Abstract
PURPOSE
The response of rectal cancers to neoadjuvant chemoradiotherapy is variable. Tumor hypoxia reduces the effectiveness of both radiation therapy and chemotherapy and is a well-known risk factor for tumor radioresistence. We hypothesized that imaging with the novel hypoxia-detecting agent, 60Cu-diacetyl-bis (N4-methylthiosemicarbazone) (60Cu-ATSM), previously validated in cervical and lung cancers, would predict the response of rectal cancers to neoadjuvant chemoradiotherapy and prognosis.
METHODS
Patients with locally invasive (T2–4) primary or node-positive rectal cancer located <12 cm from the anal verge were recruited for this pilot study. Pretreatment tumor size and stage were determined by endorectal ultrasonography, CT, and magnetic resonance imaging. Eleven patients also underwent clinical positron emission tomography with 18F-fluorodeoxyglucose at the discretion of the treating clinician. The primary tumor was imaged by positron emission tomography with 60Cu-ATSM, and accumulation of the tracer was measured semiquantitatively by determining the tumor-to-muscle activity ratio. Neoadjuvant chemoradiotherapy was then administered (within 2 weeks of 60Cu-ATSM-positron emission tomography) and consisted of 45 Gy given in 25 fractions to the pelvis with continuous intravenous infusion of 5-fluorouracil (225 mg/m2/day). Proctectomy was performed six to eight weeks after neoadjuvant chemoradiotherapy and the tumor submitted to pathology for size measurement and staging. Tumor-to-muscle activity ratios were compared with tumor 18F-fluorodeoxyglucose uptake, tumor response to neoadjuvant chemoradiotherapy, and with patient survival.
RESULTS
Nineteen patients were enrolled in the study, two of whom were excluded from final analysis (1 death during neoadjuvant chemoradiotherapy and 1 tumor perforation during neoadjuvant chemoradiotherapy requiring emergent surgery). Of the 17 remaining patients, 14 had a reduction in tumor size and 13 were downstaged. The median tumor-to-muscle activity ratio of 2.6 discriminated those with worse prognosis from those with better prognosis. Both overall and progression-free survivals were worse with hypoxic tumors (tumor-to-muscle activity ratio >2.6) than with nonhypoxic tumors (tumor-to-muscle activity ratio ≤2.6; both P<0.05). In addition, 2 of the 3 tumors with no change in size had tumor-to-muscle activity ratios >2.6 (positive predictive value 66 percent), whereas 6 of 14 with decreased size had tumor-to-muscle activity ratios >2.6 (negative predictive value 57 percent). Three of the 4 tumors not downstaged had tumor-to-muscle activity ratios >2.6 (positive predictive value 75 percent), whereas 5 of 13 downstaged tumors had tumor-to-muscle activity ratios >2.6 (negative predictive value 62 percent). The mean tumor-to-muscle activity ratio for downstaged tumors (2.2) was significantly lower than that of nondownstaged tumors (3.3) (P=0.03). The difference in mean tumor-to-muscle activity ratio between downsized (2.3) and nondownsized (2.9) tumors did not reach statistical significance (P=0.36). Tumor 18F-fluorodeoxyglucose uptake (n=11) did not correlate with 60Cu-ATSM uptake (r=0.4; P=0.9) and there was no significant difference in mean tumor 18F-fluorodeoxyglucose uptake between patients with hypoxic tumors and those with normoxic tumors (P=0.3).
CONCLUSIONS
The results of this small pilot study suggest that 60Cu-ATSM-PET may be predictive of survival and, possibly, tumor response to neoadjuvant chemoradiotherapy in patients with rectal cancer. A larger Phase II study is warranted to validate these results.
Keywords: Positron emission tomography, Cu-ATSM, Rectal cancer, Tumor hypoxia, Neoadjuvant therapy, Tumor response
Neoadjuvant therapy followed by surgical resection has become the standard treatment of advanced rectal cancers.1 This combined modality approach has led to a reduction in local recurrence rates and an increase in sphincter preservation, presumably because of tumor down-staging before surgery.2 However, patients whose tumors fail to respond to neoadjuvant therapy may not derive any benefit.3 The ability to select these patients in advance of treatment would be of practical clinical relevance. Patients with tumors deemed unlikely to respond to conventional regimens might be entered into clinical trials designed to increase the efficacy of neoadjuvant therapy.
One well-recognized cause of resistance to radiation and chemotherapy is tumor hypoxia.4 Rectal cancers that do not respond to neoadjuvant chemoradiation may be relatively hypoxic compared with responding tumors. It follows that tumor hypoxia could serve as a potential predictor of response to neoadjuvant therapy in patients with rectal cancer. Several methods have been described that can provide measures of oxygen content within tumors.5-7 The two that have received the most attention are the use of oxygen electrodes and functional imaging with hypoxia tracers. Measurement of tissue oxygen content with polarographic electrodes is not readily suited to clinical use because the procedure is invasive, tech nically demanding, and subject to sampling errors.8 Functional imaging strategies, however, may be ideal. These studies rely on the preferential uptake of specific radiotracers by hypoxic cells and are noninvasive, easily performed, and capable of a global assessment of oxygen content throughout the tumor.7 The NCI has recognized the potential of functional imaging of hypoxia and has made its development a high priority.9
The most thoroughly studied hypoxia tracer is 18F-fluoromisonidazole (FMISO), which can be imaged clinically by positron emission tomography (PET).7 However, FMISO has some important limitations that limit image quality, including poor contrast between hypoxic and normal tissues and slow tracer clearance.
Fujibayashi et al.10 have developed copper-labeled diacetyl-bis (N4-methylthio-semicarbazone) (Cu-ATSM) as an alternative to FMISO. Cu-ATSM seems to be better suited for imaging hypoxic tissues because its uptake occurs more rapidly and the hypoxic/normoxic tissue activity ratio is greater than that of FMISO.11 The in vivo kinetics of 60Cu-ATSM have been evaluated in patients with nonsmall-cell lung cancer (NSCLC) and cervical cancer by using PET.12,13 Tumor uptake of 60Cu-ATSM was readily assessed with quantitative and semiquantitative techniques, and preliminary data suggest that the degree of tracer uptake is inversely related to both radio-responsiveness of the tumor and patient survival.
Given the apparent promise of this technique, we performed a pilot study to determine whether 60Cu-ATSM uptake is predictive of response to neoadjuvant therapy in patients with rectal cancer. To the best of our knowledge, this represents the first application of this novel technique in such patients. Our hypothesis was that hypoxic rectal cancers, as determined by 60Cu-ATSM-PET, would not be downstaged or downsized by neoadjuvant chemoradiotherapy (NCRT) and survival would be significantly better in patients with nonhypoxic tumors than in those with hypoxic tumors. A secondary purpose was to compare the tumor uptake of 18F-fluorodeoxyglucose (FDG) and 60Cu-ATSM in a subset of patients who had FDG-PET ordered by their treating clinician as part of the preoperative staging workup.
PATIENTS AND METHODS
Patients with ultrasonography-stage T2–4 primary adenocarcinoma of the rectum within 12 cm of the anal verge who were candidates for NCRT followed by surgical resection were recruited for this pilot study. Patients with T1 tumors or metastatic disease, those with contra-indications to chemoradiation therapy or proctectomy, and those who were unable or unwilling to give consent were excluded from the study. This investigation was approved by the Human Studies Committee and the Radioactive Drug Research Committee of Washington University School of Medicine and by the Protocol Review and Monitoring Committee of the Siteman Cancer Center. All patients gave informed consent before entry into the study. Whole-body PET imaging with FDG was performed as part of initial clinical staging in 11 of the 17 patients. This was done at the discretion of the treating clinician and was not part of the study protocol.
Pretreatment primary tumor (T) stage was determined by endorectal ultrasonography using a 360° rotating ultrasound probe with a 7-MHz or 10-MHz transducer. Pretreatment tumor dimensions were measured primarily by proctoscopy and were confirmed by endorectal ultrasonography. If there was discrepancy, tumor dimensions also were assessed by CT or MRI and the average between all measurements was used. Tumor area was calculated from these measurements and recorded.
All patients underwent 60Cu-ATSM-PET (as described below) two weeks or less before the initiation of NCRT, which consisted of 45 Gy radiation given in 1.8 Gy fractions to the pelvis with continuous intravenous infusion of 5-fluorouracil (225 mg/m2/day). Radiation was delivered with an equally weighted four-field technique in the prone position. Standard total mesorectal excision of the tumor was performed by a board-certified colorectal surgeon six to eight weeks after completion of NCRT. The surgical specimen was submitted for determination of pathologic tumor stage (AJCC) and tumor dimensions by a pathologist who was blinded with respect to the pretreatment T stage, tumor dimensions, and 60Cu-ATSM-PET results. Downstaging and downsizing were determined by comparison of the pretreatment clinical/radiologic stage and tumor size with the final pathologic stage and tumor size. Downstaging was defined as a reduction in one T stage (e.g., T3 to T2) and downsizing was defined as a 50 percent reduction in tumor area.
Radiopharmaceutical Synthesis
60Cu (T1/2 24.5-min, 93 percent decay by positron emission) was produced in the Cyclotron Corporation CS15 cyclotron at the Washington University Medical Center, as previously described.12,14-17 60Cu-ATSM was produced based on methods previously described.10,12,18-20
PET Imaging
PET was performed with a CTI/Siemens ECAT HR+ scanner (Siemens-CTI, Knoxville, TN). The performance specifications of this scanner have been previously reported12,21,22; the scanner has a spatial resolution of 4.5 mm full-width half-maximum (FWHM).
For 60Cu-ATSM-PET studies, the level of imaging was determined by centering the tumor in the scanner field of view using positioning lasers by reference to anatomic landmarks (e.g., pubic symphysis) seen on the CT or the MRI (or the clinical FDG-PET study). After completion of a 10 to 15 minute transmission scan, approximately 13 mCi of 60Cu-ATSM was injected intravenously. All patients then underwent a 60 minute dynamic study (10×3 second frames, 10×6 second frames, 3×10 second frames, 6×30 second frames, 6× 1 minutes, and 10×5 minutes). 60Cu-ATSM-PET images were reconstructed by filtered back-projection using measured attenuation factors from the transmission scans, with smoothing to approximately 8 mm FWHM.
Image Analysis
The 60Cu-ATSM-PET images were evaluated subjectively by an experienced nuclear medicine physician in correlation with the clinical CT, MR, or FDG-PET images. In addition, regions of interest were drawn around the primary rectal cancer and both gluteal muscle groups in multiple planes, on the 30 to 60 minute summed images, again guided by the clinical staging images. The overall tumor uptake of 60Cu-ATSM was assessed semiquantitatively by determining the tumor/muscle activity (T/M) ratio.
FDG uptake within the primary tumors was assessed semiquantitatively on the clinical FDG-PET/CT images by determination of the maximum standardized uptake value (SUVmax).23
Statistical Analysis
The primary end point of this study was whether the tumor uptake of 60Cu-ATSM was predictive of tumor downstaging and downsizing after NCRT. Secondary end points were whether the tumor uptake of 60Cu-ATSM was predictive of tumor recurrence and patient survival. In addition, the relationship between tumor uptake of 60Cu-ATSM and that of FDG was evaluated by linear regression for the 11 patients who had both studies. The PET results were correlated with the results of clinical follow-up. Patients were followed after surgery according to the standard protocol in use at the Siteman Cancer Center. This consisted of a clinic visit, determination of the carcinoembryonic antigen (CEA) level, and proctoscopy every three months. Imaging studies were obtained only if clinical suspicion of recurrence was present. The physician, who assessed the patients for disease progression, as well as the surgeon and radiation oncologist were blinded to the results of the 60Cu-ATSM studies. Likewise, the nuclear medicine physician who read both the 60Cu-ATSM and FDG-PET studies was not aware of the surgical pathology report. The study was designed as a single-arm, nonrandomized pilot study with the goal of determining whether there is preliminary evidence that unresponsiveness to therapy and poor survival are associated with higher levels of tumor hypoxia. Hypoxia was defined as a T/M ratio greater than the median value (2.6). Similar studies of hypoxia in NSCLC and cervical cancer12,13 indicated that T/M ratios of 60Cu-ATSM uptake are reasonably symmetrically distributed. T/M ratios were compared among responders and nonresponders by using the unpaired t-test. Progression-free survival and overall survivals were determined by the Kaplan-Meier method and tested with the log-rank (Mantel-Cox) statistic.
RESULTS
Patients
Nineteen patients were enrolled in the study. Two were excluded from the final analysis; one patient died during NCRT and another patient’s tumor perforated before completion of NCRT necessitating emergency surgery. The demographic characteristics and study results for the remaining 17 patients are summarized in Table 1. Their mean age was 57.2 (range, 32–73) years, and there were 11 men and 6 women. All tumors were in the lower two-thirds of the rectum, and the mean distance from the anal verge was 6 cm (±3.45) in the group. Surgical resection was by low anterior resection (LAR) with coloanal anastomosis in 12 patients, LAR with colostomy in 1 patient, and by abdominoperineal resection in 4 patients. Radial margins were clear in 14 of 17 patients. There was no operative mortality. Based on the final pathology, the tumors were well differentiated in 1 patient, moderately differentiated in 11, and poorly differentiated in 3. Tumor differentiation was not reported in two patients (1 with a complete pathologic response and 1 with only a few scattered cells present within pools of mucin). Four patients had mucinous tumors.
Table 1.
Patient demographic data, pretreatment and posttreatment stages, pretreatment and posttreatment tumor sizes, survival, and PET results
| Patient | Age | Sex | TRUS stage | Path. stage | Downstaged? | Pretherapy tumor area (cm2) | Posttherapy tumor area (cm2) | Downsized? | T/M ratio | Pretherapy FDG (SUVmax) | Status (mo follow-up) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | T3N0 | T4N0 | no | 20 | 31.5 | no | 3.7 | 5.6 | DOD (12) |
| 2 | 73 | M | T2N+ | T2N0 | yes | 16 | 1.2 | yes | 3.7 | 3.5 | NED (42) |
| 3 | 48 | M | T3N+ | T3N1 | no | 20 | 4.8 | yes | 3.7 | 13.2 | NED (42) |
| 4 | 58 | M | T3N0 | T3N0 | no | 9 | 3 | yes | 2.6 | 6.2 | NED (40) |
| 5 | 61 | M | T3N+ | T3N0 | yes | 32.5 | 0.75 | yes | 2.7 | 7.2 | DOD (13) |
| 6 | 51 | M | T3N+ | T2N0 | yes | 24 | 4 | yes | 2.9 | 21.3 | DOD (13) |
| 7 | 49 | M | T3N0 | T1N0 | yes | 16 | 1 | yes | 2.3 | 11.1 | NED (39) |
| 8 | 32 | M | T3N+ | T3N1 | no | 13.5 | 20 | no | 3.1 | 13.6 | AWD (39) |
| 9 | 68 | F | T3N+ | T2N0 | yes | 20 | 9 | yes | 3.0 | 5.0 | NED (33) |
| 10 | 66 | M | T3N+ | T0N0 | yes | 16 | 0 | yes | 2.0 | 6.5 | DOD (33) |
| 11 | 49 | F | T3N+ | T3N0 | yes | 14 | 5.3 | yes | 2.8 | 5.7 | NED (38) |
| 12 | 59 | F | T3N0 | T1N0 | yes | 16 | 10 | yes | 1.0 | ND | NED (61) |
| 13 | 64 | M | T3N0 | T2N0 | yes | 16 | 6.3 | yes | 2.2 | ND | NED (66) |
| 14 | 59 | F | T3N+ | T2N0 | yes | 4 | 1 | yes | 1.0 | ND | NED (67) |
| 15 | 51 | F | T3N+ | T2N0 | yes | 16 | 4 | yes | 2.2 | ND | NED (66) |
| 16 | 57 | M | T3N0 | T2N0 | yes | 16 | 16 | no | 1.9 | ND | NED (62) |
| 17 | 57 | F | T3N+ | T2N1 | yes | 12 | 0.04 | yes | 1.0 | ND | NED (60) |
PET=positron emission tomography; TRUS=transrectal ultrasonography; Path=pathologic; T/M=tumor-to-muscle uptake ratio; FDG=18F-fluorodeoxyglucose; SUVmax = standardized uptake ratio, ND=not done; DOD=dead of disease; NED=no evidence of disease; AWD=alive with disease.
The mean (± standard deviation) 60Cu-ATSM T/M for the 17 rectal cancers was 2.5±0.9). The median value was 2.6, and this threshold was used to dichotomize the patients for comparisons of response and survival in relation to 60Cu-ATSM uptake.
Fourteen patients had a reduction in tumor size (mean reduction in tumor area 14.2±8.4 cm) and 13 were downstaged (Table 1). Two of the 3 tumors with no change in size had T/M>2.6 (positive predictive value (PPV) 66 percent), whereas 6 of 14 with decreased size had T/M>2.6 (negative predictive value (NPV) 57 percent). Three of the 4 tumors not downstaged had T/M>2.6 (PPV 75 percent), whereas 5 of 13 downstaged tumors had T/M>2.6 (NPV 62 percent). The mean T/M ratio for downstaged tumors (2.2±0.8) was significantly lower than that of nondownstaged tumors (3.3±0.5; P= 0.03). However, the difference in mean T/M ratio between downsized (2.4±.0.9) and nondownsized (2.9± 0.9) tumors did not reach statistical significance (P= 0.36). No correlation was found between tumor size and stage before therapy and 60Cu-ATSM uptake. Of interest, the three patients with involved radial margins on final pathology all had hypoxic tumors with T/M ratios of 3.7, 3.0, and 2.9, respectively.
The mean follow-up time for those patients alive at the time of last follow-up was 4.1 years. None of the patients with normoxic tumors (T/M≤2.6) developed recurrent disease or died as a result of their tumor. The progression-free survival at three years was 50 percent for patients with hypoxic tumors (T/M>2.6), and their overall survival was 63 percent (both P<0.05; Fig. 1).
FIGURE 1.
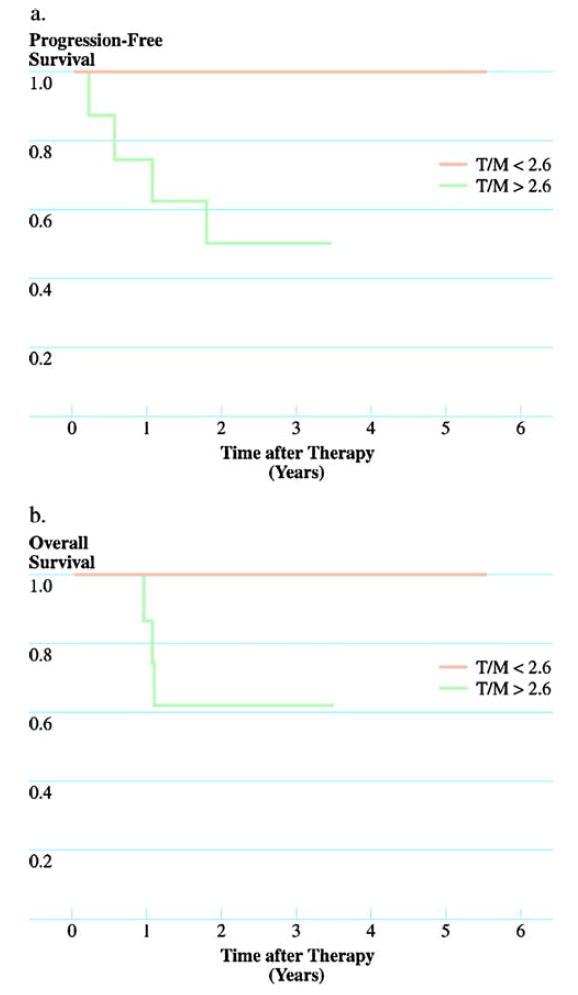
Progression-free (A) and overall (B) survival based on 60Cu-ATSM uptake using Kaplan-Meier method. Patients with hypoxic tumors (tumor-to-muscle uptake ratio (T/M) >2.6) had significantly better progression-free and overall survival than did patients with normoxic tumors (tumor-to-muscle uptake ratio ≤2.6; both P<0.05).
There was no significant difference in tumor SUVmax for FDG of patients with hypoxic tumors (7.8±3.5) and those with normoxic tumors (11.9±8.9; P=0.3). No significant correlation was found between tumor FDG uptake and T/M ratios for 60Cu-ATSM (r=0.04; P=0.9).
DISCUSSION
Rectal cancer is a common oncologic problem. It is estimated that there will be nearly 41,420 new cases of rectal cancer in the United States in 2007.24 In locally advanced rectal cancer, preoperative NCRT has become the standard of care.3 The benefits of effective NCRT include treatment of mesorectal lymph node disease and downstaging and downsizing of the primary tumor, which may improve the proportion of curative surgery and reduce the rate of local recurrence. However, not all patients respond to NCRT and benefit from such therapy; thus, nonresponders are subjected to the toxicity and cost of an ineffective treatment. To avoid treatment toxicity in nonresponding patients, prediction of response before initiation of therapy could be an important tool in tailoring preoperative therapy.25 The current clinical tools are limited in predicting responsiveness to NCRT. A number of histologic and molecular markers, such as p53 nuclear staining and thymidylate synthase expression, have been identified as predictors of pathologic response.26,27 However, these markers have not been shown consistently to be accurate in predicting response to NCRT. More recently, gene expression profiling using microarrays, has been shown to be potentially of value in discriminating responders from nonresponders.28 However, even in responders a complete pathologic response after preoperative chemoradiation does not reliably predict late outcome.29
Hypoxia has been recognized as a major obstacle to tumor radiotherapy and often chemotherapy. Hypoxic tumors are less responsive to therapy, behave more aggressively, and have poorer prognosis. Tumor hypoxia has been most extensively studied in carcinomas of the head and neck and cervix, where it has been shown to be an important predictor of poor response to chemoradiotherapy.18,30,31 The presence of hypoxia in colorectal cancers has been documented and likely is an important factor in determining responsiveness to therapy and prognosis in these patients as well.32,33
Several methods have been described that can provide measures of oxygen content within tumors. Commercially available polarographic oxygen electrodes (Eppendorf GMbH, Hamburg, Germany) are capable of direct measurement of oxygen content of the tumor, and this method is considered the “standard.”34 However, oxygen electrodes are not used in routine clinical practice because the procedure is invasive, technically demanding, and subject to sampling errors. Functional imaging strategies, as an alternate to oxygen electrodes, may be ideal for clinical evaluation of tumor oxygenation.7,34
Recently, we have shown that PET imaging with 60Cu-ATSM is a reliable method for predicting response to therapy in NSCLC12,13 as well as in predicting disease-free and overall survival in patients with cervical cancer.13 60Cu-ATSM is a positron-emitting radiopharmaceutical that accumulates selectively in hypoxic cells, clears rapidly from the blood, and washes out rapidly from normoxic cells. In vitro studies of tumor cells in culture have shown that the uptake of 64Cu-ATSM was clearly dependent on the oxygen concentration in the medium and its uptake was significantly greater than the uptake of FMISO, a well-known hypoxic tracer, which peaked after a much longer incubation time (2 hours vs. 15 minute).11 Thus, 60Cu-ATSM has a better hypoxic-to-normoxic uptake ratio.
In the current study, we have shown that 60Cu-ATSM uptake, as measured by T/M ratio, was reasonably accurate in predicting downstaging after NCRT, although less successful in predicting change in the tumor size. No correlation was found between tumor size and stage before therapy and 60Cu-ATSM uptake. More significantly, perhaps, 60Cu-ATSM uptake also allowed for discrimination of patients with poor and good prognosis. The overall and progression-free survivals were worse in patients with hypoxic tumors (T/M>2.6) than in those with nonhypoxic tumors (T/M≤2.6). In addition, the three patients with involved radial margins at resection all had hypoxic tumors as measured by 60Cu-ATSM-PET. However, it should be stressed that this was a pilot study with a small number of patients and limited follow-up. Therefore, the ability of 60Cu-ATSM-PET to predict prognosis must still be considered largely unknown and will require further study. Although this is the first report to assess hypoxia imaging as a predictor of tumor response and patient outcome in rectal carcinoma, a previous report from Box et al.35 linked anemia with the same end points in a group of 100 patients with rectal cancer. Those authors reported that patients with normal hemoglobin levels during NCRT achieved better tumor response, less local recurrence, and improved overall survival compared with anemic patients. They speculated that correcting anemia before NCRT might improve tumor response and oncologic outcomes.
There have been a number of recent reports examining the role of FDG-PET in the evaluation of patients with primary cancers. It has been shown that the SUV for FDG uptake is reflective of tumor aggressiveness and is a predictor of patient outcome in several tumor types.14,15,19,36,37 Specific to rectal cancer, Gearhart et al.37 have suggested that FDG-PET improves the accuracy of staging in patients with low rectal cancer and may result in changes to the treatment plan in a significant percentage. Other authors have used FDG-PET to assess response to neoadjuvant therapy based on reduction in tumor SUV after treatment.38-41 However, the potential role of 60Cu-ATSM-PET in patients with rectal cancer differs from that of FDG-PET. Our findings suggest that 60Cu-ATSM-PET may be able to predict response to neoadjuvant therapy before it is given. This may allow tailoring of the treatment plan at a very early stage, perhaps by directing patients predicted to be nonresponders into clinical trials designed to increase the efficacy of standard NCRT. Our finding of no significant correlation between the uptake measures for the two tracers also supports the idea that 60Cu-ATSM-PET provides unique prognostic information that cannot be derived from FDG-PET (Fig. 2)
FIGURE 2.
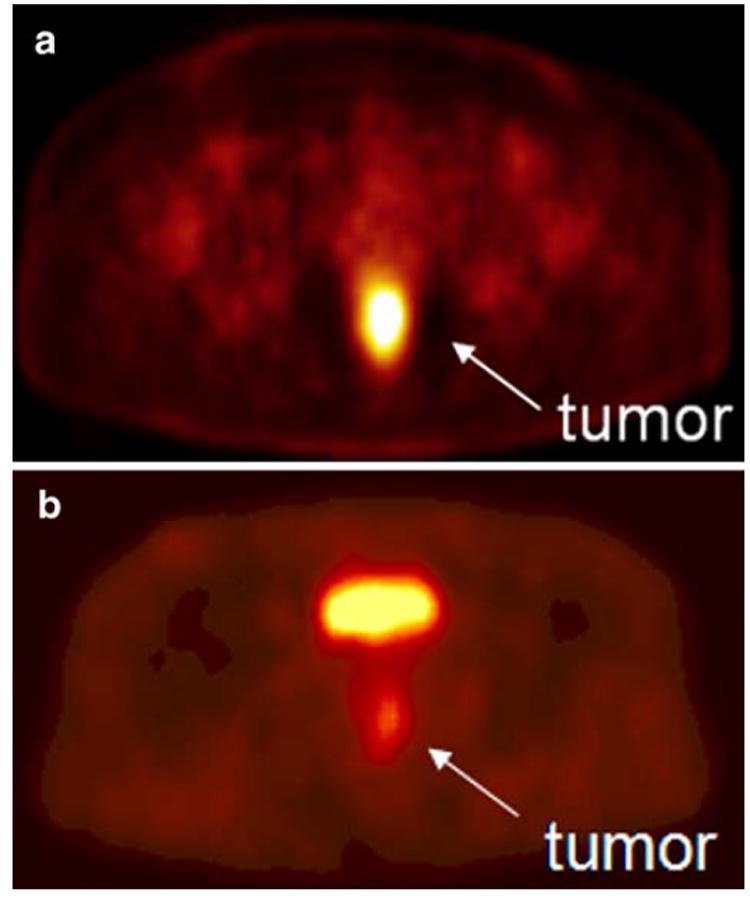
A. 60Cu-ATSM-positron emission tomography scan showing a hypoxic tumor (tumor-to-muscle uptake ratio=3.1). The tumor has increased tracer uptake compared with surrounding tissues because the 60Cu-ATSM is reduced and retained more avidly in hypoxic tissues. This tumor did not respond to neoadjuvant chemoradiation therapy. B. 60Cu-ATSM-positron emission tomography scan showing a normoxic tumor (tumor-to-muscle activity ratio= 2.3). This tumor responded to neoadjuvant chemoradiation therapy. The bright spot above the tumor is the bladder.
CONCLUSIONS
In this current pilot study, we have shown that 60Cu-ATSM-PET is a promising predictor of survival and, to a lesser extent, tumor response to NCRT in patients with rectal cancer. The NCI has recognized the potential of functional imaging of hypoxia and has made its development a high priority.42 Our studies on Cu-ATSM led to a NCI DCIDE application (Cu-ATSM: Preclinical Development via the DCIDE Program, PI: J. S. Lewis) that was used for a successful application to the FDA for an IND for 64Cu-ATSM PET imaging of tumor hypoxia in humans. Larger studies are now needed to validate the threshold that best differentiates hypoxic from nonhypoxic tumors.
Acknowledgments
The authors thank Dr. Benjamin Tan, Division of Oncology, Department of Medicine, and Drs. Matthew Mutch, Elisa Birnbaum, Ira Kodner, and James Fleshman, Section of Colon and Rectal Surgery, Department of Surgery at Washington University School of Medicine for their help and support in recruiting patients.
Supported by an American Cancer Society Institutional Research Grant (ACS-IRG #58–010–46).
Footnotes
Poster presentation at the meeting of The American Society of Colon and Rectal Surgeons, St. Louis, Missouri, June 2 to 6, 2007.
References
- 1.Han N, Galandiuk S. Induction chemoradiation for rectal cancer. Arch Surg. 2006;141:1246–53. doi: 10.1001/archsurg.141.12.1246. [DOI] [PubMed] [Google Scholar]
- 2.Matrai Z, Lovey J, Hitre E, et al. Histologic response after neoadjuvant therapy in rectal adenocarcinoma: own experience and review of the literature. Orv Hetil. 2006;147:2011–20. [PubMed] [Google Scholar]
- 3.Cervantes A, Chirivella I, Rodriguez-Braun E, Campos S, Navarro S, Garcia Granero E. A multimodality approach to localized rectal cancer. Ann Oncol. 2006;17(Suppl 10):x129–34. doi: 10.1093/annonc/mdl250. [DOI] [PubMed] [Google Scholar]
- 4.Hoogsteen IJ, Marres HA, van der Kogel AJ, Kaanders JH. The hypoxic tumour microenvironment, patient selection and hypoxia-modifying treatments. Clin Oncol (R Coll Radiol) 2007;19:385–96. doi: 10.1016/j.clon.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–9. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Milosevic M, Fyles A, Hedley D, Hill R. The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin Radiat Oncol. 2004;14:249–58. doi: 10.1016/j.semradonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Padhani A. PET imaging of tumour hypoxia. Cancer Imaging. 2006;6:S117–21. doi: 10.1102/1470-7330.2006.9018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Williams KJ, Parker CA, Stratford IJ. Exogenous and endogenous markers of tumour oxygenation status: definitive markers of tumour hypoxia? Adv Exp Med Biol. 2005;566:285–94. doi: 10.1007/0-387-26206-7_38. [DOI] [PubMed] [Google Scholar]
- 9.Hoskins WJ, Urban N, Trimble EC. Priorities of the Gynecologic Cancer Progress Review Group. National Institutes of Health. 2001 Nov; [Google Scholar]
- 10.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–60. [PubMed] [Google Scholar]
- 11.Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999;40:177–83. [PubMed] [Google Scholar]
- 12.Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response: a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55:1233–8. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 13.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003;30:844–50. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsuka T, Nomori H, Watanabe K, et al. Prognostic significance of [(18)F]fluorodeoxyglucose uptake on positron emission tomography in patients with pathologic stage I lung adenocarcinoma. Cancer. 2006;107:2468–73. doi: 10.1002/cncr.22268. [DOI] [PubMed] [Google Scholar]
- 15.Hatano E, Ikai I, Higashi T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736–41. doi: 10.1007/s00268-005-0791-5. [DOI] [PubMed] [Google Scholar]
- 16.Bass LA, McCarthy DW, Jones LA, et al. High purity production and potential applications of copper-60 and copper-61. J Labeled Compd Radiopharm. 1997;40:325–7. [Google Scholar]
- 17.McCarthy DW, Bass LA, Cutler PD, et al. High purity production and potential applications of copper-60 and copper-61. Nucl Med Biol. 1999;26:351–8. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 18.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–9. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzbach MH, Hinz U, Dimitrakopoulou-Strauss A, et al. Prognostic significance of preoperative [18-F] fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with resectable soft tissue sarcomas. Ann Surg. 2005;241:286–94. doi: 10.1097/01.sla.0000152663.61348.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young H, Carnochan P, Zweit J, Babich J, Cherry S, Ott R. Evaluation of copper(II)-pyruvaldehyde bis (N-4-methylthiosemicarbazone) for tissue blood flow measurement using a trapped tracer model. Eur J Nucl Med. 1994;21:336–41. doi: 10.1007/BF00947969. [DOI] [PubMed] [Google Scholar]
- 21.Pillot G, Siegel BA, Govindan R. Prognostic value of fluorodeoxyglucose positron emission tomography in non-small cell lung cancer: a review. J Thorac Oncol. 2006;1:152–9. [PubMed] [Google Scholar]
- 22.Adam LE, Zaers J, Ostertag H, et al. Performance evaluation of the whole-body PET scanner ECAT EXAT HR+following the IEC standard. IEEE Trans Nucl Sci. 1997;44:1172–9. [Google Scholar]
- 23.Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6:10–21. doi: 10.1053/SRAO0060010. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 25.Ceelen W, Pattyn P, Boterberg T, Peeters M. Pre-operative combined modality therapy in the management of locally advanced rectal cancer. Eur J Surg Oncol. 2006;32:259–68. doi: 10.1016/j.ejso.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Saw RP, Morgan M, Koorey D, et al. p53, deleted in colorectal cancer gene, and thymidylate synthase as predictors of histopathologic response and survival in low, locally advanced rectal cancer treated with preoperative adjuvant therapy. Dis Colon Rectum. 2003;46:192–202. doi: 10.1007/s10350-004-6524-2. [DOI] [PubMed] [Google Scholar]
- 27.Chang HJ, Jung KH, Kim DY, et al. Bax, a predictive marker for therapeutic response to preoperative chemoradiotherapy in patients with rectal carcinoma. Human pathology. 2005;36:364–71. doi: 10.1016/j.humpath.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Ghadimi BM, Grade M, Difilippantonio MJ, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23:1826–38. doi: 10.1200/JCO.2005.00.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glynne-Jones R, Mawdsley S, Pearce T, Buyse M. Alternative clinical end points in rectal cancer–are we getting closer? Ann Oncol. 2006;17:1239–48. doi: 10.1093/annonc/mdl173. [DOI] [PubMed] [Google Scholar]
- 30.Brizel DM, Scully SP, Harrelson JM, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56:5347–50. [PubMed] [Google Scholar]
- 31.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 32.Goethals L, Debucquoy A, Perneel C, et al. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys. 2006;65:246–54. doi: 10.1016/j.ijrobp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Lu XG, Xing CG, Feng YZ, Chen J, Deng C. Clinical significance of immunohistochemical expression of hypoxia-inducible factor-1alpha as a prognostic marker in rectal adenocarcinoma. Clin Colorectal Cancer. 2006;5:350–3. doi: 10.3816/ccc.2006.n.005. [DOI] [PubMed] [Google Scholar]
- 34.Isa AY, Ward TH, West CM, Slevin NJ, Homer JJ. Hypoxia in head and neck cancer. Br J Radiol. 2006;79:791–8. doi: 10.1259/bjr/17904358. [DOI] [PubMed] [Google Scholar]
- 35.Box B, Lindsey I, Wheeler JM, et al. Neoadjuvant therapy for rectal cancer: improved tumor response, local recurrence, and overall survival in nonanemic patients. Dis Colon Rectum. 2005;48:1153–60. doi: 10.1007/s10350-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 36.Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 37.Pillot G, Siegel BA, Govindan R. Prognostic significance of fluorodeoxyglucose positron emission tomography in non-small cell lung cancer: a review. J Thorac Oncol. 2006;1:152–9. [PubMed] [Google Scholar]
- 38.Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Melton GB, Lavely WC, Jacene HA, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg. 2007;11:961–9. doi: 10.1007/s11605-007-0170-7. [DOI] [PubMed] [Google Scholar]
- 40.Guillem JG, Puig-La Calle J, Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxy-glucose positron emission tomography. Dis Colon Rectum. 2000;43:18–24. doi: 10.1007/BF02237238. [DOI] [PubMed] [Google Scholar]
- 41.Denecke T, Rau B, Hoffmann KT, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658–66. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- 42.Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]


