Abstract
Mitochondria have a number of essential roles in neuronal function. Their complex mobility patterns within neurons are characterized by frequent changes in direction. Mobile mitochondria can become stationary or pause in regions that have a high metabolic demand and can move again rapidly in response to physiological changes. Defects in mitochondrial transport are implicated in the pathogenesis of several major neurological disorders. Research into the mechanisms that regulate mitochondrial transport is thus an important emerging frontier.
Mitochondria are essential for neuronal function and survival1. Mitochondrial ATP production supports many important functions, including the mobilization of synaptic vesicles for exocytosis and recycling2, the assembly of the actin cytoskeleton for presynaptic development3 and the generation of axonal and synaptic membrane potentials4. In addition, mitochondria have a tremendous capacity to sequester Ca2+ transients elicited by brief trains of action potentials5. During tetanic neuronal stimulation, synaptic mitochondria maintain Ca2+ homeostasis by buffering extra intracellular Ca2+ and releasing Ca2+ after stimulation to prolong the residual Ca2+ levels6. Through this mechanism, synaptic mitochondria are thought to be involved in maintaining and regulating neurotransmission7–10 or certain types of short-term synaptic plasticity11,12.
Neurons are polarized cells that consist of three distinct structural and functional domains: the cell body (soma), a long axon and thick dendrites with many branches and elaborate dendritic arbors. Owing to their unique metabolic requirements, these areas do not display a uniform mitochondrial distribution (reviewed in REF. 13). Areas with high demands for ATP — such as presynaptic and postsynaptic terminals, active growth cones or axonal branches, and nodes of Ranvier — contain more mitochondria than other cellular domains14–20. Although the biogenesis of mitochondria can occur locally within the axon21, it is thought that most new mitochondria are generated in the soma and that dysfunctional mitochondria also return to the soma for degradation by the autophagy–lysosomal system. Although little direct evidence for this hypothesis exists, the idea is based on the fact that the cellular machineries for DNA replication, mRNA and protein synthesis, and membrane protein sorting and trafficking, as well as degradation organelles such as lysosomes, are all predominantly localized in the soma. Thus, it is likely that neurons require specialized mechanisms to transport mitochondria from the soma to their destinations and to ensure that the mitochondria remain stationary in particular regions to support various neuronal functions.
Mitochondrial transport in neurons is regulated in response to acute application of glutamate22,23 or to elevated neuronal Ca2+ levels caused by the application of the calcium ionophore calcimycin24. Activation of glutamate receptors with exogenous or synaptically released glutamate recruits mitochondria to synapses23. In cardiac myoblasts, mitochondrial movement is also arrested when the intracellular Ca2+ concentration is increased by applying the Ca2+-mobilizing hormone vasopressin25 or during spikes in the cytosolic free Ca2+ concentration mediated by the inositol trisphosphate receptor or the ryanodine receptor26. Given the dynamic nature of neuronal activity patterns, efficient regulation of mitochondrial mobility is required to enable the rapid redistribution of mitochondria to different areas in order to meet increased metabolic requirements. Similarly, mitochondrial transport is regulated during neuronal development16,18. Defective mitochondrial transport is thought to contribute to the pathogenesis of some neurodegenerative diseases (reviewed in REFS 27–30). Thus, understanding the mechanisms that regulate mitochondrial mobility and distribution in response to neuronal activity and various physiological and pathological states will advance our knowledge of processes that are essential for neuronal function and may shed light on disease mechanisms. Here, we provide an overview of the mechanisms that regulate mitochondrial transport and distribution and discuss how defects of these mechanisms affect axonal and synaptic homeostasis, mitochondrial quality control and neurodegeneration.
Mitochondrial transport machinery
When visualized using time-lapse imaging approaches (BOX 1), neuronal mitochondria can be observed to undergo dynamic, bidirectional transport along neuronal processes, frequently changing direction, pausing or switching to persistent docking12,13,16,31,32. These complex mitochondrial mobility patterns are a result of mitochondrial coupling to anterograde and retrograde motor proteins and to docking and anchoring machinery (TABLE 1). Mitochondria attach to the motors by associating with their respective motor adaptor proteins directly or through mitochondrial receptors. These motor–adaptor–receptor complexes ensure targeted trafficking of mitochondria and precise regulation of their mobility.
Table 1.
Molecular motor–adaptor complexes and regulators of mitochondrial transport
| Protein | Role | Organism(s) | Refs |
|---|---|---|---|
| KIF5 | Microtubule motor | Drosophila melanogaster and mammals | 35,37,40,41 |
| KIF1Bα | Microtubule motor | Mammals | 42 |
| KLP6 | Microtubule motor | Mammals | 43 |
| Dynein–dynactin | Microtubule motor | D. melanogaster and mammals | 37,60 |
| MIRO | KIF5 receptor | D. melanogaster and mammals | 45–47,49 |
| Milton | KIF5 adaptor | D. melanogaster | 36,44,48 |
| TRAKs (OIP106, GRIF1) | KIF5 adaptor | Mammals | 46,50,51,55,56 |
| Syntabulin | KIF5 adaptor | Mammals | 41 |
| FEZ1 | KIF5 adaptor | Mammals | 57,58 |
| RANBP2 | KIF5 adaptor | Mammals | 59 |
| APLIP1 | KIF5 adaptor | D. melanogaster | 69 |
| Syntaphilin | Static anchor | Mammals | 12,81 |
| Mitofusins | MIRO–TRAK regulator | Mammals | 70 |
| Tau | Microtubule stability | Mammals | 93 – 99 |
| MAP1B | Microtubule stability | Mammals | 100 |
| Myosin XIX | Actin motor | Mammals | 72 |
| Myosin V | Actin motor | D. melanogaster neurons | 75 |
| Myosin VI | Actin motor | D. melanogaster neurons | 75 |
| WAVE1 | Actin polymerization | Mammals | 82 |
APLIP1, APP-like-interacting protein1; FEZ1, fasciculation and elongation protein-ζ1; KIF, kinesin superfamily protein; MAP1B, microtubule-associated protein 1B; MIRO, Mitochondrial rho; RANBP2, RAN-binding protein 2; WAVE1, WASP family verprolin homologous protein 1.
Long-range mitochondrial transport from the soma to distal axonal and dendritic regions depends on the polarity and organization of neuronal microtubules. Microtubules are formed from the polymerization of α- and β-tubulin and are arranged in a polarized manner with plus and minus ends. Kinesin superfamily proteins (KIFs) and cytoplasmic dynein are the main microtubule-based motor proteins. They drive long- distance transport of mitochondria and other membranous organelles or cargoes through mechanisms that require ATP hydrolysis (reviewed in REF. 33). Whereas most KIF motors move towards the microtubule plus end, cytoplasmic dynein motors mediate microtubule minus-end-directed transport. Axonal microtubules are uniformly arranged so that their minus ends are directed towards the soma and their plus ends are directed distally. Thus, in axons, the minus-end-directed cytoplasmic dynein motors are responsible for retrograde movement towards the soma, whereas plus-end-directed KIF motors drive anterograde transport to distal axonal regions and synaptic terminals (reviewed in REFS 33,34)(FIG. 1). As dendritic microtubules exhibit mixed polarity in the proximal regions, KIFs and dynein motors can drive cargo transport in dendrites in either an anterograde or retrograde direction depending on the microtubule polarity.
Figure 1. Mitochondrial transport in neurons.
a | Neurons have three distinct functional and structural domains: a cell body (soma), a long axon and thick dendrites with many branches and elaborate dendritic arbors. Owing to the complex geometry of neurons, specialized mechanisms are required to transport mitochondria to their destinations and to ensure that mitochondria remain stationary in regions with a high demand for energy production and Ca2+ homeostatic capacity. The figure highlights the molecular mechanisms that are involved in transporting mitochondria to three specific neuronal locations: the presynaptic terminal, the axon and the dendritic spine. Long-range mitochondrial transport from the soma to distal axonal and dendritic regions depends on the polarity and organization of neuronal microtubules. Axonal microtubules are arranged so that their plus end (+) is directed distally and their minus end (−) is directed towards the soma. Thus, in axons, cytoplasmic dynein motors are responsible for returning mitochondria to the soma, whereas kinesin motors of the KIF5 family drive anterograde mitochondrial transport to distal axonal regions and synaptic terminals. For dendritic processes, where microtubules exhibit mixed polarity in proximal regions, kinesins and dynein motors can transport mitochondria in either the anterograde or the retrograde direction depending on the microtubule polarity. KIF5 motors link to the mitochondria that they transport via motor adaptors. Myosin motors probably mediate short-range movement in presynaptic terminals, growth cones and dendritic spines, where actin filaments form the major cytoskeletal architecture. Mobile mitochondria can also be recruited to stationary pools via dynamic interactions between the docking receptor syntaphilin and microtubules or via actin-based anchoring machinery. Mitochondrial docking mechanisms ensure that stationary mitochondria are adequately distributed within axons and at synapses. b | Mitochondrial movement and accumulation near a node of Ranvier in a peripheral nervous system myelinated axon. A high density of Na+ channels and Na+/K+ ATPases at the nodes of Ranvier is essential for myelinated axons to conduct high-velocity nerve impulses and to permit repetitive firing. Thus, regions near nodes of Ranvier have a high energy demand. KIF5 and dynein motors drive either anterograde or retrograde mitochondrial transport towards the node of Ranvier. Mobile mitochondria become stationary at the node and serve as energy sources to support Na+ channels and Na+/K+ ATPases.
Anterograde transport motors
At least 45 different KIF motor genes, which are classified into 14 families, have been identified in humans and mice33. Members of the kinesin-1 family (collectively known as KIF5) have a key role in the anterograde transport of neuronal mitochondria35–37. Each KIF5 heavy chain contains an amino-terminal motor domain, whereas its carboxy-terminal domain mediates an association with kinesin light chains or directly interacts with cargoes or cargo adaptors such as the mitochondrial adaptor proteins (reviewed in REFS 33,38). Mammals have three KIF5 motor isoforms (namely, KIF5A, KIF5B and KIF5C). KIF5B is expressed by most cell types, whereas KIF5A and KIF5C are found only in neurons39 (BOX 2), where they are associated with various membranous organelles, including mitochondria40. Mutations in the murine Kif5b gene result in the clustering of mitochondria close to the nucleus in undifferentiated extra-embryonic cells, whereas in wild-type conditions mitochondria are transported towards the cell periphery35. Drosophila melanogaster that has mutations in Khc (which encodes the sole KIF5 homologue in D. melanogaster) also exhibits impaired mitochondrial transport in larval motor axons37. Furthermore, overexpressing the KIF5 cargo-binding domain in hippocampal neurons disrupts the linkage between KIF5 and mitochondrial adaptor proteins and consequently impairs anterograde mitochondrial transport41.
Although mutation of the D. melanogaster Khc gene severely reduces anterograde mitochondrial transport in motor neurons, it does not abolish it entirely37, suggesting that other kinesin motors also drive mitochondrial transport. KIF1Bα is a brain-enriched member of the monomeric kinesin-3 motor family that colocalizes with mitochondria in vivo42. Purified KIF1Bα can transport mitochondria along microtubules in vitro. Mutation of Klp6, a newly identified member of the kinesin motor family, inhibits anterograde transport of mitochondria into axonal neurites43. However, the proposed roles of KIF1Bα and KLP6 in anterograde mitochondrial transport in neurons require further investigation.
Motor adaptors
The D. melanogaster protein Milton is a well-characterized motor adaptor protein that is associated with KIF5 and seems to be involved in neuronal mitochondrial transport. Milton is linked indirectly to mitochondria through an interaction with Mitochondrial rho (MIRO), a RHO family GTPase that is present in the mitochondrial outer membrane44–47. MIRO contains two EF hand Ca2+-binding motifs and two GTPase domains and connects Milton and KIF5 to mitochondria. Milton binds directly to the C-terminal cargo-binding domain of the kinesin heavy chain48. KIF5 is recruited to the mitochondrial surface by the Milton–MIRO adaptor independently of the kinesin light chain. Thus, KIF5, Milton and MIRO constitute an antero-grade transport system that is specific for mitochondria (FIG. 2a). Mutation of the milton or Miro genes in D. melanogaster results in impaired anterograde mitochondrial transport and the depletion of mitochondria in distal synaptic terminals36,44,49.
Figure 2. KIF5-driven mitochondrial transport.
a | Mitochondrial transport driven by kinesins of the KIF5 family requires the mitochondrial rho (MIRO)–Milton (or MIRO–TRAK) adaptor complex. MIRO is a mitochondrial outer membrane protein of the RHO GTPase family. In Drosophila melanogaster, Milton recruits KIF5 to mitochondria by binding to MIRO. In a similar way, TRAK1 and TRAK2 (mammalian Milton orthologues) can bind to MIRO1 and MIRO2 (mammalian orthologues of MIRO). In hippocampal neurons, the MIRO1–TRAK2 complex is an important regulator of mitochondrial trafficking. b | KIF5 also associates with mitochondria and mediates mitochondrial anterograde transport via syntabulin, a KIF5 adaptor that binds to mitochondria via its carboxy-terminal transmembrane domain. Fasciculation and elongation protein-ζ1 (FEZ1) and RAN-binding protein 2 (RANBP2) are additional kinesin adaptors that may contribute to mitochondrial transport. c | There are two proposed models of the role of MIRO in the regulation of mitochondrial mobility. MIRO contains two Ca2+-binding regions (EF-hand motifs), which allow it to regulate mitochondrial mobility in response to synaptic activity and Ca2+ signalling pathways. The C-terminus of the KIF5 motor attaches to mitochondria through an interaction with the MIRO–Milton (or MIRO–TRAK) complex in the absence of Ca2+, whereas its amino-terminal motor domain binds to microtubules and drives transport. In the ‘motor–MIRO binding’ model24, Ca2+ binds to the EF hands of MIRO and induces the KIF5 motor domain to bind to MIRO instead of microtubules and thus prevents motor–microtubule engagement. d | Alternatively, in the ‘motor-releasing’ model23, Ca2+ binding releases KIF5 from MIRO-bound mitochondria. Thus, Ca2+ influx following synaptic activity could cause mobile mitochondria to become stationary.
In mammals, two Milton orthologues (TRAK1 and TRAK2) and two MIRO orthologues (MIRO1 and MIRO2) have been identified. TRAK1 and TRAK2 — also termed OIP106 (106kDa O-GlcNAc transferase-interacting protein) and GRIF1 (GABA A receptor-interacting factor 1), respectively — were shown to interact with KIF5 (REFS 50,51). Interestingly, TRAKs are required not only for mitochondrial transport but also for the trafficking of other organelles, including endosomes52–54. TRAKs bind to the first GTPase domain of MIRO1 and MIRO2 (REF. 46). In hippocampal neurons, MIRO1 acts as the major mitochondrial acceptor site for TRAK2 and the MIRO1– TRAK2 complex has been shown to be a key regulator of mitochondrial transport55. Elevating MIRO1 levels enhances the recruitment of TRAK2 to mitochondria and facilitates mitochondrial transport55. Knocking down TRAK1 or expressing dominant-negative TRAK1 mutants in hippocampal neurons results in impaired mitochondrial mobility in axons, thus providing evidence for the roles of the endogenous TRAK family in the regulation of mitochondrial mobility56.
Syntabulin is another prominent KIF5 motor adaptor for mitochondria41. It binds directly to the cargo-binding domain of KIF5 motors and attaches to the outer mitochondrial membrane via its C-terminal domain. Depleting syntabulin using small interfering RNA (siRNA) or blocking the syntabulin–KIF5 interaction by introducing transgenes that interfere with the interaction reduces anterograde mitochondrial transport along axons in cultured hippocampal neurons41.
Several other proteins have also been proposed to be adaptors that link KIF5 motors to mitochondria. These proteins include fasciculation and elongation protein-ζ1 (FEZ1), which is a brain-specific protein involved in axonal outgrowth. FEZ1 has been shown to serve as a kinesin motor adaptor and is essential for axonal mitochondrial transport57,58. Knocking down FEZ1 using RNA interference (RNAi) reduces anterograde mitochondrial movement into the tips of growing neurites of both hippo campal neurons and PC12 cells. Thus, FEZ1 is proposed to participate in the establishment of neuronal polarity by controlling mitochondrial motility along axons. RAN-binding protein 2 (RANBP2) is another mitochondrial adaptor candidate. It has been shown to mediate the interaction between mitochondria and KIF5B or KIF5C (but not KIF5A)59. Inhibition of the interaction between RANBP2 and KIF5B or KIF5C in non-neuronal cell lines causes perinuclear clustering of mitochondria. However, whether this protein has a role in neuronal mitochondrial transport remains unclear.
Why do neurons require multiple KIF5 adaptors to mediate mitochondrial transport? One attractive hypothesis is that KIF5 motors physically attach to their transport cargoes via different adaptor complexes, thus allowing neurons to regulate mitochondrial transport through separate mechanisms in response to different physiological signals. The identification of the ATP/ADP and Ca2+ sensors that are involved in the response to these signals will help to determine how neuronal mitochondrial transport and distribution are coordinated so precisely by neuronal function and metabolic demands. It is worth noting that future identification of new candidate motor adaptors and transport regulators should require a higher standard of evidence than has previously been possible. Biochemical evidence showing interactions between the candidate adaptor or regulator protein and endogenous motor proteins should be combined with an analysis of the effects of the adaptor or regulator on cell biology in live cells. Following overexpression of exogenous candidate adaptor or regulator proteins or knock-down of the endogenous levels of these proteins, it is necessary to observe altered mitochondrial transport in neurons in order to verify their physiological roles. In addition, loss-of-function analysis (such as interference with the motor–adaptor interactions and motor–mitochondrion tethering because of overexpression of binding-domain mutants or dominant-negative mutants) should also be used to justify any new candidate motor adaptors or regulators for mitochondrial transport.
Retrograde transport motors
Cytoplasmic dynein drives the retrograde movement of mitochondria in axons (BOX 2), whereas in dendrites, where micro-tubule polarity is mixed, it is involved in transporting mitochondria to both the periphery and the cell body. Cytoplasmic dynein is composed of two dynein heavy chains and several dynein intermediate chains, dynein light intermediate chains and dynein light chains. Dynein heavy chains function as motors, and the association of the dynein motor with cargoes and the regulation of its motility involve various other polypeptides. In the D. melanogaster nervous system, mutations of dynein heavy chain genes alter various aspects of mitochondrial transport in axons, including retrograde transport velocity and run length37. The mechanisms that link dynein to mitochondria are not well characterized. In contrast to the kinesin superfamily, few dynein heavy chains have been identified. Thus, accessory proteins probably mediate cargo-binding specificity. One model suggests that dyneins link to organelle membrane-associated proteins via their light and intermediate chains. TCTEX1, which is a dynein light chain protein, is thought to associate with mitochondria by binding to the mitochondrial outer membrane protein voltage-dependent anion- selective channel 1 (VDAC1)60. Dynactin, a large 11-subunit complex, binds directly to cytoplasmic dynein and to microtubules through its p150Glued component, thus enhancing the processivity of the dynein motor or regulating its interactions with its cargo61. Both dynein and dynactin have been shown to associate with purified D. melanogaster mitochondria and to be essential for mediating mitochondrial retrograde transport37. However, an in vivo analysis showed that dynactin has a crucial role in regulating and/or coordinating the bidirectional motility of membrane organelles and is not required to link dynein to membranes62. Snapin was recently reported as a dynein motor adaptor that targets the late endocytic membrane and the snapin–dynein intermediate chain linkage contributes to dynein-mediated retrograde transport of late endocytic organelles in neurons63. Thus, the question still remains whether the dynein motor complex associates with mitochondrial membranes directly or indirectly via a linkage by an unidentified dynein adaptor. Dynein-mediated retrograde mitochondrial transport in D. melanogaster axons also appears to require the action of MIRO64. Loss of the Miro gene impairs kinesin- and dynein-mediated mitochondrial transport and overexpressing MIRO also alters mitochondrial transport. Although a MIRO–dynein association has not been demonstrated, it seems that MIRO may not simply be an adaptor for KIF5 but may also be required for dynein-mediated retrograde mitochondrial transport.
Interplay of motor proteins
The bidirectional transport of mitochondria suggests that both anterograde and retro grade motors may be simultaneously associated with an individual mitochondrion. Indeed, dynein has been observed to colocalize both with mitochondria that are moving in the anterograde direction and with those moving in the retrograde direction65. However, disruptions to kinesin-driven movement do not seem to lead to a simple domination of mitochondrial movement by dynein23,24. Thus, the kinesin and dynein motor proteins probably act to coordinate bidirectional transport rather than simply competing against one another66,67. This makes understanding their regulation an interesting challenge. Although it mostly promotes movement towards the microtubule minus end, dynein can move bidirectionally68. In D. melanogaster mutants lacking the dynactin complex, dynein displays normal associations with membrane compartments. However, anterograde and retrograde organelle movement is disrupted, suggesting that dynactin may coordinate the activity of opposing motors62. Mutations in the dynein heavy chain or dynactin p150Glued subunit disrupt fast bidirectional organelle transport and result in axonal swellings that contain mitochondria and other types of cargo34. Similarly, the inhibition of kinesin-1 also reduces both anterograde and retrograde mitochondrial movement, suggesting that kinesin-1 is required for dynein-driven retrograde transport37.
It remains unclear whether the direction in which an individual mitochondrion moves is determined by a ‘tugof-war’ between opposing kinesin and dynein motors. If this were the case, any changes in the activity of one motor would be inversely correlated to changes in the opposing motor. The more powerful motor would dictate the ultimate transport direction. Alternatively, bidirectional transport may depend on the processivity of the motors. Any change in motor processivity should alter the directional bias and the time spent in stationary phases.
Several lines of evidence support a potential coupling between kinesin adaptors and dynein motor activity. First, APP-like-interacting protein 1 (APLIP1), a kinesin-binding scaffold protein of the JIP family, influences mitochondrial transport in D. melanogaster axons69. A mutation in Aplip1 impaired retrograde mitochondrial transport, suggesting that dynein activity was inhibited. Second, MIRO regulates both anterograde and retrograde axonal mitochondrial transport64. When Miro was mutated, the distance and duration of movement in the main direction of net transport was reduced and the duration of pauses was increased. Thus, MIRO may promote either kinesin- or dynein-mediated movement in response to a signal that determines the net transport direction of mitochondria. Third, the mitochondrial fusion proteins mitofusin 1 (MFN1) and MFN2 have a physical coupling with the mouse MIRO proteins, through which they regulate axonal mitochondrial transport. Deleting Mfn2 or expressing mutant MFN2 in neurons resulted in longer pauses and slower anterograde and retrograde movements70. This study may highlight a role of the MIRO2–MFN2 complex in regulating the processivity of kinesin or in coordinating the switch between kinesin and dynein. However, further studies are necessary to determine whether MIRO2 facilitates crosstalk between kinesin and dynein and to elucidate the mechanisms by which bidirectional mitochondrial movement is coordinated in response to changes in neuronal physiology.
Short-range myosin motors
Although long-range mitochondrial transport along processes is driven by microtubule-based kinesin and dynein motors, short-range movement in nerve terminals and growth cones — where actin filaments form the major cytoskeletal architecture — is probably mediated by myosin motors. Axonal mitochondria have been shown to travel along both microtubules and actin microfilaments in cultured cells, but with different velocities and net transport properties71. Although mitochondrial association with myosin motors and myosin-driven mitochondrial transport have not been directly demonstrated in neurons, it has been shown that cargoes associated with myosin V move along axons at a rate that is markedly slower than that of most axonal organelles, but similar to that of some mitochondria. Thus, myosin motors may drive short-range mitochondrial movement in certain regions where actin filaments are enriched (FIG. 1). Myosin XIX is a newly identified mitochondrion-associated myosin that is involved in actin-based mitochondrial dynamics72. Expression of a green fluorescent protein (GFP)–myosin XIX fusion protein in A549 cells (a human lung adenocarcinoma epithelial cell line) induces a dramatic increase in actin-based mitochondrial motility. Given that myosin XIX is targeted to mitochondria by its tail domain and is widely expressed, including in neurons, it is likely that myosin XIX may also be involved in mitochondrial transport along neuronal processes and at synaptic terminals. As myosin V can form hetero-motor complexes by interacting with a dynein light chain73, a coupled ‘dual motor complex’ may coordinate mitochondrial long-range transport and short-range movement. If so, one outstanding question is: how do neurons precisely control the transitions between microtubule- and actin-based transport?
Given that mitochondrial movement is enhanced in the absence of actin71 and that actin is required for mitochondrial docking along axons74, an interesting hypothesis is that myosin V competes with dynein for binding to organelles, causing them to be displaced from microtubule tracks onto actin filaments during retrograde movements. A recent study using D. melanogaster neurons and RNAi demonstrates that myosin V and VI modulate axonal mitochondrial transport75. Myosin V depletion increased mitochondrial velocity in both directions, whereas knocking down myosin VI expression selectively increased retrograde mitochondrial transport in axons. These results suggest that myosin V and VI may compete with microtubule-based motor proteins. Alternatively, myosins may promote mitochondrial docking along the actin-based cytoskeleton by moving mitochondria away from microtubule tracks (FIG. 3d). According to this model, fine and dynamic coordination of microtubule- and actin-based motor proteins, as well as of docking and anchoring receptors, probably contributes to the complex, saltatory mobility patterns of neuronal mitochondria. Although these two models are certainly attractive, further investigation is needed to determine how myosin motors regulate microtubule-based motor activity and whether they are required for synapse-directed mitochondrial transport or for mitochondrial docking at regions of axons and dendrites with high metabolic demands.
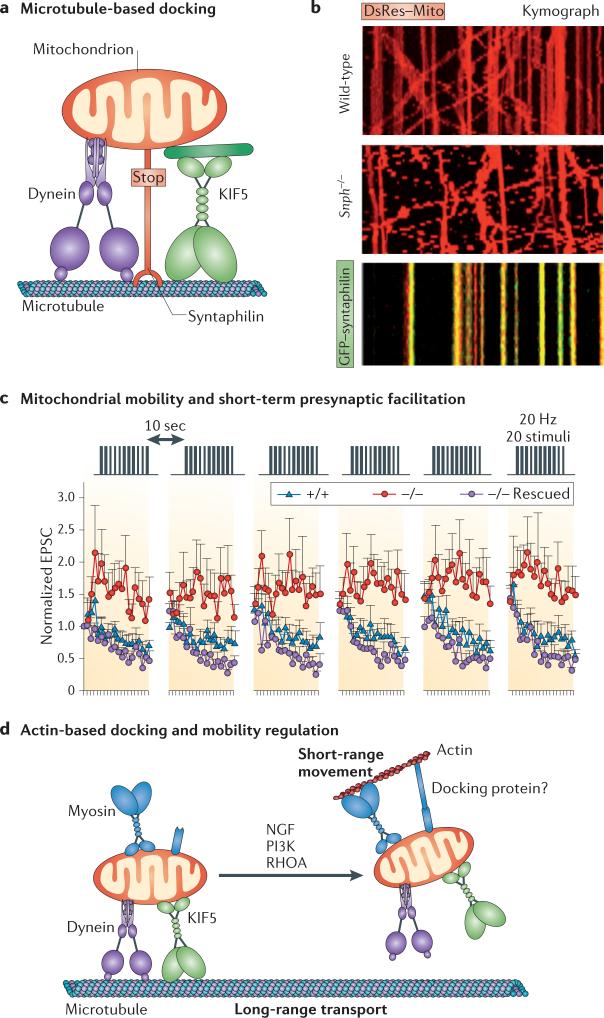
Figure 3. Mitochondrial docking and synaptic homeostasis.
a | Microtubule-based docking. Mitochondria have complex mobility patterns that suggest that they are coupled to two opposing molecular motors, namely kinesin-1 (KIF5) and dynein, as well as to docking machinery. Whereas KIF5 motors are responsible for anterograde mitochondrial transport, cytoplasmic dynein motors are the driving force behind retrograde movement. Syntaphilin, a neuron-specific and axon-targeted protein that associates with the mitochondrial outer membrane, acts as an anchor for axonal mitochondria by binding to microtubules. b | Representative kymographs showing relative mitochondrial mobility in axons. The upper panel shows wild-type neurons transfected with the mitochondrial marker DsRed–Mito (red). The middle panel shows syntaphilin-deficient neurons transfected with DsRed–Mito. The lower panel shows wild-type neurons co-transfected with DsRed–Mito and green fluorescent protein (GFP)–syntaphilin (green). In kymographs, vertical lines represent stationary mitochondria, whereas slanted or curved lines indicate mobile ones. Overexpressing GFP–syntaphilin in wild-type neurons abolishes axonal mitochondrial transport (lower kymograph). Conversely, deleting the murine syntaphilin gene (Snph) results in a dramatic increase in the percentage of axonal mitochondria in dynamic mobile states (76 ± 20%) (middle kymograph; Supplementary information S2 (movie)) relative to wild-type neurons (36 ± 15%) (upper kymograph; Supplementary information S1 (movie)). c | Increased mitochondrial mobility in Snph−/− neurons induces short-term presynaptic facilitation during prolonged stimulation. A 20 Hz, 1 second stimulus train was delivered at 10-second intervals. Normalized excitatory postsynaptic current (EPSC) amplitudes were plotted against stimulus number. Persistent facilitation in synaptic responses occurred only in Snph−/− neurons (red circles). Reintroducing syntaphilin into the mutant presynaptic neurons (purple circles) eliminated the short-term presynaptic facilitation and fully rescued the wild-type phenotype (blue triangles). d | Schematic model of the transition from long-range, microtubule-based mitochondrial transport to myosin-driven short-range, actin-based movement and of subsequent stationary docking on actin through unknown docking and anchoring receptors. Actin-based docking is facilitated by nerve growth factor (NGF), phosphoinositide 3-kinase (PI3K) or RHOA signalling pathways. Part c is modified, with permission, from REF. 12 © (2008) Elsevier.
Mitochondrial docking mechanisms
Mitochondrial docking maintains the required numbers of stationary mitochondria in regions that particularly rely on energy production and Ca2+-buffering capacity. ATP has a limited diffusion capacity in the intracellular environment76 and particularly within long neuronal processes; thus, stationary mitochondria are needed to provide local sources of ATP, which is necessary to maintain the activity of Na+/K+ ATPases, as well as fast spike propagation and synaptic transmission. Mitochondria, which can sequester intracellular Ca2+, also have an important role in maintaining Ca2+ homeostasis at synapses5–7,11,12. We are now beginning to understand the mechanisms that neurons use to modify the numbers of mobile and stationary mitochondria in response to changes in neuronal and synaptic activity.
Microtubule-based docking
Approximately two-thirds of the mitochondrial population have been shown to be stationary in mature neurons. It is likely that these immobilized mitochondria either have dissociated from transport motors or are anchored to the cytoskeleton. A recent study identified syntaphilin as a ‘static anchor’ for axonal mitochondria12. Syntaphilin is a neuron-specific protein that binds to the mitochondrial outer membrane through its C-terminal domain (FIG. 3a). Deleting the murine syntaphilin gene (Snph) resulted in a substantial increase in the percentage of mobile axonal mitochondria relative to that of wild-type neurons (FIG. 3b; Supplementary information S1,S2 (movies)). Conversely, overexpressing syntaphilin abolished axonal mitochondrial transport. Syntaphilin docks mitochondria by anchoring to microtubules, and this provides an explanation for the previous observations of biochemical interactions between neuronal mitochondria and micro-tubules and of morphological crossbridges between axonal mitochondria and microtubules77–80.
The identification of syntaphilin as an axonal mitochondrial docking receptor has led to the discovery of further molecules involved in the regulation of the temporal and spatial distribution of mitochondria in axons. LC8, a dynein light chain, can recruit mobile mitochondria into the stationary pool by stabilizing the syntaphilin–microtubule interaction81. This finding suggests that mitochondrial mobility can be regulated by dynamic interactions between docking receptors and the axonal cytoskeleton. Syntaphilin contains 12% serine residues and several phosphorylation sites, which suggests that its docking function can be regulated through diverse signal transduction pathways. Thus, syntaphilin may be an interesting molecular target to use to further investigate how mobile mitochondria are recruited to the stationary pool in response to changes in axonal activity and synaptic modification. In addition, Snph-knockout mice provide a genetic tool to address whether enhanced mitochondrial mobility contributes to the recruitment of dysfunctional mitochondria from distal axons and synapses for turnover by mitophagy.
Actin-based docking
Both axonal and dendritic mitochondria have been shown to dock along actin filaments74, although the docking receptors involved have yet to be identified. Actin is enriched in growth cones, presynaptic terminals and dendritic spines, where ATP consumption is high. Nerve growth factor (NGF) can influence the interactions between mitochondria and actin and thus regulate mitochondrial mobility74 (FIG. 3d). Mitochondria in dendritic spines have a crucial role in spine morphogenesis and synaptic transmission19. WASP family verprolin homologous protein 1 (WAVE1) regulates actin polymerization and is essential for activity-dependent mitochondrial transport to dendritic spines82. Early electron microscopy imaging analyses revealed morphological evidence showing the crossbridges between axonal mitochondria and neurofilaments77. Neurofilaments bind to mitochondria through the neurofilament heavy chain. This interaction is affected by the phosphorylation status of neurofilaments and is dependent on the mitochondrial membrane potential83. Inducing mitochondrial depolarization releases mitochondria from neurofilaments. These observations suggest that healthy mitochondria remain stably anchored along neurofilaments, whereas dysfunctional mitochondria are transported back to the soma for recycling.
Regulation of mitochondrial transport
Synaptic activity-dependent regulation
The transport and spatial distribution of mitochondria in neurons is directly correlated with synaptic activity (reviewed in REF. 38). Mitochondria are often retained in presynaptic terminals and postsynaptic dendritic spines during sustained synaptic activity19,23. Mitochondria are transported to activated synapses in response to two intracellular signals that control their velocity and their recruitment into the stationary pool. The first signalling pathway involves the detection of ATP and ADP levels: mitochondrial velocity increases when the mitochondria enter regions with high ATP levels and decreases when they are close to areas of local ATP depletion, such as synapses84. The consumption of synaptic ATP following the application of 1 mM glutamate to the medium, which results in local production of ADP, facilitates the recruitment of more mitochondria to the activated synapses. The second signalling pathway involves alterations in intracellular Ca2+ concentration. Elevated intracellular Ca2+ levels inhibit mitochondrial mobility23,24. Enhanced Ca2+ influx — as a result of either KCl-induced activation of voltage-dependent Ca2+ channels at presynaptic terminals or activation of NMDA receptors by glutamate at postsynaptic terminals — decreases mitochondrial movement in cultured neurons22,85. Thus, it is important to understand the mechanisms by which mobile mitochondria are recruited to the stationary pool at synapses in response to these signals.
Identification of the KIF5–Milton–MIRO complex provides molecular targets to address this issue. Three elegant studies independently identified MIRO as a Ca2+ sensor, providing a potential mechanism underlying Ca2+-dependent regulation of mitochondrial mobility23–25. MIRO is located at the outer mitochondrial membrane and has two Ca2+-binding EF hands45. Functional assembly of the anterograde transport complex KIF5–Milton–MIRO (in D. melanogaster neurons) or KIF5–TRAK2–MIRO (in mammalian cells) allowed mitochondria to move along dendrites23 and axons24 of hippocampal neurons or along microtubules in a cardiac cell line25. Elevated cytosolic Ca2+ levels inhibited this mitochondrial mobility. However, this Ca2+-induced cessation of mitochondrial movement was effectively abolished in neurons expressing mutant MIRO EF hands that cannot bind Ca2+. Thus, these studies suggest a MIRO-mediated Ca2+-sensing pathway through which mitochondria are recruited into activated synapses in response to increased action potential firing rates or the activation of glutamate receptors.
Two possible mechanisms have been proposed to explain how Ca2+ sensing by MIRO mediates the suppression of mitochondrial mobility. KIF5 attaches to mitochondria through an interaction between its C-terminus and the MIRO–Milton (or MIRO–TRAK2) complex in the absence of Ca2+ and this leaves its N-terminal motor domain free to bind microtubules and drive transport. In the ‘motor–MIRO binding’ model24, Ca2+ binding to MIRO induces the motor domain of KIF5 to bind to MIRO instead of to microtubules (FIG. 2c). Alternatively, in the ‘motor-releasing’ model23, Ca2+ binding to MIRO releases KIF5 from MIRO-bound mitochondria (FIG. 2d). Thus, through this MIRO-mediated Ca2+-sensing pathway, mobile mitochondria are recruited to and retained at activated synapses86.
Although these models are attractive, several issues must be addressed. First, the mechanisms that underlie Ca2+- and MIRO-dependent inactivation of the KIF5 transport machinery are not clear. Second, although Ca2+- and MIRO-dependent suppression of mitochondrial mobility affects both anterograde and retrograde transport, it is unclear how this pathway affects the retrograde dynein transport complex. As described previously, when KIF5-driven movement is impaired, retrograde movement does not necessarily dominate23–25. Thus, the inactivation of the KIF5 transport machinery that is induced by MIRO and Ca2+ probably occurs through a static docking mechanism. Third, further investigation is required into whether the motor–adaptor complexes and docking receptors share a single regulatory system and how docking interactions physically displace or compete with the motor–adaptor complex. Furthermore, what are the ATP/ADP and Ca2+ sensors that coordinate these interactions? It is hoped that the use of genetic mouse models will enable further dissection of the molecular mechanisms involved.
Whereas cytoplasmic Ca2+ has a vital role in regulating mitochondrial motility in neurons, a recent study suggested that mitochondrial matrix Ca2+ content is also an intrinsic signal for modulating mitochondrial transportation in hippocampal neurons87. Interestingly, the Ca2+ sensor MIRO is probably involved in this process, as the MIRO1 EF hand mutant was able to block Ca2+ entry into the mitochondrial matrix. Although the mechanism by which intra-mitochondrial Ca2+ affects the KIF5–MIRO1 transport machinery is not known, this signalling pathway may integrate cellular energy demands and ATP production with subcellular mitochondrial distribution in neurons. In addition to the effect of Ca2+ signalling on mitochondrial movement, Ca2+ influx through voltage-dependent Ca2+ channels can induce mitochondrial membrane fission, thus having profound effects on mitochondrial membrane dynamics88. Neuronal Ca2+ influx facilitates mitochondrial fragmentation by activating Ca2+/calmodulin-dependent protein kinase I (CaMKI), which subsequently phosphorylates the fission protein dynamin-related protein 1 (DRP1). The phosphorylation of DRP1 increases its interaction with mitochondrial fission 1 protein (FIS1), thus enhancing the mitochondrial fission process.
Neuronal signalling-mediated regulation
In dorsal root ganglion neurons, NGF can act as a docking signal, causing axonal mitochondria to accumulate close to an external source of NGF74. Actin-based mechanisms seem to have a role in this phenomenon. When neurons are treated with inhibitors of phosphoinositide 3-kinase (PI3K) or latrunculin B, an agent that destabilizes filamentous actin, mitochondria are not recruited to the NGF stimulation site, highlighting a crucial role for the PI3K signalling cascade in NGF-induced regulation of mitochondrial mobility (FIG. 3d). However, it remains to be determined whether the NGF signalling pathway recruits mitochondria to the stationary pool through a mitochondrion–actin interaction or indirectly through an unidentified docking receptor.
A study has shown that axonal mitochondrial transport can also be stimulated by activation of the serotonin receptor 5-HT1A and the AKT–glycogen synthase kinase 3β (GSK3β) pathway89. Although the molecular targets of the AKT–GSK3β signalling pathway are not clear, this highlights the possibility that serotonin (also known as 5-HT) acts as an extracellular modulator that regulates neuronal ATP distribution by controlling axonal mitochondrial trafficking. By contrast, another study showed that dopamine or D2 dopamine receptor agonists inhibit mitochondrial movement in hippocampal neurons via the same AKT–GSK3β signalling cascade90. This implies that the distribution of neuronal mitochondria occurs through a conserved regulatory mechanism. Nitric oxide can also modulate mitochondrial mobility by inhibiting mitochondrial function91,92. Nitric oxide targets mitochondria and inhibits respiration and ATP synthesis. Nitric oxide treatment of cultured forebrain neurons causes a rapid cessation of mitochondrial movement. Similarly, elevated nitric oxide levels produced by the nitric oxide donors propylamine propylamine NONOate and diethylamine–NO complex result in rapid immobilization of mitochondria.
Regulation of mitochondrial transport by microtubule-associated proteins
Microtubules are dynamic structures and are stabilized by microtubule-associated proteins (MAPs). Whereas MAP2 is specifically distributed in dendrites, MAP1B and tau are mainly axon-targeted MAPs. In addition to stabilizing axonal microtubules, tau has been shown to contribute to the regulation of the axonal transport of membrane organelles, including mitochondria93. Overexpressing tau in N2a and NB2a/d1 neuroblastoma cell lines, primary cortical neurons and retinal ganglion neurons selectively inhibits kinesin-driven anterograde mitochondrial transport93–95. In these experiments, dynein-mediated retrograde transport became dominant and mitochondria accumulated in the soma instead of being delivered to neuronal processes. These studies suggested that tau preferentially competes with kinesin motors for binding to microtubules because mitochondrial transport velocity was not altered when tau was overexpressed96. This hypothesis was further supported by a study that involved co-expression of tau and MARK, a microtubule affinity-regulating kinase that is particularly efficient in detaching tau from micro-tubules by phosphorylating the K-X-G-S motifs of tau. This revealed that the tau-mediated inhibition of axonal mitochondrial transport can be rescued by tau phosphorylation by MARK97. These findings show that increased binding of tau to microtubules is crucial to the impairment of mitochondrial transport in axons and that MARK-mediated tau phosphorylation could efficiently remove excess tau obstacles on the surface of microtubules to clear the path for motor proteins. Interestingly, complete loss (in Tau−/− mice) or partial reduction (in Tau−/+ mice) of tau expression in mutant neurons prevented amyloid-β-mediated defects in mitochondrial transport98, suggesting that the role of amyloid-β in inhibiting axonal mitochondrial mobility is dependent on tau expression levels. Tau therefore probably contributes to the general spatiotemporal regulation of axonal transport in both healthy and degenerating neurons. The binding of tau to microtubules can reverse the direction in which dynein moves, whereas kinesin tends to detach when moving along tau-decorated microtubules99. Thus, perturbing tau distribution in axons would impair axonal transport, leading to neurodegeneration. In addition to tau, MAP1B was also reported to negatively regulate axonal retrograde transport of mitochondria100.
Mitochondrial mobility at nodes of Ranvier
Axonal depolarization and action potential conduction depend on the activation of voltage-gated Na+ channels. Axons repolarize rapidly via Na+/K+ ATPases. A high density of Na+ channels and Na+/K+ ATPases at nodes of Ranvier is essential for myelinated axons to conduct high-velocity nerve impulses and to permit repetitive firing. Thus, regions near nodes of Ranvier have a high energy demand (FIG. 1b). Compared with the well-characterized synaptic recruitment of mitochondria during neuronal activity, little is known about how mitochondria are recruited to nodes of Ranvier. Recent studies have begun to address this issue. A study examined mitochondrial distribution and mobility in myelinated CNS axons using time-lapse imaging in cerebellar organotypic slice cultures101. This revealed that repetitive axonal firing increases the degree of mitochondrial accumulation and decreases the velocity of mitochondrial transport in nodal and paranodal, but not internodal, axoplasm. Mitochondrial recruitment to the nodal axoplasm increases in response to axonal electrical activity and elevated axoplasmic Ca2+ levels. Removing extracellular Ca2+ or pharmacologically blocking P/Q-type and N-type Ca2+ channels abolishes the activity-induced reduction of mitochondrial mobility and blocks the expansion of the mitochondrial stationary pool in nodal and paranodal regions.
These findings are consistent with those of another recent study, which demonstrated Ca2+ nodal signalling in myelinated frog sciatic nerves20. Ca2+ imaging analysis showed that a brief train of action potentials caused a highly localized increase in axonal Ca2+ levels at individual nodes of Ranvier. This repetitive stimulation also slowed mitochondrial mobility, as revealed by the reduced number and velocity of mobile mitochondria and the increased duration of mitochondrial pauses. Interestingly, this study demonstrated that the physiological activation of Na+/K+ ATPases also immobilizes mitochondria. Blocking Na+/K+ ATPases prevents action potentials from stopping mitochondrial movement, highlighting a possible Na+/K+ ATPase-related signalling pathway that controls mitochondrial transport at nodes of Ranvier. Although an axonal increase in Ca2+ levels probably influences mitochondrial movement via the MIRO Ca2+ sensor23–25, the mechanism that couples Na+/K+ ATPase activation to mitochondrial immobilization remains unclear. One hypothesis proposes that, following Na+/K+ ATPase activation, ATP depletion and increased ADP intracellular concentration gradients can recruit mitochondria to nodes of Ranvier84. Further investigation is necessary to determine whether the two signalling pathways act synergistically or independently to meet metabolic needs.
Loss of myelin presents a major metabolic challenge to nerves. Following demyelination, Na+ channels are dispersed along axons102. To restore nerve conduction, axons must drive Na+/K+ ATPases and thus must consume more ATP. It is therefore not surprising that there is an increase in mitochondrial density in demyelinated axons103,104. Live imaging of dorsal root ganglion axons demonstrates that the size of stationary mitochondrial sites was significantly increased by both myelination and demyelination but decreased by remyelination105. In addition, demyelination increased the transport velocity of motile mitochondria. This study suggests that myelination, demyelination and remyelination could modulate axonal stationary mitochondrial site size by changing the transport and distribution of mitochondria, thus representing an initial adaptive or stress response to balance axonal ATP production and energy requirements.
Mitochondrial mobility and synaptic function
Mitochondria are commonly found in synaptic terminals106,107, where they help to maintain neurotrans-mission by producing ATP and buffering Ca2+ at synapses5–12. Mitochondria in dendrites support synapse density and plasticity19, and the loss of mitochondria from these regions can inhibit synaptic transmission owing to insufficient ATP supply or altered Ca2+ dynamics during intensive synaptic activity.
Transport defects and neurotransmission
Factors that inhibit mitochondrial transport result in the loss of mitochondria from synaptic terminals. For example, D. melanogaster photoreceptors that express mutant Milton have lower numbers of mitochondria at synapses and exhibit dysfunctional synaptic transmission44. The absence of mitochondria in presynaptic terminals may reduce local ATP supply and thus affect ATP-dependent processes, such as the pump that establishes the proton gradient necessary for vesicle neurotransmitter loading, the Na+/Ca2+ exchanger that removes Ca2+ from nerve terminals and myosin motors that transport synaptic vesicles. In D. melanogaster, mutation of the gene encoding the mitochondrial fission protein DRP1 also leads to dramatic defects in the synaptic localization of mitochondria and impaired mobilization of the reserve synaptic vesicle pool, thus resulting in the faster depletion of synaptic vesicles during prolonged trains of stimulation pulses2. The addition of ATP partially rescues these defects, indicating that ATP production by mitochondria in nerve terminals is required for myosin-driven mobilization of vesicles from the reserve pool to the readily releasable pool during intense synaptic activity.
Using cultured mature superior cervical ganglion neurons, a recent study showed that syntabulin has a crucial role in the maintenance of presynaptic function108. Loss of syntabulin function reduces basal synaptic activity, accelerates the synaptic depression that occurs during high-frequency firing and slows the rate of synapse recovery after the depletion of synaptic vesicles. The loss of syntabulin function also reduces the number of mitochondria in neuronal processes. These defects can be reversed by the application of ATP to pre-synaptic neurons. This study indicates that syntabulinand KIF5-driven mitochondrial transport is vital for the onset of synaptic function in developing neurons and for the maintenance of synaptic function in mature neurons.
As neurotransmitter release is triggered by Ca2+ influx through voltage-gated Ca2+ channels, any factors that regulate presynaptic Ca2+ transients may influence the probability of synaptic vesicle release. Although presynaptic Ca2+ levels are elevated during low-frequency stimulation, a high level of accumulation of Ca2+ in presynaptic boutons occurs only during prolonged periods of repetitive stimulation. Using electrophysiological recording combined with imaging of cytoplasmic and mitochondrial Ca2+ at the calyx of Held (a well-characterized glutamatergic synapse), mitochondria were found to rapidly sequester substantial quantities of cytoplasmic Ca2+ and consequently to influence neurotransmitter release on the millisecond timescale7. This study suggests that mitochondria can modulate short-term presynaptic plasticity by buffering presynaptic Ca2+ levels. At neuro-muscular junctions, presynaptic mitochondria maintain Ca2+ homeostasis by sequestering excess intracellular Ca2+ during repetitive stimulation. D. melanogaster Miro mutants exhibit defective transport of mitochondria, resulting in a lack of mitochondria at neuromuscular junctions. This reduces Ca2+ buffering and impairs neurotransmitter release during prolonged stimulation49.
Mobile mitochondria and synaptic homeostasis
In mature neurons, approximately one-third of axonal mitochondria are mobile at any one time, either passing by or entering and exiting presynaptic boutons85. This raises a fundamental question: do these mobile axonal mitochondria influence synaptic homeostasis and synaptic plasticity? A study that used Snph−/− mice recently provided genetic and cellular evidence that changes in axonal mitochondrial mobility can affect short-term presynaptic plasticity12. Although basal synaptic transmission was not affected by Snph deletion, persistent enhanced short-term facilitation in response to short stimulus trains was observed in mutant presynaptic neurons (FIG. 3c). The phenotype was fully rescued by the introduction of a Snph transgene. Furthermore, the disruption of syntaphilin-mediated docking altered presynaptic Ca2+ dynamics during intense, prolonged stimulation. It is possible that intracellular Ca2+ may rapidly build up in presynaptic terminals during intense stimulation and that stationary mitochondria are able to sequester this Ca2+ most effectively. Furthermore, although mobile mitochondria may supply sufficient ATP for basal synaptic transmission, this may not be sufficient to pump Ca2+ out of the cell, and higher levels of ATP supplied by synaptically stationary mitochondria may be required. These experiments suggest that alterations to mitochondrial mobility could affect synaptic homeostasis.
Mitochondrial transport and quality control
The half-life of neuronal mitochondria is estimated to be ~30 days109,110. Elaborate quality-control systems maintain mitochondrial integrity and function (BOX 3). These mechanisms include transport, fusion, fission and turnover via mitophagy and constitute an interdependent system of mitochondrial dynamics. Neurons have a unique dependence on the precise control of mitochondrial dynamics, which are crucial for mitochondrial transport to distal locations, signal recognition, the maintenance of morphology and content, and quality control. Mitochondrial fusion enables the exchange of contents between mitochondria and allows damaged mitochondria to acquire components from healthy mitochondria. Mitochondrial fission is also essential for mitochondrial function. A damaged mitochondrion can generate a daughter mitochondrion to avoid a potentially catastrophic rupture. This may help to segregate damaged segments of mitochondria and promote mitophagy. Perturbations in any of these processes can lead to neurological defects111.
Transport and mitochondrial fusion and fission
As the size of an organelle affects its mobility, defects in membrane fission can influence mitochondrial transport. DRP1 is a mitochondrial dynamin-like GTPase that is essential for mitochondrial membrane fission. DRP1 is required for embryonic development of the mouse brain and synapse formation between cultured neurons112. In cultured hippocampal neurons, defects in DRP1-mediated mitochondrial fission result in the accumulation of mitochondria in the cell body and reduced dendritic mitochondrial content19. DRP1 also maintains the distribution of mitochondria near D. melanogaster neuromuscular junctions2. This suggests that it may not be possible for the highly interconnected mitochondria that are present in fission-deficient neurons to be efficiently transported to distal neuronal processes. Deletion of GEM1 (the yeast Miro homologue) also alters mitochondrial morphology in yeast cells, resulting in the accumulation of collapsed and globular mitochondria47. Loss of MIRO function also suppresses mitochondrial mobility and causes mitochondrial fragmentation25,46,113. By contrast, overexpressing MIRO not only enhances mitochondrial movement but also increases mitochondrial interconnection and their volume intensity in dendrites23,25,46. Moreover, the dynein–dynactin retrograde motor complex was shown to have a role in the regulation of mitochondrial morphology. Disruption of dynein function increases the formation of highly branched mitochondrial structures by controlling the recruitment of DRP1 (REF. 114).
Fusion requires the apposition of two adjacent organelles. Mitochondrial transport along neuronal processes is particularly important for the fusion events. Deletion or mutation of the gene encoding the fusion protein MFN2 impairs both anterograde and retrograde mitochondrial transport115, suggesting crosstalk between the fusion and transport machinery. In fusion-deficient cells, mitochondria exhibit reduced mobility116. Motor neurons derived from transgenic mice with mutant MFN2 showed improper mitochondrial distribution117. Mitochondria in conditional Mfn2-knockout mice are fragmented and are not found in long or branched neurites, indicating that fusion also influences mitochondrial transport and distribution118. A recent study showed that an association between MFN2 and the MIRO–Milton complex is necessary for axonal mitochondrial transport70. Therefore, mitochondrial transport may directly affect mitochondrial morphology by regulating the fusion and fission machinery. Conversely, deficiency of mitochondrial fusion or fission impairs mitochondrial mobility and distribution. This may be due to altered mitochondrial respiration or a defective association in the mitochondrial transport complex. These findings indicate that interplay between mitochondrial mobility and the membrane fusion and fission machineries may regulate mitochondrial shape, function and distribution.
Mitochondrial dynamics and mitophagy
A direct connection between mitochondrial mobility and mitophagy has not yet been established. However, it is known that PTEN-induced putative kinase protein 1 (PINK1) is targeted to mitochondria by an N-terminal targeting sequence and is involved in mitochondrial dynamics through interaction with the MIRO–Milton complex119 or the fusion and fission machineries120. Parkin, a cytosolic E3 ubiquitin ligase, translocates to depolarized mitochondria for autophagic clearance. PINK1 and parkin can cooperate in the process of mitophagy, and PINK1 is required to induce the recruitment of cytosolic parkin to dysfunctional mitochondria121–127. Recent results from non-neuronal cells show widespread degradation of mitochondrial outer membrane proteins, including MIRO1 and MIRO2, by the parkin-activated ubiquitin–proteasome system during the early phase of mitophagy128,129. This indicates that a suppression of mitochondrial anterograde transport may occur during mitophagy. A recent study demonstrated that PINK1 can phosphorylate MIRO and activate the proteasomal degradation of MIRO in a parkin-dependent manner. As a result, mitochondrial movement is arrested owing to the detachment of kinesin motors from mitochondria. These results suggest that the PINK1–parkin pathway also regulates mitochondrial transport, which may help to quarantine parkin-labelled damaged mitochondria for clearance by mitophagy130.
Mitochondrial membrane dynamics influence the selective degradation of damaged mitochondria via the autophagy–lysosomal pathway. Several studies suggest that the PINK1–parkin pathway has a key role in maintaining mitochondrial stability and function. In flies and in some mammalian cells, PINK1 and parkin have been shown to promote mitochondrial fission and/or to inhibit fusion by downregulating MFN proteins and mitochondrial dynamin-like 120 kDa protein (also known as OPA1), the latter of which is a protein required for mitochondrial inner membrane fusion120,131–135. However, conflicting observations in mammalian cells were also reported136–140. Recently, several groups demonstrated that parkin ubiquitylates MFN proteins, thereby promoting mitochondrial fragmentation and possibly mitophagy128,141–144. It is thought that parkin inhibits the re-incorporation of damaged mitochondria into healthy ones, thereby segregating impaired mitochondria for mitophagy.
Mitochondrial transport and membrane potential
It has been reported that the mitochondrial membrane potential influences the direction of mitochondrial transport. Mitochondria with high membrane potentials undergo anterograde transport towards distal processes, whereas damaged mitochondria return to the cell body following acute depolarization145. It has therefore been proposed that damaged or dysfunctional mitochondria are transported to the soma for repair and/or degradation. As most mature, acidic lysosomes are predominantly located in the somatodendritic regions and the proximal axon, it is likely that organelles destined for degradation must be delivered to these compartments63,146–148. Thus, it is likely that dynein-mediated retrograde transport facilitates mitochondrial fission and promotes the targeting of segregated mitochondria to the soma. However, another study suggests that there is no difference in the membrane potential of mobile and stationary mitochondria under physiological conditions149. Therefore, the molecular interplay between mitochondrial transport, fusion, fission and mitophagy must be further investigated to advance our understanding of mitochondrial quality control in neurons. This represents an important field of research, as various neurodegenerative disorders are associated with mitochondrial dysfunction.
Transport defects and neurodegeneration
It is well documented that mitochondrial dysfunction, changes in mitochondrial dynamics and mobility, and perturbation of mitochondrial turnover are involved in the pathology of some neurodegenerative and neurological disorders (reviewed in REFS 27,30,111). In particular, damaged mitochondria not only fail to produce ATP and to buffer Ca2+ levels but also release apoptotic cell death signals (reviewed in REF. 150). Defects in mitochondrial transport may cause local energy depletion and toxic changes in Ca2+ buffering that may trigger synaptic dysfunction and loss, thus contributing to neurodegenerative disorders (BOX 4).
Conclusions and perspectives
The recent discovery of several proteins involved in the mitochondrial transport machinery, as well as of docking receptors, has boosted our understanding of the molecular mechanisms that regulate mitochondrial mobility and distribution in response to neuronal activity and intracellular signalling. However, many questions remain. For example, precisely how a MIRO- and Ca2+-dependent mechanism prevents both anterograde and retrograde mitochondrial transport is unknown. It seems likely that the opposing actions of kinesin and dynein are coordinated through their adaptors or by other unknown linkers. If so, how does dynein mechanistically or physically couple to MIRO, and does Ca2+ sensing also inactivate dynein motors? The discovery of syntaphilin and its characterization as a mitochondrial anchoring receptor raise additional questions. Do motor–adaptor complexes and docking machineries physically displace one another as the mitochondria change from mobile to stationary phases? How do mobile mitochondria become stationary (and vice versa) in response to changes in mitochondrial membrane potential and neuronal physiological state, and in pathological conditions? Does a single independent pathway or multiple simultaneous cellular signals regulate the activity of motor–adaptor complexes and docking machineries? What are the signalling pathways that coordinate the interactions between transport machineries and docking receptors? Additional investigations will determine how myosin motors coordinate microtubule-based motors, mitochondrial transport and/or stationary docking. Answering many of these questions will probably involve the use of genetic mouse models.
Investigation into how the mobility of mitochondria coordinates their quality control in neurons represents an important emerging area for basic and disease- oriented research. Throughout the neuronal lifetime, aged and damaged mitochondria undergo dynamic recycling through fusion and fission processes or degradation via mitophagy. Dysfunctional mitochondria not only produce energy less efficiently but also release potentially harmful reactive oxygen species and initiate apoptotic signalling cascades. Thus, damaged mitochondria at synapses must be recognized and transported back to the soma, where lysosomes are predominantly localized. Microtubule-based retrograde transport is probably an important process for damaged mitochondria or autophagic cargo to be transported back to the soma and delivered to lysosomes. Proper sequestration of damaged mitochondria into autophagosomes and subsequent degradation within the lysosomal system may also serve to limit the leakage of these potentially deleterious mediators. Identification of the molecules involved in linking mitochondrial transport, fusion, fission and mitophagy will advance our understanding of the cellular mechanisms that regulate mitochondrial quality control. It will be important to examine whether defective mitochondrial transport plays any part in neurodegeneration or simply reflects the sequelae of general transport alterations. Does impaired transport of mitochondria in neurodegenerative disease models have any effect on the removal of dysfunctional mitochondria from synapses and distal axons by mitophagy or on the recycling of damaged mitochondria through membrane fusion or fission? If this is the case, then it will be interesting to determine whether a rescue of mitochondrial transport defects could enhance mitochondrial quality control processes in both slow- and rapid-onset neuronal degeneration mouse models. Future studies using neurons from diseased mice and live cell imaging of mobile mitochondria will help to elucidate the molecular and cellular mechanisms that regulate mitochondrial mobility, distribution and turnover. Mechanistic insights into these fundamental neuronal processes will advance our understanding of human neurodegenerative diseases.
Supplementary Material
Box 1 | Visualizing mitochondrial transport in neurons.
Recent studies applying time-lapse imaging of live cultured neurons and in vivo studies in genetically engineered mice have revealed the complex nature of mitochondrial transport along neuronal processes12,31,32. Mitochondria move bidirectionally, and frequently pause or switch to persistent docking (Supplementary information S1 (movie)). In mature cultured neurons, only one-third of axonal mitochondria are mobile and the remaining 65–80% are stationary12. Saltatory and bidirectional movements result in mean mitochondrial velocities between 0.32 and 0.91 μm per second23,71.
To visualize mitochondrial transport in live cultured neurons, mitochondria can be labelled by transfection of DsRed–Mito (a fluorescently labelled, mitochondrion- targeted protein) or by directly loading neurons with MitoTracker Green FM dye. Time-lapse imaging is performed using a confocal microscope. Applying a relatively long interval (10 seconds) between each time-lapse scan can minimize laser-induced cellular damage. Kymographs are used to quantify relative mitochondrial mobility12,145 (see FIG. 3b). As a general rule, a mitochondrion is considered to be stationary if it remains immobile for the entire recording period; a mobile mitochondrion is counted only if its displacement is at least 5 μm during the same period12.
Although this approach provides an important tool for studying the cellular and molecular mechanisms that regulate mitochondrial transport, it also has some limitations, as most neuron cultures must be prepared from embryos or early postnatal mice. Furthermore, the morphology of cultured neurons can differ from that of neurons in vivo. An elegant tool to visualize axonal mitochondrial transport in living mice and explanted nervous tissue was developed32 in transgenic mouse lines in which mitochondrion-targeted cyan fluorescent protein or yellow fluorescent protein are selectively expressed in neurons. Thus, time-lapse recordings can directly monitor mitochondrial transport in acute nerve–muscle explants or in living adult mice. In explants, 87% of axonal mitochondria were shown to be stationary (which is slightly higher than in cultured neurons), two-thirds of mobile mitochondria were shown to move in the anterograde direction and the remaining third were shown to move in the retrograde direction. Living mice displayed similar mitochondrial mobility patterns, although both anterograde and retrograde transport rates were slightly slower.
Box 2 | Structures of motor proteins for mitochondrial transport.
Kinesin-1 (also known as KIF5) motor proteins contain two kinesin heavy chains (KHCs) and two kinesin light chains (KLCs)33 (see the figure, part a). Their motor function arises from homodimer heavy chain associations in the coiled-coil regions of the stalk domain. Each KHC contains an amino-terminal motor domain that has ATPase activity and binds directly to microtubules, whereas its carboxy-terminal cargo-binding domain mediates an association with a KLC or directly interacts with cargoes or cargo adaptors, such as mitochondrial adaptor proteins.
Cytoplasmic dynein is composed of two dynein heavy chains (DHCs) and several dynein intermediate chains (DICs), dynein light intermediate chains (DLICs) and dynein light chains (DLCs)151 (see the figure, part b). DHCs function as motors, and the association of the dynein motor with cargoes and the regulation of its motility involve various other polypeptides.
Myosin motor proteins require ATP hydrolysis to provide the energy required to generate force and movement along actin filaments. There are 18 classes of myosin motor proteins152. Myosin V, one candidate motor for driving mitochondrial movement, forms a dimer and consists of a motor domain, a stalk region and a tail region (see the figure, part c). Figure is modified, with permission, from REF. 153 © (2011) Springer.
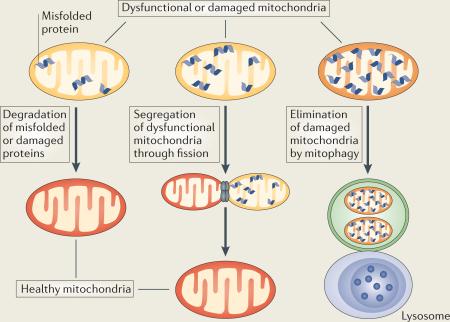
Box 3 | Mitochondrial quality control.
Mitochondrial quality control involves surveillance and protection strategies to limit mitochondrial damage and ensure mitochondrial integrity. This quality control occurs at the following molecular, organellar and cellular levels (see the figure):
Degradation of misfolded or damaged mitochondrial proteins
The molecular level of defence is supported by the proteolytic system. Molecular chaperones and ATP-dependent proteases in the matrix and inner membrane of mitochondria degrade damaged proteins, stabilize misfolded proteins (thus preventing their aggregation) and/or dissolve protein aggregates (and thereby promote proteolysis)154. In addition, the cytosolic ubiquitin–proteasome system can also participate in the quality control of mitochondrial proteins155.
Segregation of dysfunctional mitochondria through fission
Mitochondrial fusion and fission provide additional protection against mitochondrial damage. Damaged mitochondria can be repaired by fusion with healthy mitochondria111,156,157, which allows the contents of healthy and dysfunctional mitochondria to be mixed. Fission, however, sequesters mitochondria that have become irreversibly damaged or are fusion-incompetent and results in their subsequent elimination by autophagy.
Elimination of damaged mitochondria by mitophagy
In the event that the two quality control pathways described above are ineffective, damaged mitochondria are eliminated by autophagy. One type of cargo-specific autophagy is mitophagy, which selectively removes damaged mitochondria. Mitophagy requires the specific labelling of damaged mitochondria and their subsequent recruitment into isolation membranes, and this can occur through two mechanisms. First, outer mitochondrial membrane proteins, such as NIP3-like protein X (NIX; also known as BNIP3L) in mammalian cells (autophagy-related protein 32 (Atg32) in yeast), bind to LC3 (Atg8 in yeast) on the isolated membranes, which mediate the sequestration of damaged mitochondria into autophagosomes158. Second, when mitochondria are damaged by losing their membrane potential, PTEN-induced putative kinase protein 1 (PINK1) recruits the E3 ubiquitin ligase parkin from the cytosol to the damaged mitochondria, where it ubiquitinates mitochondrial proteins and causes mitochondria to become engulfed by isolation membranes that then fuse with lysosomes. If the levels of damage exceed the capacity of all three quality control pathways, damaged mitochondria can rupture, leading to the release of pro-apoptotic factors and cell death.
Box 4 | Mitochondrial transport defects in neurodegenerative diseases.
Alzheimer's disease
Several lines of evidence support the hypothesis that impaired axonal transport has an important role in the pathogenesis of Alzheimer's disease29,159. Briefly exposing cultured hippocampal neurons to amyloid-β results in impaired mitochondrial transport98,160. Axonal degeneration in patients with Alzheimer's disease is characterized by regions of swelling in which abnormal amounts of organelles (including mitochondria) accumulate161. Furthermore, defective axonal transport of mitochondria and other organelles may lead to synaptic dysfunction and loss.
Amyotrophic lateral sclerosis (ALS)
Neurons from patients with ALS and from mice with ALS-like manifestations (owing to expression of mutant superoxide dismutase 1 (SOD1)) display impaired axonal mitochondrial transport162–165. Altered mitochondrial transport has also been observed after overexpression of alsin or TAR DNA-binding protein 43 (TDP43), two proteins which, when mutated, cause familial ALS166,167. Misfolded wild-type SOD1 species that have been immunopurified from patients with sporadic ALS inhibit fast axonal transport driven by KIF5 motors168. The loss of mitochondria or presence of dysfunctional mitochondria in distal motor axons in ALS might result from reduced mitochondrial movement and quality control and may contribute to motor degeneration. Thus, increasing mitochondrial transport might help to deliver healthy mitochondria to axons and/or to return damaged mitochondria to somas for proper degradation. However, it was recently demonstrated that the twofold increase in axonal mitochondrial mobility in crossed mice carrying both mutant SOD1 (SOD1G93A) and a syntaphilin deficiency does not slow ALS-like disease progression169, suggesting that the reduced mitochondrial mobility seen in SOD1G93A mice has a minimal role in the rapid onset of ALS-linked pathology. Further investigation is necessary to determine whether increased mitochondrial transport in this crossed mouse model helps to improve the turnover of dysfunctional mitochondria in motor neurons.
Huntington's disease
Recent studies indicate that impaired mitochondrial transport plays an important part in the pathology of Huntington's disease. Huntingtin (HTT) acts as a scaffolding protein by binding to huntingtin-associated protein 1 (HAP1), which mediates the association of HTT with kinesin and dynein–dynactin and stimulates the trafficking of various membrane-bound organelles, including mitochondria, late endosomes and ER–Golgi trafficking vesicles170. HTT can also interact directly with the dynein intermediate chain and facilitate dynein- and dynactin-mediated vesicle transport171. Phosphorylation of HTT serves as a molecular switch for anterograde versus retrograde mitochondrial movement172. Whereas wild-type HTT promotes both anterograde and retrograde mitochondrial transport, mutant HTT disrupts the formation of transport complexes and impairs mitochondrial movement173–175. These effects are probably due to the polyglutamine expansion at the amino-terminal region of HTT, which associates with mitochondria and affects their transport in cultured neurons176.
Parkinson's disease
Direct evidence for defective mitochondrial transport in Parkinson's disease neurons has not been demonstrated. The link between familial Parkinson's disease-related gene products and defects in mitochondrial transport is still speculative. Mutations in the genes encoding PTEN-induced putative kinase protein 1 (PINK1) and the E3 ubiquitin ligase parkin are linked to autosomal recessive familial parkinsonism, and mutations in the leucine-rich repeat kinase 2 gene (LRRK2) can cause autosomal dominant Parkinson's disease. Interestingly, PINK1 is reported to form a complex with MIRO–Milton119, suggesting a role in the regulation of mitochondrial transport. Expressing α-synuclein in dorsal root ganglion neurons disrupts the microtubule network177. As both LRRK2 and parkin modulate microtubule stability178,179, these proteins may have an indirect role in mitochondrial transport.
Nodes of Ranvier.
Regularly spaced gaps in the myelin sheath that surrounds myelinated axons. They expose the axonal plasma membrane to the extracellular fluid. Nodes of Ranvier contain large numbers of voltage-gated ion channels and thus enable conduction of the action potential.
Autophagy–lysosomal system.
A primary cellular route for the breakdown of organelles and the degradation of cytoplasmic components. Following the sequestration of organelles and cytoplasm within a double-membrane-bound vacuole (autophagosome), fusion with lysosomes occurs. Lysosomal hydrolases in these ‘autolysosomes’ degrade their contents.
EF hand.
A Ca2+-binding domain that was originally identified in parvalbumin. EF hands are also known as helix–turn–helix domains.
Small interfering RNA.
(siRNA). A sequence-specific gene-silencing tool used in RNA interference. siRNAs are short fragments of synthetic double-stranded RNA with 21–23 pairs of nucleotides that have sequence specificity to the gene of interest (the target). These small double-stranded RNAs trigger degradation of the target RNA, thereby creating a partial loss-of-function.
RNA interference.
(RNAi). A method by which double-stranded RNA that is encoded in an exogenous vector can be used to interfere with normal RNA processing, causing rapid degradation of the endogenous RNA and thereby precluding translation. This provides a simple way of studying the effects of the absence of a gene product in simple organisms and cells.
Processivity.
Motor proteins move stepwise along microtubules without detachment over long distances at the expense of ATP. This movement, termed hand-over-hand motility, is based on the coordinated action of two motor heads that bind one after another to microtubules. This mechanism requires precise coordination of the microtubule affinity of the two motor domains.
Saltatory mobility patterns.
The complex nature of mitochondrial transport along neuronal processes, whereby mitochondria move bidirectionally, pause and start moving again, slow down and speed up, and frequently change direction.
Na+/K+ ATPases.
Also known as Na+/K+ pumps, these membrane proteins use ATP hydrolysis to move Na+ and K+ in opposite directions across the plasma membrane. They are responsible for maintaining transmembrane concentration gradients for both Na+ and K+ and have a particularly important role in enabling neurons to respond to stimuli and transmit impulses.
Short-term facilitation.
A transient increase in synaptic strength occurs when two or more action potentials invade the presynaptic terminal in close succession or as a result of a high-frequency burst of presynaptic action potentials. Facilitation results in more neurotransmitter being released in response to each succeeding action potential owing to prolonged elevation of presynaptic Ca2+ levels following synaptic activity.
Acknowledgements
The authors thank M. Davis for editing and other members of the Sheng laboratory for their assistance and discussion. This work was supported by the Intramural Research Program of the US National Institute of Neurological Disorder and Stroke (NINDS), US National Institutes of Health (NIH) (Z.-H.S.) and the NIH Pathway to Independence Award K99 (Q.C.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Zu-Hang Sheng's homepage: http://intra.ninds.nih.gov/Lab.asp?Org_ID=56
SUPPLEMENTARY INFORMATION
See online article: S1 (movie) | S2 (movie)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 2.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [This paper shows that in D. melanogaster with mutant DRP1, the loss of mitochondria from neuromuscular junctions results in faster depletion of synaptic vesicles during prolonged pulse train stimulation owing to a specific defect in mobilizing reserve pool vesicles.] [DOI] [PubMed] [Google Scholar]
- 3.Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol. Biol. Cell. 2008;19:150–158. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J. Neurosci. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 7.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medler K, Gleason EL. Mitochondrial Ca2+ buffering regulates synaptic transmission between retinal amacrine cells. J. Neurophysiol. 2002;87:1426–1439. doi: 10.1152/jn.00627.2001. [DOI] [PubMed] [Google Scholar]
- 9.David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J. Physiol. 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot JD, David G, Barrett EF. Inhibition of mitochondrial Ca2+ uptake affects phasic release from motor terminals differently depending on external [Ca2+]. J. Neurophysiol. 2003;90:491–502. doi: 10.1152/jn.00012.2003. [DOI] [PubMed] [Google Scholar]
- 11.Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- 12.Kang JS, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [By using genetic mouse models and time-lapse imaging this study identifies syntaphilin as a ‘static anchor’ for axonal mitochondria.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogan N, Cabot JB. Light and electron microscopic analyses of intraspinal axon collaterals of sympathetic preganglionic neurons. Brain Res. 1991;541:241–251. doi: 10.1016/0006-8993(91)91024-u. [DOI] [PubMed] [Google Scholar]
- 15.Fabricius C, Berthold CH, Rydmark M. Axoplasmic organelles at nodes of Ranvier. II. Occurrence and distribution in large myelinated spinal cord axons of the adult cat. J. Neurocytol. 1993;22:941–954. doi: 10.1007/BF01218352. [DOI] [PubMed] [Google Scholar]
- 16.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 17.Mutsaers SE, Carroll WM. Focal accumulation of intra-axonal mitochondria in demyelination of the cat optic nerve. Acta Neuropathol. 1998;96:139–143. doi: 10.1007/s004010050873. [DOI] [PubMed] [Google Scholar]
- 18.Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang CL, Ho PL, Kintner DB, Sun D, Chiu SY. Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J. Neurosci. 2010;30:3555–3566. doi: 10.1523/JNEUROSCI.4551-09.2010. [This study demonstrates a highly localized elevation of axonal Ca2+ levels and reduced mitochondrial mobility at individual nodes of Ranvier during a brief train of action potentials.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev. Neurobiol. 2008;68:1348–1361. doi: 10.1002/dneu.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [This study shows that mobile mitochondria are recruited to stationary pools in response to acute application of glutamate to cultured neurons. Mitochondria are also changed from an elongated to a rounded morphology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macaskill AF, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saotome M, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl Acad. Sci. USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [References 23, 24 and 25 independently identified MIRO as a Ca2+ sensor, providing a mechanism for the underlying Ca2+-dependent regulation of mitochondrial mobility.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog. Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Stokin GB, Goldstein LS. Axonal transport and Alzheimer's disease. Annu. Rev. Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- 30.Schon EA, Przedborski S. Mitochondria: the next (neurode) generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller KE, Sheetz MP. Direct evidence for coherent low velocity axonal transport of mitochondria. J. Cell Biol. 2006;173:373–381. doi: 10.1083/jcb.200510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nature Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [This study develops an elegant tool to visualize axonal mitochondrial transport in living mice and explanted nervous tissue.] [DOI] [PubMed] [Google Scholar]
- 33.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, et al. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka Y, et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [This is the first genetic mouse study showing that KIF5 motors are essential for mitochondrial transport.] [DOI] [PubMed] [Google Scholar]
- 36.Górska-Andrzejak J, et al. Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J. Comp. Neurol. 2003;463:372–388. doi: 10.1002/cne.10750. [DOI] [PubMed] [Google Scholar]
- 37.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [This study provides the genetic evidence in the D. melanogaster nervous system that dynein motors have a crucial role in mitochondrial retrograde transport in axons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Kanai Y, et al. KIF5C, a novel neuronal kinesin enriched in motor neurons. J. Neurosci. 2000;20:6374–6384. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirokawa N, et al. Kinesin associates with anterogradely transported membranous organelles in vivo. J. Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [This study shows that KIF5 motors are attached to brain mitochondria.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Q, Gerwin C, Sheng Z-H. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J. Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [This study identifies syntabulin as a second prominent KIF5 motor adaptor for mitochondria. Syntabulin loss-of-function or interference of the syntaphilin–KIF5 interaction reduces anterograde, but not retrograde, mitochondrial transport along axons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nangaku M, et al. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Sugiura Y, Ichishita R, Mihara K, Oka T. KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells. J. Cell Sci. 2011;124:2457–2465. doi: 10.1242/jcs.086470. [DOI] [PubMed] [Google Scholar]
- 44.Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [This paper reports that D. melanogaster photoreceptors that express mutant Milton show aberrant synaptic transmission owing to a reduced distribution of mitochondria at synapses.] [DOI] [PubMed] [Google Scholar]
- 45.Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 46.Fransson S, Ruusala A, Aspenström P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 47.Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J. Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo X, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [In this paper, the authors show that a MIRO mutant results in the chronic loss of mitochondria from neuromuscular junctions, a reduction in Ca2+ buffering capacity and impaired neurotransmitter release during prolonged stimulation.] [DOI] [PubMed] [Google Scholar]
- 50.Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J. Biol. Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- 51.Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J. Biol. Chem. 2006;281:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- 52.Grishin A, Li H, Levitan ES, Zaks-Makhina E. Identification of γ-aminobutyric acid receptor-interacting factor 1 (TRAK2) as a trafficking factor for the K+ channel Kir2.1. J. Biol. Chem. 2006;281:30104–30111. doi: 10.1074/jbc.M602439200. [DOI] [PubMed] [Google Scholar]
- 53.Kirk E, Chin LS, Li L. GRIF1 binds Hrs and is a new regulator of endosomal trafficking. J. Cell Sci. 2006;119:4689–4701. doi: 10.1242/jcs.03249. [DOI] [PubMed] [Google Scholar]
- 54.Webber E, Li L, Chin LS. Hypertonia-associated protein Trak1 is a novel regulator of endosometo-lysosome trafficking. J. Mol. Biol. 2008;382:638–651. doi: 10.1016/j.jmb.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacAskill AF, Brickley K, Stephenson FA, Kittler JT. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell. Neurosci. 2009;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J. Biol. Chem. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [References 55 and 56 provide evidence that elevating MIRO1 levels enhances the recruitment of the TRAK2–KIF5 transport complex to mitochondria and that knocking down TRAK1 or expressing dominant-negative TRAK1 mutants results in impaired mitochondrial mobility in axons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikuta J, et al. Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem. Biophys. Res. Commun. 2007;353:127–132. doi: 10.1016/j.bbrc.2006.11.142. [DOI] [PubMed] [Google Scholar]
- 58.Fujita T, et al. Axonal guidance protein FEZ1 associates with tubulin and kinesin motor protein to transport mitochondria in neurites of NGF-stimulated PC12 cells. Biochem. Biophys. Res. Commun. 2007;361:605–610. doi: 10.1016/j.bbrc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 59.Cho KI, et al. Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function. Traffic. 2007;8:1722–1735. doi: 10.1111/j.1600-0854.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 60.Schwarzer C, Barnikol-Watanabe S, Thinnes FP, Hilschmann N. Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int. J. Biochem. Cell Biol. 2002;34:1059–1070. doi: 10.1016/s1357-2725(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 61.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nature Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 62.Haghnia M, et al. Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol. Biol. Cell. 2007;18:2081–2089. doi: 10.1091/mbc.E06-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai Q, et al. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron. 2010;68:73–86. doi: 10.1016/j.neuron.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo GJ, et al. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J. Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [This study suggests that MIRO promotes either kinesin- or dynein-mediated movement during a neuronal signal that dictates the net transport direction of mitochondria.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J. Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ligon LA, Tokito M, Finklestein JM, Grossman FE, Holzbaur EL. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J. Biol. Chem. 2004;279:19201–19208. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- 67.Welte MA. Bidirectional transport along microtubules. Curr. Biol. 2004;14:R525–R537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 68.Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr. Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 69.Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr. Biol. 2005;15:2137–2141. doi: 10.1016/j.cub.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J. Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [This study highlights a role of the MIRO2–MFN2 complex in regulating the processivity of kinesin or in coordinating the switch between kinesin and dynein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quintero OA, et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr. Biol. 2009;19:2008–2013. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naisbitt S, et al. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J. Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [References 74 and 75 provide compelling evidence that NGF can regulate mitochondrial mobility by influencing static interactions between mitochondria and actin and that inhibiting actin-based myosin motors results in increased mitochondrial mobility.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hubley MJ, Locke BR, Moerland TS. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim. Biophys. Acta. 1996;1291:115–121. doi: 10.1016/0304-4165(96)00053-0. [DOI] [PubMed] [Google Scholar]
- 77.Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J. Cell Biol. 1982;94:129–142. doi: 10.1083/jcb.94.1.129. [This study provides the first morphological evidence for the crossbridges between axonal mitochondria and microtubules or neurofilaments.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindén M, Nelson BD, Loncar D, Leterrier JF. Studies on the interaction between mitochondria and the cytoskeleton. J. Bioenerg. Biomembr. 1989;21:507–518. doi: 10.1007/BF00762522. [DOI] [PubMed] [Google Scholar]
- 79.Jung D, Filliol D, Miehe M, Rendon A. Interaction of brain mitochondria with microtubules reconstituted from brain tubulin and MAP2 or TAU. Cell Motil. Cytoskeleton. 1993;24:245–255. doi: 10.1002/cm.970240405. [DOI] [PubMed] [Google Scholar]
- 80.Price RL, Lasek RJ, Katz MJ. Microtubules have special physical associations with smooth endoplasmic reticula and mitochondria in axons. Brain Res. 1991;540:209–216. doi: 10.1016/0006-8993(91)90509-t. [DOI] [PubMed] [Google Scholar]
- 81.Chen YM, Gerwin C, Sheng Z-H. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J. Neurosci. 2009;29:9429–9438. doi: 10.1523/JNEUROSCI.1472-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sung JY, et al. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc. Natl Acad. Sci. USA. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner OI, et al. Mechanisms of mitochondria-neurofilament interactions. J. Neurosci. 2003;23:9046–9058. doi: 10.1523/JNEUROSCI.23-27-09046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mironov SL. ADP regulates movements of mitochondria in neurons. Biophys. J. 2007;92:2944–2952. doi: 10.1529/biophysj.106.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [This study demonstrates that mitochondrial mobility is regulated in response to changes in synaptic activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai Q, Sheng ZH. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron. 2009;61:493–496. doi: 10.1016/j.neuron.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang KT, Niescier RF, Min KT. Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc. Natl Acad. Sci. USA. 2011;108:15456–15461. doi: 10.1073/pnas.1106862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han XJ, et al. CaM kinase I α-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen S, Owens GC, Crossin KL, Edelman DB. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol. Cell. Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Chen S, et al. Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS ONE. 2008;3:e2804. doi: 10.1371/journal.pone.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rintoul GL, Bennett VJ, Papaconstandinou NA, Reynolds IJ. Nitric oxide inhibits mitochondrial movement in forebrain neurons associated with disruption of mitochondrial membrane potential. J. Neurochem. 2006;97:800–806. doi: 10.1111/j.1471-4159.2006.03788.x. [DOI] [PubMed] [Google Scholar]
- 92.Zanelli SA, et al. Nitric oxide impairs mitochondrial movement in cortical neurons during hypoxia. J. Neurochem. 2006;97:724–736. doi: 10.1111/j.1471-4159.2006.03767.x. [DOI] [PubMed] [Google Scholar]
- 93.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubey M, Chaudhury P, Kabiru H, Shea TB. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil. Cytoskeleton. 2008;65:89–99. doi: 10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- 95.Stoothoff W, et al. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J. Neurochem. 2009;111:417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- 97.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J. Cell Biol. 2004;167:99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vossel KA, et al. Tau reduction prevents Aβ-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiménez-Mateos EM, González-Billault C, Dawson HN, Vitek MP, Avila J. Role of MAP1B in axonal retrograde transport of mitochondria. Biochem. J. 2006;397:53–59. doi: 10.1042/BJ20060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohno N, et al. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J. Neurosci. 2011;31:7249–7258. doi: 10.1523/JNEUROSCI.0095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Craner MJ, et al. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl Acad. Sci. USA. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrews H, et al. Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. J. Neurosci. Res. 2006;83:1533–1539. doi: 10.1002/jnr.20842. [DOI] [PubMed] [Google Scholar]
- 104.Hogan V, et al. Increase in mitochondrial density within axons and supporting cells in response to demyelination in the Plp1 mouse model. J. Neurosci. Res. 2009;87:452–459. doi: 10.1002/jnr.21867. [DOI] [PubMed] [Google Scholar]
- 105.Kiryu-Seo S, Ohno N, Kidd GJ, Komuro H, Trapp BD. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J. Neurosci. 2010;30:6658–6666. doi: 10.1523/JNEUROSCI.5265-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J. Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma H, Cai Q, Lu W, Sheng Z-H, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J. Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gross NJ, Getz GS, Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J. Biol. Chem. 1969;244:1552–1562. [PubMed] [Google Scholar]
- 110.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J. Biol. Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- 111.Chen H, Chan DC. Mitochondrial dynamics — fusion, fission, movement, and mitophagy — in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishihara N, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nature Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 113.Liu X, Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro–Milton complex. Int. J. Biochem. Cell Biol. 2009;41:1972–1976. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varadi A, et al. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J. Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 115.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J. Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Detmer SA, Vande Velde C, Cleveland DW, Chan DC. Hindlimb gait defects due to motor axon loss and reduced distal muscles in a transgenic mouse model of Charcot-Marie-Tooth type 2A. Hum. Mol. Genet. 2008;17:367–375. doi: 10.1093/hmg/ddm314. [DOI] [PubMed] [Google Scholar]
- 118.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 119.Weihofen A, et al. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu W, Sun Y, Guo S, Lu B. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum. Mol. Genet. 2011;20:3227–3240. doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narendra D, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 125.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in Parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matsuda N, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl Acad. Sci. USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chan NC, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [This study demonstrated that the PINK1–parkin pathway also regulates mitochondrial transport, which may help to quarantine parkin-labelled damaged mitochondria for clearance by mitophagy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl Acad. Sci. USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl Acad. Sci. USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl Acad. Sci. USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park J, Lee G, Chung J. The PINK1–Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem. Biophys. Res. Commun. 2009;378:518–523. doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 135.Mortiboys H, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wood-Kaczmar A, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dagda RK, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sandebring A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lutz AK, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J. Biol. Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ziviani E, Tao RN, Whitworth AJ. Drosphila Parkin requires PINK1 for mitochondrial translocation and ubiquitinates Mitofusin. Proc. Natl Acad. Sci. USA. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gegg ME, et al. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Poole AC, et al. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS ONE. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tanaka A, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [This study demonstrates that mitochondria with a high membrane potential are transported anterogradely towards distal processes, whereas damaged mitochondria return to the cell body following acute depolarization.] [DOI] [PubMed] [Google Scholar]
- 146.Katsumata K, et al. Dynein- and activity-dependent retrograde transport of autophagosomes in neuronal axons. Autophagy. 2010;6:378–385. doi: 10.4161/auto.6.3.11262. [DOI] [PubMed] [Google Scholar]
- 147.Cai Q, Sheng ZH. Uncovering the role of Snapin in regulating autophagy-lysosomal function. Autophagy. 2011;7:445–447. doi: 10.4161/auto.7.4.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J. Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J. Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [This paper provides evidence that mobile and stationary mitochondria show no difference in membrane potential under physiological conditions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Green DR, Houten BV. SnapShot: mitochondrial quality control. Cell. 2011;147:950–950e1. doi: 10.1016/j.cell.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Karki S, Holzbaur EL. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 152.Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc. Natl Acad. Sci. USA. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cai Q, Sheng Z-H. In: Mitochondrial Dynamics and Neurodegeneration. Lu B, editor. Springer; Dordrecht: 2011. pp. 139–168. [Google Scholar]
- 154.de Castro IP, Martins LM, Tufi R. Mitochondrial quality control and neurological disease: an emerging connection. Expert Rev. Mol. Med. 2010;19:e12. doi: 10.1017/S1462399410001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nature Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 157.Westermann B. Mitochondrial fusion and fission in cell life and death. Nature Rev. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 158.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X, et al. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J. Neurochem. 2009;109:153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by β-amyloid peptides in hippocampal neurons. J. Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stokin GB, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 162.Sasaki S, Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:535–540. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- 163.De Vos KJ, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum. Mol. Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Magrané J, et al. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum. Mol. Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shi P, Ström AL, Gal J, Zhu H. Effects of ALS-related SOD1 mutants on dynein- and KIF5-mediated retrograde and anterograde axonal transport. Biochim. Biophys. Acta. 2010;1802:707–716. doi: 10.1016/j.bbadis.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Millecamps S, et al. Alsin is partially associated with centrosome in human cells. Biochim. Biophys. Acta. 2005;1745:84–100. doi: 10.1016/j.bbamcr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 167.Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc. Natl Acad. Sci. USA. 2010;107:16325–16330. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nature Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu YB, Sheng ZH. Increased axonal mitochondrial mobility does not slow amyotrophic lateral sclerosis (ALS)-like disease in mutant SOD1 mice. J. Biol. Chem. 2011;286:23432–23440. doi: 10.1074/jbc.M111.237818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Caviston JP, et al. Huntingtin facilitates dynein/ dynactin-mediated vesicle transport. Proc. Natl Acad. Sci. USA. 2007;104:10045–10050. doi: 10.1073/pnas.0610628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Colin E, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Trushina E, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol. Cell. Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol. Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 175.Song W, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nature Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Orr AL, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J. Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of α-synuclein. Eur. J. Neurosci. 2006;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 178.Yang F, et al. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J. Biol. Chem. 2005;280:17154–17162. doi: 10.1074/jbc.M500843200. [DOI] [PubMed] [Google Scholar]
- 179.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability — a point of convergence in Parkinsonian neurodegeneration? J. Neurochem. 2009;110:1514–1522. doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.