Keywords: nerve regeneration, spinal cord injury, secondary injury, statins, atorvastatin, autophagy, Beclin-1, light chain 3B, neuroprotection, apoptosis, motor function recovery, neural regeneration
Abstract
Atorvastatin, a lipid-lowering medication, provides neuroprotective effects, although the precise mechanisms of action remain unclear. Our previous studies confirmed activated autophagy following spinal cord injury, which was conducive to recovery of neurological functions. We hypothesized that atorvastatin could also activate autophagy after spinal cord injury, and subsequently improve recovery of neurological functions. A rat model of spinal cord injury was established based on the Allen method. Atorvastatin (5 mg/kg) was intraperitoneally injected at 1 and 2 days after spinal cord injury. At 7 days post-injury, western blot assay, reverse transcription-polymerase chain reaction, and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining results showed increased Beclin-1 and light chain 3B gene and protein expressions in the spinal cord injury + atorvastatin group. Additionally, caspase-9 and caspase-3 expression was decreased, and the number of TUNEL-positive cells was reduced. Compared with the spinal cord injury + saline group, Basso, Beattie, and Bresnahan locomotor rating scale scores significantly increased in the spinal cord injury + atorvastatin group at 14–42 days post-injury. These findings suggest that atorvastatin activated autophagy after spinal cord injury, inhibited apoptosis, and promoted recovery of neurological function.
Introduction
Although spinal cord injury (SCI) comprises primary injuries and secondary injuries, the secondary injury plays a more important role in the recovery of neurological function after spinal cord injury. Secondary injury is mainly caused by oxidative stress, immune dysfunction, the occurrence of apoptosis, and autophagy inhibition (Beattie, 2004). In recent years, an increasing number of studies have focused on the neuroprotective effects of drugs with regard to neurological function and mechanisms of action after SCI. For example, rapamycin promotes recovery of neurological function by activating nerve cell autophagy, and melatonin promotes recovery of neurological function by inhibiting oxidative stress and apoptosis (Chen et al., 2013; Naseem and Parvez, 2014). However, the specific molecular mechanisms of functional recovery after SCI and treatment measures have not been clearly stated. In recent years, researchers have questioned the efficacy of methylprednisolone, the first-line therapeutic drug for SCI (Liu et al., 2009; Tesiorowski et al., 2013; Fehlings et al., 2014; Harrop, 2014). Methylprednisolone increases the risk of SCI complications, such as infection, pulmonary embolism, and even death (Hurlbert et al., 2015), which highlights the urgency of a novel treatment strategy for SCI.
Statins are one of the first-line therapeutic drugs for treating diseases such as high cholesterol, coronary atherosclerosis, and atherosclerosis (Komukai et al., 2014; Pordal et al., 2015). The statins mainly comprise atorvastatin, simvastatin, and lovastatin (Pursnani et al., 2015; Schultz et al., 2015). Some studies have focused on the non-cholesterol-lowering effect of statins. For instance, lovastatin exhibits antioxidant properties, suppresses apoptosis and schwannoma cell differentiation, and induces autophagy (Sane et al., 2010; Abdanipour et al., 2014). Simvastatin activates Wnt signaling in nerve cells, inhibits apoptosis and inflammatory reactions, and accelerates expression of neurotrophic factors (Holmberg et al., 2006; Esposito et al., 2012; Gao et al., 2015a). Atorvastatin works by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. In recent years, the diverse effects of atorvastatin in the central nervous system have been a concern. Atorvastatin inhibits early apoptosis after thoracic SCI and promotes recovery of neurological function (Dery et al., 2009). Atorvastatin also activates autophagy in mesenchymal stem cells via the AMPK/mTOR signaling pathway, suppresses apoptosis, and protects cells against injury (Zhang et al., 2012). Some studies have reported that atorvastatin promotes axonal regeneration and nerve growth (Aktas et al., 2003; Chen et al., 2003). Unfortunately, the specific role of atorvastatin on recovery of neurological function after SCI has not been clearly verified.
Autophagy plays an important role in secondary injury after SCI (Goldshmit et al., 2015; Wang et al., 2015a). Autophagy is a survival-promoting pathway that captures, degrades, and recycles intracellular proteins and organelles in lysosomes. The autophagosome fuses with a lysosome and the contents are degraded and recycled. The resulting breakdown products are inputs of cellular metabolism for the maintenance of cellular homeostasis (Komatsu et al., 2006; Lee et al., 2012). During the formation of autophagy bodies, light chain 3 (LC3) shifts from the non-esterified form (LC3A) to the esterified form (LC3B). The formation of LC3B leads to the formation of autophagosomes (Kabeya et al., 2000; Chen et al., 2012). Beclin-1 protein is also used as a marker for autophagy (Levine and Kroemer, 2008).
Wang et al. (2015a) showed activated autophagy and inhibited apoptosis after SCI, which led to recovery of neurological function. Therefore, in the present study, we associated atorvastatin with autophagy after SCI, and hypothesized that atorvastatin exerts a neuroprotective effect by influencing autophagy.
Materials and Methods
Animals
A total of 54 male 8–12-week-old Sprague-Dawley rats weighing 260–300 g, were purchased from Charles River, Beijing, China (license No. SCXK (Jing) 2012-0001). The rats were housed in individual cages in the Specific Pathogen-Free Animal Experimental Center of Jinzhou Medical University of China under a 12-hour light/dark cycle and in a dry and ventilated room at 23–25°C, with free access to food and water. All surgery was performed under anesthesia, and all efforts were made to minimize pain and distress in the experimental animals. All procedures were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986). The protocols were approved by the Animal Ethics Committee of Liaoning Medical University of China.
Model establishment and intervention
A total of 54 male rats were equally and randomly allocated to the SCI + atorvastatin (SCI + Ato group), SCI + saline, and sham groups. In the SCI + Ato and SCI + saline groups, rat models of SCI were established using the method described previously (Gao et al., 2015b). In the SCI + Ato group, atorvastatin (5 mg atorvastatin was dissolved in 1 mL saline; 5 mg/kg; Pfizer, New York, NY, USA) was intraperitoneally injected immediately after model establishment. An equal volume of atorvastatin was injected again at 1 and 2 days after SCI (Dery et al., 2009). In the SCI + saline group, saline (1 mL; 1 mL/kg) was intraperitoneally injected at the same time. In the sham group, rats only underwent laminectomy, but did not receive SCI.
Establishing models of SCI
SCI models were established based on the method by Allen (Gao et al., 2015b). The rats were intraperitoneally anesthetized with 10% chloral hydrate (0.3 mL/kg). The T9/10 spinous process was exposed and clamped with rongeur forceps. After removal of the vertebral plate, the spinal cord was exposed. A 10-g 2-mm diameter impactor was vertically dropped from a 2.5-cm height, which directly impacted the spinal cord at T9/10. Congestion at the injury site, rapid contraction, tremor of the lower limbs, and incontinence confirmed successful establishment of the model. Any rats where the model was not established were eliminated from the study. The wound was washed with warm saline, and the tissue was sutured layer by layer. The surface was covered with sterile gauze. After surgery, the rats were housed in the Specific Pathogen-Free Animal Experimental Center of Liaoning Medical University of China, and injected with antibiotics for 3 consecutive days. Urinary bladder massage and extrusion were performed three times daily until autonomic function of urination resumed.
Evaluation of motor function
Motor function was assessed in six rats from each group before surgery, and then 1, 3, 7, 14, 21, 28, 35, and 42 days after surgery using the Basso, Beattie, and Bresnahan (BBB) scale (Basso et al., 1995). The BBB scores ranged from 0 to 21, where 21 = normal and 0 = complete paralysis.
Western blot assay
At 7 days post-injury, the rats were intraperitoneally anesthetized with 10% chloral hydrate 0.3 mL/kg (n = 4 per group). After exposing the spinal cord, a 3-mm spinal cord section from the center of the injury site was collected and lysed with radioimmune precipitation assay buffer, which contained 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, and 1 mM ethylenediamine tetraacetic acid. Protein concentrations were determined using the Bicinchoninic Acid Protein Assay, and samples were diluted to a concentration of 2 μg/μL. Protein samples (40 μg) were then separated by electrophoresis on 12% separation gels. The separated proteins were transferred onto polyvinylidene difluoride membranes and then blocked with confining liquid (5% skim milk powder and 0.1% Tween-20 in PBS) at room temperature for 2 hours, and incubated overnight at 4°C with hybridization solution-diluted primary antibody: rabbit anti-Beclin-1, LC3B, caspase-9, caspase-3, and β-actin polyclonal antibodies (1:1,000; Abcam, Cambridge, UK). The following day, the membranes were incubated with hybridization solution-diluted secondary antibody (goat anti-rabbit IgG; 1:2,000; Abcam) at room temperature for 2 hours. Images were collected using the UVP gel imaging system (UVP, Upland, CA, USA). Gray values of specific bands were measured using the ImageJ2x system (National Institutes of Health, Bethesda, MD, USA). Results represented the optical density ratio of the target protein to β-actin.
Reverse transcription- polymerase chain reaction (RT-PCR)
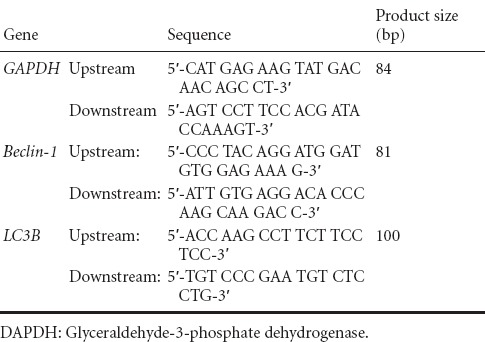
At 7 days post-injury, the rats were intraperitoneally anesthetized with 10% chloral hydrate 0.3 mL/kg (n = 4 per group). After exposing the spinal cord, a 3-mm spinal cord section from the center of the injury site was collected and total RNA was isolated using Trizol (Invitrogen, Grand Island, CA, USA). From each sample, 1 μg total RNA was converted to cDNA using the TaKaRa RNA PCRTM Kit (AMV 8) Ver. 3.0. (TaKaRa Bio Inc, Shiga, Japan). With the cDNA serving as a template, the cDNA was amplified in a gradient cycler (Mastercycler Gradient; Eppendorf, Hamburg, Germany) with the following conditions: one cycle of 5 minutes at 94°C; 30 cycles of 30 seconds at 94°C (denaturalization), 30 seconds at 60°C (annealing), and 30 seconds at 72°C (extension); and one cycle of 10 minutes at 72°C (amplification). The sequences of primers used for RT-PCR are shown in Table 1. RT-PCR products were electrophoretically separated on agarose gels. Images were collected using the UVP gel imaging system (UVP). Gray values of specific bands were measured using the ImageJ2x system (National Institutes of Health). Results represented the optical density ratio of the target gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 1.
Primer sequences
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
At 7 days post-injury, the rats were intraperitoneally anesthetized with 10% chloral hydrate 0.3 mL/kg (n = 4 per group). The rats were perfused with 0.9% saline and 4% paraformaldehyde. After exposing the spinal cord, a spinal cord tissue (1 cm) section was collected from the center of the injury site. The tissue was then fixed with 4% paraformaldehyde for 3 days, and dehydrated with 30% sucrose for 3 days. An approximately 3-mm section of the spinal cord proximal to the injury site on the side of the head was sliced into frozen sections using a freezing microtome (Leica CM3050S; Heidelberg, Germany). The sections on the slides were incubated in fixative at room temperature for 20 minutes, washed with phosphate-buffered saline (PBS) for 30 minutes, permeabilized with 0.1% Triton X-100 for 2 minutes at 4°C, washed twice with PBS, and then incubated with TUNEL staining mixture (In Situ Cell Death Detection Kit, TMR red; Roche, Mannheim, Germany) at 37°C in the dark for 1 hour. Afterwards, samples were incubated with 4′,6-diamidino-2-phenylindole (1:1,000) in the dark for 2 minutes, and mounted with glycerol. Apoptosis was observed using a fluorescence microscope. Apoptotic cells were labeled by TUNEL (red; DNA fragments after apoptosis) and 4′,6-diamidino-2-phenylindole (blue; nucleus). The proportion of TUNEL-positive cells was calculated by the number of apoptotic cells/total number of cells.
Statistical analysis
Data were expressed as the mean ± SD, and analyzed using the Graph Prism Program, Version 5.0 software (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance and the least significant difference test were used to compare differences in intergroup data. A value of P < 0.05 was considered statistically significant.
Results
Atorvastatin effects on recovery of neurological function after SCI
No significant difference in motor function was detected in any rats prior to model establishment. On day 1 post-injury, rats from the SCI + Ato and SCI + saline groups scored 0 (complete paralysis), and these BBB scores increased with prolonged time. During the two weeks immediately following injury, there was no significant difference in BBB scores between the SCI + Ato group and SCI + saline group (P > 0.05). Between 2 and 6 weeks post-injury, the BBB scores were significantly increased in the SCI + Ato group compared with the SCI + saline group (P < 0.05, P < 0.01, P < 0.001; Figure 1).
Figure 1.
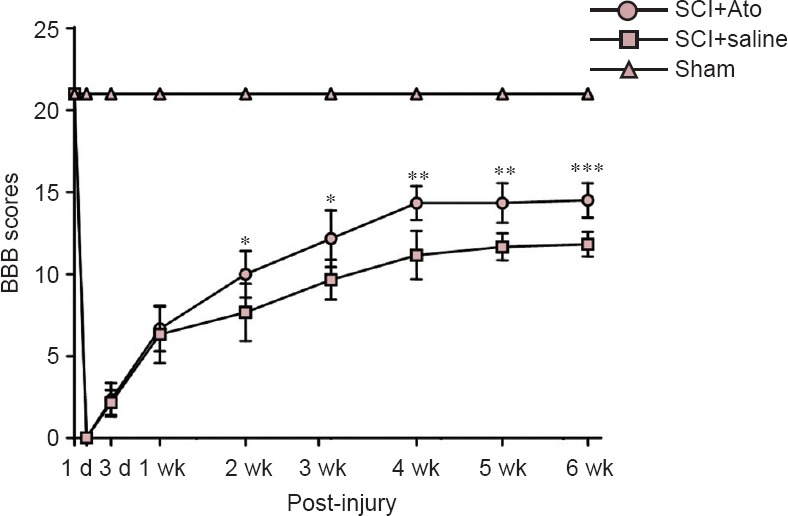
Effects of atorvastatin on motor function in rats with SCI.
*P < 0.05, **P < 0.01, ***P < 0.001, vs. SCI + saline group (mean ± SD, n = 6, one-way analysis of variance and the least significant difference test). Ato: Atorvastatin; SCI: spinal cord injury; BBB: Basso, Beattie, and Bresnahan locomotor rating scale; d: day(s); wk: week(s).
Effects of atorvastatin on apoptosis in rats with SCI
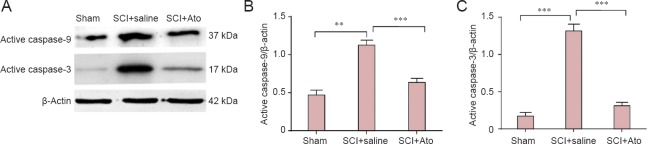
At 7 days post-injury, western blot assay results revealed increased caspase-9 and caspase-3 expression, respectively, in the SCI + saline group (P < 0.001). Caspase-9 and caspase-3 expression was significantly inhibited in the SCI + Ato group, respectively (P < 0.01, P < 0.001), suggesting that atorvastatin significantly suppressed apoptosis after SCI (Figure 2).
Figure 2.
Effects of atorvastatin on caspase-9 and caspase-3 expression in the injured spinal cord.
(A) At 7 days post-injury, caspase-9 and caspase-3 expression in the SCI + Ato, SCI + saline, and sham groups (western blot assay). (B, C) Optical density ratio of caspase-9 and caspase-3 to β-actin, respectively. **P < 0.01, ***P < 0.001 (mean ± SD, n = 4, one-way analysis of variance and the least significant difference test). Ato: Atorvastatin; SCI: spinal cord injury.
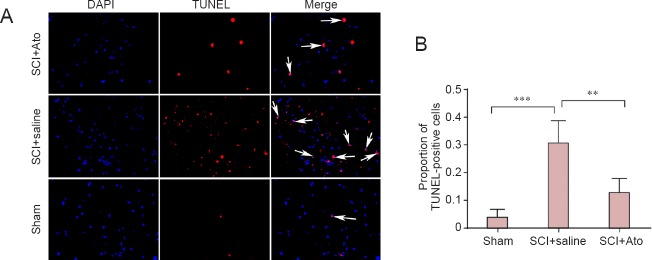
TUNEL results showed significantly more TUNEL-positive cells in the SCI + saline group compared with the sham group (P < 0.001). The number of TUNEL-positive cells was significantly less in the SCI + Ato group than in the SCI + saline group (P < 0.01; Figure 3).
Figure 3.
Effects of atorvastatin on apoptosis after SCI.
(A) At 7 days post-injury, apoptosis in the anterior horn of the spinal cord in the SCI + Ato, SCI + saline, and sham groups was determined using TUNEL assay. DAPI (blue): nuclei; TUNEL (red): DNA fragments after apoptosis; merge: overlapping cells. Arrows point to DAPI/TUNEL-positive cells. (B) The proportion of apoptotic cells in total cells in each group. **P < 0.01, ***P < 0.001 (mean ± SD, n = 4, one-way analysis of variance and the least significant difference test). Ato: Atorvastatin; SCI: spinal cord injury; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; DAPI: 4′,6-diamidino-2-phenylindole.
Effects of atorvastatin on autophagy after SCI
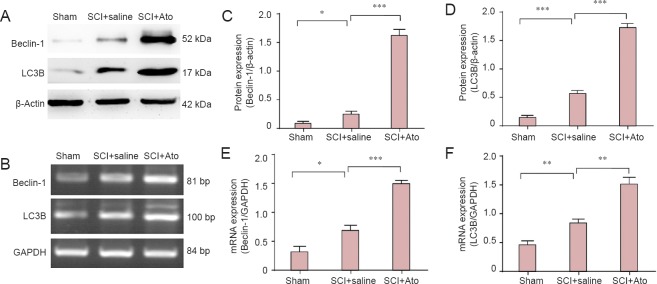
At 7 days post-injury, western blot assay and RT-PCR results revealed significantly increased Beclin-1 and LC3B protein and gene expression, respectively, in the SCI + saline group compared with the sham group (P < 0.05, P < 0.01, P < 0.001). Compared with the SCI + saline group, Beclin-1 and LC3B protein expression was significantly higher in the SCI + Ato group (P < 0.01, P < 0.001; Figure 4).
Figure 4.
Effects of atorvastatin on protein and mRNA expression of Beclin-1 and LC3B, respectively, in rats with SCI.
At 7 days post-injury, Beclin-1 and LC3B protein and mRNA expression was determined using western blot assay (A) and RT-PCR (B), respectively. (C, D) Optical density ratio of Beclin-1 and LC3B protein expression to β-actin in each group. (E, F) Optical density ratio of Beclin-1 and LC3B mRNA expression to GAPDH in each group. *P < 0.05, **P < 0.01, ***P < 0.001 (mean ± SD, n = 4, one-way analysis of variance and the least significant difference test). Ato: Atorvastatin; SCI: spinal cord injury; RT-PCR: reverse transcription-polymerase chain reaction; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Discussion
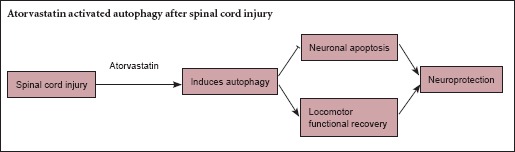
Results from the present study confirmed that atorvastatin significantly up-regulated Beclin-1 and LC3B gene and protein expression, respectively, after SCI, suggesting that atorvastatin activated autophagy. Atorvastatin also suppressed caspase-9 and caspase-3 expression. Similarly, atorvastatin significantly reduced the proportion of apoptotic cells in the anterior horn of the spinal cord after SCI and promoted cell survival. These results verified that atorvastatin exerted neuroprotective effects after SCI by activating autophagy and inhibiting apoptosis.
To test the effects of atorvastatin on functional recovery of motor nerves after SCI, BBB scores were used to observe motor function. Results demonstrated that after 2 weeks, the BBB scores were significantly higher in the SCI + Ato group than in the SCI + saline group (P < 0.05). BBB scores in the SCI + Ato group peaked between 5 and 6 weeks. These findings showed that atorvastatin significantly improved motor function following SCI, suggesting that atorvastatin could provide neuroprotective effects.
To further verify the neuroprotective effect of atorvastatin, we measured apoptosis. (Yuan and Yankner, 2000) showed that apoptosis played an important role in the occurrence and development of central nervous system diseases, such as Alzheimer's disease. When they looked at the effects of SCI, the occurrence of apoptosis seriously affected recovery of neurological function, showing that neuronal apoptosis is negatively associated with SCI recovery (Yuan and Yankner, 2000). In some studies, decreased caspase-3 and caspase-9 expression after SCI suggested inhibition of apoptosis, which likely reduced neuronal loss and promoted recovery of neurological function (Gong et al., 2014; Ozdemir et al., 2015). Our results demonstrated that atorvastatin inhibited apoptosis after SCI, promoted cell survival, provided a favorable environment for the recovery of motor nerve function in rats, and resulted in the recovery of neurological function. Nevertheless, very little is known about the precise molecular mechanisms that are involved in the neuroprotective effect of atorvastatin after SCI.
Chen et al. (2013) showed that rapamycin induces autophagy by suppressing the mTOR signaling pathway, and accelerates recovery of neurological function following SCI. Methylprednisolone has also been shown to exert neuroprotective effects by inhibiting neuronal autophagy (Chen et al., 2012). Our previous study confirmed that vascular endothelial growth factor inhibits the inflammatory response by activating autophagy, which ultimately promotes functional recovery of motor nerves (Wang et al., 2015a). Another study verified that atorvastatin activates autophagy through the Akt/mTOR signaling pathway, leading to improved left ventricular function and hypertension (Wang et al., 2015b). Atorvastatin has also been shown to prevent vascular smooth muscle calcification by inducing autophagy and promoting apoptosis of prostate cancer cells (Liu et al., 2014). However, further studies are needed to better understand the relationship between atorvastatin and autophagy after SCI. Our results suggested that atorvastatin significantly increased Beclin-1 and LC3B gene and protein expression, respectively by 7 days after SCI, indicating that atorvastatin activated autophagy, which may promote recovery of neurological function.
Our experiments not only verify the protective effect of atorvastatin on motor nerves after SCI, but also show that atorvastatin exerts neuroprotective effects by activating autophagy. Our findings may provide a novel molecular mechanism for the clinical application of atorvastatin in the treatment of SCI. Unfortunately, our experiments only validated the effect of atorvastatin on autophagy in whole cells of spinal cord tissue after SCI, and we did not observe autophagy in neurons and glial cells. Further studies are required to investigate the neuroprotective effects of atorvastatin on SCI at different time points (acute stage and chronic stage), to observe the effect of atorvastatin on autophagy in various neural cells, and to provide a theoretical and experimental basis for the treatment of SCI with atorvastatin.
Acknowledgments
We are very grateful to Xi-fan Mei from Department of Orthopedics, First Affiliated Hospital of Jinzhou Medical University of China for technical support.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81471854.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Maxwell R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Abdanipour A, Tiraihi T, Noori-Zadeh A, Majdi A, Gosaili R. Evaluation of lovastatin effects on expression of anti-apoptotic Nrf2 and PGC-1alpha genes in neural stem cells treated with hydrogen peroxide. Mol Neurobiol. 2014;49:1364–1372. doi: 10.1007/s12035-013-8613-5. [DOI] [PubMed] [Google Scholar]
- Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, Sallach S, Endres M, Brocke S, Nitsch R, Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chen HC, Fong TH, Lee AW, Chiu WT. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine. 2012;37:470–475. doi: 10.1097/BRS.0b013e318221e859. [DOI] [PubMed] [Google Scholar]
- Chen HC, Fong TH, Hsu PW, Chiu WT. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res. 2013;179:e203–210. doi: 10.1016/j.jss.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Dery MA, Rousseau G, Benderdour M, Beaumont E. Atorvastatin prevents early apoptosis after thoracic spinal cord contusion injury and promotes locomotion recovery. Neurosci Lett. 2009;453:73–76. doi: 10.1016/j.neulet.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Esposito E, Rinaldi B, Mazzon E, Donniacuo M, Impellizzeri D, Paterniti I, Capuano A, Bramanti P, Cuzzocrea S. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-alpha. J Neuroinflammation. 2012;9:81. doi: 10.1186/1742-2094-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery 61 Suppl. 2014;1:36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- Gao K, Shen Z, Yuan Y, Han D, Song C, Guo Y, Mei X. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/beta-catenin signaling pathway after spinal cord injury. J Neurochem. 2015a doi: 10.1111/jnc.13382. doi: 10.1111/jnc.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Wang YS, Yuan YJ, Wan ZH, Yao TC, Li HH, Tang PF, Mei XF. Neuroprotective effect of rapamycin on spinal cord injury via activation of the Wnt/beta-catenin signaling pathway. Neural Regen Res. 2015b;10:951–957. doi: 10.4103/1673-5374.158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82–91. doi: 10.1016/j.mcn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Gong S, Peng L, Yan B, Dong Q, Seng Z, Wang W, Lv J, He X. Bosentan reduces neuronal apoptosis following spinal cord ischemic reperfusion injury. Spinal cord. 2014;52:181–185. doi: 10.1038/sc.2013.133. [DOI] [PubMed] [Google Scholar]
- Harrop JS. Spinal cord injury: debating the efficacy of methylprednisolone. Neurosurgery 61 Suppl. 2014;1:30–31. doi: 10.1227/NEU.0000000000000391. [DOI] [PubMed] [Google Scholar]
- Holmberg E, Nordstrom T, Gross M, Kluge B, Zhang SX, Doolen S. Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma. 2006;23:1366–1378. doi: 10.1089/neu.2006.23.1366. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC, Theodore N. Pharmacological therapy for acute spinal cord injury. Neurosurgery 76 Suppl. 2015;1:S71–83. doi: 10.1227/01.neu.0000462080.04196.f7. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, Okumoto Y, Shiono Y, Orii M, Shimamura K, Ueno S, Yamano T, Tanimoto T, Ino Y, Yamaguchi T, Kumiko H, Tanaka A, Imanishi T, Akagi H, Akasaka T. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am College Cardiol. 2014;64:2207–2217. doi: 10.1016/j.jacc.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, Yang X, Gu G, Zhang J, Zheng H. Atorvastatin protects vascular smooth muscle cells from TGF-beta1-stimulated calcification by inducing autophagy via suppression of the beta-catenin pathway. Cell Physiol Biochem. 2014;33:129–141. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- Liu JC, Patel A, Vaccaro AR, Lammertse DP, Chen D. Methylprednisolone after traumatic spinal cord injury: yes or no? PM R. 2009;1:669–673. doi: 10.1016/j.pmrj.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal 2014. 2014 doi: 10.1155/2014/586270. 586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir US, Naziroglu M, Senol N, Ghazizadeh V. Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2015:11–12. doi: 10.1007/s12035-015-9292-1. [DOI] [PubMed] [Google Scholar]
- Pordal AH, Hajmiresmail SJ, Assadpoor-Piranfar M, Hedayati M, Ajami M. Plasma oxysterol level in patients with coronary artery stenosis and its changes in response to the treatment with atorvastatin. Med J Islam Repub Iran. 2015;29:192. [PMC free article] [PubMed] [Google Scholar]
- Pursnani A, Massaro JM, D’Agostino RB, Sr, O’Donnell CJ, Hoffmann U. Guideline-Based Statin Eligibility, Coronary Artery Calcification, and Cardiovascular Events. JAMA. 2015;314:134–141. doi: 10.1001/jama.2015.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane KM, Mynderse M, Lalonde DT, Dean IS, Wojtkowiak JW, Fouad F, Borch RF, Reiners JJ, Jr, Gibbs RA, Mattingly RR. A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J Pharmacol Exp Ther. 2010;333:23–33. doi: 10.1124/jpet.109.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AE, Snider MJ, Blais DM, Gulati M. Statin desensitization in a patient with probable familial hypercholesterolemia. J Clin Lipidol. 2015;9:597–601. doi: 10.1016/j.jacl.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Tesiorowski M, Potaczek T, Jasiewicz B, Sapa J, Zygmunt M. Methylprednisolone- acute spinal cord injury, benefits or risks? Postepy Hig Med Dosw (Online) 2013;67:601–609. doi: 10.5604/17322693.1054873. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang Y, Li D, Liu Z, Zhao Z, Han D, Yuan Y, Bi J, Mei X. VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem Biophys Res Commun. 2015a;464:453–458. doi: 10.1016/j.bbrc.2015.06.146. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang H, Geng QX, Wang HT, Miao W, Cheng B, Zhao D, Song GM, Leanne G, Zhao Z. Augmentation of autophagy by atorvastatin via Akt/mTOR pathway in spontaneously hypertensive rats. Hypertension Res. 2015b;38:813–820. doi: 10.1038/hr.2015.85. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang YJ, Wang H, Dong QT, Wang TJ, Qian HY, Xu H. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem cells Dev. 2012;21:1321–1332. doi: 10.1089/scd.2011.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]