Keywords: nerve regeneration, spinal cord injury, neural stem cells, Rho signaling pathway, neurite outgrowth, myelin, YAP, neural regeneration
Abstract
Spontaneous axonal regeneration of neurons does not occur after spinal cord injury because of inhibition by myelin and other inhibitory factors. Studies have demonstrated that blocking the Rho/Rho-kinase (ROCK) pathway can promote neurite outgrowth in spinal cord injury models. In the present study, we investigated neurite outgrowth and neuronal differentiation in neural stem cells from the mouse subventricular zone after inhibition of ROCK in vitro. Inhibition of ROCK with Y-27632 increased neurite length, enhanced neuronal differentiation, and upregulated the expression of two major signaling pathway effectors, phospho-Akt and phospho-mitogen-activated protein kinase, and the Hippo pathway effector YAP. These results suggest that inhibition of ROCK mediates neurite outgrowth in neural stem cells by activating the Hippo signaling pathway.
Introduction
After spinal cord injury (SCI), central nervous system neurons cannot regenerate axons spontaneously. This may be partially attributed to axon outgrowth inhibitors in the central nervous system environment, such as myelin-associated glycoprotein, myelin oligodendrocyte glycoprotein, chondroitin sulfate proteoglycans, and Nogo-66 and amino-Nogo (GrandPré et al., 2000; Prinjha et al., 2000; Fournier et al., 2001; Liu et al., 2002). Nogo-66 mainly functions as a neuron-specific inhibitor in soluble monomeric form, whereas amino-Nogo acts as a nonspecific inhibitor of neuronal and non-neuronal cells in the cluster form. Nogo-66 exerts its inhibitory function during axon regeneration by binding to the Nogo-66 receptor (Prinjha et al., 2000). The inhibitory effects of Nogo-66 and amino-Nogo can be reversed by inhibition of the Rho/Rho-kinase (ROCK) pathway in vivo, which enhances axonal regeneration and improves functional recovery after central nerve system injury.
Fournier et al. (2000) reported that actin rearrangements within the growth cone play a key role in neurite outgrowth inhibition and neurite repulsion. The Rho family of GTPases regulates the actin cytoskeleton (Kawano et al., 2014). Rho family members are present either in their inactive form, or in an active GDP-bound form. Rho GTPases regulate collapse of the growth cone and inhibit neurite outgrowth (Königs et al., 2014).
Rho family members exert their functions mainly through a variety of downstream effectors. Of interest among the downstream targets of GTP-bound Rho are p160ROCK (ROCK-I) and ROK-Rho-kinase (ROCK-II) (Guignandon et al., 2014). Once either of these is activated, the regulatory myosin light-chain phosphatase is phosphorylated (Deng et al., 2014).
A previous study demonstrated that at low concentrations the cell-permeable pyridine derivative Y-27632, a relatively specific ROCK inhibitor, decreases ROCK-I and ROCK-II activity (Ishizaki et al., 2000). Günther et al. (2014) and Roloff et al. (2015) found that in vitro application of Y-27632 to primary motoneurons and human NT2 model neurons increases neurite outgrowth. In addition, Y-27632 promotes the survival and neurite outgrowth of early postnatal cultured retinal neurocytes (Feng et al., 2013) and enhances axon regeneration in an adult rat retinal culture model only in the presence of growth-promoting factors or under high cAMP conditions (Ahmed et al., 2009). Y-27632 also promotes nerve growth factor-induced neurite outgrowth in PC12 cells; IP3 receptors and the PI3K-Akt signaling pathway might be involved in the underlying mechanisms (Minase et al., 2010). However, the mechanism of Y-27632 has not been fully explored. YAP is a major downstream effector of the Hippo pathway (Dill et al., 2015). It is markedly upregulated in many mammalian cancers, and YAP transgenic mice eventually develop liver tumors. Few reports have addressed the role of the Hippo pathway in axon regeneration after SCI.
A previous report showed that Y-27632 treatment led to functional and anatomical recovery in mice with corticospinal tract lesions (Dergham et al., 2002). The present study was designed to investigate the effects of ROCK inhibition using Y-27632 on neurite outgrowth and neuronal differentiation in neural stem cells (NSCs) and explore the underlying mechanisms.
Materials and Methods
NSC culture
Animal studies were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Southwest Medical University, China (IACUC protocol No. (2012009)), and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
For NSC isolation, 100 specific-pathogen-free neonatal Kunming mice (postnatal days 2–6) were provided by the Animal Experimental Center of Southwest Medical University, China (license No. SYXK (Chuan) 2013-181). NSC isolation and culture were performed as described previously (Lingor et al., 2008), with modifications. Briefly, a single-cell suspension was prepared from subventricular zone tissue (Paxinos and Franklin, 2013) and cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1; Invitrogen, Carlsbad, CA, USA) with B27 supplement (2%; Invitrogen), epidermal growth factor (20 ng/mL; Invitrogen) and basic fibroblast growth factor (20 ng/mL; Invitrogen). After 5–7 days, neurospheres were broken into small clusters and cultured in the same medium at a ratio of 1:3. Proliferative NSCs formed three-dimensional cell clusters. NSCs at passages 2–4 were used for subsequent experiments.
To investigate NSC differentiation, medium containing DMEM/F12 (1:1), B27 (2%) and retinoic acid (5 μM; Sigma-Aldrich, St. Louis, MO, USA) was used to culture cell clusters on cover slips pre-coated with 0.1 mg/mL poly-D-lysine. The culture medium was refreshed every other day. Six days later, neurobasal medium containing 2% B27 and 20 ng/mL nerve growth factor (Invitrogen) was used.
Detection of cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay
A single-cell suspension made by cell clusters was seeded into a 96-well plate at a density of 3.0 × 103 NSCs per 100 μL of proliferation-inducing medium, and then cultured with the same medium containing different concentrations of Y-27632 (Cytoskeleton Inc., Denver, CO, USA) for 4, 8 or 12 hours. Four hours later, cells were incubated with MTT detergent (10 μL/well; Sigma-Aldrich) at 37°C for 2–4 hours in the dark. Optical density at 570 nm was read on a microplate fluorescence reader (FL600; BioTek, Winooski, VT, USA).
Neurite outgrowth measurement
Neurite outgrowth was measured as described previously (Hirose et al., 1998; Sandvig et al., 2004). To detect inhibition of ROCK, the dissociated cell clusters were incubated with proliferation-inducing medium containing 10 μM Y-27632 or an equivalent volume of sterile H2O and then plated onto 10 μg/mL laminin (Sigma-Aldrich) for 1–2 hours. Cells treated with Y-27632 were spread onto the myelin substrate. Neurite outgrowth was quantified using ImageJ software (NIH, Bethesda, MD, USA).
Western blot assay
Western blot assay was performed as described previously (Ivanov et al., 2009). Cells were collected after centrifugation and then lysed in radioimmune precipitation assay buffer containing a protein inhibitor cocktail (Sigma-Aldrich). Protein content was quantified by bicinchoninic acid assay (Pierce, Rockford, IL, USA), and equal amounts of protein were separated by polyacrylamide gel electrophoresis. The proteins were then transferred to a polyvinylidene fluoride membrane (BioRad, Hercules, CA, USA). The membrane was incubated for 1 hour at room temperature in Tris-buffered saline with Tween-20 containing 5% bovine serum albumin to block nonspecific binding, then at 4°C overnight with antibodies against ROCK-II (1:1,000; BD Transduction Laboratories, San Diego, CA, USA), YAP (1:1,000; BD Transduction Laboratories), Akt (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-Akt (1:1,000; Cell Signaling Technology, Danvers, MA, USA), phospho-p44/42 mitogen-activated protein kinase (MAPK; Thr202/Tyr204; 1:1,000; Cell Signaling Technology), and GAPDH (1:5,000; Sigma-Aldrich). All primary antibodies were rabbit anti-mouse monoclonal antibodies. The membrane was rinsed, and the enhanced chemiluminescence method (ThermoScientific, Hudson, NH, USA) was used to visualize the bands, using goat anti-rabbit HRP-conjugated antibodies (1:400; BioRad) at room temperature for 2 hours. Signal intensity (optical density) was quantified using ImageJ software. Relative optical density was calculated relative to GAPDH.
Statistical analysis
Raw data were imported into Origin 9.0 software (OriginLab Corporation, Northampton, MA, USA) for graphing and fitted with Sigma Plot (Systat Software Inc., San Jose, CA, USA). Data, expressed as the mean ± SEM, were analyzed using Student's two-tailed t-test between two groups, or one-way analysis of variance followed by Tukey's post-hoc test for multiple comparisons. P < 0.05 was considered statistically significant.
Results
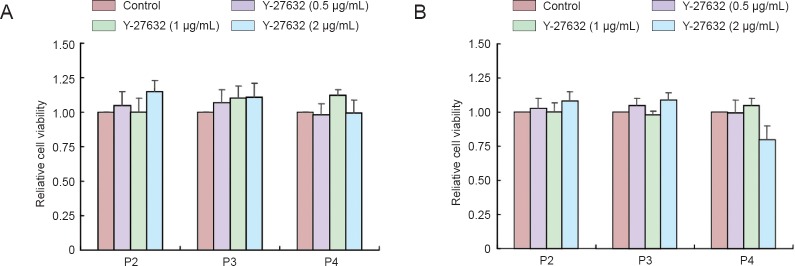
Effects of Y-27632 on NSC viability
MTT assay showed that the viability of NSCs at passages 2–4 was not reduced after incubation with Y-27632 at 0.5–2 μg/mL for 4 hours (Figure 1A) or 1 μg/mL for 4 and 8 hours (Figure 1B). Therefore, in subsequent experiments, NSCs were treated with 1 μg/mL Y-27632 for 4 hours.
Figure 1.
Changes in viability of NSCs after Y-27632 exposure (MTT assay).
(A) P2, P3 and P4 NSCs were treated with 0.5, 1.0 or 2.0 μg/mL Y-27632 for 4 hours. (B) P2, P3 and P4 NSCs were treated with 1.0 μg/mL Y-27632 for 4, 8 or 12 hours. Y-27632 treatment for 12 hours decreased cell viability by 22% (P < 0.05 vs. control group; one-way analysis of variance followed by Tukey's post-hoc test). Relative cell viability was expressed as the optical density normalized to that of the control group. Data are expressed as the mean ± SEM of at least four experiments. NSC: Neural stem cells; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide; P: passage; h: hours.
Y-27632 increased neurite outgrowth in NSCs
Treatment of NSCs with Y-27632 for 4 hours significantly increased neurite length during subsequent neuronal differentiation. Y-27632 dose-dependently promoted NSC neurite outgrowth, with the greatest effect observed at 2 μg/mL (Figure 2A). After 1 day of culture, the average neurite length in the NSCs was significantly greater in the three groups treated with Y-37632 than in the control group (150 ± 20 μm vs. 63 ± 6 μm;P < 0.05). At 5 days, the average neurite length in the three Y-27632 groups was more than twice that in the control group (Figure 2B).
Figure 2.
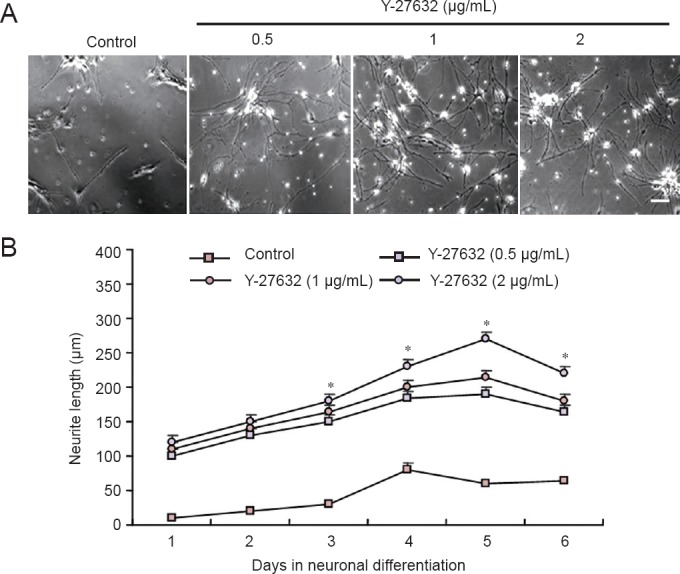
Neurite outgrowth of NSCs after treatment with Y-27632 for 4 hours.
(A) Phase contrast images of NSCs treated with Y-27632 at different concentrations after 5 days in culture. Scale bar: 200 μm. (B) Average neurite length over time in culture. Data are expressed as the mean ± SEM of five NSCs. *P < 0.05 vs. control group (Con) (one-way analysis of variance followed by Tukey's post-hoc test). MTT assay was performed at least six times for each test. NSCs: Neural stem cells; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide.
Y-27632 increased YAP protein expression in NSCs
After a 4-hour treatment with 1 μg/mL Y-27632, western blots were performed to assess the changes in two important signaling pathway effectors, p-Akt and p-MAPK. After Y-27632 treatment, ratios of p-Akt/Akt and p-MAPK/MAPK were significantly elevated (Figure 3). Interestingly, YAP protein expression was also significantly upregulated, and accompanied by a downregulation of ROCKII expression. These findings suggest that hyperactivation of YAP in NSCs contributes to neurite outgrowth.
Figure 3.
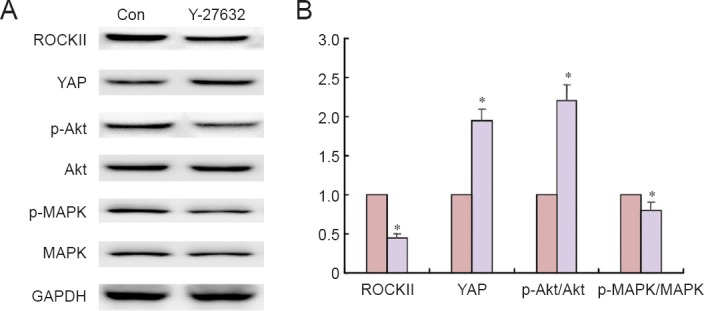
YAP protein expression in NSCs after Y-27632 treatment (western blot assay).
(A) Immunoblots of MAPK, p-MAPK, Akt, p-Akt, ROCKII and YAP. (B) Quantification of p-MAPK/MAPK, p-Akt/Akt, ROCKII and YAP protein expression in NSCs treated with 1.0 μg/mL Y-27632 for 4 hours, normalized against GAPDH and expressed as the ratio to the control group. Western blot assay was performed four times for each measurement. Data are expressed as the mean ± SEM. *P < 0.05, vs. control group (Con) (Student's two-tailed t-test). NSCs: Neural stem cells; (p-)MAPK: (phosphorylated) mitogen-activated protein kinase.
Discussion
Blocking the ROCK pathway promotes neurite outgrowth in SCI models (Fournier et al., 2003; Kubo et al., 2007; Boadas-Vaello and Verdú, 2015; Hou et al., 2015). Here, we investigated the inhibitory effects of ROCK on NSC differentiation and neurite outgrowth. Our results showed that ROCK inhibition significantly promoted neuronal differentiation and neurite outgrowth in mouse NSCs. These observations provide insights into stem cell therapy and functional recovery of axonal growth.
Myelin is an important inhibitor of axon regeneration after SCI. It is secreted by oligodendrocytes in injured nervous tissue (Sandvig et al., 2004). In the central nervous system, Nogo-A is the most ubiquitous myelin-associated protein (McGee and Strittmatter, 2003). By binding to the Nogo-66 receptor, Nogo activates the Rho/ROCK pathway, which in turn activates PI3K/Akt signaling and cytoskeleton organization effectors (Liao et al., 2007; Schmandke et al., 2007), leading to neurite elongation (Hirose et al., 1998). There is evidence that cell survival, proliferation and regeneration can be enhanced by inhibition of the Rho/ROCK signaling pathway, and partially through the MAPK and Akt signaling pathways (Lingor et al., 2008). Our results also showed that phospho-Akt and MAPK were elevated in Y-27632-treated NSCs, indicating that these molecules are important in axonal regeneration after SCI. The Rho/ROCK signaling pathway is also suggested to play a key role in neuronal differentiation (Sandvig et al., 2004). Our results show that neuronal differentiation was significantly enhanced in NSCs after Y-27632 treatment. These results were consistent with a previous study that showed that ERK1/2 phosphorylation is necessary for early neuronal differentiation and embryonic stem cell survival (Li et al., 2006).
Interestingly, our results showed that while Y-27632 upregulated p-Akt and MAPK expression in NSCs, it also significantly increased that of the Hippo signaling pathway mediator YAP. All components of the Hippo signaling pathway have one or more vertebrate orthologs. Human cancers have been linked to mutations in these genes (McClatchey and Giovannini, 2005; Harvey and Tapon, 2007). Cao et al. (2008) reported that the Hippo signaling pathway greatly contributes to the regulation of neural progenitor cell number by controlling cell proliferation and apoptosis. Our results showed that ROCKII inhibition induced YAP to regulate the neurite outgrowth of vertebrate NSCs. In the mouse blastocyst, inhibition of ROCK by Y-27632 significantly enhances inner cell mass by activating Hippo signaling pathway (Kono et al., 2014). Taken together, our data suggest that Y-27632 mediates NSC outgrowth partially through activation of Hippo signaling pathway, thus enhancing axon regeneration after SCI.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China (General Program), No. 30872602.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy J, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Ahmed Z, Berry M, Logan A. ROCK inhibition promotes adult retinal ganglion cell neurite outgrowth only in the presence of growth promoting factors. Mol Cell Neurosci. 2009;42:128–133. doi: 10.1016/j.mcn.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Boadas-Vaello P, Verdú E. Epigallocatechin-3-gallate treatment to promote neuroprotection and functional recovery after nervous system injury. Neural Regen Res. 2015;10:1390–1392. doi: 10.4103/1673-5374.165502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Huang Z, Yuan M, Yang K, Pang Y. Baculovirus induces host cell aggregation via a Rho/Rok-dependent mechanism. J Gen Virol. 2014;95:2310–2320. doi: 10.1099/vir.0.066811-0. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill PE, Liang N, Pende M. New insights into the pathophysiology of the tuberous sclerosis complex: crosstalk of mTOR- and hippo-YAP pathways in cell growth. Rare Dis. 2015;3:e1016701. doi: 10.1080/21675511.2015.1016701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng PL, Wang J, Yang ZJ, Liu XH, Zhong YS. Effect of Y-27632 on the cultured retinal neurocytes of rats. Int J Ophthalmol. 2013;6:15–18. doi: 10.3980/j.issn.2222-3959.2013.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol. 2000;149:411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther R, Saal KA, Suhr M, Scheer D, Koch JC, Bähr M, Lingor P, Tönges L. The rho kinase inhibitor Y-27632 improves motor performance in male SOD1(G93A) mice. Front Neurosci. 2014;8:304. doi: 10.3389/fnins.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Guignandon A, Faure C, Neutelings T, Rattner A, Mineur P, Linossier MT, Laroche N, Lambert C, Deroanne C, Nusgens B, Demets R, Colige A, Vico L. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 2014;28:4077–4087. doi: 10.1096/fj.14-249714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway-an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XL, Chen Y, Yin H, Duan WG. Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone. Neural Regen Res. 2015;10:1836–1840. doi: 10.4103/1673-5374.170314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs V, Jennings R, Vogl T, Horsthemke M, Bachg AC, Xu Y, Grobe K, Brakebusch C, Schwab A, Bähler M, Knaus UG, Hanley PJ. Mouse macrophages completely lacking Rho subfamily GTPases (RhoA, RhoB, and RhoC) have severe lamellipodial retraction defects, but robust chemotactic navigation and altered motility. J Biol Chem. 2014;289:30772–30784. doi: 10.1074/jbc.M114.563270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kaneko-Kawano T, Shimamoto K. Rho family GTPase-dependent immunity in plants and animals. Front Plant Sci. 2014;5:522. doi: 10.3389/fpls.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev Biol. 2014;394:142–155. doi: 10.1016/j.ydbio.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Hata K, Yamaguchi A, Yamashita T. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr Pharm Des. 2007;13:2493–2499. doi: 10.2174/138161207781368657. [DOI] [PubMed] [Google Scholar]
- Li Z, Theus MH, Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev Growth Differ. 2006;48:513–523. doi: 10.1111/j.1440-169X.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Minase T, Ishima T, Itoh K, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by the ROCK inhibitor Y-27632: a possible role of IP3 receptors. Eur J Pharmacol. 2010;648:67–73. doi: 10.1016/j.ejphar.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. San Diego: Elsevier; 2013. [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Neurobiology: Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Roloff F, Scheiblich H, Dewitz C, Dempewolf S, Stern M, Bicker G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS One. 2015;10:e0118536. doi: 10.1371/journal.pone.0118536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]