Preclinical stroke research has introduced a number of potential interventions that can be utilized to enhance recovery after stroke (Lo and Rosenberg, 2009). Many of these potential interventions targeted a single component of the neurovascular unit, but the majority of these interventions failed to prove effective in clinical studies. Additionally, Madri et al. (2009) demonstrated that improving stroke outcome requires a well-orchestrated prosurvival response in the neurovascular unit. This includes promoting angiogenesis, neurogenesis and neuroplasticity. Accordingly, it is essential to identify novel targets that can positively affect different components of the neurovascular unit.
Brain-derived neurotrophic factor (BDNF) is a 13 kDa protein that belongs to the neurotrophin family (Kermani and Hempstead, 2007). The biological effects of BDNF include angiogenesis, neurogenesis, and neuroplasticity (Kermani and Hempstead, 2007; Madri, 2009). BDNF is expressed in different components of the neurovascular unit, including brain microvascular endothelial cells. Madri et al. (2009) demonstrated an essential role of BDNF in recovery after ischemic central nervous system (CNS) insults. Unfortunately, clinical utility of this protein has been limited by its pharmacokinetic and chemical properties (Kermani and Hempstead, 2007). Interestingly, Krikov et al. (2008) suggested the ability of candesartan, an angiotensin receptor blocker (ARB), to enhance BDNF-mediated signaling through upregulating its receptor (Tropomyosin receptor kinase B – TrkB) expression. This upregulation was detected in the brain without documenting the source of this upregulation. Additionally, Kozak et al. (2009) demonstrated the ability of candesartan to induce a sustained proangiogenic response in the brain after ischemic stroke. Accordingly, attention was directed to assess the effect of ARB on BDNF expression in brain endothelial cells and its involvement in ARB-mediated beneficial effects.
Candesartan increased BDNF expression in both the brain and the cultured brain endothelial cells (Alhusban et al., 2013). This increase was independent of its hypotensive effect. Additionally, candesartan induced a proangiogenic response in the brain derived-endothelial cells (Alhusban et al., 2013), encompassing migration, proliferation and tube formation (Alhusban et al., 2013). This response was blunted by inhibiting BDNF receptor-mediated signaling via a blocking antibody (Alhusban et al., 2013). A similar response was achieved when TrkB-Fc, a fusion protein of the extracellular domain of TrkB and the Fc portion of IgG that is used to inhibit BDNF signaling, was used to inhibit BDNF signaling (Alhusban et al., 2013). Interestingly, inhibiting BDNF-mediated signaling blunted the candesartan-induced proangiogenic response (Alhusban et al., 2013). These findings introduced BDNF as a novel angiogenic mediator in the brain and a key regulator of brain angiogenesis under normoxic conditions. It was still unknown whether BDNF plays a role in candesartan-induced angiogenesis and recovery after stroke.
BDNF has been found to be essential during embryogenesis, making gene deletion lethal and complicating the determination of the exact biologic role in recovery after stroke (Ploughman et al., 2009). To overcome this, we decided to use a short hairpin RNA (shRNA)-based knocking down strategy in adult animals. Lentiviruses expressing shRNA directed against BDNF were injected bilaterally in the ventricles (Fouda et al., 2016). We adopted this strategy after demonstrating its efficiency in knocking down more than 70% of the BDNF in whole brain sections (Fouda et al., 2016). Lentiviruses were allowed 14 days to fully integrate their genetic content in the brain cells (Fouda et al., 2016). Following this integration period, we induced stroke using middle cerebral artery occlusion (MCAO) for 90 minutes (Fouda et al., 2016). Animals were then randomized to receive either a single dose of candesartan or saline at the time of reperfusion and followed up for 14 days (Fouda et al., 2016). Consistent with our previous reports (Kozak et al., 2009), candesartan reduced the stroke-induced neurologic deficit and improved functional recovery. This benefit was observed 24 hours after stroke and was sustained for 14 days (Fouda et al., 2016). Knocking down BDNF blunted both short term and long-term candesartan-induced benefits (Fouda et al., 2016).
Angiogenesis and neuroplasticity has been shown to be essential for improving stroke outcome (Madri, 2009). To assess the involvement of BDNF in candesartan-induced angiogenic response, we evaluated vascular density and vascular endothelial growth factor (VEGF) expression in the brain sections. Candesartan induced a two-fold increase in the vascular density and about a 40% increase in VEGF immunoreactivity (Fouda et al., 2016). This proangiogenic response was completely prevented by knocking down BDNF expression (Fouda et al., 2016). Similarly, candesartan increased synaptic density whereas knocking down BDNF blunted this candesartan-induced effect (Fouda et al., 2016). Interestingly, candesartan-induced increased synaptic density was observed in both hemispheres which suggest an ischemia-independent effect of candesartan on neuroplasticity (Fouda et al., 2016). Collectively, these results introduce BDNF as a key mediator of the candesartan prorecovery effect after stroke (Fouda et al., 2016).
Vascular endothelial growth factor is the most well studied angiogenic factor. Kozak et al. (2009) demonstrated that the candesartan-induced proangiogenic response after stroke was partially mediated through increased VEGF expression. In contrast, blocking BDNF signaling in human cerebromicrovascular endothelial cells (hCMECs) completely blocked the candesartan-induced proangiogenic response (Alhusban et al., 2013). Furthermore, knocking down BDNF after stroke inhibited candesartan-induced increased VEGF expression (Fouda et al., 2016). These findings suggest that BDNF acts as an upstream regulator of VEGF expression in the brain after stroke.
BDNF has a well-established involvement in recovery following CNS ischemic insults (Ploughman et al., 2009). Accordingly, it is logical to assume that knocking down BDNF would impair the recovery after stroke. Surprisingly, knocking down BDNF in saline-treated animals did not affect functional recovery as compared to vector-injected saline treated animals. Similar findings were found on the molecular level, as these two groups had similar levels of angiogenesis and synaptic density. This discrepancy highlights the possibility that spontaneous brain recovery after ischemic stroke involves different molecular pathways from those involved in rehabilitation-induced recovery. The majority of papers that have assessed the BDNF involvement in recovery after stroke included a rehabilitation element or BDNF overexpression (Ploughman et al., 2009; Fouda et al., 2016). Ploughman et al. (2009) used an oligonucleotide-based BDNF knockdown program to assess its involvement in motor recovery after stroke. In their study, they used an enrichment and exercise program as an intervention and they demonstrated the essential role of BDNF in the intervention-induced prorecovery effect. In agreement with our findings, there was no difference between the animals that received anti BDNF oligonucleotide or vehicle and were not rehabilitated (Ploughman et al., 2009).
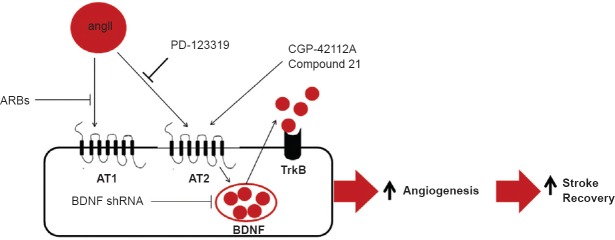
In light of these results, BDNF is an essential requirement for ARB-induced prorecovery effect after stroke. ARBs mediate their effects through blocking the angiotensin II receptor type 1 (AT1), which leads to unopposed stimulation of the other angiotensin II receptors, mainly AT2 (Alhusban et al., 2013). Iwai et al. (2004) demonstrated that knocking down AT2 receptor blunts the ARB prorecovery effect after stroke. Accordingly, it is reasonable to hypothesize that ARB-induced BDNF expression is mediated through unopposed stimulation of the AT2 receptor. To test this hypothesis, we treated hCMECs with candesartan or PD123319, an AT2 antagonist, or their combination (Alhusban et al., 2013). Blocking AT2 reversedthe candesartan-induced proangiogenic effect as well as the ARB-induced BDNF expression in hCMECs (Alhusban et al., 2013). Furthermore, direct AT2 stimulation via CGP-42112A, a selective AT2 peptide agonist, increased the migration rate of endothelial cells and BDNF expression in endothelial cells (Alhusban et al., 2013). These findings suggested the potential of AT2 agonists to increase BDNF expression and consequently improve stroke outcome. To assess the ability of AT2 agonists to increase BDNF expression and improve stroke outcome, we randomized rats to receive either Compound 21, a water solublenon-peptide selective AT2 agonist, or at the time of reperfusion after stroke (Alhusban et al., 2015). Compound 21 reduced infarct size and improved functional recovery (Alhusban et al., 2015). This prorecovery effect was associated with increased BDNF expression, an anti-apoptotic effect, and increased survival signaling in the ischemic hemisphere (Alhusban et al., 2015). Additionally, C21 induced a proangiogenic response in the brain. This proangiogenic response was inhibited with BDNF neutralization in hCMECs (Alhusban et al., 2015).
In conclusion, BDNF plays an indispensable role in ARB-induced prorecovery effect after stroke. Furthermore, the ARB-induced prorecovery effect is associated with a proangiogenic response in the brain that is dependent on BDNF signaling. Additionally, the prorecovery effect of ARBs is mediated through the unopposed AT2 stimulation that leads to increased BDNF expression following stroke. These findings highlight an intriguing novel pathway that can be used to improve stroke outcome (Figure 1).
Figure 1.
Angiotensin II receptor blockers (ARB) improve functional outcome after stroke through unopposed stimulation of angiotensin II receptor type 2 (AT2).
This unopposed stimulation upregulates brain-derived neurotrophic factor (BDNF) expression which induces a proangiogenic state. The proangiogenic state is associated with improved stroke outcome.
This work was supported by RO1-NS063965, R21-NS088016, VA Merit Review-BX000891 and Jowdy Professorship to SCF.
References
- Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344:348–359. doi: 10.1124/jpet.112.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhusban A, Fouda AY, Bindu P, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens. 2015;33:170–180. doi: 10.1097/HJH.0000000000000364. [DOI] [PubMed] [Google Scholar]
- Fouda AY, Alhusban A, Ishrat T, Pillai B, Eldahshan W, Waller JL, Ergul A, Fagan SC. Brain-derived neurotrophic factor knockdown blocks the angiogenic and protective effects of angiotensin modulation after experimental stroke. Mol Neurobiol. doi: 10.1007/s12035-015-9675-3. doi:10.1007/s12035-015-9675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40:1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikov M, Thone-Reineke C, Muller S, Villringer A, Unger T. Candesartan but not ramipril pretreatment improves outcome after stroke and stimulates neurotrophin BNDF/TrkB system in rats. J Hypertens. 2008;26:544–552. doi: 10.1097/HJH.0b013e3282f2dac9. [DOI] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40:S2–3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol 60 Suppl. 2009;4:95–104. [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]