Abstract
Current treatments for osteoarthritis (OA) are largely palliative until the joints become totally dysfunctional and prosthetic replacement becomes necessary. Effective methods are needed for diagnosing OA and monitoring its progression during its early stages, when the effects of therapeutic drugs or biological agents are most likely to be effective. Theranostic nanosomes and nanoparticles have the potential to noninvasively detect, track and treat the early stages of OA. As articular cartilage does not regenerate once it is degraded, cell-based treatments aided by superparamagnetic iron oxide nanoparticle tracking are attractive future treatment modalities for the later stages of OA progression, when significant cartilage replacement is needed. This article will describe the current and future translational approaches for the detection and noninvasive treatment of degenerative OA.
Keywords: liposome, nanosome, noninvasive imaging, osteoarthritis, quantum dot, siRNA carrier, smart nanoparticle, SPIO, theranostic technology
Osteoarthritis (OA), the most prevalent form of arthritis and a leading cause of disability in the USA, is a painful, disabling, multifactorial disease whose etiology is largely unknown. It is currently not diagnosed until it becomes symptomatic, often decades after its original onset. No proven disease-modifying therapy exists for OA and the current options for chronic OA pain are insufficient. The pain associated with OA reduces patient proclivity to exercise, as well as limiting physical activities. In addition to quality-of-life issues, OA impacts on individuals’ life expectancy and leads to work disability, affecting work force and global healthcare expenditures. Theranostic methods, which are techniques that may be used to diagnose, track the progression of and treat disease, involving nanosized particles are being developed for the detection of early-onset OA and the intervention thereof. Permutations of the same technology could be used to diagnose pathology (e.g., antibody-targeted nanosomes encapsulating fluorescent dyes) and then track and treat the disease (e.g., fluorescent nanosomes loaded with therapeutic/biologic agents). The use of targeted nanotechnology allows for the delivery of drugs or siRNAs in a site-specific manner in order to decrease undesirable side effects on nontarget tissues and to reduce the quantities of drugs needed for therapeutic effects. In addition, these noninvasive, targeted techniques can also be used for the evaluation of disease progression, drug efficacy or cell-based therapies. They have the potential to delay and/or eliminate the necessity for total joint replacements by treating the underlying cartilage degeneration that is characteristic of OA.
Background
OA results from a combination of trauma, repetitive mechanical stress and aging. Propagated by proteinases produced by superficial articular chondrocytes in response to an offset in their homeostatic environment, early OA lesions often result in irreversible damage. Some of the major biological factors that regulate cartilage degeneration in OA include, but are not limited to: NF-κB, HIF-2α, the families of matrix metalloproteinases (MMPs) and ADAMTS [1–4]. Chondrocytes, the resident cells within articular cartilage, are activated by cellular signaling molecules evoked by high-intensity exercise (stress), cytokines, inflammation or toxins and produce transcriptional factors NF-κB, HIF-2α and their target genes. The target genes include the families of MMPs and ADAMTS, among others, enzymes that play important roles in osteoarthritic degradation of the cartilage matrix and other catabolic processes [1–7]. NF-κB regulates upwards of 150 genes including, but not limited to, those involved in inflammation, cell proliferation, antiapoptosis and the negative feedback of the NF-κB signal, while HIF-2α elicits the expression of COL10A1, MMP13 and VEGFA, all of which are markers of OA [1–3]. Once transduced to the nucleus, these transcriptional factors interact with specific DNA-binding elements, triggering the expression or repression of mRNA synthesis [5]. For example, inflammation, mechanical stress and the hypoxic response present in surgical models of OA or traumatic joint injuries lead to the production of HIF-1α and HIF-2α [1,2,8]. The transcription factor NF-κB has also been identified as a likely player in OA pathogenesis and has been suggested to be linked to the induction of HIF-2α and pathological cartilage destruction and osteophyte formation [1,7]. In addition, studies performed in human OA chondrocytes have demonstrated that NF-κB mediates MMP expression induced by TNF-α and IL-1β, and the upregulation and activation of NF-κB results in pathological cartilage destruction [1–3]. For these reasons, NF-κB, HIF-2α and downstream products (i.e., MMPs) have been targeted by researchers focused on impeding the progression of arthritis (and other disease states) in its early stages [9].
Cartilage is an avascular and relatively acellular tissue with a dense, highly charged extracellular matrix [10]. These characteristics serve as barriers to treating diseased cartilage, because high systemic concentrations of drugs are necessary in order to ensure access and permeation of the drug into the tissue. Some of the mediators and pathways in arthritis, however, play beneficial roles in cellular survival and the metabolic processes of other cells. For this reason, high doses of antiarthritic drugs may create undesirable side effects when used systemically [11]. Other, less toxic therapeutic agents that show efficacy in vitro, such as curcumin, may have low bioavailability or may be quickly degraded by the body [12,13]. For these reasons, a targeted delivery method for antiarthritic drugs is attractive.
In order to successfully treat OA, disease progression should be identified early, when articular cartilage erosions are small. One of the obstacles for evaluating pharmacological intervention involves the identification and quantification of early OA. However, cartilage is radiolucent by conventional x-ray, which makes disease monitoring difficult to evaluate without expensive MRI measurements that may span the course of a decade (or longer) and are not useful for small animal models [13,14]. Ideally, disease progression would be tracked at the same time that interventional drugs are being delivered. Promising new findings in the field of nanomedicine suggest that the simultaneous detection and treatment of arthritis can be achieved using, for example, theranostic nanoscale liposomes (nanosomes) [15]. These nanosomes contain a near infrared (NIR) fluorescent dye component that makes them visible with optical imaging. Due to their surface-bound antibody to type II collagen (CII), they recognize and bind to CII, which is unmasked by the proteolytic activity of MMPs or other proteinases early in the development of the OA lesion in articular cartilage [16]. Alternatively, some groups have worked to improve existing technologies, such as MRI, using nanoparticle technologies particularly for the study of disease in small animal models [17].
Antibody-targeted or MMP-activated nanosized particles that specifically recognize damaged articular cartilage can simultaneously be used to deliver therapeutic agents (e.g., NF-κB inhibitors or siRNAs) in order to modulate the overactive, catabolic pathways of arthritis [15]. After binding to the CII or activation via MMP proteolysis, the nanoparticles degrade, releasing the encapsulated drugs locally at the site of damage [15]. This unique targeting approach has been used with nanoparticles that are activated by MMP proteolysis in order to deliver siRNA molecules directly to cells in proximity-activated targeted smart polymeric nanoparticles (PAT-SPNs) [18].
Nanosomes, smart nanocarriers, nanodiscs, quantum dots (QDs), superparamagnetic iron oxides (SPIOs) and many other nanoscale therapeutic or diagnostic devices are now being investigated for their potential to detect and/or treat arthritis in its early stages [15,18–21]. Furthermore, nanoparticles/liposomes have been used in other disease models that could be adapted for use in arthritis. This article presents the work of researchers working in the field of regenerative medicine who seek to engineer biocompatible, biodegradable, nanosized devices in order to diagnose, influence and/or utilize living cells so as to target and treat degenerative cartilage diseases [15,17,18].
Liposomes
Liposomal formulations are widely used in the pharmaceutical field as drug-delivery systems due to their biodegradability, biocompatibility, low toxicity, their ability to entrap both lipophilic and hydrophilic drugs and their highly sensitive, cost-effective, nonionizing and noninvasive real-time optical imaging features in vivo [22]. This type of formulation has been used for the intra-articular delivery of several nonsteroidal anti-inflammatory drugs in order to avoid problems of gastric ulceration and increased risk of death from cardiovascular events and stroke with their systemic administration [22]. Ghanarzadeh et al. engineered liposomes consisting of phosphatidylcholine (PC) and cholesterol in order to deliver naproxen [23]. They found that the cholesterol content of the carrier was inversely related to the amount of drug that could be loaded. However, lower cholesterol levels in the nanosome membrane resulted in decreased stability, with increasing leakage of the entrapped drug over time. PC and cholesterol-based liposomes have also been used to deliver a COX-2 inhibitor (celecoxib) or celecoxib embedded in a hyaluronate gel in a surgical model of OA in the rabbit knee [24]. The drug-loaded liposomes were introduced into the intra-articular space. Both types of liposomal treatments improved the ability of the animals to bear weight [24]. No targeting method was provided for the aforementioned liposomes other than intra-articular delivery. Topical application methods have also been attempted in animal models; however, some studies describe a lack of effect [25].
In healthy joints, the synovial space is filled with viscous synovial fluid containing hyaluronic acid and a glycoprotein called lubricin [26,27]. This lubricating layer is deficient in OA [28]. Phospholipids and hyaluronic acid physically facilitate a low-friction environment for articular cartilage in the synovial fluid and have been proposed as exogenous supplements for the lubrication of articular cartilage surfaces [29,30]. Design considerations, such as the clearance and stability of these agents, must be taken into consideration when creating joint lubricants [31]. For example, Sivan et al. investigated the use of PCs, a major constituent of the synovial fluid, for lubricating intra-articular cartilage [32]. The group screened various PC-based multilamellar vesicles composed of lipid:cholesterol ratios as cartilage lubricants and identified liposomes composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) to be the most effective single-component lubricant under various loads and temperatures. These lubricating liposomes were able to overcome many of the engineering hurdles for a successful intra-articular joint lubricant. The DMPC–multilamellar vesicle variant was able to reduce the friction coefficient in the joint space, had the best retention time in cartilage and remained the most stable over a variety of temperatures. Based on their results, the researchers proposed that intra-articular injections of DMPC liposomes or DMPC/1,2-dipalmitoyl-sn-glycero-3-phosphocholine liposomes could be used in order to improve cartilage lubrication in joint dysfunctions [32]. Their work is supported by recent in vivo studies in sheep joints as well as in vitro work identifying the anti-inflammatory effects of PCs [33,34]. The use of a simple physical liposomal barrier is an attractive option for preclinical and clinical development as cartilage lubricants.
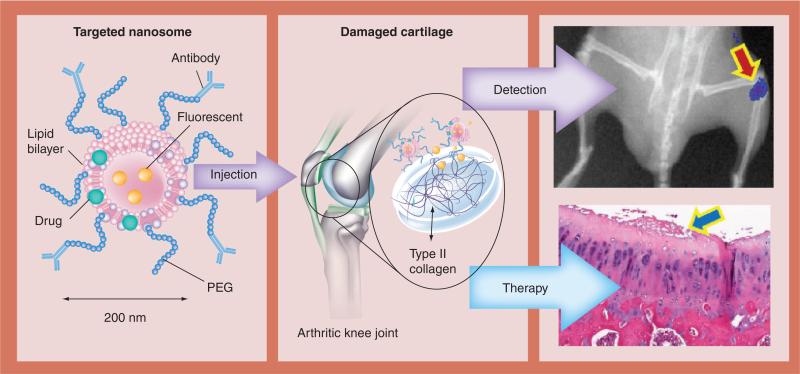
While there are effective disease-modifying drugs for rheumatoid arthritis and several related rheumatic diseases, as well as palliative drugs to partially deal with the pain of OA progression, no efficacious disease-modifying OA drugs are yet available in order to consistently halt or alter the progression of OA [35,36]. This lack of progress is surprising, as much is known about the disease process. However, it is difficult to evaluate drug therapy in patients as the disease typically progresses slowly, taking decades to fully manifest. Monitoring OA progression and identifying early onset is critical for establishing and evaluating the efficacy of new treatment modalities. Researchers working in the field of regenerative medicine are seeking to overcome these hurdles by creating therapeutic and diagnostic (theranostic) nanodevices that can be quantitatively evaluated for the degree of OA progression, even is small animals, and bind specifically to the damaged cartilage. This would allow for intravenous administration instead of intra-articular injection. Cho et al. have developed fluorescent, targeted nanosomes (200-nm liposomes) as a way of diagnosing and treating the early stages of OA [15]. These nanosomes were created using the extrusion of a lipid solution through polycarbonate track-etched filters with uniform cylindrical pores resulting in uniformly sized (average diameter of 200 nm), unilamellar liposomes. The nanosomes are covalently linked to a monoclonal antibody (mAb) recognizing native CII and also containing near-infrared (NIR)-emitting fluorescent dyes (Xenofluor™ 680 and 750 dyes [Caliper Life Sciences Inc., MA, USA]) that can be quantitatively visualized in vivo (Figure 1). CII is not available for antibody binding in normal cartilage and is only exposed when the surface of the cartilage is proteolytically damaged, as in arthritis [16]. Specific binding of the NIR-mAbCII-labeled nanosomes was confirmed by ELISA on plates coated with type I or II collagen. Only nanosomes with CII antibody significantly bound to CII, but not type I collagen, while immunonanosomes labeled with an isotype control antibody (NIR-mAbCon) did not significantly bind to either. In order to prove the efficacy of this approach in vivo, animals with different types of arthritis resulting from post-traumatic (mechanical loading of the mouse knee) or inflammatory autoimmune arthritis in mice (collagen-induced arthritis) were injected with immunonanosomes. In all of the mice that were examined, early damage could be identified by in vivo by fluorescent imaging instruments (IVIS® Lumina II XR, Caliper Life Sciences Inc., MA, USA), while normal mice showed no localization of the fluorescence in the joints. For mice, intra-articular injections proved to be problematic, with escape of the nanosomes into the circulation and binding to the damaged contralateral control knee [15]. In order to evaluate immunonanosome performance in a larger model, spontaneously occurring OA was evaluated in young and old Dunkin–Hartley guinea pigs with intraarticularly injected NIR-mAbCII or NIR-mAbCon nanosomes encapsulating the NIR dye, Xenofluor 750 [15]. At 24 h postinjection, the NIR dye was visualized by IVIS, showing that the fluorescence was only localized to joints if the nanosomes were targeted to CII. Importantly, contralateral control knees did not show staining with the targeted nanosomes, even if damage was present, showing that the injected nanosomes were contained within the synovial space. Older guinea pigs displayed a higher degree of nanosome binding in the knee joints than younger ones, and they also showed histopathological correlation of OA. IVIS imaging of the dissected knee tissues of the guinea pigs showed binding to the articular cartilage, even with very small early lesions, but no soft-tissue fluorescence [15]. The ability to track these nanocarriers for diagnosis and treatment monitoring of OA and their potential as delivery systems offers a promising future for this approach.
Figure 1. Theranostic approach for early osteoarthritis utilizing antibody-targeted fluorescent nanosomes.
These nanosomes bind to type II collagen that is unmasked in damaged cartilage, providing a quantifiable fluorescent signal and delivering therapeutic agents.
Micellar nanocarriers
Gene silencing by siRNA represents a new paradigm for the treatment of degenerative diseases such as OA [18,37,38]. However, siRNA treatments have been largely unsuccessful in clinical trials due to delivery barriers in vivo [39,40]. When delivered systemically, free siRNA molecules are rapidly cleared through kidney filtration [41,42], have susceptibility to degradation by nucleases and are too large and negatively charged to efficiently pass through cellular and endolysosomal membranes. Nanotechnology has shown promise for overcoming these barriers by protecting siRNAs from degradation, enhancing their half-life and targetability and providing a mechanism for endolysosomal escape [38,43–45]. The targeted delivery of siRNA is crucial in order to maximize bioavailability and avoid off-target effects that could potentially compromise the therapeutic index and clinical applications [46]. This is typically achieved through the functionalization of nanocarriers with ligands that bind to receptors on a targeted cell population [47]. More recently, approaches have been engineered for the environmental targeting of nanoparticles that preferentially accumulate based on environmental (i.e., pH or enzymatic) cues that are unique to the pathological site of interest [18,48,49]. These nanoparticles are designed to intelligently ‘sense’ pathological environmental signals and enzymatic activities that are unique to diseased regions of the body in order to trigger the targeting and release of therapeutic agents.
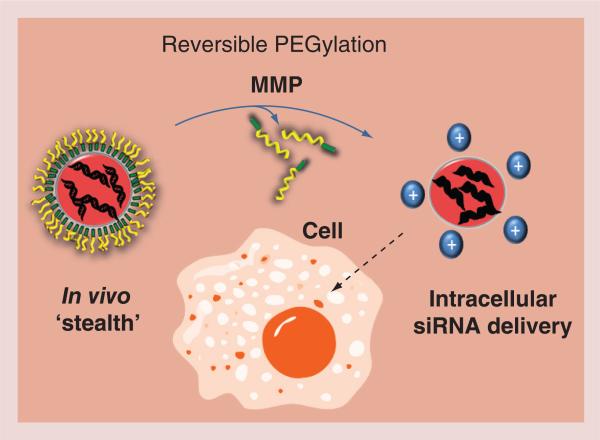
In one application, a multilayered micellar nanocarrier has been engineered that activates in response to elevated MMP activity in order to target the delivery of the siRNA cargo [18]. MMPs, which are known players in the pathophysiology of arthritis, are also widely present in cancerous tissues, such as primary breast tumors, and are thought to play a role in metastasis [1,50–51]. For this purpose, environmentally responsive micellar nanocarriers were developed that are capable of MMP-7 proximity-activated targeting for siRNA delivery and target gene knockdown specifically at sites that are characterized by elevated MMP activity (Figure 2) [38]. The base delivery system consists of a SPN that is capable of packaging and efficiently delivering the siRNA cargo into cells and escaping from endolysosomal pathways in order to gain cytoplasmic access [52]. The MMP PAT-SPN design incorporates surface PEGylation that is shed upon MMP-7 proteolysis. Prior to proteolytic activation, the surface PEGylation of the PAT-SPNs provides particle ‘stealth’ and increased blood circulation times in vivo. Enzymatic cleavage of the surface layer of the PAT-SPNs exposes an underlying cationic layer, which triggers a significant increase in the nanoparticle ξ-potential and facilitates transport into the cell. The core of the internalized PAT-SPN responds to endolysosomal pH ranges (equal to or below pH 6.2) in order to disrupt the lysosomal membrane and release siRNA into the cytosol without causing significant cytotoxicity [38]. Environmentally demanded delivery of siRNA allows for the controlled and targeted release of therapeutic agents, which reduces undesirable side effects and minimizes dosages [53]. Advanced delivery techniques that have been developed for cancer, such as the PAT-SPNs, have potential for translation into treatment for OA, which is also characterized by pathologically elevated levels of MMPs that degrade the cartilage matrix. Modification of the delivery system in order to respond to the MMPs that are characteristic of OA and the therapeutic silencing of targets such as NF-κB, HIF-2α or MMPs could be explored for the translation of PAT-SPNs for OA therapy. A recent report supports the potential use of siRNAs to knockdown NF-κB in chondrocytes, which would concurrently decrease the expression of other downstream genes, including the gene encoding the enzyme MMP-9 [54], but such approaches have not been efficiently translated in vivo due to the difficulty in the targeting and efficient local delivery of siRNAs to sites of OA.
Figure 2. A proximity-activated targeted smart polymeric nanoparticle.
A multilayered micellar nanocarrier is shown that is engineered for ‘on-demand’ triggering by elevated MMP activity that degrades its PEG surface shielding, exposing a cationic core and facilitating transport into the cell. The endolysosomal pH shift results in lysosomal membrane lysis by the core of the internalized proximity-activated targeted smart polymeric nanoparticle and release of the siRNA. The siRNA delivery results in the knockdown of MMPs and other target genes.
MMP: Matrix metalloproteinase.
High-density lipoprotein nanodiscs
Structurally, high-density lipoprotein (HDL) is a natural ‘mesoparticle’ approximately 10 nm in diameter, which is composed of the lipid-binding proteins ApoA-I and/or ApoA-II complexed with cholesterol and phospholipids [55]. Its initial discoidal form is called ‘nascent HDL’ or a nanodisc; this can be easily synthesized in vitro by mixing recombinant ApoA-I protein with phospholipids in the presence of a detergent. The detergent is removed by dialysis [55]. HDL effectively incorporates hydrophobic drugs into its phospholipid core; hydrophilic agents can be incorporated into HDL after chemical modification with cholesterol. The size of the HDL particle partially dictates the target of the HDL particle. Membrane modifications as well as drug loading can alter the size distribution of HDL nanodiscs [55]. Targeting can be achieved by tethering proteins and possibly antibodies to the shell of the HDL particles [55].
HDL does not need any modification (e.g., PEG grafting) in order to become biocompatible, and cytotoxicity of HDL has not yet been reported [55]. There are many ways to tailor HDL for use as a drug-delivery carrier. Chemical and genetic modification of ApoA-I, insertion of functional molecules containing hydrophobic moieties that anchor to the phospholipid bilayer and coinsertion of charged phospholipids into the bilayer are ways that HDL can be functionalized [55]. Customization of HDL may occur via the insertion of a hydrophobic moiety containing functional molecules that anchor to the phospholipid bilayer, coinsertion of charged phospholipids into the bilayer and/or chemical and genetic modification of the ApoA-I component [55]. Therapeutic drugs, such as curcumin, and lipophilic siRNAs can be loaded into this drug-delivery system [56,57]. For example, Wolfrum et al. have optimized HDL nanoparticles for the in vivo delivery of lipophilic siRNAs [57]. Their study demonstrated that cholesterol-conjugated siRNAs are taken up in vivo by a lipoprotein-dependent mechanism [57]. The importance of lipoproteins in the delivery of cholesterol-bound siRNAs in vivo was examined by comparing the in vivo silencing activity of HDL-bound cholesterol–siRNA–ApoB1 with equal amounts of unbound cholesterol–siRNA–ApoB1. Wolfrum et al. found that HDL-bound cholesterol–siRNA–ApoB1 was approximately five-times more efficient in cleaving the ApoB transcript in mice compared with free cholesterol–siRNA–ApoB1 [57]. In addition, the group demonstrated that the enhanced cleavage of the ApoB transcript resulted in a more than eightfold improvement for lipoprotein-bound cholesterol–siRNA–ApoB versus unbound cholesterol–siRNA–ApoB in reducing ApoB protein levels in the liver, gut and blood, demonstrating that the lipoprotein binding of these siRNAs is favorable for the targeted delivery of siRNA therapeutic agents [57]. The delivery of siRNA particles to damaged cartilage for the purposes of silencing NF-κB or HIF-2α expression and their downstream stimulation of MMPs is just one way that HDL nanoparticles could be utilized in order to treat OA. The tailorability and biocompatibility of HDL makes it an attractive material platform for theranostic technology development. Biomimetic HDL particles offer great potential for theranostic devices for the treatment of a plethora of diseases, but more work is still needed in order to adapt this technology for use in vivo [58].
In addition to targeted therapeutic delivery, HDL also can be modified to carry imaging agents, such as for MRI (e.g., gadolinium chelates); fluorescent probes can also be loaded onto a HDL platform (e.g., rhodaminephosphotidylethanolamine) and are used in current nanodisc research [58,59]. For example, Chen et al. demonstrated that the nonspecific accumulation of unmodified, reconstituted HDL (rHDL) nanoparticles can give rise to optical and MR contrast within tumors at longer time points when compared with other nano particles. Molecular imaging of the vasculature and angiogenesis was achieved via rerouting the nanoparticles to endothelial cells by the conjugation of a cyclic pentamer RGD peptide that was specific for an integrin protein. Different accumulation/binding kinetics for the rHDL compared with the rHDL–RGD nanoparticles was observed by optical imaging, showing that the combination of a targeting agent (RGD protein) enabled more specific delivery of the HDL nanoparticles [59]. Unlike low-density lipoprotein, which is mainly taken up by the liver, HDL is able to locate to many different tissues (e.g., the kidneys, liver and ovaries) [60,61]. Their ability to target the delivery of therapeutic agents makes them an attractive option for the delivery of drugs with high levels of toxicity, low bioavailability (e.g., curcumin) or high degradability (e.g., siRNA).
Quantum dots
QDs, typically coated with streptavidin and conjugated with biotinylated peptide ligands, have been used for fluorescence resonance energy transfer (FRET) analyses in order to profile MMPs that are upregulated in diseased cartilage and cancerous tissue [62]. Most QDs have a core–shell structure and are 2–8 nm in diameter. They have broad absorption spectra and also show narrow emission spectra, including those in NIR emission spectra that are desirable for the exclusion of autofluorescence [62]. QDs are also highly photostable. They are effectively synthetic fluorescence semiconductor nanocrystals that can be activated in vivo by the presence of exogenous factors (e.g., MMPs) [19]. This is a highly sensitive technology: QDs have been developed that detect MMP-2 at sub-picogram per milliliter levels in human serum [63]. QDs that detect proteinase activity have also been modified with gold nanoparticles, where the gold nanoparticles suppress QD luminescence until proteolytically activated. A collagenase-susceptible sequence was incorporated between the QDs and the gold nanoparticles in order to achieve proteinase-activated imaging, while a PEG coating was applied to the QDs in order to enhance enzyme activity. To date, QDs hold promise as a noninvasive, theranostic technology, but limited in vitro and in vivo testing has been carried out [19,62].
Bajpayee et al. have investigated QDs for use in OA remediation; their study defined the optimal QD size and charge for therapeutic use [64]. Specifically, these researchers found that deep penetration into undamaged cartilage required particle diameters of <10 nm. For superficial cartilage penetration or deep zones of proteoglycan-depleted cartilage, 15-nm particle diameters were recommended for use. Bajpayee et al. suggest that the larger QDs could be functionalized in order to specifically bind within the superficial zone of cartilage, which is often damaged in OA [64]. There, the particles could release drugs over time. Taking advantage of cartilage's highly negative, fixed charge density, the positively charged avidin molecule was used as a proof-of-concept element. Avidin penetrated through the full thickness of cartilage explants within 24 h, while the same-sized neutral counterpart, NeutrAvidin™ (Thermo Scientific, MA, USA), took 4 days to penetrate into half the thickness. Furthermore, avidin was retained within the cartilage for at least 15 days, while NeutrAvidin was largely released when explants were placed in a phosphate buffered saline desorption bath for 24 h [64]. Using this concept, positively charged QDs of less than 15 nm in diameter could deliver drugs to deeper zones of the damaged cartilage, utilizing the properties of the cartilage's extracellular matrix [64].
Outside the realm of ‘typical’ QD engineering, Smith et al. discovered a conjugation approach that utilizes a QD base with a fluorescently cleavable peptide substrate, resulting in a multifunctional nanoscale construct with unique characteristics [21]. The QD serves as a second fluorescent beacon for performance assessment, while PEG moieties serve to control nonspecific binding and shield internal ligands for the binding of drugs. In Smith et al.'s model, MMP-7, a factor that is elevated in areas populated by cancerous tissues, serves as the unmasking agent for the PEG moiety and subsequent drug release [21,65]. The modular structure of these QDs provides multiple parameters for performance optimization, with various factors presumably modulating the overall activity of the nanodevice. PEG length is one variable with the potential to significantly affect multiple characteristics by influencing the nonspecific binding limitation, effective ligand concealment, particle mobility and enzyme susceptibility [21]. The ligand surface density, ligand selection and enzyme substrate configuration of the QDs could be tailored in order to suit the needs of the disease state.
The use of MMP-activated diagnostic nanodevices, such as QDs, presents a promising method for monitoring the cartilage degeneration that is found in arthritic cartilage. In early OA lesions, large amounts of MMPs are present in damaged cartilage. Their activity results in the gradual erosion of the articular cartilage due to excessive ECM breakdown, loss of chondrocytes and, ultimately, the painful outcome of bone-on-bone contact. These diagnostic devices would elicit an MMP activation response of QDs at sites with heightened levels of MMPs (e.g., degenerative cartilage in the joint space). Applicable to OA, the overexpression of MMP family members could be quantified using this technology. QDs could also be used as indicators of DNA presence and/or DNA damage, which are additional markers of OA [19].
Magnetic nanoparticles
Researchers are currently seeking to harness cell-based therapies for the repair/regeneration of the articular cartilage in OA. In order to track cell/tissue implants, a number of noninvasive imaging modalities have been employed both in mice and humans, such as PET and MRI [40,66]. Due to the fact that SPIOs, such as ferumoxides, have already been used clinically for cell labeling and in vivo cell tracking in dermal oncology, neural degeneration and pancreatic islet transplantation with no reported negative effects, researchers are looking to this technology for use in regenerative treatments for OA. While some reports suggest genomic changes result from iron oxide labeling, it has been shown that SPIO labeling does not significantly affect chondrocyte behavior in culture, which may illustrate the potential for the use of SPIOs for the in vivo tracking of supplemental chondrocytes [66]. This demonstrates the potential for the SPIO-based tracking of cell-based therapies for advanced OA lesions in which significant amounts of cartilage have been eroded. Further studies must be conducted in order to improve the profit-ability of this technology. Some permutations of SPIOs (e.g., iron oxide nanoparticles directly administered to the patient) are already approved by the US FDA; it should be noted, however, that no FDA-approved SPIO nanoparticles are directly available in the US market (largely due to low demand). Tests performed in vitro have proven that SPIOs can be used in order to image human mesenchymal stem cell survival in cartilage explants using MRI [67,68]. In vivo tests using SPIO nanoparticles (some of which are FDA approved) suggest that cell tracking could be applied to the in vivo monitoring of stem cell therapies [69].
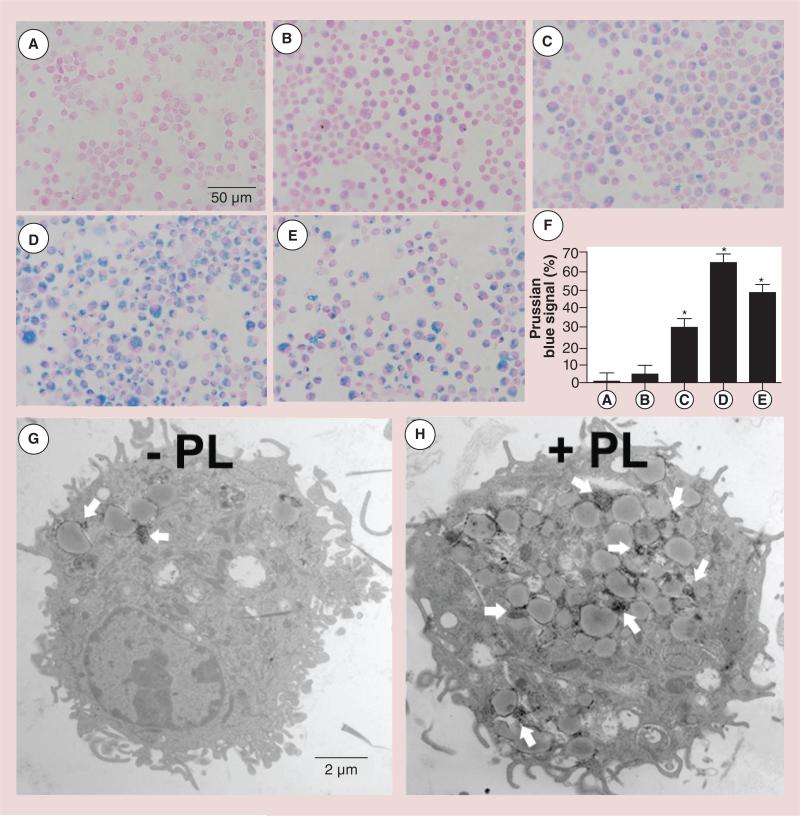
Farrel et al. and van Buul et al. found that SPIO labeling appears to be safe without influencing cell behavior [70,71]. van Buul et al. labeled and visualized the cells in the intra-articular environment and quantified the cellular population of cells seeded in cartilage defects [71]. Terminally differentiated cells, such as chondrocytes, present an especially viable target for cell tracking. Pham's group at Vanderbilt University (TN, USA) has developed such a technology for dendritic cells, another terminally differentiated cell type. MRI tracking of dendritic cells using iron oxide nanoparticles offers advantages over other imaging techniques in terms of resolution and the intrinsic anatomical features associated with this technique [72]. SPIO nanoparticles engineered with polylysine in order to facilitate cell uptake were used for the noninvasive monitoring of cells in vivo [72,73]. Currently, T2-mediated MRI using SPIO nanoparticles is the preferred technique, given its clinical relevance. Optimization of cell-labeling methods in order to ensure labeling reproducibility, the ability of cells to retain their functional viability and so that the process does not perturb the target cells are all necessary factors for consideration. Toki et al. described the creation of SPIOs, nanometer-sized iron oxide particles that are first coated with dextran, resulting in an overall size of 30 nm [72]. Dendritic cells are isolated and incubated with these SPIO nanoparticles in a cell culture dish before being injected into the host for T2-weighted and T2*-weighted imaging due to reduction of T2 and T2* relaxation times [72,74]. Since the process is time dependent, a polylysine transfection agent was utilized in order to enhance the cellular uptake of nanoparticles. This allows for a shorter incubation period. The utilization of polylysine was previously reported to cause cell death at concentrations higher than 10 ug/ml. The results from Pham's laboratory found by calibration that the addition of SPIO nanoparticles to bone marrow dendritic cells that had been exposed to 20 μg/ml of polylysine improved cell viability at SPIO concentrations below 1000 μg/ml (i.e., the addition of SPIO nanoparticles rescued dendritic cells from polylysine-induced death; Figure 3) [72,74]. High concentrations of SPIO nanoparticles have the capacity to upregulate cytokine release in bone marrow dendritic cells; this action was found to be independent of polylysine or dextran T-10. Toki et al. reasoned that the positively charged polylysine addition would facilitate easier uptake by negatively charged cells and thus serve as a transfection agent for the uptake of SPIO nanoparticles by changing their surface charge [72]. This rationale could easily be applied to the negatively charged chondrocyte surface and cartilage matrix [65,72]. SPIO particles also have potential for use in the MRI monitoring of mesenchymal stem cell-based therapies of OA amelioration [69,75]. It should be noted that SPIO labeling methods have the potential to affect cell behavior as the procedures could damage cells, and each cell type must be calibrated individually with respect to labeling conditions. Cell-viability studies should be integral in the development of studies aimed at improving SPIO technologies. The longer-term effects of this type of treatment are poorly defined.
Figure 3. Prussian Blue staining to assess the effect of polylysine-induced internalization of superparamagnetic iron oxide nanoparticles by immature bone marrow dendritic cells that had been labeled for 12 h (Nuclear Fast Red counterstain).
(A) Nonlabeled bone marrow dendritic cells, (B) 500 μg/ml superparamagnetic iron oxide (SPIO), (C) 500 μg/ml SPIO plus 10 μg/ml PL mixture, (D) 500 μg/ml SPIO nanoparticles coated with PL during a 3-h preincubation and (E) 100 μg/ml SPIO plus 20 μg/ml PL mixture. (F) Quantitative analysis of the uptake of SPIO nanoparticles shown in (A–E) by MATLAB® (MathWorks, Inc., MA, USA). Data were expressed as means ± SE. (G & H) PL-mediated delivery of SPIO nanoparticles (white arrows) was confirmed by transmission electron microscopy.
*p < 0.05.
PL: Polylysine.
Reproduced with permission from [72].
As mentioned previously, due to the lack of vasculature in cartilage, high systemic concentrations of drugs are needed in order to achieve therapeutic effects. Magnetic nanocarriers could be engineered for targeted drug delivery to inflammatory sites under the guidance of a magnetic field, assuring therapeutic concentrations, avoiding side effects and targeting the delivery of therapeutic agents [76]. Circulating magnetic nanoparticles (MNPs) respond to the application of a magnetic field by aggregating in the desired region. A number of basic MNPs have already been approved by the FDA as MRI contrast agents [77]. They have been targeted using antibodies, coated with stealth substances (e.g., PEG) and used to deliver therapeutic agents (e.g., apamers, DNA and RNA) [77]. Specifically, Xu et al. have created nanocomposites combining optical and magnetic properties; they are synthesized via a microemulsion approach with nontoxic components [78]. They can be detected via optical and MRI techniques. Although Xu et al. have demonstrated their in vivo potential for a specific chemotherapeutic drug (i.e., doxorubicin), other varieties of chemotherapeutic drugs specifically target NF-κB [78]. MNPs thus have potential for use in degenerative joint diseases. Arias et al. recognized the promise of the application of magnetic colloids in musculoskeletal disorders and engineered iron–ethylcellulose (core–shell) nanoparticles loaded with diclofenac sodium, a nonsteroidal anti-inflammatory drug with potential anti-inflammatory and analgesic activities [76]. The technology has a loading efficiency of approximately 54%, releases over approximately 60 h and responds to weak magnetic fields [76]. Although in its early stages, in vivo work could elucidate new and reliable treatment modalities using magnet technologies. It has even been suggested that MNPs could provide a means of remotely controlling cellular positioning using a magnetic field in the future [79].
Conclusion & future perspective
Nanotechnology devices show promise for improving the diagnostic assessment and therapeutic treatment of OA. Table 1 summarizes the technologies that have been reviewed in this article. Future work must be conducted in order to validate the in vivo potential for both the therapeutic and diagnostic capabilities of any nanosized delivery device in order to guarantee its success as a treatment modality. Nanoparticle encapsulation or architectural enfolding for use in OA seems ideal for presenting therapeutic agents to the body in a way that avoids rapid clearance and can be targeted to or injected into the synovial cavity or articular cartilage of the joint, enabling diagnostic and longitudinal studies of OA progression. By utilizing targeted delivery, the dosage of necessary therapeutic agents could be greatly reduced. During the early stages of OA, great potential exists for the targeted, theranostic delivery of therapeutic agents via nanoparticle delivery for the prevention of the degradation of existing cartilage. In the later stages of OA, when cartilage is largely absent, nanoparticle delivery offers more of a venue for targeted cell-based therapies that could potentially be monitored by SPIOs. Both of these approaches will hopefully lead to an extension and improved quality of life in patients with OA.
Table 1.
Nanotechnology that is applicable for osteoarthritis research.
| Nanotechnology | Size | Type of therapy | Targeting/activation method | Detection method | Ref. |
|---|---|---|---|---|---|
| Liposome | 5–200 nm | Diagnostic and therapeutic | Antibody | Fluorescence | [15,22–24,32] |
| Micellar nanocarrier | 46 nm | Therapeutic | Proteolytic | – | [18,38] |
| HDL nanodisc | 5–100 nm | Diagnostic and therapeutic | Protein/peptide, antibody targeting | MRI | [55,57–59] |
| Quantum dot (and constructs) | ~0.9–18.2 nm | Diagnostic and therapeutic | Proteolytic | FRET, fluorescence | [19,21,62–65] |
| Magnetic nanoparticles | ~30 nm–1 μm | Diagnostic and therapeutic | Magnetic | MRI, PET | [67–74,76–78] |
FRET: Fluorescence resonance energy transfer.
Executive summary.
Liposomes
Liposomes are used as drug-delivery systems because they can be engineered to be biocompatible, biodegradable and have minimal toxic effects.
Liposomes can encapsulate both lipophilic and hydrophilic drugs.
Liposomes can be diagnostic and/or used as carriers for the targeted delivery of therapeutic agents.
Liposomes can be targeted using antibodies, peptides and other small molecules and shielded from nonspecific binding with PEG.
Micellar nanocarriers
Micellar nanocarriers are useful for siRNA delivery.
‘smart’ carriers are nanoparticles that have been constructed with a layered design. The outer layer responds to environmental factors (e.g. matrix metalloproteinases) for the targeted delivery of therapeutic agents, while the inner layer facilitates cellular uptake.
Micellar nanocarriers can be constructed so the low-pH environment of the lysosome results in the lysis of the organelle membrane by the nanoparticle and the release of the siRNA into the cytoplasm.
High-density lipoprotein nanodiscs
High-density lipoprotein (HDL) nanodiscs mimic natural high-density lipoprotein conglomerates from the in vivo environment.
HDL nanodiscs are composed of the lipid-binding proteins ApoA-I and/or ApoA-II complexed with cholesterol and phospholipids.
HDL nanodiscs can be used to specifically deliver therapeutic agents and for diagnostic imaging.
Quantum dots
Quantum dots have a core–shell structure and tend to be 2–8 nm in diameter.
Quantum dots have broad absorption spectra and also show narrow emission spectra.
Quantum dots are used as imaging agents and are sometimes activated by high concentrations of specific factors, such as matrix metalloproteinases.
Magnetic nanoparticles
Superparamagnetic iron oxides are used for in vivo cell tracking for cell-based therapies.
Superparamagnetic iron oxides are also used as MRI agents.
Magnetic nanocarriers are engineered for guided delivery to inflammatory sites under the influence of a magnetic field that is applied topically.
This guided delivery ensures that a therapeutic concentration in the proximity of the desired site is reached and limits unwanted side effects elsewhere.
Acknowledgments
This work supported with resources and the use of facilities at the Veterans Affairs Medical Center (VAMC) at Memphis, TN, USA. This research was supported by a VA Merit Review award from the Department of Veterans Affairs and NIH R21AR060408 (KA Hasty).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1•.Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2a during skeletal growth and osteoarthritis development. Nat. Med. 2010;16:678–686. doi: 10.1038/nm.2146. [Connected HIF-2α to the degradative pathway that is characteristic of osteoarthritis. These results linked the production of MMP-13 and VEGFA release to induction of the transcription factor HIF-2α.] [DOI] [PubMed] [Google Scholar]
- 2•.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–693. doi: 10.1038/nm.2153. [Connected HIF-2α to the degradative pathway that is characteristic of osteoarthritis. These results linked the production of MMP-13 and VEGFA release to induction of the transcription factor HIF-2α.] [DOI] [PubMed] [Google Scholar]
- 3•.Mitchell P, Magna H, Reeves L, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Invest. 1996;97(3):761–768. doi: 10.1172/JCI118475. [Identified the proteolytic enzymes that are largely responsible for cartilage matrix degeneration. MMP-13 along with ADAMTS-4 and ADAMTS-5 were identified as critical components for the respective breakdown of collagen and aggrecan in arthritis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9(6):539–552. doi: 10.1053/joca.2001.0427. [Identified the proteolytic enzymes that are largely responsible for cartilage matrix degeneration. MMP-13 along with ADAMTS-4 and ADAMTS-5 were identified as critical components for the respective breakdown of collagen and aggrecan in arthritis.] [DOI] [PubMed] [Google Scholar]
- 5.Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000;43(1):195–205. doi: 10.1002/1529-0131(200001)43:1<195::AID-ANR24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Lee S-H, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliver. Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 8.Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through regulation of HIF-1a. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB ctivation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 11.Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 13.Shakibaei M, Shulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondroctyes from IL-1β-induced inhibition of colllagen type II and B1-integrin expression and activation of caspase-3: an immunomorphological study. Anal. Anat. 2005;187:487–497. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Leblond F, Davis S, Valdes P, Pogue B. Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J. Photochem. Photobiol. B. 2010;98(1):77–94. doi: 10.1016/j.jphotobiol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Cho H, Stuart JM, Magid R, et al. Theranostic immunoliposomes for osteoarthrits. Nanomedicine. 2013;10(3):619–627. doi: 10.1016/j.nano.2013.09.004. [Presents the creation of a nanotechnology that exemplifies the goals of researchers who are working to find solutions that halt disease progression. This devices homes to the necessary sites and is traceable via imaging devices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novorki K, Koshino T, Takaqi T, Okamoto R, Jasin HE. Binding characterisitics of antitype II collagen antibody to the surface of diseased human cartilage as a probe for tissue damage. J. Rheumatol. 1994;21(2):293–296. [PubMed] [Google Scholar]
- 17.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat. Med. 2007;13(3):372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 18•.Li H, Yu SS, Miteva M, et al. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv. Funct. Mater. 2013;23(24):3040–3052. doi: 10.1002/adfm.201202215. [Presents the creation of a nanotechnology that exemplifies the goals of researchers who are working to find solutions that halt disease progression. This devices is proximity activated and is traceable via imaging devices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy CJ, Coffer JL. Quantum dots: a primer. Appl. Spectrosc. 2002;56(1):A16–A27. [Google Scholar]
- 20.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regnerative medicine. J. Cell. Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 21•.Smith RA, Sewell SL, Giorgio TD. Proximity-activated nanoparticles: in vitro performance of specific structural modification by enzymatic cleavage. Int. J. Nanomed. 2008;3(1):95–103. doi: 10.2147/ijn.s2485. [Presents the creation of a nanotechnology that exemplifies the goals of researchers who are working to find solutions that halt disease progression. This devices homes to the necessary sites, is proximity activated and is traceable via imaging devices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil YP, Jadhav S. Novel methods for liposome preparation. Chem. Phys. Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Ghanarzadeh S, Khorramib A, Arami S. Preparation of optimized Naproxen nano liposomes using response surface methodology. J. Pharmaceut Invest. 2014;44:33–39. [Google Scholar]
- 24.Dong J, Jiang D, Wang Z, Wu G, Miao L, Huang L. Intra-articular delivery of liposomal celecoxib–hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int. J. Pharm. 2013;441:285–290. doi: 10.1016/j.ijpharm.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Schleining JA, McClure SR, Evans RB, Hyde WG, Wulf LW, Kind AJ. Liposome-based diclofenac for the treatment of inflammation in an acute synovitis model in horses. J. Vet. Pharmacol. Ther. 2008;31:554–561. doi: 10.1111/j.1365-2885.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 26.Nitzan DW, Nitzan U, Dan P, Yedgar S. The role of hyaluronic acid in protecting surface-active phospholipids from lysis by exogenous phospholipase A2. Rheumatology. 2001;40:336–340. doi: 10.1093/rheumatology/40.3.336. [DOI] [PubMed] [Google Scholar]
- 27.Swann DA, Slayter HS, Silver FH. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J. Biol. Chem. 1981;256(11):5921–5925. [PubMed] [Google Scholar]
- 28.Lotz M. Osteoarthritis year 2011 in review: biology. Osteoarthritis Cartilage. 2012;20(3):192–196. doi: 10.1016/j.joca.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y-H, Jones SA, Forbes B, Martin GP, Brown MB. Hyaluronan: pharmaceutical characterization and drug delivery. Drug Deliv. 2005;12:327–342. doi: 10.1080/10717540590952555. [DOI] [PubMed] [Google Scholar]
- 30.Hills BA, Butler BD. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann. Rheum. Dis. 1984;43:641–648. doi: 10.1136/ard.43.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang ML, Im G-I. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin. Drug Deliv. 2014;11(2):269–282. doi: 10.1517/17425247.2014.867325. [DOI] [PubMed] [Google Scholar]
- 32.Sivan S, Schroeder A, Verberne G, et al. Liposomes act as effective biolubricants for friction reduction in human synovial joints. Langmuir. 2010;26(2):1107–1111. doi: 10.1021/la9024712. [DOI] [PubMed] [Google Scholar]
- 33.Edwards SH, Cake MA, Spoelstra G, Read RA. Biodistribution and clearance of intra-articular liposomes in a large animal model using a radiographic marker. J. Liposome Res. 2007;17(3–4):249–261. doi: 10.1080/08982100701557129. [DOI] [PubMed] [Google Scholar]
- 34.Treede I, Braun A, Sparla R, et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007;282(37):27155–27164. doi: 10.1074/jbc.M704408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter DJ. Pharmacologic therapy for osteoarthritis – the era of disease modification. Nat. Rev. Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 36.Matthews GL, Hunter DJ. Emerging drugs for osteoarthritis. Expert Opin. Emerg. Drugs. 2011;16(3):479–491. doi: 10.1517/14728214.2011.576670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saltzman MW. Therapeutic siRNA: principles, challenges, and strategies. Yale J. Biol. Med. 2012;85:187–200. [PMC free article] [PubMed] [Google Scholar]
- 38.Li LY, Quarles LD, Hong HZ, Xiao SZ. RNA interference and its application in bone-related diseases. Biochem. Biophys. Res. Commun. 2007;361:817–821. doi: 10.1016/j.bbrc.2007.07.123. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Lu Z, Wientjes M, Au J. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musacchio T, Torchilin V. siRNA delivery: from basics to therapeutic applications. Front. Biosci. 2013;18:58–79. doi: 10.2741/4087. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerman JE, Choi CHJ, Han H, Davis ME. Polycation–siRNA nanoparticles can dissassemble at the kidney glomerular basement membrane. Proc. Natl Acad. Sci. USA. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naeye B, Deschout H, Caveliers V, et al. In vivo disassembly of IV administered siRNA matrix nanoparticles at the renal filtration barrier. Biomaterials. 2013;34(9):2350–2358. doi: 10.1016/j.biomaterials.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 43.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002;54(1):135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 44.Nelson CE, Kintzing JR, Hanna A, Shannon JM, Gupta MK, Duvall CL. Balancing cationic and hydrophilic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano. 2013;7(10):8870–8880. doi: 10.1021/nn403325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong Y, Love KT, Dorkin JR, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA. 2014;111(11):3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gullotti E, Yoon Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol. Pharmacol. 2009;6(4):1041–1051. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically adminstered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naito M, Ishii T, Matsumoto A, Miyata K, Miyahara Y, Kataoka K. A phenylboronate-functionalized polyion complex micelle for ATP-trigered release of siRNA. Angew. Chem. Int. Ed. Engl. 2012;51(43):10751–10755. doi: 10.1002/anie.201203360. [DOI] [PubMed] [Google Scholar]
- 49.Hatekeyama H, Akita H, Ito E, et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG–lipid. Biomaterials. 2011;32(18):4306–4016. doi: 10.1016/j.biomaterials.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 50.McCawley L, Matrisian L. Matrix metalloproteinase: multifunctional contriutors to tumor progression. Mol. Med. Today. 2000;6(4):149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 51.Bissell MJ, Le Beyec J, Anderson RL. Prostate cancer in bone: importance of context for inhibition of matrix metalloproteinases. J. Natl Cancer Inst. 2002;94(1):4–5. doi: 10.1093/jnci/94.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covertine A, Diab C, Prieve M, et al. Ph-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11(11):2904–2911. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnett JC, Rossi JJ. RNA-based therapeutics – current progress and future prospects. Chem. Biol. 2012;19(1):60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lianxu C, Hongti J, Changlong Y. NF-κBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthris Cartilage. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Murakami T. Phospholipid nanodisc engineering for drug delivery systems. Biotech. J. 2012;7:762–767. doi: 10.1002/biot.201100508. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodiscs: formulation and characterization. Nanomedicine. 2011;7(2):162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25(10):1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 58.Damiano MG, Mutharasan RK, Tripathy S, McMahon KM, Thaxton CS. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv. Drug Deliv. Rev. 2013;65:v649–v662. doi: 10.1016/j.addr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Jarzyna PA, van Tilborg GA, et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010;24(6):1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class b, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J. Clin. Invest. 1996;98(4):984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudling MJ, Reihner E, Einarsson K, Ewerth S, Angelin B. Low density lipoprotein receptor-binding activity in human tissues: quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo. Proc. Natl Acad. Sci. USA. 1990;87:3469–3473. doi: 10.1073/pnas.87.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knapinska A, Fields GB. Chemical biology for understanding matrix metalloproteinase function. Chembiochem. 2012;13:2002–2020. doi: 10.1002/cbic.201200298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng T, Zhang R, Zhang Q, et al. Ultrasensitive dual-channel detection of matrix metalloproteinase-2 in human serum using gold–quantum dot core–satellite nanoprobes. Chem. Commun. 2013;49(72):7863–7970. doi: 10.1039/c3cc44623a. [DOI] [PubMed] [Google Scholar]
- 64.Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35:538–549. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McIntyre JO, Matrisian LM. Molecular imaging of proteolytic activity in cancer. J. Cell. Biochem. 2003;90(6):1087–1097. doi: 10.1002/jcb.10713. [DOI] [PubMed] [Google Scholar]
- 66.Farrell E, Wielopolski P, Pavljsevic P, et al. Cell labelling with superparamagnetic iron oxide has no effect on chondrocyte behavior. Osteoarthritis Cartilage. 2009;17:961–967. doi: 10.1016/j.joca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Saldanha K, Doan R, Majumdar S. Iron oxide labelling of mesenchymal stem cells with micrometer-sized particles: applications to cartilage tissue engineering. Proc. Int. Soc. Magn. Res. Med. 2009;17:922. [Google Scholar]
- 68.Henning TD, Gawande R, Khurana A, et al. Magnetic resonance imaging of ferumoxide-labeled mesenchymal stem cells in cartilage defects: in vitro and in vivo investigations. Mol. Imaging. 2012;11(3):197–209. [PMC free article] [PubMed] [Google Scholar]
- 69.Majumdar S, Li X, Blumenkrantz G, et al. MR imaging and early cartilage degeneration and strategies for monitoring regeneration. J. Musculoskelet. Neuronal Interact. 2006;6(4):382–384. [PubMed] [Google Scholar]
- 70.Farrel E, Wielopolski P, Pavljasevic P, et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2008;369:1076–1081. doi: 10.1016/j.bbrc.2008.02.159. [DOI] [PubMed] [Google Scholar]
- 71.van Buul GM, Kotek G, Wielopolski PA, et al. Clinically translatable cell tracking and quantification by MRI in cartilage repair using superparamagnetic iron oxides. PLoS ONE. 2011;6(2):e17001. doi: 10.1371/journal.pone.0017001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Toki S, Omary RA, Wilson K, Gore JC, Peebles RS, Pham W. A comprehensive analysis of transfection-assisted delivery of iron oxide nanoparticles to dendritic cells. Nanomedicine. 2013;9(8):1235–1244. doi: 10.1016/j.nano.2013.05.010. [Presents the creation of a nanotechnology that exemplifies the goals of researchers who are working to find solutions that halt disease progression. This devices homes to the necessary sites and is traceable via imaging devices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mowat P, Franconi F, Chapon C, et al. Evaluating SPIO-labelled cell MR efficiency by three-dimensional quantitative T2* MRI. NMR Biomed. 2007;20:21–27. doi: 10.1002/nbm.1084. [DOI] [PubMed] [Google Scholar]
- 74.Morgan DM, Clover J, Pearson JD. Effects of synthetic polycations on leucine incorporation, lactate dehydrogenase release, and morphology of human umbilical vein endothelial cells. J. Cell. Sci. 1988;91:231–238. doi: 10.1242/jcs.91.2.231. [DOI] [PubMed] [Google Scholar]
- 75.Nedopil A, Klenk C, Kim C, et al. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in MASI for treatment of osteoarthritis. Invest. Radiol. 2010;45(10):634–640. doi: 10.1097/RLI.0b013e3181ed566c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arias JL, Lopez-Viota M, Lopez-Viota J, Delgado AV. Development of iron/ethylcellulose (core/shell) nanoparticles loded with diclofenac sodium for arthritis treatment. Int. J. Pharm. 2009;382:270–276. doi: 10.1016/j.ijpharm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 77.Colombo M, Carregal-Romero S, Casula MF. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012;41:4306–4334. doi: 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- 78.Xu H, Cheng L, Wang C, Ma X, Li Y, Liu Z. Polymer encapsulated upconversion nanoparticle/iron oxide nanocomposites for multimodal imaging and magnetic targeted drug delivery. Biomaterials. 2011;32:9368–9373. doi: 10.1016/j.biomaterials.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 79.Dobson J. Remote control of cellular behavior with magnetic nanoparticles. Nat. Nanotechnol. 2008;3:139–143. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]