 doi: 10.1111/adb.12266. [DOI] [PubMed] [Google Scholar]
- GEHLERT DR, CIPPITELLI A, THORSELL A, LE AD, HIPSKIND PA, HAMDOUCHI C, LU J, HEMBRE EJ, CRAMER J, SONG M, MCKINZIE D, MORIN M, CICCOCIOPPO R, HEILIG M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE O, SANDERS C, FREILING J, GRIGORYAN E, VU S, ALLEN CD, CRAWFORD E, MANDYAM CD, KOOB GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–61. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILPIN NW, KARANIKAS CA, RICHARDSON HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER YG, ZUBIETA JK, HSU DT, VILLAFUERTE S, MICKEY BJ, TRUCCO EM, BURMEISTER M, ZUCKER RA, HEITZEG MM. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J Neurosci. 2014;34:4099–107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON L, PLOJ K, NYLANDER I. Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav. 2005;81:506–16. doi: 10.1016/j.pbb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- HAMILL OP, MARTY A, NEHER E, SAKMANN B, SIGWORTH FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARVEY SC, FOSTER KL, MCKAY PF, CARROLL MR, SEYOUM R, WOODS JE, 2ND, GREY C, JONES CM, MCCANE S, CUMMINGS R, MASON D, MA C, COOK JM, JUNE HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–75. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILIG M, GOLDMAN D, BERRETTINI W, O’BRIEN CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–84. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOERBELT P, LINDSLEY TA, FLECK MW. Dopamine directly modulates GABAA receptors. J Neurosci. 2015;35:3525–36. doi: 10.1523/JNEUROSCI.4390-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU FC, ZHANG GJ, RAOL YS, VALENTINO RJ, COULTER DA, BROOKS-KAYAL AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–8. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS KN, MATHEWS TA, LOCKE JL, SZELIGA KT, FRIEDMAN DP, BENNETT AJ, JONES SR. Effects of early life stress on drinking and serotonin system activity in rhesus macaques: 5-hydroxyindoleacetic acid in cerebrospinal fluid predicts brain tissue levels. Alcohol. 2012;46:371–6. doi: 10.1016/j.alcohol.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULSHOF HJ, NOVATI A, SGOIFO A, LUITEN PG, DEN BOER JA, MEERLO P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res. 2011;216:552–60. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- HUOT RL, THRIVIKRAMAN KV, MEANEY MJ, PLOTSKY PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–73. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- JAWORSKI JN, FRANCIS DD, BROMMER CL, MORGAN ET, KUHAR MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- JUNE HL, EILER WJA. Handbook of Contemporary Neuropharmacology. John Wiley & Sons, Inc; 2007. Dopaminergic and GABAergic Regulation of Alcohol-Motivated Behaviors: Novel Neuroanatomical Substrates. [Google Scholar]
- KAMINSKI BJ, VAN LINN ML, COOK JM, YIN W, WEERTS EM. Effects of the benzodiazepine GABAA alpha1-preferring ligand, 3-propoxy-beta-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboons. Psychopharmacology (Berl) 2013;227:127–36. doi: 10.1007/s00213-012-2946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMANN WA, HUMPEL C, ALHEID GF, MARKSTEINER J. Compartmentation of alpha 1 and alpha 2 GABA(A) receptor subunits within rat extended amygdala: implications for benzodiazepine action. Brain Res. 2003;964:91–9. doi: 10.1016/s0006-8993(02)04082-9. [DOI] [PubMed] [Google Scholar]
- KOE AS, SALZBERG MR, MORRIS MJ, O’BRIEN TJ, JONES NC. Early life maternal separation stress augmentation of limbic epileptogenesis: the role of corticosterone and HPA axis programming. Psychoneuroendocrinology. 2014;42:124–33. doi: 10.1016/j.psyneuen.2014.01.009. [DOI] [PubMed] [Google Scholar]
- KOOB GF. Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism. Proc Natl Acad Sci U S A. 2008;105:8809–10. doi: 10.1073/pnas.0804354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- KORPI ER, LUDDENS H. Regional gamma-aminobutyric acid sensitivity of t-butylbicyclophosphoro[35S]thionate binding depends on gamma-aminobutyric acidA receptor alpha subunit. Mol Pharmacol. 1993;44:87–92. [PubMed] [Google Scholar]
- LEJUEZ CW, MAGIDSON JF, MITCHELL SH, SINHA R, STEVENS MC, DE WIT H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res. 2010;34:1334–45. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, YANG AR, KELLY T, PUCHE A, ESOGA C, JUNE HL, JR, ELNABAWI A, MERCHENTHALER I, SIEGHART W, JUNE HL, SR, AURELIAN L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–70. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWERY EG, THIELE TE. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWERY-GIONTA EG, NAVARRO M, LI C, PLEIL KE, RINKER JA, COX BR, SPROW GM, KASH TL, THIELE TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–13. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINELLI M, PIAZZA PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- MOFFETT MC, VICENTIC A, KOZEL M, PLOTSKY P, FRANCIS DD, KUHAR MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–30. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONROY E, HERNANDEZ-TORRES E, FLORES G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- NAIMI TS, BREWER RD, MOKDAD A, DENNY C, SERDULA MK, MARKS JS. Binge drinking among US adults. JAMA. 2003;289:70–5. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- NAMJOSHI OA, GRYBOSKI A, FONSECA GO, VAN LINN ML, WANG ZJ, DESCHAMPS JR, COOK JM. Development of a two-step route to 3-PBC and betaCCt, two agents active against alcohol self-administration in rodent and primate models. J Org Chem. 2011;76:4721–7. doi: 10.1021/jo200425m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEMEROFF CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004a;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- NEMEROFF CC. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004b;38:14–20. [PubMed] [Google Scholar]
- NIH-NIAAA. NIAAA council approves definition of binge drinking. NIAAA News. 2004;3 [Google Scholar]
- NYLANDER I, ROMAN E. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology (Berl) 2013;229:555–69. doi: 10.1007/s00213-013-3217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’MALLEY D, DINAN TG, CRYAN JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2011;214:221–9. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- OBERLIN BG, GRAHAME NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENASCO S, MELA V, LOPEZ-MORENO JA, VIVEROS MP, MARCO EM. Early maternal deprivation enhances voluntary alcohol intake induced by exposure to stressful events later in life. Neural Plast. 2015;2015:342761. doi: 10.1155/2015/342761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS TJ, REED C, PASTOR R. Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation. Genes Brain Behav. 2015;14:98–135. doi: 10.1111/gbb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIRKER S, SCHWARZER C, WIESELTHALER A, SIEGHART W, SPERK G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- RACHLIN H, GREEN L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON ES, EAGLE DM, ECONOMIDOU D, THEOBALD DE, MAR AC, MURPHY ER, ROBBINS TW, DALLEY JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ’waiting’ versus ’stopping’. Behav Brain Res. 2009;196:310–6. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- ROCERI M, HENDRIKS W, RACAGNI G, ELLENBROEK BA, RIVA MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–16. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- ROMAN E, HYYTIA P, NYLANDER I. Maternal separation alters acquisition of ethanol intake in male ethanol-preferring AA rats. Alcohol Clin Exp Res. 2003;27:31–7. doi: 10.1097/01.ALC.0000047352.88145.80. [DOI] [PubMed] [Google Scholar]
- ROMANO-LOPEZ A, MENDEZ-DIAZ M, RUIZ-CONTRERAS AE, CARRISOZA R, PROSPERO-GARCIA O. Maternal separation and proclivity for ethanol intake: a potential role of the endocannabinoid system in rats. Neuroscience. 2012;223:296–304. doi: 10.1016/j.neuroscience.2012.07.071. [DOI] [PubMed] [Google Scholar]
- RUBIO G, JIMENEZ M, RODRIGUEZ-JIMENEZ R, MARTINEZ I, AVILA C, FERRE F, JIMENEZ-ARRIERO MA, PONCE G, PALOMO T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–7. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- SIEGHART W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol. 2015;72:53–96. doi: 10.1016/bs.apha.2014.10.002. [DOI] [PubMed] [Google Scholar]
- SILVERI MM, SNEIDER JT, CROWLEY DJ, COVELL MJ, ACHARYA D, ROSSO IM, JENSEN JE. Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMER WH, RIMONDINI R, HANSSON AC, HIPSKIND PA, GEHLERT DR, BARR CS, HEILIG MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- TREUTLEIN J, KISSLING C, FRANK J, WIEMANN S, DONG L, DEPNER M, SAAM C, LASCORZ J, SOYKA M, PREUSS UW, RUJESCU D, SKOWRONEK MH, RIETSCHEL M, SPANAGEL R, HEINZ A, LAUCHT M, MANN K, SCHUMANN G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- VARGAS WM, BENGSTON L, GILPIN NW, WHITCOMB BW, RICHARDSON HN. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. J Neurosci. 2014;34:14777–82. doi: 10.1523/JNEUROSCI.3189-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLAFUERTE S, HEITZEG MM, FOLEY S, YAU WY, MAJCZENKO K, ZUBIETA JK, ZUCKER RA, BURMEISTER M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17:511–9. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, GONDRE-LEWIS MC. Prenatal nicotine and maternal deprivation stress de-regulate the development of CA1, CA3, and dentate gyrus neurons in hippocampus of infant rats. PLoS One. 2013;8:e65517. doi: 10.1371/journal.pone.0065517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q, SHAO F, WANG W. Maternal separation produces alterations of forebrain brain-derived neurotrophic factor expression in differently aged rats. Front Mol Neurosci. 2015;8:49. doi: 10.3389/fnmol.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILHELM CJ, MITCHELL SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–13. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG AR, LIU J, YI HS, WARNOCK KT, WANG M, JUNE HL, JR, PUCHE AC, ELNABAWI A, SIEGHART W, AURELIAN L, JUNE HL., SR Binge Drinking: In Search of its Molecular Target via the GABA(A) Receptor. Front Neurosci. 2011;5:123. doi: 10.3389/fnins.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG XD, LIAO XM, URIBE-MARINO A, LIU R, XIE XM, JIA J, SU YA, LI JT, SCHMIDT MV, WANG XD, SI TM. Stress during a Critical Postnatal Period Induces Region-Specific Structural Abnormalities and Dysfunction of the Prefrontal Cortex via CRF. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORRILLA EP, HEILIG M, DE WIT H, SHAHAM Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–86. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]