Abstract
Studies on a variety of highly regenerative tissues, including the central nervous system (CNS) in non-mammalian vertebrates, have consistently demonstrated that tissue damage induces the formation of an ionic current at the site of injury. These injury currents generate electric fields (EF) that are 100-fold increased in intensity over that measured for uninjured tissue. In vitro and in vivo experiments have convincingly demonstrated that these electric fields (by their orientation, intensity and duration) can drive the migration, proliferation and differentiation of a host of cell types. These cellular behaviors are all necessary to facilitate regeneration as blocking these EFs at the site of injury inhibits tissue repair while enhancing their intensity promotes repair. Consequently, injury-induced currents, and the EFs they produce, represent a potent and crucial signal to drive tissue regeneration and repair. In this review, we will discuss how injury currents are generated, how cells detect these currents and what cellular responses they can induce. Additionally, we will describe the growing evidence suggesting that EFs play a key role in regulating the cellular response to injury and may be a therapeutic target for inducing regeneration in the mammalian CNS.
Keywords: electric fields, injury current, regeneration, galvanotaxis, glia, traumatic brain injury, migration, proliferation
Traumatic injury to the mammalian central nervous system (CNS) is characterized by minimal functional recovery, in large part because wound repair generally inhibits regeneration through the formation of a cystic cavity that is an anatomical barrier to regeneration, and glial scar that is enriched with extracellular matrix molecules that inhibit sprouting axons (Silver and Miller, 2004). In contrast, many non-mammalian vertebrates demonstrate profound functional recovery following spinal cord transection, cortical stab wounds, infarcts, and even the removal of large portions of the brain through the complete regeneration of the damaged tissue (Tanaka and Ferretti, 2009; Ferreira et al., 2012). These differences in regenerative potential persist in peripheral tissues, where there are examples of non-mammalian vertebrates demonstrating regeneration of the limb, tail, cardiac ventricle, jaw, lens, and cornea (McCaig et al., 2005). The regenerative potential of injured tissues is closely linked to the intensity of injury-induced direct-current bioelectric fields (EFs), which increase upon injury and reach a greater intensity in regenerating tissues than they do in non-regenerating tissues (Borgens et al., 1979a; McCaig et al., 2005). In peripheral tissues, injury-induced EFs are both necessary and sufficient to stimulate regeneration: attenuating endogenous EFs aborts spontaneous regeneration (Borgens et al., 1979b), whereas enhancing endogenous EFs can induce regeneration in those tissues of species where regeneration is normally not expressed (Becker and Spadaro, 1972; Borgens et al., 1977).
Injury-induced EFs are an ideal signal to coordinate the cellular response to CNS injury because they are inherently directional and thus they can guide cells towards the lesion site (a process called electrotaxis), and because their intensity is proportional to the severity of the injury and thus they can stimulate an appropriately robust cellular response. Bioelectric fields are physiologically produced by all tissues because the epithelial cells lining each tissue have an asymmetric distribution of ion channels and transporters in their membrane that sustains a constitutive ionic current into the tissue (Figure 1A) (Colello and Alexander, 2003; McCaig et al., 2005). Tight junctions between epithelial cells allow Na+ to accumulate within the tissue, creating a trans-epithelial electrical potential (TEP). Ohm's law quantifies the relationship between the magnitude of the TEP (V) and both the ionic current (I) produced by the electrogenic Na+ current and the electrical resistance (R) of the epithelial cells and tight junctions such that V = IR. EFs, which are voltage gradients within tissues, are produced by variations in the TEP across the epithelial surface. EFs are relatively low in magnitude in intact tissues where they are a result of local variations in either the electrical resistivity or the ionic current across the epithelium. Large EFs arise when the electrochemical resistivity across the epithelium is lost, which locally grounds the TEP (i.e., TEP = 0 mV) and creates a large voltage gradient with the surrounding tissue where the TEP is sustained (Figure 1B). Moreover, disruption of the epithelial integrity allows Na+ to diffuse down its concentration gradient and out of the tissue, and this ionic current further contributes to the induced EF. In embryonic tissues, tight junctions between epithelial cells break down at sites of high cellular activity and growth such as at the developing limb bud, creating large EFs, which have been shown to be necessary for normal limb development. In injured tissues, physical damage disrupts the epithelial barrier, creating large EFs that are sustained throughout the healing process. Interestingly, regeneration induced by high EFs recapitulates the same physiologic mechanisms of embryogenesis, suggesting that EFs regulate both of these processes through the same mechanisms (Stewart et al., 2007). As the physiology of embryogenesis is largely conserved among vertebrates, this suggests that all vertebrates may have a latent ability to regenerate, but that regeneration is only expressed in those in which injury induced EFs reach an intensity that is sufficient enough to activate these pathways.
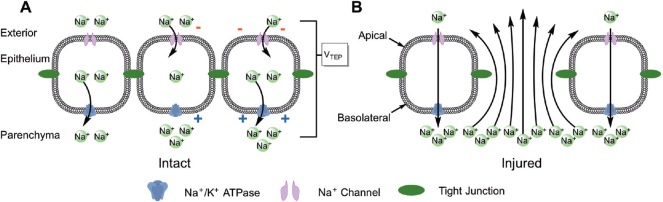
Figure 1.
Physiology of endogenous bioelectric fields.
(A) Epithelial cells have an asymmetric distribution of ion channels throughout their membrane, which allows them to produce a constitutive ionic current into the tissue. Na+/K+-ATPases in the basolateral membrane actively transport Na+ out of the cell and into the tissue parenchyma (left cell), which depletes intracellular Na+ and creates a concentration gradient. Na+ channels in the apical domain allow Na+ from outside the tissue to diffuse down this newly-established concentration gradient and into the epithelial cell (center cell). Basolateral Na+/K+-ATPases continue to export sodium (right cell), which creates a net inward Na+ current and results in a trans-epithelial Na+ concentration gradient. Tight junctions between epithelial cells prevent paracellular diffusion of Na+ down its newly-established concentration gradient, and this accumulation of Na+ within the tissue creates a trans-epithelial electrical potential (TEP). (B) When the integrity of the epithelium is compromised by injury, large bioelectric fields (EFs) are generated between the lesion site and the surrounding intact tissue through two interrelated electrochemical properties. Large voltage gradients between the injured epithelium where the TEP is grounded (i.e., 0 mV) and the surrounding intact tissue where the TEP is sustained create a static EF, which is a function of a voltage gradient over a distance (E = ΔV/d). Epithelial damage also allows Na+ ions to diffuse down their concentration gradient and out of the tissue through a lesion site, and these ionic currents also induce an EF (E = ρI/A where ρ is the resistivity of the tissue, I is the ionic current, and A is the cross-sectional area of the current).
Just as all tissues produce EFs, all cells detect these EFs through electrostatic interactions between these EFs and charged molecules both in the extracellular matrix (ECM) and in the cellular membrane (McCaig et al., 2005). In the ECM, these electrostatic forces cause those molecules with a net charge to move (a process called electrophoresis) and thus establish a concentration gradient, and they cause neutral molecules containing electrical dipoles (i.e., the local separation of charges within the molecule but no overall charge) to align their dipoles – and thus their overall molecular orientation – to be parallel to the EF (Colello and Alexander, 2003; Levin, 2014). Thus, cells may indirectly respond to external EFs through the effect that EFs have on the orientation and concentration of molecules in the surrounding environment. Cells may also directly transduce the external EF either by creating a voltage drop across the cell that affects the resting membrane potential and thus the activity of voltage-sensitive membrane proteins, or by inducing electroosmosis of membrane proteins through which they redistribute to one side of the cell in a charge-dependent manner. Together, these mechanisms allow cells to detect both the magnitude and direction of the external EF, and the non-specific electrostatic interactions allow cell-type specific responses to these EFs based on the particular ECM molecules expressed in the given tissue and on the particular proteins expressed by each cell type. Multiple pathways have been implicated in EF transduction in a variety of cell types and the general trend is that EFs affect cellular behaviors through the same physiologic mechanisms by which these cells would otherwise respond to chemical stimuli.
Recently, ex vivo recordings, which are made using a vibrating electrode to measure the current density across a tissues surface (Reid and Zhao, 2011), have demonstrated that the adult mouse cortex and hippocampus both produce endogenous EFs (Cao et al., 2013, 2015). In the CNS, epithelial cells lining the ventricles contribute to these endogenous EFs by sustaining an inward ionic current into the SVZ; while the surface of the cortex is not lined by an epithelium per se, the astrocytic end feet composing the glia limitans perform the same role in generating EFs. Interestingly, the cortical surface sustains an outward ionic current, which is explained by the fact that the astrocytic endfeet have a distribution of membrane proteins opposite of that in epithelial cells, such that the Na+/K+-ATPases are localized to the apical domain while the and Na+ channels in the basolateral domain. As EFs in the CNS increase upon injury and demonstrate other physiologic properties that are similar to those produced in peripheral tissues, this raises the possibility that, as in the periphery, EFs may be able to activate endogenous mechanisms for regeneration in the CNS. In vitro experiments, where cells are exposed to EFs by applying a direct current through the culture chamber (Song et al., 2007; Baer et al., 2015), have shown that EFs have an intensity-dependent response on multiple cell types in the CNS, affecting neural stem cell migration, proliferation, and differentiation; astrocyte migration, proliferation, and process alignment; neuronal neurite outgrowth; and microglia cyclooxygenase-2 expression and morphologic markers of reactivity (Ariza et al., 2010; Cao et al., 2013; Pelletier et al., 2014; Baer et al., 2015). In general, these EF-induced behavioral responses emerge only at intensities associated with injured and regenerating tissues, and they become more robust at higher EF intensities. In vivo, applying EFs using implanted current-stimulating electrodes has a modest effect on promoting axon sprouting after spinal cord injury in Guinea Pigs (Borgens and Bohnert, 1997). These effects were found both when the EF orientation was constant, and when the EF polarity was reversed every 45 minutes to try and further enhance axon outgrowth towards the lesion by taking advantage of the fact that, upon EF exposure in vitro, spinal neurons extend neuritis towards the cathode faster than they retract those facing the anode (Jaffe and Poo, 1979). Taken together, these observations demonstrate that EFs can induce a behavioral phenotype that is associated with regeneration among multiple cell types from the mammalian CNS, and they suggest that EFs may be able to therapeutically induce regeneration.
The importance of EFs is that they are an endogenous signal generated at an injury site that, upon reaching a certain threshold, may activate an innate regenerative response pre-programmed within the cells that is characterized by migration, proliferation, and changes in cellular differentiation. Once activated, the regenerative physiology occurs spontaneously and thus would require minimal – if any – subsequent therapeutic intervention. In order to demonstrate that endogenous EFs drive these physiologic responses, experimental design needs to consider not only the EF intensities both that are endogenously present in mammals and that are necessary to induce regeneration in nonmammalian vertebrates, but also the time frame over which the cellular behaviors putatively induced by EFs emerge both during the normal injury response and during a successful regenerative response in vivo. Previous research hints that EFs may induce a regenerative response in the CNS, and suggests that EFs may as have an intensity dependent effect on inducing wound repair and regeneration (Borgens et al., 1981, 1987). However, these studies are limited because they do not consider the EF-intensities found in the injured mammalian CNS in vivo, the EF-intensities associated with successful regeneration, the range of cellular behaviors necessary for a regenerative response, or the time frame over which these behaviors develop following an injury.
More recently, our laboratory has been exploring the physiologic role of EFs in driving the cellular response to injury in the mammalian CNS by using an in vitro approach to test a series of interrelated hypotheses: that EFs associated with intact tissues maintain a basal cellular response characteristic of that within the adult CNS, that EFs associated with injured mammalian tissues drive a cellular response that is characterized by cellular reactivity, and that EFs associated with regenerating non-mammalian vertebrate tissues induce a cellular response characteristic of regeneration. The astrocytic response to CNS injury in mammals is non-regenerative, in that astrocytes create a glial scar that inhibits sprouting axons from extending past the lesion, while the astrocytic response in regenerating non-mammalian vertebrates is regenerative, in that astrocytes form a bridge across the lesion site and actively guides regenerating axons towards their original targets (Silver and Miller, 2004; Floyd and Lyeth, 2007; Tanaka and Ferretti, 2009). Thus, we initially assessed whether EFs induce each of the behaviors associated with these responses using purified cortical astrocytes, and we compared the duration over which these behaviors emerge in vitro relative to the timeline over which these same astrocytic responses occur following injury in vivo (Baer et al., 2015). We found that EFs associated with intact tissues (4 mV/mm) had no behavioral effects as compared to unexposed controls. In contrast, EFs associated with both injured mammalian tissues (40 mV/mm) and regenerating non-mammalian vertebrate tissues (400 mV/mm) induced a robust increase in astrocyte migration and in proliferation; whereas the effects on migration were almost immediate, the increase in proliferation emerged only after 48 hours of exposure. Moreover, these effects were transient among astrocytes exposed to EFs associated with the injured mammalian CNS, while they were more robust and sustained among those cells exposed to EFs associated with regeneration. Most interestingly, only EFs associated with regeneration induced morphological changes in astrocytes whereby they developed elongated, highly aligned processes; these highly aligned bipolar astrocytes are morphologically similar to astrocytes in the regenerating CNS that guide sprouting axons. Furthermore, in previous studies, our group and others have found that these EF-exposed aligned astrocytes facilitate robust axon outgrowth in vitro as compared to cells exposed to no EF (Alexander et al., 2006). The fact that the same behaviors necessary for the normal astrocytic response to injury both in mammalian and non-mammalian vertebrates are induced by physiologic EFs associated with injury and regeneration, respectively, together with the observation that these behaviors emerge over the same time period as they do in vivo, suggests that injury-induced EFs may be a crucial regulator of repair.
In conclusion, physiologic EFs may play an important role in the cellular response to injury and in regulating regeneration in the mammalian CNS. This hypothesis, if confirmed by further studies, suggests that the absence of significant regeneration in the mammalian CNS is due to the insufficient intensity of the injury-induced EFs. Thus, it may be possible to induce regeneration by therapeutically enhancing EFs such that they pass the threshold that is required to activate the necessary endogenous physiologic mechanisms. Moreover, as EFs are a major component of the stimulus driving a regenerative response, applied EFs should be able to induce regeneration regardless of the delay between the initial injury and the start of therapy. However, it is essential to establish a better understanding of the intensity of injury-induced EFs within the mammalian CNS in vivo and how these EFs change throughout the wound repair process. Moreover, as the mechanisms by which cells detect these EFs have yet to be elucidated, it is unclear what magnitude and duration of EF stimulation is necessary to induce regeneration. Nevertheless, growing evidence suggests that EFs play a likely role in regulating the cellular response to injury and may be a therapeutic target for inducing regeneration in the mammalian CNS.
Footnotes
Conflicts of interest: None declared.
References
- Alexander J, Fuss B, Colello R. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2:93–103. doi: 10.1017/S1740925X0600010X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza CA, Fleury AT, Tormos CJ, Petruk V, Chawla S, Oh J, Sakaguchi DS, Mallapragada SK. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev. 2010;6:585–600. doi: 10.1007/s12015-010-9171-0. [DOI] [PubMed] [Google Scholar]
- Baer ML, Henderson SC, Colello RJ. Elucidating the role of injury-induced electric fields (EFs) in regulating the astrocytic response to injury in the mammalian central nervous system. PLoS One. 2015;10:e0142740. doi: 10.1371/journal.pone.0142740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RO, Spadaro JA. Electrical stimulation of partial limb regeneration in mammals. Bull N Y Acad Med. 1972;48:627–641. [PMC free article] [PubMed] [Google Scholar]
- Borgens RB, Bohnert DM. The responses of mammalian spinal axons to an applied DC voltage gradient. Exp Neurol. 1997;145:376–389. doi: 10.1006/exnr.1997.6499. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science. 1987;238:366–369. doi: 10.1126/science.3659920. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Roederer E, Cohen MJ. Enhanced spinal cord regeneration in lamprey by applied electric fields. Science. 1981;213:611–617. doi: 10.1126/science.7256258. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Role of subdermal current shunts in the failure of frogs to regenerate. J Exp Zool. 1979a;209:49–56. doi: 10.1002/jez.1402090106. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. J Exp Zool. 1979b;209:377–386. doi: 10.1002/jez.1402090304. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Bioelectricity and regeneration. I. initiation of frog limb regeneration by minute currents. J Exp Zool. 1977;200:403–416. doi: 10.1002/jez.1402000310. [DOI] [PubMed] [Google Scholar]
- Cao L, Pu J, Scott RH, Ching J, McCaig CD. Physiological electrical signals promote chain migration of neuroblasts by up-regulating P2Y1 purinergic receptors and enhancing cell adhesion. Stem Cell Rev. 2015;11:75–86. doi: 10.1007/s12015-014-9524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013;14:184–190. doi: 10.1038/embor.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello RJ, Alexander JK. Chapter 6 - electrical fields: Their nature and influence on biological systems. In: Morkoc H, editor. Advanced semiconductor and organic nano-techniques. San Diego: Academic Press; 2003. p. 319. [Google Scholar]
- Ferreira LM, Floriddia E, Quadrato G, Di Giovanni S. Neural regeneration: Lessons from regenerating and non-regenerating systems. Mol Neurobiol. 2012;46:227–241. doi: 10.1007/s12035-012-8290-9. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Lyeth BG. Astroglia: Important mediators of traumatic brain injury. Prog Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Poo MM. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209:115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol. 2014;592:2295–2305. doi: 10.1113/jphysiol.2014.271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: Current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Lagace M, St-Amour I, Arsenault D, Cisbani G, Chabrat A, Fecteau S, Levesque M, Cicchetti F. The morphological and molecular changes of brain cells exposed to direct current electric field stimulation. Int J Neuropsychopharmacol. 2014;18:pyu090. doi: 10.1093/ijnp/pyu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Zhao M. Measurement of bioelectric current with a vibrating probe. J Vis Exp. 2011;47:e2358. doi: 10.3791/2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Song B, Gu Y, Pu J, Reid B, Zhao Z, Zhao M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc. 2007;2:1479–1489. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- Stewart S, Rojas-Muñoz A, Izpisúa Belmonte JC. Bioelectricity and epimorphic regeneration. Bioessays. 2007;29:1133–1137. doi: 10.1002/bies.20656. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci. 2009;10:713–723. doi: 10.1038/nrn2707. [DOI] [PubMed] [Google Scholar]