Abstract
The generation of cardiomyocytes from human induced pluripotent stem cells (hiPSCs) provides a source of cells that accurately recapitulate the human cardiac pathophysiology. The application of these cells allows for modeling of cardiovascular diseases, providing a novel understanding of human disease mechanisms and assessment of therapies. Here, we describe a stepwise protocol developed in our laboratory for the generation of hiPSCs from patients with a specific disease phenotype, long-term hiPSC culture and cryopreservation, differentiation of hiPSCs to cardiomyocytes, and assessment of disease phenotypes. Our protocol combines a number of innovative tools that include a codon-optimized mini intronic plasmid (CoMiP), chemically defined culture conditions to achieve high efficiencies of reprogramming and differentiation, and calcium imaging for assessment of cardiomyocyte phenotypes. Thus, this protocol provides a complete guide to use a patient cohort on a testable cardiomyocyte platform for pharmacological drug assessment.
Keywords: Cardiomyocytes, Disease modeling, Human induced pluripotent stem cells, Calcium imaging
1 Introduction
The heart is uniquely at risk to genetic disease due to the dependence on structural proteins for function and requirement for high levels of mitochondrial activity. Techniques to model human cardiac diseases have progressed significantly in the last five years with the development of patient-specific human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) (1), which has been shown to accurately recapitulate a large number of genetic and environmentally acquired cardiac diseases (2, 3).
To reach this point, significant advances are required in human pluripotent culture, reprogramming, and cardiac differentiation. For the reprogramming of human somatic cells into hiPSCs, progress has been made in the methodology for gene delivery, starting with retroviral and lentiviral expression and progressing through self-replicating episomal plasmids to a single codon optimized mini intronic plasmid (CoMIP) (4) that is demonstrated here. In CoMiP, the human OCT4, SOX2, and KLF4 cDNA sequences have been replaced with those most suited for high level expression, and a plasmid has been used with a minimal-size backbone to enhance transfection efficiency. This technique allows for reprogramming without the integration of exogenous DNA sequences, thus maintaining the integrity of the target cell genome.
A second major development has been the discovery that a simple, chemically defined, serum/albumin-free medium consisting of just eight components (E8) can be used to culture hiPSCs (5), which can be modified to be compatible for reprogramming (E7). The advent of this media substantially improves the quality of hiPSC cultures (i.e., by eliminating spontaneously differentiating cells) and reduces the cost and complexity of reprogramming and culture. Recently, it has been demonstrated that hiPSC-CM differentiation can be performed using a chemically defined medium and small molecules, without the need for serum or growth factors, as demonstrated here. This methodology has proven to be reliable and reproducible for the differentiation of a large number of hiPSC lines (6). Finally, it has been demonstrated that immunofluorescent staining for TNNT2 (troponin T) and ACTN2 (α-actinin) can detect a known structural phenotype, and that we can detect a functional phenotypes using calcium imaging with Fluo-4AM.
Here we demonstrate that by combining all three of these advances (i.e., CoMIP, chemically defined reprogramming, and chemically defined differentiation), somatic cells can be isolated from a patient, reprogrammed to hiPSCs, and differentiated to hiPSC-CMs. The cells can be phenotypically characterized using immunofluorescence and calcium imaging. The aim of this study is to reproduce the data published earlier using lentiviral-derived dilated cardiomyopathy (DCM) hiPSCs (7), now derived with a non-integrating technique (CoMiP). We concentrated on this disease model as it is one of the first hiPSC-CM disease models that demonstrated a phenotype that was not just electrophysiological. We show that the published DCM phenotype caused by a mutation of TNNT2 (R173W) is independent of the integrated lentivirus, and that this mutation causes the phenotypic perturbations previously seen (i.e., elimination of sarcomeric alignment and reduction in calcium handling).
2 Materials
2.1 Patient Fibroblast Isolation and Growth
Lidocaine HCl 1 % and epinephrine 1:100,000 injection.
1 mL SafetyGlide TB syringe.
3 mm Tri-punch disposable skin punch biopsy punch.
Sterile non-latex gloves.
Alcohol wipes.
ChloraPrep One-Step (2 % chlorhexidine gluconate and 70 % isopropyl alcohol).
Chlorhexidine gluconate cloth.
15 and 50 mL polypropylene conical tubes.
MycoAlert kit.
Fibroblast medium (see formula 500 mL DMEM high glucose, with GlutaMAX, HEPES, 10 % FBS, filter-sterilized).
Collagenase II solution (see formula 50 mg collagenase II, 50 mL DMEM, filter-sterilized).
Matrigel-coated 6-well plates, 100 mm dishes and T225 flasks (see protocol).
Dimethyl Sulfoxide (DMSO).
Fetal Bovine Serum (FBS).
Cryovials.
CoolCell LX.
2.2 Somatic Cell Reprogramming
CoMiP plasmid (1 μg/μL, provided by S. Diecke and J. Wu upon request).
TrypLE Express.
Fibroblast medium (see formula 500 mL DMEM high glucose, with GlutaMAX, HEPES, 10 % FBS, filter-sterilized).
E7 medium (see formula Essential 6, 100 ng/mL FGF2).
Essential 8 medium.
E8Y medium (see formula Essential 8, 10 μM Y27632).
Neon transduction device with 100 μL tips.
Sodium butyrate.
L-ascorbic acid 2-phosphate.
Matrigel-coated 100 mm dishes and 6-well plates (see protocol).
0.5 mM EDTA.
Bambanker.
Cardiomyocyte Differentiation Kit.
RPMI 1640 (without glucose).
B-27 with insulin supplement.
2.3 Cell Dissociation and Calcium Imaging
TrypLE Express.
100 μm cell strainer.
8-chamber coverslips.
Fluo-4 AM.
Pluronic F-127, 0.2 μm filtered (10 % solution in water).
Tyrode’s salts.
2.4 Equipment
Cell culture incubator capable of 37 °C, 5 % CO2, 85 % relative humidity.
Automated cell counter.
Centrifuge −86 °C ultralow temperature freezer.
Liquid nitrogen storage.
Inverted cell culture microscope (such as LEICA DMi1).
Confocal microscope (such as Zeiss LSM 510Meta).
3 Methods
3.1 Patient Fibroblast Isolation, Growth, and Cryopreservation
Obtain local Institutional Review Board (IRB) approval that complies with local and national laws and guidelines.
Informed consent forms signed by patient.
To be performed by MD or RN: Swab area to be biopsied (hip/upper cheek area, not exposed to sun) twice with iodine, inject lidocaine, using sterile gloves, take two 3 mm skin punch biopsies and place in ~5 mL of fibroblast medium.
Transport back to lab in a 15 mL Falcon tube.
Fill two wells of a 6-well plate with 2 mL of 200 U/mL collagenase II and transfer each skin biopsy from Falcon tube to a well using a P1000 tip. Using a round blade scalpel, cut the biopsy into approximately 0.5–1 mm pieces.
Incubate for 4 h at 37 °C, during this time prepare Matrigel-coated plate (see protocol).
Transfer cells/collagenase to a 15 mL Falcon tube, top up with fibroblast medium, centrifuge at 200 × g for 4 min, resuspend pellet in 2 mL fibroblast medium, and transfer each biopsy to one well of a Matrigel-coated 6-well plate.
Every 3–4 days remove the medium and transfer to a 15 mL Falcon tube, top up the well with 2 mL fibroblast medium, centrifuge the spent medium, and return any pelleted cells to the well.
Cell clumps will attach after ~5 days and after ~15 days wells will be ~50 % confluent and ready to passage.
To passage, aspirate medium, add 1 mL per well TrypLE Express and incubate at 37 °C for 2 min. Transfer dissociated cells to a 15 mL Falcon tube and top up with fibroblast media, and centrifuge at 200 × g for 4 min. Resuspend pellet in 45 mL fibroblast media and transfer cells to a Matrigel-coated T225 flask. Change media every other day.
After ~5–7 days, cells will be confluent and ready to be cryopreserved.
Passage cells as above, centrifuge and resuspend cells in 10 mL cold (4 °C) 10 % DMSO/90 % FBS.
Aliquot cells at 1 mL per cryovial, 10 vials total, these will be passage 2 when thawed.
Transfer vials to CoolCell and place at −86 °C overnight.
Transfer vials to liquid nitrogen tank (see Note 1).
3.2 Coating Tissue Culture Plates and Flasks with Matrigel
Thaw a bottle of growth factor-reduced Matrigel overnight at 4 °C and store it at 4 °C. There is no need to aliquot or leave it on ice.
Add 250 μL of Matrigel to 50 mL of 4 °C DMEM/F12 in a 50 mL Falcon tube. Return the Matrigel bottle to 4 °C quickly as it will gel at >10 °C.
Mix the tube by inversion and plate at 1 mL per well of a 12-well plate, 2 mL per well of a 6-well plate, or 45 mL into a T225 flask.
Place plates/flasks at 37 °C for at least 30 min. Plates/flask may be kept here at 37 °C for up to 4 weeks.
Before use, aspirate diluted Matrigel. Washing with D-PBS is not required.
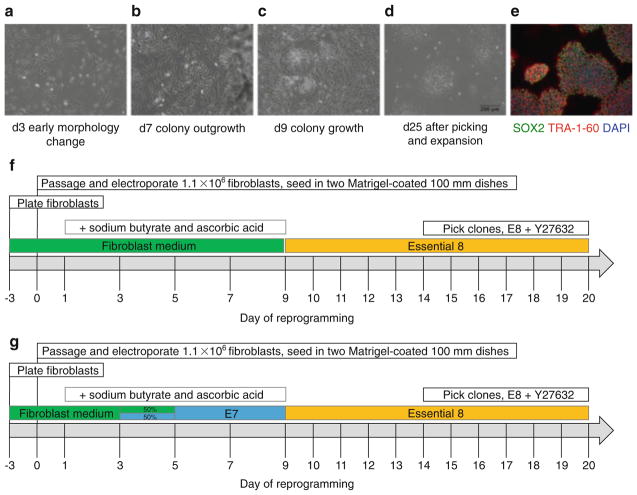
3.3 Reprogramming of Fibroblasts with CoMiP (Fig. 1)
Fig. 1.
Stepwise protocol for the reprogramming of fibroblasts to human induced pluripotent stem cells (hiPSCs) using a single codon-optimized mini-intronic plasmid (CoMiP). Day of reprogramming represents the days at which the medium is changed. (a–d) Phase-contrast images of the five major stages of reprogramming demonstrating the progression from the fibroblast morphology, through early hiPSC colonies and cells ready for picking, and cells at passage 1. (e) Immunofluorescent staining of CoMiP-derived hiPSCs for the common pluripotency markers SOX2 and TRA-1-60. (f–g) Two different strategies for reprogramming of fibroblasts: one approach uses continued culture in fibroblast medium (containing FBS) up until day 9 of reprogramming before switching to E8 medium, and a second approach uses a gradual transfer from fibroblast medium through chemically defined E7 medium (E8 without TGFβ1) to E8 medium
The target fibroblasts cell line should be thawed and cultured for at least 3 days before starting the reprogramming experiment.
Thaw a vial of fibroblasts for ~1 min in a 37 °C water bath, transfer cells to a 15 mL Falcon tube and top up to 10 ml with fibroblast medium, centrifuge at 200 × g for 4 min and plate in a 100 mm Matrigel-coated dish. Grow to confluence (~2–3 days) and passage with TrypLE Express as above.
Count cells using an automated cell counter, place 1.1 × 106 cells in a 15 mL Falcon tube and centrifuge at 200 × g for 4 min.
Prepare the Neon transfection device following the manufacturer’s instructions using Buffer E2.
Aspirate the supernatant and resuspend the cell pellet in 100 μL Neon Buffer R; add 10 μg of CoMiP plasmid and resuspend gently. Load the cells mixed with the DNA into the 100 μL Neon transfection tip (make sure to avoid introducing air bubbles in the tip as this will result in poor transfection efficiency and reduced viability of the cells).
Perform the transfection using 100 μL Neon tip, making sure it is properly inserted into the transfection tube (see Instruction manual) under the following settings: 1,600 V, 10 ms, and 3 pulses (transfection efficiency should be more than 50 %).
Mix the transfected cells with 30 mL of fibroblast medium.
Plate the cells equally distributed onto two Matrigel-coated 100 mm plates (5.5 × 105 cells); name the plates Plate 1 and Plate 2.
Day 1: Change medium on both plates to fibroblast medium with sodium butyrate (NaB, 200 μM) and L-ascorbic acid 2-phosphate (AA2-P, 64 μg/mL)
Day 3: Change medium on Plate 1 to fibroblast medium with NaB and AA2-P. Change medium on Plate 2 to 50 % fibroblast medium; 50 % E7 with NaB
Day 5: Change medium on Plate 1 to fibroblast medium with NaB and AA2-P. Change medium on Plate 2 to E7 medium with NaB.
Day 7 onwards: Change medium on Plate 1 (fibroblast medium with NaB and AA2-P) and plate 2 (E7 medium with NaB) every other day
Around day 9 onwards: After you recognize the first colonies, switch to E8 medium and change medium every day.
Around day 14–20, the hiPSC colonies should be big enough for manual picking under the microscope. Pick six individual hiPSC clones and transfer them into six different wells of a Matrigel-coated 12-well plate in E8 medium with 10 μM Y27632.
If you have problems detaching the hiPSC colonies from the surrounding fibroblasts, you can scrape the fibroblasts away to make space for the outgrowing hiPSC colony. After two days, the free hiPSC clone should be large enough for easy picking.
Once cells are picked they can be clump passaged with 0.5 mM EDTA, gradually increasing the split ratio at each passage from 1:3 to 1:12 (see Note 2).
3.4 Long-Term Culture of hiPSCs
Ideally cells should have reached 75–95 % confluence in 4 days. Adjust split ratio ~1:6 to 1:12 to achieve this, as higher split ratios (1:12) will result in more efficient differentiations.
Aspirate culture medium from well.
Add ~950 μL of 0.5 mM EDTA per well, incubate for 6 min at RT (in hood)
During this time, set up a 50 mL Falcon tube with E8T (i.e., for a 1:12 split add 24 mL for 1 well).
Remove EDTA from the well with a P1000 tip, eject tip.
Using a P1000 tip, add 1 mL of the E8T medium from the Falcon tube containing E8T and blast against cell surface to dissociate cells. Cells should come off easily. Turn plate 180° to detach all cells. Transfer cell suspension to the Falcon tube containing E8Y.
Aspirate un-gelled Matrigel from Matrigel-coated 6-well plate.
Invert capped E8T tube containing cells to mix and plate out cells at 2 mL per well. Use no larger than a 10 mL pipette to improve consistency in number of cells per well.
After 24 h, aspirate used medium and replace with E8.
Repeat media change at 48 and 72 h.
At 96 h either passage cells or begin differentiation (see Note 3).
Staining of hiPSCs for pluripotency markers can be performed as described in the Life Technologies Pluripotent Stem Cell 4-Marker Immunocytochemistry Kit.
3.5 Cryopreservation of hiPSCs
When cells are 75–95 % confluent (96 h), aspirate culture medium from well, add ~950 μL of 0.5 mM EDTA per well, and incubate for 6 min at RT (in hood).
Using a P1000 tip, add 1 mL of cold (4 °C) Bambanker and blast against cell surface to dissociate cells. Cells should come off easily.
Turn plate 180° to detach all cells. Aliquot cells at 1 mL per cryovial.
Transfer vials to CoolCell and place at −86 °C overnight.
Transfer vials to liquid nitrogen tank.
3.6 Cardiac Differentiation
Grow hiPSCs to 80 % confluence in 96 h as described above (see Note 4).
Day 0: Aspirate medium in wells and replace with 2 mL per well of Cardiomyocyte Differentiation Medium A (Fig. 2).
Day 2 Aspirate medium in wells and replace with 2 mL per well of Cardiomyocyte Differentiation Medium B.
Day 4 then every other day up to day 15: aspirate medium in wells and replace with 2 mL per well of Cardiomyocyte Maintenance Medium.
Contracting cardiomyocytes should appear from day 9 to 11 of differentiation.
Cardiomyocytes can be purified using metabolic selection by replacing the Cardiomyocyte Maintenance Medium with RPMI 1640 no glucose supplemented with 2 % B-27 for day 10 to day 15.
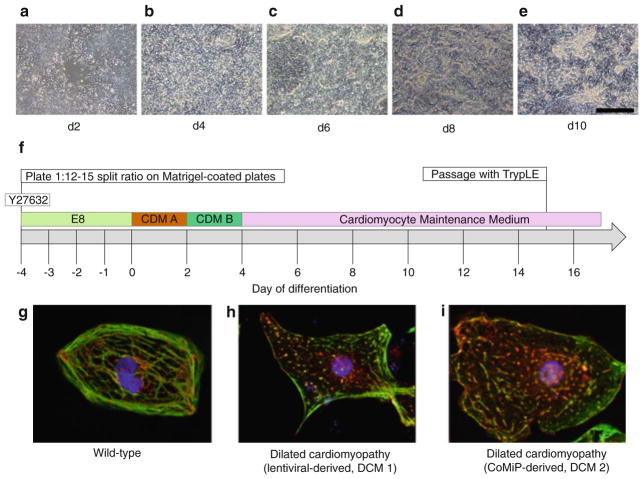
Fig. 2.
Stepwise protocol for the differentiation of human pluripotent stem cells into cardiomyocytes (hiPSC-CMs). (a–e) Phase-contrast images of the five major stages of differentiation from the initial epithelial to mesenchymal transition through the acquisition of the refractory cardiomyocyte morphology. (f) Timeline of cardiac differentiation. Day of differentiation represents the days at which the medium is changed. (g–i) Immunofluorescent images of cardiomyocytes from three hiPSC lines stained for the cardiomyocytes structural markers TNNT2 (troponin T, green) and ACTN2 (α-actinin, red ). (g) Cardiomyocytes from a wild-type hiPSC line demonstrating aligned sarcomeres. (h) Cardiomyocytes from a hiPSC line (DCM 1) derived from a DCM patient with a MYH6 mutation, which we reprogrammed using lentivirus (as published in ref. (7)) showing punctate, non-aligned TNNT2 staining. (i) Cardiomyocytes from the same fibroblast cell line as DCM 1 that are reprogrammed using the non-integrating CoMiP (DCM 2), showing similar punctate, non-aligned TNNT2 staining. Scale bar, 200 μm
3.7 Dissociation of Cardiomyocytes
Aspirate medium from wells.
Add 1 mL of TrypLE Express and incubate for 10–15 min at 37°C.
Pipette up and down with a P1000 to dislodge cells and to break up aggregates. Avoid forming bubbles.
Transfer cells into a 15 mL Falcon tube and top up with Cardiomyocyte Maintenance Medium. Centrifuge at 200 × g for 5 min.
Resuspend in 1 mL Cardiomyocyte Maintenance Medium and pipette up and down 10 times to release single cells, top up to ~ >1 × 106 cells/mL. One well of a 6-well plate will provide ~1–3 × 106 cells.
Pass through a 100 μm cell strainer into a 50 mL Falcon tube.
Count cells with Automated Cell Counter.
Dilute to 1 × 106 per mL.
Plate cells at 4 × 104 cells/cm2 on Matrigel-coated 8-chamber coverslips.
3.8 Calcium Imaging
Wait for cells to begin contraction, around 3–5 days.
Treat with 5 μM Fluo-4 AM and 0.02 % Pluronic F-127 in Tyrode’s solution for 5 min at 37 °C.
Wash with Tyrode’s solution.
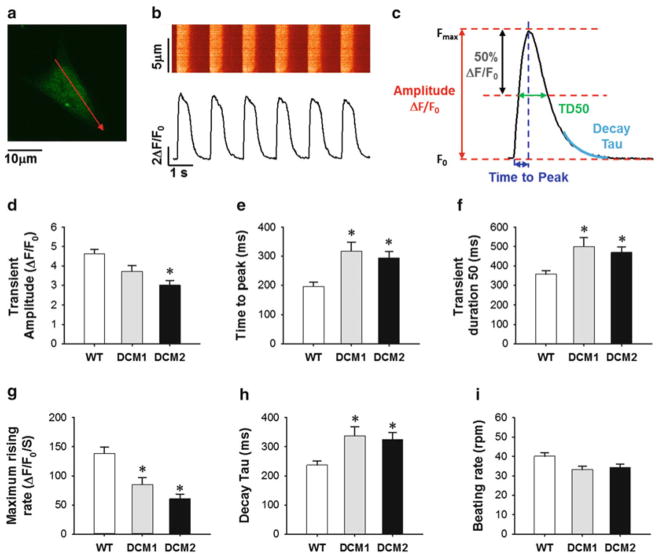
Conduct Ca2+ imaging using Carl Zeiss LSM 510 Meta confocal microscope with an oil immersion 63× objective (Plan-Apochromat 63× /1.40 Oil DIC M27) and analyze using Zen imaging software. Record spontaneous Ca2+ transients at 37°C using a single-cell line scan mode (see Note 5) (Fig. 3). For analysis of the data, subtract extracellular background signal from calcium signals and normalize the calcium signal to the intracellular basal line (F0). Transient amplitude is expressed as ΔF/F0. Decay Tau (mS) is calculated by mono-exponential curve fitting.
Fig. 3.
Comparison of calcium handling properties between wild type (WT) and dilated cardiomyopathy (DCM) hiPSC-CMs demonstrating that lentiviral- and CoMiP-derived hiPSCs from the same DCM patient produce cardiomyocytes with similar functional abnormalities. (a) Line-select of time-lapse calcium imaging. (b) Representative recording of spontaneous calcium transient. (c) Measurement of calcium handling parameters from recording data. (d–i) Comparison of calcium transient amplitude, time to peak, transient duration 50, maximum rising rate, decay Tau, and beating rate among WT (n = 53), DCM1 (n = 30), and DCM2 (n = 35) hiPSC-CMs. *P < 0.05 vs. WT group by Student’s T-test
Acknowledgments
This work was supported by the American Heart Association Beginning Grant-in-Aid 14BGIA20480329 and US National Institutes of Health K99 HL121177 to P.W.B.; American Heart Association Predoctoral Fellowship 13PRE15770000, National Science Foundation Graduate Research Fellowship Program DGE-114747 to A.S.; American Heart Association Established Investigator Award 14420025, Foundation Leducq, the National Institutes of Health U01 HL099776, P01 GM099130, R01 HL113006, R01 HL123968, and R24 HL117756 to J.C.W.
Footnotes
Successful isolation should yield 10 vials of passage 2 fibroblasts suitable for reprogramming.
Typically, the hiPSC reprogramming process will take up to 1 month. After hiPSC colonies are obtained, they should be passaged up to 20 times before optimal differentiation can occur. This may take an additional 2 months. The cardiac differentiation process will produce beating cardiomyocytes after about 10 days.
For ideal differentiations, culture hiPSCs to at least passage 20. For most lines, there may be a passage “window” for optimal differentiation. This will allow for sustained, continuous, high-quality differentiations for many passages. This passage “window” likely varies from line to line. Continue passaging hiPSCs until high-efficiency differentiations (greater than 80 % cardiomyocytes) are obtained.
During cardiac differentiation, initial hiPSC monolayer confluency is a key factor. For most lines, hiPSC monolayers must be between 60 and 90 % confluent at the start of differentiation. This is somewhat variable from line to line, as lower or higher confluence may be required during differentiation. However, allowing hiPSCs to grow to full confluency during maintenance prior to cardiac differentiation is not recommended, as this may reduce the efficiency of differentiation.
Age of cardiomyocytes upon calcium imaging: cell characteristics may be dependent on maturation level or disease progression.
References
- 1.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsa E, Burridge PW, Wu JC. Human stem cells for modeling heart disease and for drug discovery. Sci Transl Med. 2014;6(239):239ps236. doi: 10.1126/scitranslmed.3008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Marceau C, Hamaguchi R, Burridge PW, Rajarajan K, Churko JM, Wu H, Sallam KI, Matsa E, Sturzu AC, Che Y, Ebert A, Diecke S, Liang P, Red-Horse K, Carette JE, Wu SM, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ Res. 2014;115(6):556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diecke S, Lu J, Lee J, Termglinchan V, Kooreman NG, Burridge PW, Ebert AD, Churko JM, Sharma A, Kay MA, Wu JC. Novel codon-optimized mini-intronic plasmid for efficient, inexpensive, and xeno-free induction of pluripotency. Sci Rep. 2015;5:8081. doi: 10.1038/srep08081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4(130):130–147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]