Galectins are a family of endogenous β glycan-binding proteins that play an important role in the modulation of inflammation-associated with neurodegeneration as seen in various neurological disorders such as dementia, multiple sclerosis (MS), Alzheimer's disease (AD) (Chen et al., 2014). Members of the galectin family specifically galectin-1, -3 and -9 are involved in neuromodulation via cytokine production contributing to central nervous system (CNS) pathology and/or neuro-preservation. Galectins are expressed by activated microglia/infiltrating macrophages and astrocytes and recently we have shown that they are also expressed by brain microvascular endothelial cells that constitute the blood-brain barrier (BBB) (Parikh et al., 2015). Several studies have shown that galectins are important modulators participating in homeostasis of the CNS and neuroinflammation. Galectins contribute to CNS pathophysiology by either controlling the inflammatory response or by remodeling damaged CNS tissues. Since galectins are pro-inflammatory mediators in the CNS, either modulation of their expression or activity could be a sound therapeutic strategy for a variety of neurological disorders mentioned above.
HIV-1 targets the CNS early in infection, specifically in subcortical brain regions resulting in cognitive disturbances linked both to viral activity and inflammatory response leading to the neuronal apoptosis causing a spectrum of HIV-associated neurocognitive disorders (HAND). Long-term HIV replication in the brain occurs in astrocytes and microglia, allowing the sequestered virus to compromise neuronal function. Galectin-1 promotes HIV-1 binding to susceptible CD4+ cells enhancing viral infection suggesting that CD4 is one of the host ligand of galectin-1 (Mercier et al., 2008; St-Pierre et al., 2011). It is believed that galectin-1 facilitates HIV-1 infection through direct cross-linking of HIV gp120 and host cell surface CD4 receptor. Additionally, galectin-1 promotes infection of HIV-1 X4, X4R5, and R5 variants in susceptible cells as well. HIV-1 susceptible CNS cells may have affinity for galectin-1 due to the presence of glycosylated protein receptors thus responding to the immunomodulatory effects mediated by galectin-1. Microglial cells are the resident macrophages of the brain and act as the first line of defense in the CNS microglia constitute the lesser portion of the total glial cell population within the brain and are found in a resting state in the healthy CNS. Under pathological conditions microglia get activated and undergo morphological changes in addition to inducing expression of proinflammatory cytokines like interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-α (TNF-α), chemokines, reactive oxygen and nitrogen species, all of which cause neuronal damage (Kettenmann et al., 2011). A variety of microglia receptors are involved in triggering an inflammatory response, specifically via receptor for advanced glycosylation endproducts (RAGE), complement receptor C5aR and certain toll like receptors (TLRs). C5a is a highly proinflammatory molecule generated in the process of complement activation. CD88 is a receptor for C5a expressed on the surface of innate immune cells, including microglia. The interaction between C5a and CD88 leads to the production of inflammatory cytokines and reactive oxygen species. (Woodruff et al., 2010). Microglial expression of TLR-2, -4 and -9, have been associated with microglial activation and neurotoxicity highlighting their role in neuropathology (Lehnardt, 2010). It will be interesting to investigate if galectin-1 modulates the expression of RAGE, C5aR or TLRs thereby altering inflammatory response. Our current studies are focused on exploring these mechanisms of galectin-1 action.
In the context of HAND, activation of TLRs on microglial cells by morphine and HIV protein TAT has been shown to contribute to the increased neuropathophysiology (Dutta et al., 2012). HIV-1 Tat or gp120 alone and in combination with morphine modulate the expression of TLR-2, -4 in astrocytes resulting in the release of inflammatory cytokines (El-hage et al., 2011). Although the role of these microglial receptors in the neuroinflammatory response is established, limited information is available on the role of endogenous ligands such as galectins that may trigger activation of TLRs, C5aR or RAGE. A recent report showed galectin-3-dependent-TLR4 activation could contribute to sustained microglia activation, prolonging the inflammatory response in the brain (Burguillos et al., 2015).
Activated microglia contribute to CNS pathology or repair, depending on the prevalent microenvironment and their mode of activation, typically activated microglia are associated with neurodegeneration. When endogenous galectin-1 is absent, classical microglial activation occurs, concurrent with an increase in demyelination, axonal loss and a reduction in endogenous synaptic repair. Within CNS inflamed tissues, Iba1+ cells are represented by microglia and when galectin-1 is present, there is a downregulation of activation markers, proinflammatory cytokines, and inducible nitric oxide synthase (iNOS) expression.
The underlying mechanisms of galectin-1 mediated neuroprotection occur by inactivation of microglia, via the establishment of galectin-1-N-linked glycan interactions on microglial cells that may provide an endogenous mechanism to limit neuropathology. Galectin-1-N-linked-glycan interactions can adjust thresholds of cellular activation and survival through modulation of endocytosis, trafficking, and signaling of canonical receptors (Starossom et al., 2012). Therefore, targeting the galectin1-N-linked glycan interactions will result in decrease of inflammation-associated neurodegeneration and may represent a new therapeutic approach for neurodegenerative diseases like HIV-1 dementia.
Our research is focused on evaluating mechanism(s) that contribute to neuroinflammation and neurodegeneration in HIV-1 infection in the context of drug abuse and we have shown that galectin-1 is neuroprotective (Reynolds et al., 2012; Parikh et al., 2015). We are currently examining the effect of galectin-1 treatment on inflammatory response and cellular apoptosis in vitro in normal and HIV infected primary human microglia. For these studies we use primary human microglial cells CHME-5 and HIV-latently infected CHME-5 cells (generously donated by Dr. J. Karn, CWRU, Cleveland, OH). CHME-5 cell line was created by transfecting human fetal microglia with the large T antigen of the simian virus 40 and both CHME-5/CHME-5 HIV microglia are maintained in Dulbecco's minimal essential medium, high glucose (DMEM HG) supplemented with 5% FBS and 1% penicillin/streptomycin. Treatment of CHME-5 and CHME-5/HIV with LPS (50 ng/mL) stimulated the release of proinflammatory cytokines resulting in an increase in oxidative stress as measured by reactive oxygen species (ROS) production. ROS includes superoxide (O2), hydrogen peroxide (H2O2) and hydroxyl radical (OH), which under physiological conditions are generated at low levels and play important roles in signaling and metabolic pathways. Increased oxidative stress causes the generation of ROS, which are potentially toxic for cells further contributing to neuroinflammation. We observed increased Iba1+ expression in HIV transfected CHME-5/HIV microglia as compared to untransfected CHME-5 cells indicative of increased microglial activation in HIV infected cells (Figure 1A, B). We hypothesize that galectin-1 will reduce neuroinflammation in both human microglia cell line CHME-5 and CHME-5/HIV. Our results (Figure 1C, D) showed that treatment with galectin-1 (1 µM) decreased microglial activation in both CHME-5 and CHME-5/HIV cells. Histograms shown in Figure 1E, F show the Image J quantitation of fluorescence signal intensity in pixel units of representative fluorescent images shown in Figure 1A, B and Figure 1C, D respectively. Treatment of microglia with pro-inflammatory cytokines LPS resulted in a 60% increase (P < 0.01) in oxidative stress in CHME-5 and a 26% increase (P < 0.05) in CHME5/HIV respectively compared to the untreated controls. Further, treatment of microglia with galectin-1 (1 µM) reduced oxidative stress by ~25% (P < 0.05) in CHME5 by ~60% (P < 0.01) in CHME-5/HIV when compared to the LPS treated cells (Figure 2). Our data suggests that galectin-1 treatment could reduce neuroinflammation via decreased oxidative stress and we propose that mechanism such as the nitric oxide-arginase network may potentially play a neuroprotective role in HAND. Our current research is focused on further exploring the mechanism by which galectin-1 mediates CNS homeostasis.
Figure 1.
Immunofluorescence staining using Iba-1 antibody.
Primary antibody is a mouse monoclonal and fluorescence labeled secondary antibody (yellow) (Alexa Fluor 647 rabbit anti-mouse). Nuclear stain DAPI (blue). The fluorescent signal intensity analyzed using image J software Imaging done using EVOS® FL Cell Imaging System. Iba-1 is a microglial marker. Increased Iba-1 expression in CHME-5/HIV indicating microglial activation in the HIV transfected microglia. DAPI: 4′6-diamidino-2-phenylindole; Iba-1: ionized calcium-binding adapter molecule 1.
Figure 2.
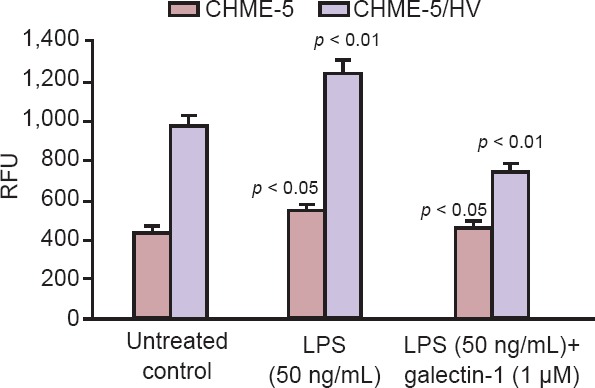
ROS/RNS assay (Cell Biolabs kit).
The assay employs a proprietary quenched fluorogenic probe DiOxyQ (DCFH-DiOxyQ), which is a specific ROS/RNS probe. Briefly, the DCFH-DiOxyQ probe is first primed with a quench removal reagent, and subsequently stabilized in the highly reactive DCFH form. In this reactive state, ROS and RNS species react with DCFH, which is rapidly oxidized to the highly fluorescent DCF. The amount of DCF expressed as the fluorescence intensity is proportional to the total ROS/RNS levels within the sample. Treatment of CHME-5/HIV with LPS resulted in 60 % increase (P < 0.01) in oxidative stress in CHME-5 and a 26% increase (P < 0.05) in CHME5/HIV respectively compared to the untreated control. Treatment of microglia with galectin-1 (1 mM) reduced oxidative stress by ~25% (P < 0.05) in CHME5 and by ~60% in CHME-5/HIV as compared to the LPS treated cells. DCF: 2′,7′-dichlorofluorescin; ROS/RNS: reactive oxygen species/reactive nitrogen species.
Currently, a galectin-1 inhibitor (OTX008) is undergoing phase I clinical trials and is believed to have potential anti-angiogenic and antineoplastic activities. A galectin-3 inhibitor GR-MD-02 is also in early phase I clinical trials, is shown to be safe and well tolerated, in patients with fatty liver disease/nonalcoholic steatohepatitis (NASH) and advanced liver fibrosis. In light of the neuroprotective properties of galectin-1, its targeted overexpression would be a novel therapeutic approach for several neurodegenerative pathologies.
References
- Burguillos MA, Svensson M, Schulte T, Boza-Serrano A, Garcia-Quintanilla A, Kavanagh E, Santiago M, Viceconte N, Oliva-Martin MJ, Osman AM, Salomonsson E, Amar L, Persson A, Blomgren K, Achour A, Englund E, Leffler H, Venero JL, Joseph B, Deierborg T. Microglia-secreted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. 2015;9:1626–1638. doi: 10.1016/j.celrep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Chen HL, Liao F, Lin TN, Liu FT. Galectins and neuroinflammation. Adv Neurobiol. 2014;9:517–542. doi: 10.1007/978-1-4939-1154-7_24. [DOI] [PubMed] [Google Scholar]
- Dutta R, Krishnan A, Meng J, Das S, Ma J, Banerjee S, Wang J, Charboneau R, Prakash O, Barke RA, Roy S. Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae. J Neurosci. 2012;32:9917–9930. doi: 10.1523/JNEUROSCI.0870-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest. 2011;40:498–522. doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. 2008;371:121–129. doi: 10.1016/j.virol.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Parikh N, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Mammen MJ, Schwartz SA, Mahajan SD. Galectin-1 suppresses methamphetamine induced neuroinflammation in human brain microvascular endothelial cells: Neuroprotective role in maintaining blood brain barrier integrity. Brain Res. 2015;1624:175–187. doi: 10.1016/j.brainres.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Yong KT, Hui R, Prasad PN, Schwartz SA. Nanoparticle based galectin-1 gene silencing, implications in methamphetamine regulation of HIV-1 infection in monocyte derived macrophages. J Neuroimmune Pharmacol. 2012;7:673–685. doi: 10.1007/s11481-012-9379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, Bassil R, Croci DO, Cerliani JP, Delacour D, Wang Y, Elyaman W, Khoury SJ, Rabinovich GA. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012;37:249–263. doi: 10.1016/j.immuni.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol. 2011;85:11742–11751. doi: 10.1128/JVI.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TM, Ager RR, Tenner AJ, Noakes PG, Taylor SM. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromolecular Med. 2010;12:179–192. doi: 10.1007/s12017-009-8085-y. [DOI] [PubMed] [Google Scholar]



