Abstract
Zirconium-89 has an ideal half-life for use in antibody-based PET imaging; however, when used with the chelator DFO, there is an accumulation of radioactivity in the bone, suggesting that the 89Zr4+ cation is being released in vivo. Therefore, a more robust chelator for 89Zr could reduce the in vivo release and the dose to nontarget tissues. Evaluation of the ligand 3,4,3-(LI-1,2-HOPO) demonstrated efficient binding of 89Zr4+ and high stability; therefore, we developed a bifunctional derivative, p-SCN-Bn-HOPO, for conjugation to an antibody. A Zr-HOPO crystal structure was obtained showing that the Zr is fully coordinated by the octadentate HOPO ligand, as expected, forming a stable complex. p-SCN-Bn-HOPO was synthesized through a novel pathway. Both p-SCN-Bn-HOPO and p-SCN-Bn-DFO were conjugated to trastuzumab and radiolabeled with 89Zr. Both complexes labeled efficiently and achieved specific activities of approximately 2 mCi/mg. PET imaging studies in nude mice with BT474 tumors (n = 4) showed good tumor uptake for both compounds, but with a marked decrease in bone uptake for the 89Zr-HOPO-trastuzumab images. Biodistribution data confirmed the lower bone activity, measuring 17.0%ID/g in the bone at 336 h for 89Zr-DFO-trastuzumab while 89Zr-HOPO-trastuzumab only had 2.4%ID/g. We successfully synthesized p-SCN-Bn-HOPO, a bifunctional derivative of 3,4,3-(LI-1,2-HOPO) as a potential chelator for 89Zr. In vivo studies demonstrate the successful use of 89Zr-HOPO-trastuzumab to image BT474 breast cancer with low background, good tumor to organ contrast, and, importantly, very low bone uptake. The reduced bone uptake seen with 89Zr-HOPO-trastuzumab suggests superior stability of the 89Zr-HOPO complex.
Graphical Abstract
INTRODUCTION
Antibodies possess exquisite specificity and affinity for their antigens,1 and as a consequence, positron emission tomography (PET) using targeted antibodies is a molecular imaging technique at the forefront of cancer diagnosis and treatment management.1–6 Zirconium-89 (89Zr), a positron-emitting radionuclide, possesses excellent physical properties for PET imaging when paired with antibodies, namely, an ideal 78.41 h half-life and low energy positron (βavg = 395.5 keV), and is readily attracting attention for this purpose.7–14 In the past several years, a wide variety of preclinical studies have been published15–21 and a number of 89Zr-based antibody imaging agents have been translated into the clinic, including a number of current clinical trials in the US alone.2–4,22–25
These clinical studies and all preclinical studies use the current standard bifunctional chelator for 89Zr: desferrioxamine B (DFO).16 DFO, a natural bacterial siderophore, is a hexadentate ligand with three hydroxamate groups which provide six oxygen donors for metal binding.26 It possesses an amine tail that can be derivatized for facile conjugation to antibodies and other biomolecular vectors. Although image quality is generally very good, DFO is not the optimal ligand for 89Zr. This is revealed by the observed uptake of radioactivity in the bone.7,16,27,28 This uptake is evidence of in vivo release of 89Zr4+ from the chelator. When unbound, the osteophilic 89Zr4+ cation is readily mineralized into the skeleton.28,29 This accumulation of 89Zr4+ in the bone can dramatically increase radiation dose to the bone marrow, an especially radiosensitive tissue. While the extent of this uptake is less established in the clinic, it is still being investigated and may be of particular concern since 89Zr-immunoPET agents have found specific use in the detection of bone metastasis.30 This concern over in vivo stability sparks the need to develop an improved bifunctional chelator for Zr that will significantly improve 89Zr-antibody PET imaging by providing an improved alternative to DFO, reducing absorbed doses to healthy tissues and therefore safer PET imaging, and enhanced image quality.
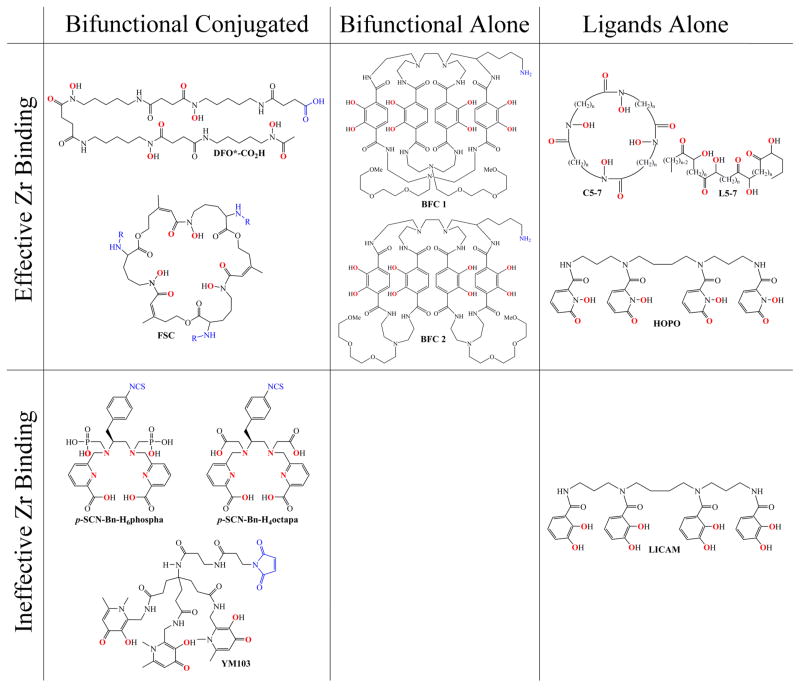
Recently, there has been a surge of interest in the development of an alternative chelator for 89Zr4+ to replace DFO, with several novel ligand systems being reported within the past year or so (Figure 1).31–37 While multiple studies demonstrate the issue of bone uptake seen with 89Zr-DFO complexes and stress the need for improved chelators,16,27,28,34 the first investigation toward designing a better chelator of Zr4+ came from Guérard et al.38 This work examined the coordination chemistry of the Zr4+ cation and confirmed the advantage of an octa-coordinate zirconium complex as Zr4+ was shown to preferentially form complexes with eight oxygen donors contained within four bidentate hydroxamate groups. This study opened the door for the investigation of octadentate ligands to replace the hexadentate DFO chelator with the goal of improving in vivo stability. Thus, far, however, there has been no reporting of a new ligand for 89Zr4+ that has been demonstrated to be viable in vivo for a sufficient length of time for antibody imaging. Several potential ligands require additional development while others simply require further evaluation. Herein, we present the first successful demonstration of an alternative chelator for 89Zr that includes PET imaging and biodistribution data that shows improved stability over DFO across a period of several days in vivo.
Figure 1.
Chemical structures of newly developed ligands evaluated with 89Zr4+. The binding donor groups are highlighted in red and the conjugation points of the bifunctional ligands are shown in blue. The molecules are divided by their progress in development as monofunctional ligand which cannot be attached to a targeting vector, bifunctional ligands that have yet to be attached to a targeting vector, and bifunctional chelators which have been shown to bind a metal and have been conjugated to a targeting molecule. The ligands are further divided between those that were found to effiectively bind Zr and those that were found to be unsuitable.
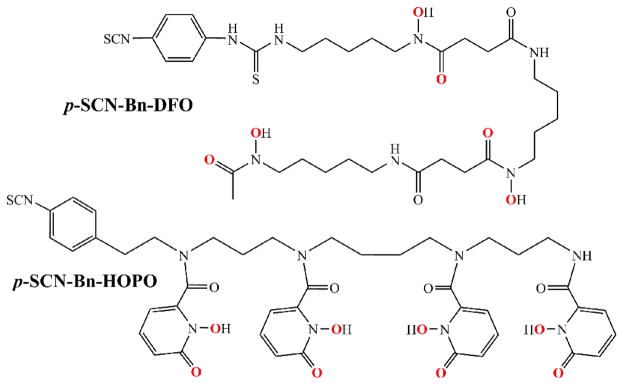
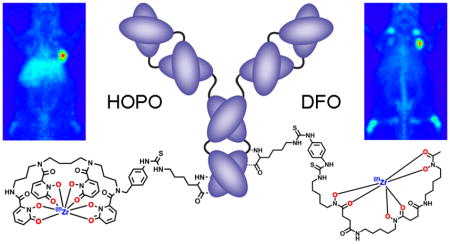
We investigated the potential of a nonhydroxamate-based ligand—3,4,3-(LI-1,2-HOPO) or HOPO—which has four 1,2-hydroxypyridinone groups for metal binding and comes from the actinide sequestration literature.39 As we postulated, the HOPO ligand labeled efficiently and 89Zr-HOPO exhibited equal or superior stability compared to 89Zr-DFO in all chemical and biological assays.34 Not only did the 3,4,3-(LI-1,2-HOPO) ligand show tremendous promise in our preliminary evaluation, but even more recently, stability constants for Zr-HOPO were determined to be on the order of log β = 43, the highest recorded for any Zr complex which attests to the superior stability.40 Therefore, we endeavored to develop a bifunctional variant of the HOPO ligand for further evaluation and application in antibody-based PET imaging. The result of this venture is the bifunctional chelator: p-SCN-Bn-HOPO (Figure 2). This molecule is the HOPO ligand with a para-benzyl-isothiocyanate pendant arm added to one of the secondary amides in order to be directly comparable with the currently most used bifunctional chelator: p-SCN-Bn-DFO (Figure 2). We also report the crystal structure of Zr-HOPO which corroborates the high stability.
Figure 2.
Chemical structures of the current standard bifunctional chelator, p-SCN-Bn-DFO, and our proposed alternative chelator, p-SCN-Bn-HOPO, based on the previously described 3,4,3-(LI-1,2-HOPO) ligand. The binding oxygen donor groups are highlighted in red.
RESULTS AND DISCUSSION
Synthesis and Characterization
Zr-HOPO Crystal Structure
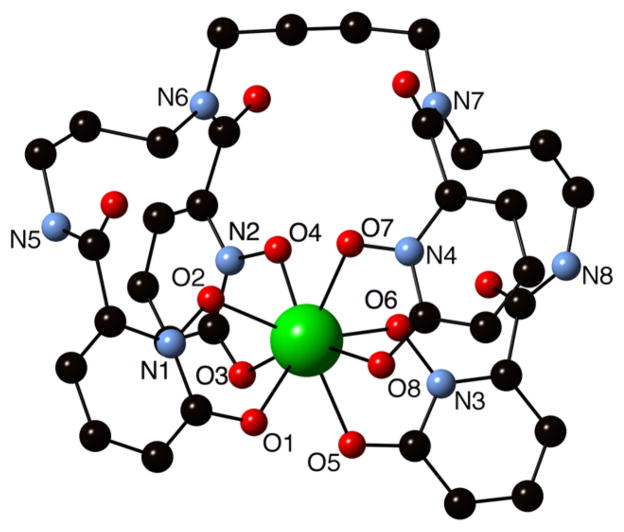
Our past work demonstrated the stability of the Zr-HOPO complex in vitro, in vivo, and in silico, which led to the advancement of the HOPO ligand into a bifunctional chelator. However, efforts were also made to confirm the calculated structure. These efforts came to fruition with the successful crystal growth and crystal structure determination of the Zr-HOPO complex (Figure 3). This structure confirmed that the central Zr4+ ion is bonded to eight oxygen donor atoms from the four 1,2-HOPO units to form a neutral complex. The immediate coordination geometry about the Zr is identical to that of the crystal structure reported by Guérard et al. of Zr(Me-AHA)4, where Zr is chelated by four bidentate N-methyl acetohydroxamic acid groups.38 The bond lengths of Zr-HOPO and Zr(Me-AHA)4 are similar as well (Table S.1). Figure S.1 shows the variation of bond lengths in these two crystal structures and in the DFT calculated structure of Zr-HOPO (Zr-HOPOcalc).34
Figure 3.
Crystal structure of the Zr-HOPO complex. The amide nitrogen atoms connecting the 1,2-HOPO moieties to the spermine backbone, the oxygen donor groups from the 1,2-HOPO units, and nitrogen atoms from the hydroxypyridinone groups are labeled.
The Zr-HOPOcalc structure is more contorted and possesses slightly longer Zr–O bond lengths than the single crystal structure (Figure S.2). Optimization of the structure in the gas phase showed several local energy minima, and the lowest energy structure possesses an unfavorable gauche orientation of the –(CH2)4– linker between the middle two chelating groups of the HOPO ligand. In contrast, the Zr-HOPO crystal structure displays an open orientation of the –(CH2)4– linker between the middle two chelating groups. These features point to the flexibility of HOPO backbone and versatility toward metal coordination. The differences in overall structure may likely be a result of crystal packing forces, which the calculations do not take into account. A detailed comparison of the Zr-HOPO crystal structure to the DFT structure as well as to a recently published Eu-HOPO− structure41 is included in the Supporting Information.
Bifunctional Ligand Synthesis
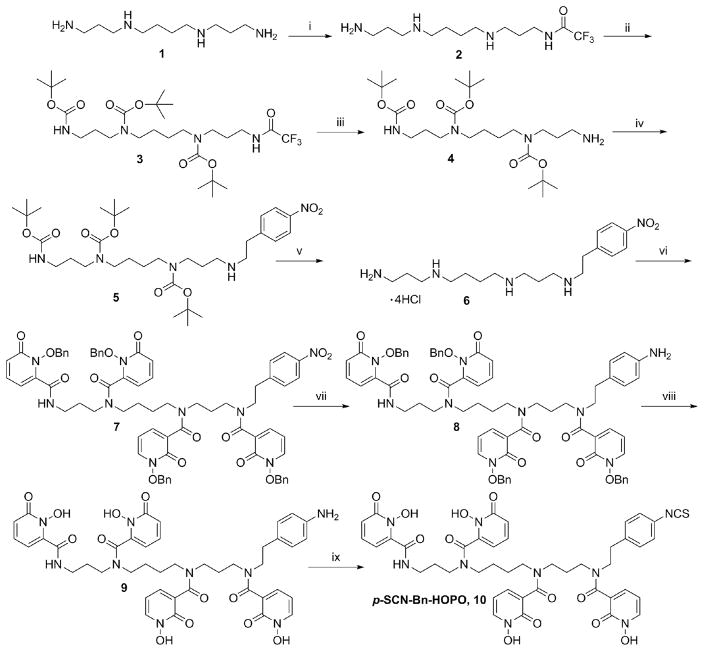
Initial attempts were made to attach a linker arm directly to one of the secondary amides of the original 3,4,3-(LI-1,2-HOPO) ligand in order to make it bifunctional; however, our efforts were unsuccessful. Coupling to a single secondary amide proved to be impractical and so we developed a novel synthesis to rebuild the ligand from scratch (Scheme 1). The new method built the pendant arm into the backbone itself before coupling the hydroxypyridinone groups onto it. The synthesis of the bifunctional chelator proved to be challenging, with a particular difficulty in the deprotection and purification steps, but it was ultimately achieved. The final product, p-SCN-Bn-HOPO, was purified by HPLC and characterized by NMR, IR, and HRMS.
Scheme 1. Synthetic Scheme for p-SCN-Bn-HOPOa.
a(i) Ethyl trifluoroacetate, MeOH, −78 °C, 1 h; (ii) (BOC)2O, MeOH, r.t, 18 h; (iii) conc. NH4OH, r.t, 15 h (28% over 3 steps); (iv) 4-Nitro phenylethyl bromide, K2CO3, DMF, 60 °C, 12 h, 38%; (v) 4 M HCl in Dioxane, r.t, 2 h; (vi) 1-(benzyloxy)-6-oxo-1,6-dihydropyridine-2-carboxylic acid chloride, NEt3, DCM, 0–25 °C, 12 h, 56% (over 2 steps); (vii) Raney Ni, H2, MeOH, 3 h; (viii) 1:1 (AcOH: HCl), 50 °C; (ix) di-2-pyridyl thiocarbonate, NEt3, CH3CN, H2O, r.t, 1 h.
Ligand–Antibody Conjugation
p-SCN-Bn-DFO was conjugated to antibodies through the formation of a thiourea bond with the amine side chain of a lysine residue. The p-SCN-Bn-HOPO ligand was designed to be attached in the very same way. Both ligands were conjugated to trastuzumab at a ratio of 5:1 ligand:antibody in the reaction mixture. The average number of chelates per antibody was determined to be 2.0 ± 0.5 for p-SCN-Bn-DFO and 2.8 ± 0.2 for p-SCN-Bn-HOPO through a simplified isotopic dilution assay.
Radiolabeling
All compounds were radiolabeled under mild conditions using a 89Zr-oxalate solution at pH 7 and room temperature. Reaction progress was monitored using radio-TLC. First, the bifunctional chelators p-SCN-Bn-HOPO and p-SCN-Bn-DFO were radiolabeled on their own without being attached to any targeting vectors to compare their Zr binding ability. Both ligands labeled quantitatively within 1 h without issues. This confirmed that the benzyl isothiocyanate linker arm did not interfere with the metal binding. Next, the chelator-modified trastuzumab complexes were radiolabeled under the same conditions. Both complexes labeled within 1–3 h at room temperature and achieved specific activities of approximately 2 mCi/mg. Radiolabeled antibody conjugates were purified via size exclusion chromatography and spin filtration.
Serum Stability
The 89Zr-ligand complexes alone as well as the 89Zr-ligand-antibody complexes were evaluated for stability in human serum at 37 °C. Both 89Zr-ligand complexes showed great stability with 97.7 ± 0.2% of the p-SCN-Bn-DFO complex and 97.5 ± 0.5% of the p-SCN-Bn-HOPO complex intact after 7 d. When the ligands were conjugated to trastuzumab and then labeled, both complexes demonstrated slight decreases in stability, with the 89Zr-DFO-tratuzumab complex showing 94.7 ± 0.7% stability and the 89Zr-HOPO-tratuzumab complex showing 89.2 ± 0.9% stability after 7 d. While 89.2% for the HOPO complex is still reasonably stable, it is notably less than the 94.7% stability of the DFO conjugate. The reason for the change in stability between the 89Zr-ligand complexes and the 89Zr-ligand-antibody complexes is currently unknown, but may be due to the influence of the antibody side chains altering the chelation environment of the metal either during radiolabeling or during the serum incubation.
Immunoreactivity
The viability of the 89Zr-labeled trastuzumab complexes was assayed against BT474 cells to ensure that the conjugation of the chelators did not disrupt the biological activity of the antibody. The 89Zr-DFO-trastuzumab and 89Zr-HOPO-trastuzumab conjugates were found to have immunoreactive fractions of 88.6 ± 2.1% and 92.4 ± 6.8%, respectively.
In Vivo Studies
Imaging
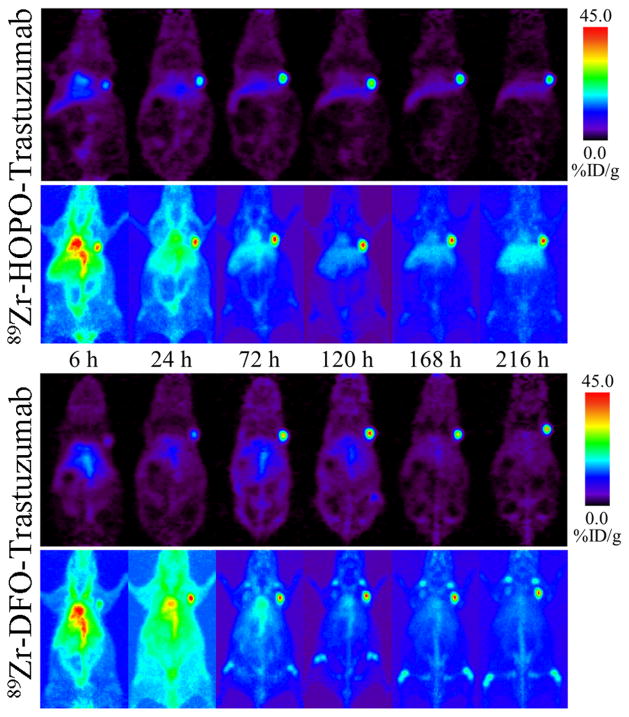
PET imaging was carried out in order to directly compare the in vivo behavior and pharmacokinetics of the DFO- and HOPO-based 89Zr-trastuzumab radioimmunoconjugates. Female athymic nude mice with subcutaneous BT474 xenografts in their right shoulders were injected with either 89Zr-DFO-trastuzumab or 89Zr-HOPO-trastuzumab (n = 4 for each compound) and imaged over 9 d. The resulting images showed good tumor uptake for both compounds, but with a marked decrease in the appearance of bone uptake for the 89Zr-HOPO-trastuzumab images (Figure 4). While the liver is more visible in the 89Zr-HOPO-trastuzumab images, particularly the maximum intensity projections, this may be due to how the images are scaled individually and not directly comparable in terms of intensity. The reduced bone uptake seen with 89Zr-HOPO-trastuzumab suggests superior stability of the 89Zr-HOPO complex. The difference in in vivo performance in contrast to the in vitro stability study highlights the inadequacy of the serum stability assay alone. This demonstrates the successful use of 89Zr-HOPO-trastuzumab to image BT474 breast cancer with low background, good tumor to organ contrast, and, importantly, very low bone uptake.
Figure 4.
PET images of 89Zr-HOPO-trastuzumab (top) and 89Zr-DFO-trastuzumab (bottom) in female athymic nude mice with BT474 xenografts on their right shoulders (9.25–9.99 MBq [250–270 μCi] in 200 μL 0.9% sterile saline). Representative images are shown for each compound following a single mouse over 9 d with coronal slice images above the corresponding maximum intensity projection images. Both compounds show good tumor to background contrast, but the 89Zr-DFO-trastuzumab shows evidence of bone uptake suggesting in vivo release of 89Zr4+.
Biodistribution
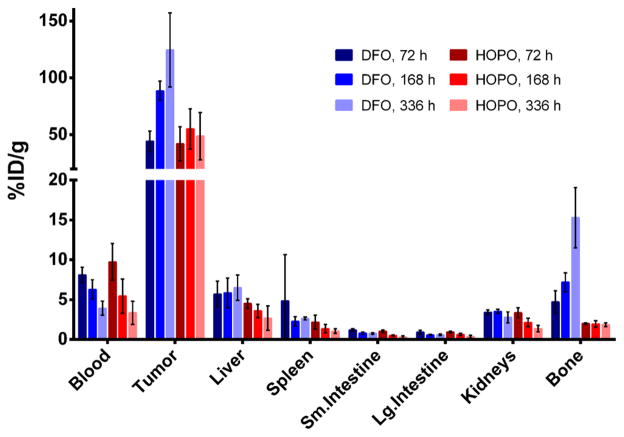
Acute biodistribution experiments were performed to further probe the localization and uptake of 89Zr-DFO-trastuzumab and 89Zr-HOPO-trastuzumab. These results corroborate the observations from the PET images with the activity associated with all collected tissues, except the tumors and the bone, decreasing over time (Figure 5). The biodistribution data reveals the liver uptake to be essentially the same for both compounds which suggests that the difference in appearance seen in the images is due to differences in scaling rather than a difference in actual uptake. Both compounds showed good uptake in the tumor with the DFO complex achieving even higher uptake than the HOPO compound (138.2 ± 35.3 vs 61.9 ± 26.4%ID/g at 336 h, Table 1). The difference in tumor uptake between the two compounds is not easily understandable as the immunoreactivity was not significantly different and they are using the same targeting method. Biodistribution data confirmed the lower bone activity of the HOPO conjugate, measuring 17.0 ± 4.1%ID/g in the bone for the 89Zr-DFO-trastuzumab, while the 89Zr-HOPO-trastuzumab only had 2.4 ± 0.3%ID/g at 336 h. The amount of activity seen in the bone with 89Zr-HOPO-trastuzumab is consistently less than the residual blood activity, which means it is possible that there is no specific bone accumulation since the %ID/g values do not increase over time (Figure 6). This is particularly striking when compared with the constantly increasing bone uptake seen with 89Zr-DFO-trastuzumab, which is indicative of accumulation of 89Zr4+ in the skeleton.
Figure 5.
Selected biodistribution data of 89Zr-HOPO-trastuzumab (red) and 89Zr-DFO-trastuzumab (blue) in female athymic nude mice with BT474 xenografts (0.59–0.74 MBq [16–20 μCi] in 200 μL 0.9% sterile saline). Both compounds successfully target and accumulate in the BT474 tumors with good tumor to background contrast, but 89Zr-DFO-trastuzumab has ~2.2 times the absolute uptake in the tumor. The distribution pattern is very similar for all nontarget organs except for the bone, where 89Zr-DFO-trastuzumab has ~7 times the uptake in the bone. The 89Zr-DFO-trastuzumab mice show an increasing level of activity in the bone, suggesting in vivo release of 89Zr4+ and accumulation in the bone, whereas the 89Zr-HOPO-trastuzumab mice show only a low level of activity in the bone which is below the level of the blood and does not increase over time.
Table 1.
Biodistribution Data with Values Expressed as %ID/ga
| 24 h
|
72 h
|
120 h
|
168 h
|
216 h
|
336 h
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOPO | DFO | HOPO | DFO | HOPO | DFO | HOPO | DFO | HOPO | DFO | HOPO | DFO | |
| Blood | 13.6 ± 2.4 | 14.8 ± 1.4 | 12.5 ± 2.9 | 9.4 ± 1.0 | 8.9 ± 1.6 | 10.2 ± 0.8 | 6.9 ± 2.7 | 7.1 ± 1.4 | 3.5 ± 2.2 | 4.8 ± 0.9 | 4.3 ± 1.8 | 4.4 ± 0.9 |
| Tumor | 29.0 ± 11.4 | 22.4 ± 14.3 | 54.7 ± 19.5 | 51.4 ± 10.4 | 68.8 ± 18.8 | 95.0 ± 16.7 | 70.4 ± 23.5 | 99.1 ± 8.7 | 39.6 ± 21.2 | 74.9 ± 29.9 | 61.9 ± 26.4 | 138.2 ± 35.3 |
| Heart | 3.7 ± 0.4 | 3.9 ± 0.7 | 2.7 ± 0.5 | 3.7 ± 2.3 | 2.4 ± 0.5 | 3.0 ± 0.3 | 1.7 ± 0.6 | 2.0 ± 0.3 | 1.0 ± 0.4 | 1.4 ± 0.3 | 1.0 ± 0.4 | 1.4 ± 0.2 |
| Lungs | 5.9 ± 1.0 | 7.2 ± 1.6 | 6.0 ± 1.7 | 4.3 ± 2.2 | 4.6 ± 1.2 | 5.9 ± 0.8 | 3.7 ± 1.2 | 4.8 ± 1.0 | 1.7 ± 0.9 | 3.0 ± 0.4 | 2.1 ± 0.8 | 3.4 ± 1.0 |
| Liver | 5.2 ± 0.4 | 5.6 ± 1.1 | 5.8 ± 0.8 | 6.6 ± 1.9 | 9.2 ± 3.2 | 5.7 ± 0.5 | 4.5 ± 1.0 | 6.6 ± 2.1 | 4.7 ± 0.9 | 4.9 ± 2.2 | 3.4 ± 1.9 | 7.2 ± 1.8 |
| Spleen | 3.6 ± 1.2 | 2.8 ± 0.7 | 2.9 ± 1.1 | 2.3 ± 0.2 | 1.9 ± 0.2 | 3.3 ± 0.3 | 1.8 ± 0.7 | 2.6 ± 0.7 | 1.3 ± 0.3 | 2.9 ± 0.7 | 1.4 ± 0.4 | 3.0 ± 0.2 |
| Pancreas | 1.6 ± 0.1 | 1.5 ± 0.5 | 1.4 ± 0.4 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.4 ± 0.2 | 0.8 ± 0.4 | 1.0 ± 0.2 | 0.5 ± 0.3 | 0.9 ± 0.2 | 0.5 ± 0.2 | 0.8 ± 0.1 |
| Stomach | 0.8 ± 0.4 | 1.2 ± 0.2 | 0.6 ± 0.2 | 1.3 ± 0.6 | 0.5 ± 0.4 | 1.3 ± 0.4 | 0.6 ± 0.4 | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.7 ± 0.2 |
| Sm. Int. | 1.6 ± 0.4 | 2.1 ± 0.6 | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.4 | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.4 ± 0.2 | 0.8 ± 0.2 | 0.4 ± 0.2 | 0.9 ± 0.1 |
| Lg. Int. | 1.4 ± 0.6 | 1.2 ± 0.3 | 1.2 ± 0.1 | 1.1 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.1 |
| Kidneys | 4.4 ± 0.8 | 4.6 ± 0.4 | 4.4 ± 0.8 | 4.0 ± 0.3 | 3.4 ± 0.5 | 4.3 ± 0.2 | 2.7 ± 0.7 | 4.0 ± 0.3 | 1.9 ± 0.6 | 2.6 ± 0.3 | 1.8 ± 0.5 | 3.1 ± 0.8 |
| Muscle | 1.3 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.0 ± 0.1 | 0.8 ± 0.3 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.5 | 0.4 ± 0.1 | 0.6 ± 0.1 |
| Bone | 2.6 ± 0.6 | 2.4 ± 0.7 | 2.7 ± 0.1 | 5.5 ± 1.7 | 2.0 ± 0.2 | 6.1 ± 0.7 | 2.5 ± 0.5 | 8.1 ± 1.4 | 2.5 ± 0.3 | 10.7 ± 1.3 | 2.4 ± 0.3 | 17.0 ± 4.1 |
| Tail | 2.9 ± 0.6 | 2.4 ± 0.9 | 2.2 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.5 | 1.8 ± 0.4 | 1.1 ± 0.4 | 1.7 ± 0.4 | 0.9 ± 0.3 | 1.5 ± 0.2 |
Studies were performed in BT474 tumor-bearing female athymic nude mice administered 89Zr-HOPO-trastuzumab (0.59–0.67 MBq [16–18 μCi] in 200 μL 0.9% sterile saline) or 89Zr-DFO-trastuzumab (0.67–0.74 MBq [18–20 μCi] in 200 μL 0.9% sterile saline) via intravenous tail vein injection (n = 4 per group).
Figure 6.
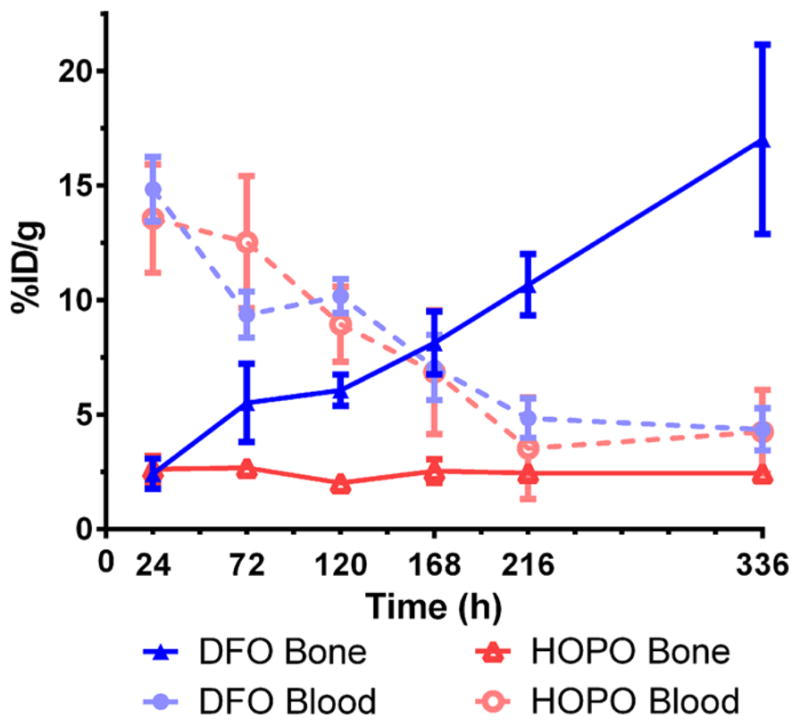
Comparison of the levels of radioactivity in the blood and bone of BT474 tumor-bearing female, athymic nude mice after injection of either 89Zr-HOPO-trastuzumab (red) or 89Zr-DFO-trastuzumab (blue) (0.59–0.74 MBq [16–20 μCi] in 200 μL 0.9% sterile saline). While the activity in the blood starts high and decreases over time, the activity in the bone is very different for the two compounds. The 89Zr-HOPO-trastuzumab mice show a near-constant level (~2.5%ID/g) of activity in the bone which never exceeds the level of activity remaining in the blood. The 89Zr-DFO-trastuzumab mice show an increasing amount of radioactivity in the bone over time, which far surpasses the level in the blood and suggests release of the 89Zr4+ cation in vivo which is known to preferentially accumulate in the bone.
While 89Zr-DFO-trastuzumab has a better tumor:blood ratio than 89Zr-HOPO-trastuzumab (31.4 vs 14.4 at 336 h, respectively), the 89Zr-HOPO-trastuzumab complex has a drastically improved tumor:bone ratio of 25.8 at 336 h compared to that of 89Zr-DFO-trastuzumab (8.1). Both compounds show a high contrast between the tumor and the general background as represented by the blood activity, but 89Zr-HOPO-trastuzumab provides a much better contrast between the tumor and the bone specifically. This benefit of the improved stability of the p-SCN-Bn-HOPO ligand could make a meaningful difference in clinical imaging by enabling easier distinction of bone metastasis.
Comparison with Other Ligands
The search for an alternative chelator for zirconium has led to a number of novel ligand systems based on hydroxamates, picolinates, various hydroxypyridinones, catechols, and terephthalamide groups. Thus, far, however, no single chelator has been fully tested and found to be an improvement over DFO in the context of actual antibody-based long imaging. Several potential ligands have been proposed, but each still requires either further development or evaluation as described below.
Hydroxamate-Based Chelators
These have been a major focus in the development of a new ligand since DFO itself contains such groups. Most recently, Zhai et al.37 have reported a competitive hexadentate ligand based on a derivatized fusarinine C (FSC) molecule (Figure 1). This is a cyclic ligand with a hydroxamate structure very similar to DFO. FSC was conjugated to an RGD peptide and showed improved stability over DFO.37 The prepared 89Zr-FSC-RGD complexes demonstrated improved stability over 89Zr-DFO-RGD in in vitro transchelation studies (93.9% stability vs 42.2%, respectively). The cyclic nature of the ligand likely provides greater kinetic stability as it is more difficult for competing materials to access the metal. However, providing only six donor groups for the binding of Zr4+ allows for the possibility of additional molecules being needed to satisfy the coordination sphere. Currently, the 89Zr-FSC-RGD complex has only been evaluated with short-term in vivo experiments (4 h biodistribution, 24 h PET image) which do not give a full picture of its long-term in vivo stability. Further experiments with longer circulating targeting molecules such as antibodies are necessary to reveal the practicality of this chelator.
The natural next step, to move toward octadentate hydroxamate-based ligands, has been taken by several groups as well. For example, a series of cyclic (C5–C7) and acyclic (L5–7) tetrahydroxamate ligands (Figure 1) were developed for evaluation with Zr4+. Of the synthesized ligands, the pair with the largest spacer, a seven carbon alkyl chain between hydroxamates, was found to be the best chelator both experimentally in comparison with DFO and computationally. The cyclic ligand C7 in particular showed promise in the stability of the resulting Zr complex, but further development to bifunctionalize the ligand is necessary to prove its utility. In a similar study, an octadentate derivative of DFO called DFO* (Figure 1), containing an additional hydroxamate group was also shown to chelate zirconium well.35 The complex of Zr4+ with this octadentate DFO* was predicted to be more stable than that with hexadentate DFO by DFT calculations. A bifunctional derivative, DFO*–CO2H, was also created and conjugated to bombesin for preliminary evaluation and was shown to be significantly more stable than 89Zr-DFO-bombesin in in vitro challenge studies over 24 h. No additional stability experiments were reported for longer time points or conditions. The potential of this chelator has yet to be determined in vivo or in serum, or in any experiments for longer than 24 h.
Nonhydroxamate-Based Chelators
These have also been investigated. H6phospa and H4octapa, a pair of octadentate picolinate-based N4O4 ligands (Figure 1), were evaluated for zirconium chelation, but found to be ineffective for radio-labeling with 89Zr.31 The reliance on nitrogen donors for the oxophilic Zr4+ cation was the most likely source of incompatibility.
YM103 (Figure 1) is a bifunctional isothiocyanate derivative of the hexadentate ligand CP256 which is made up of three 3-hydroxy-4-pyridinone (HPO) groups. YM103 was shown to bind 89Zr4+ and performed well in in vitro studies showing >95% stability in serum; however, the 89Zr-YM103-trastuzumab complex was demonstrated to be unstable in vivo with nearly 30%ID/g of the 89Zr localized in the bone. The release of the 89Zr4+ cation suggested by the high bone uptake is likely due to the lability of a six coordinate Zr complex.
A pair of nonhydroxamate-based ligands, abbreviated only as BFC 1 and 2 (Figure 1), containing four terephthalamide (TAM) binding groups in large dimacrocyclic structures with built-in amine pendant arms, as well as two short PEG units, were investigated as well.36 While both showed improved stability, BFC 1 was chosen as the preferred ligand due to its superior clearance profile. Follow-up studies will need to be done to evaluate the ability to conjugate this ligand onto a targeting vector and the long-term in vivo stability of the resulting immunoconjugate.
Additionally, our lab has investigated a catechol-based version of the HOPO ligand: 3,4,3-LICAM (LICAM) (Figure 1, previously unpublished), which was also taken from the actinide literature.39 This variant was ultimately found to be unsuitable for 89Zr4+ due to the incompatibility between the pKa of the catechol group and the solubility of the Zr4+ cation. Despite being octadentate and oxygen-rich, the maximum radiolabeling yield reached for the LICAM ligand was <30%, despite varying temperature, pH, and reaction time. We believe the issue to lie in the fact that the pKa for full deprotonation of the catechol group is 13.0, which requires a higher pH for proper labeling; however, the Zr4+ cation is only soluble at lower pH, and antibody radiolabeling is typically performed at pH ≈ 7. This leads to a precarious balance between keeping the 89Zr4+ in solution and trying to get the coordinating oxygen atoms of the catechol moiety deprotonated. Unfortunately, an appropriate compromise could not be found, so the ligand and catechol groups in general were dropped from further development.
The current study confirms the stability of the 1,2-hydroxypyridinone-based HOPO chelator and further presents a bifunctional ligand based on the HOPO scaffold. The work reported herein on p-SCN-Bn-HOPO represents the first evidence of a new ligand for 89Zr that demonstrates in vivo stability of an antibody-conjugated complex over an extended period of time.
CONCLUSIONS
The 3,4,3-(LI-1,2-HOPO) ligand was found to exhibit excellent stability for 89Zr complexes in a previous study.34 The development of a bifunctional derivative of the HOPO ligand was the next logical step and led to the design and synthesis of p-SCN-Bn-HOPO. This bifunctional ligand was shown to bind 89Zr as expected and was evaluated for conjugation to an antibody, radiolabeling of the ligand–antibody complex, and application of the radioimmunoconjugate in vivo. The p-SCN-Bn-HOPO was comparable to the standard p-SCN-Bn-DFO chelator, achieving specific activity ~2 mCi/mg and remaining ~90% stable through a 7 d incubation in human serum. The greatest distinction between the two compounds was the amount of bone uptake seen in the imaging and biodistribution experiments; the activity measured in the bone for 89Zr-HOPO-trastuzumab was more than 7 times lower than for 89Zr-DFO-trastuzumab. While the absolute uptake in BT474 breast cancer tumors was just over 2 times higher for 89Zr-DFO-trastuzumab, the tumor:bone ratio was more than 3 times higher for 89Zr-HOPO-trastuzumab. This improved contrast between tumor and bone could be advantageous for the detection of bone metastasis and for the general clarity of the images. Furthermore, the lower bone uptake is the ultimate proof that the p-SCN-Bn-HOPO ligand forms a more stable complex with 89Zr4+ than p-SCN-Bn-DFO and reduces the release of free 89Zr4+ in vivo.
The bifunctional chelator p-SCN-Bn-HOPO was shown to be an effective alternative for the chelation of 89Zr4+ for immunoPET applications. It successfully reduces the levels of radioactivity in the bones of mice compared with the use of p-SCN-Bn-DFO. This study not only led to a superior ligand for 89Zr, but also demonstrates the power of inorganic chemical principles to suggest the best chelation system for a metal which can actually be seen to make a difference in biological applications.
EXPERIMENTAL SECTION
Materials and Methods
All chemicals, unless otherwise noted, were acquired from Sigma-Aldrich (St. Louis, MO) and used as received without further purification. All instruments were calibrated and maintained in accordance with standard quality-control procedures. High-resolution mass spectrometry was carried out through electrospray ionization using an Agilent 6520 QTOF instrument. 1H and 13C NMR spectra were recorded at varying temperatures on a Bruker Avance III spectrometer equipped with a triple resonance inverse cryoprobe, with 1H and 13C resonance frequencies of 600.13 and 150 mHz or a Bruker DRX spectrometer equipped with a 1H, 13C cryoprobe, with respective resonance frequencies of 500.13 and 125.76 MHz with Topspin software. The NMR spectra are expressed on the δ scale and were referenced to residual solvent peaks and/or internal tetramethylsilane. The HPLC system used for analysis and purification of compounds consisted of a Rainin HPXL system with a Varian ProStar 325 UV–vis Detector monitored at 254 nm. Analytical chromatography was carried out using a Waters Symmetry C18 Column, 100 Å, 5 μm, 4.6 mm × 100 mm at a flow rate of 1.0 mL/min and purification was done with a preparatory Waters Symmetry C18 Prep Column, 100 Å, 5 μm, 19 mm × 100 mm at a flow rate of 17.059 mL/min. IR spectroscopy was performed on a solid sample using an attenuated total reflectance attachment on a PerkinElmer Spectrum 2 FT-IR spectrometer with a UATR Two attachment.
89Zr was produced at Memorial Sloan Kettering Cancer Center on a TR19/9 cyclotron (Ebco Industries Inc.) via the 89Y(p,n)89Zr reaction and purified to yield 89Zr with a specific activity of 196–496 MBq/mg. Activity measurements were made using a CRC-15R Dose Calibrator (Capintec). For the quantification of activities, experimental samples were counted on an Automatic Wizard (2) γ-Counter (PerkinElmer). The radiolabeling of ligands was monitored using salicylic acid impregnated instant thin-layer chromatography paper (ITLC-SA) (Agilent Technologies) and analyzed on a Bioscan AR-2000 radio-TLC plate reader using Winscan Radio-TLC software (Bioscan Inc., Washington, DC). All in vivo experiments were performed according to protocols approved by the Memorial Sloan Kettering Institutional Animal Care and Use Committee (protocol 08–07–013). Purity of greater than 95% was confirmed using quantitative HPLC analysis for nonradioactive compounds (HOPO and Zr-HOPO) and radio-TLC for radioactive compounds (89Zr-HOPO).
Crystal Structure
X-ray quality single crystals of Zr-HOPO were obtained from a reaction of ZrCl4 and HOPO in methanol, heating to 55 °C, and slowly cooling the reaction mixture over 2 days. A colorless plate-like crystal with approximate dimensions of 0.04 mm × 0.10 mm × 0.36 mm was selected for geometry and intensity data collection with a Bruker SMART APEXII CCD area detector on a D8 goniometer at 100 K. The temperature during the data collection was controlled with an Oxford Cryosystems Series 700+ instrument. Preliminary lattice parameters and orientation matrices were obtained from three sets of frames. Data were collected using graphite-monochromated and 0.5 mm-Mono-Cap-collimated Mo Kα radiation (γ= 0.71073 Å) with the ω scan method.42 Data were processed with the INTEGRATE program of the APEX2 software42 for reduction and cell refinement. Multiscan absorption corrections were applied by using the SCALE program for the area detector. The structure was solved by the direct method and refined on F2 (SHELX).43 Some solvent molecules, MeOH and H2O, which cocrystallize with the Zr-HOPO, are disordered. The constraints and restraints were applied to keep the geometries and atomic displacements of their groups close to the theoretical values. Non-hydrogen atoms in the whole structure were refined with anisotropic displacement parameters, and hydrogen atoms on carbons were placed in idealized positions (C–H = 0.95–1.00 Å) and included as riding with Uiso(H) = 1.2 or 1.5 Ueq(non-H). The hydrogen atoms on the oxygen and nitrogen atoms were refined isotropically with restrained O–H and N–H distances of 0.84 and 0.86 Å, respectively. The selected crystallographic parameters were listed in Table S.3. The crystallographic information file can be found in the Supporting Information.
Synthesis
Detailed analytical and spectral data are included in the Supporting Information.
(N1,N4,N9-Tri-tert-butoxycarbonyl)-1,12-diamino-4,9-dia-zadodecane (4)
The tri-BOC-protected spermine was prepared according to Geall et al.44 To a flask containing spermine (1) (2.02 g, 10 mmol) in 150 mL methanol at −78 °C under argon was added dropwise ethyl trifluoroacetate (1.42 g, 10 mmol) in 100 mL of methanol over 30 min while stirring. Stirring was continued for another 30 min and the reaction mixture was allowed to come to 0 °C. An excess of di-tert-butyl dicarbonate (60 mmol) in 100 mL methanol was added over a period of 1 h. The reaction mixture was stirred at room temperature for 18 h. Concentrated ammonium hydroxide solution was added to the reaction mixture until the pH reached 11 and the reaction was stirred for another 15 h at room temperature. The methanol was evaporated under reduced pressure and the resulting liquid was dissolved in methylene chloride, washed with water, dried over anhydrous sodium sulfate, and evaporated to dryness. The crude compound was purified by silica column chromatography using CH2Cl2:MeOH:conc NH3 70:10:1 to 50:10:1 (v/v/v) yielding the desired product 4 (yield 28%). Products 2 and 3 were used directly and not isolated.
1H NMR (CDCl3, 400 MHz): δ 3.07–3.21 (m, 10H), 2.68 (t, 2H), 2.08 (bs, 2H), 1.59–1.70 (m, 4H), 1.41–1.46 (m, 31H). 13C NMR (CDCl3, 100 MHz): δ 156.04, 155.55, 79.48, 78.88, 46.80, 43.79, 38.76, 37.35, 32.46, 30.9, 28.42. HRMS calculated for C25H50N4O6 ([M + H]+), 503.38, found 503.3817.
tert-Butyl(4-((tert-butoxycarbonyl)(3-((4-nitrophenethyl)-amino)propyl)amino)butyl) (3-((tert-butoxycarbonyl)-amino)propyl)carbamate (5)
A solution of 4-nitrophenylethyl bromide (0.126 g, 0.55 mmol) in DMF (2 mL) was added to a suspension of 4 (0.201 g, 0.5 mmol) and K2CO3 (0.138 g, 1 mmol) in DMF (5 mL) under N2. The resulting reaction mixture was stirred at 60 °C for 12 h. Solvent was removed under vacuum and the resulting residue was dissolved in methylene chloride, washed with water, dried over anhydrous sodium sulfate, and evaporated to dryness. The crude compound was purified by silica column chromatography using 1% methanol in methylene chloride to give compound 5 as a gummy solid. (Yield = 30%).
1H NMR (500 MHz, CDCl3): (mixture of rotamers) δ 8.10–8.09 (d, 2H), 7.36 (d, 2H), 3.28–2.58 (m, 20H), 1.80 (bs, 2H), 1.58 (bs, 2H), 1.38–1.36 (m, 27H). 13C NMR (500 MHz, CDCl3): (mixture of rotamers) δ 156.1, 155.9, 129.8, 123.9, 79.3, 78.7, 78.3, 49.85, 49.83, 49.80, 49.76, 49.72, 47.1, 47.0, 46.94, 46.91, 46.89, 46.86, 46.77, 46.72, 46.67, 46.60, 46.52, 46.46, 46.41, 46.38, 46.33, 46.27, 46.26, 46.23, 46.20, 45.69, 45.66, 44.28, 44.20, 43.88, 43.83, 43.78, 43.52, 43.47, 43.40, 43.38, 43.32, 37.76, 37.68, 37.64, 37.60, 37.56, 37.51, 37.48, 37.38, 34.45, 34.40, 34.15, 28.45; HRMS calculated for C33H57N5O8 ([M + H]+), 651.4207, found 652.4387.
N1-(3-Aminopropyl)-N4-(3-((4-nitrophenethyl)amino)-propyl)butane-1,4-diamine (6)
A solution of 4 M HCl in dioxane (5 mL) was added to a stirring solution of 5 (0.17g, 0.5 mmol) in CH2Cl2 (10 mL), under nitrogen, at 25 °C. After 2 h, the solution was concentrated in vacuo and co-distilled with toluene (3 × 5 mL) (poly-HCl salt). This compound was not isolated, but rather used directly in the next step.
1-(Benzyloxy)-N-(3-(1-(benzyloxy)-6-oxo-1,6-dihydropyri-dine-2-arboxamido)propyl)-N-(4-(1-(benzyloxy)-N-(3-(1-(benzyloxy)-N-(4-nitrophenethyl)-2-oxo-1,2-dihydropyridine-3-carboxamido)propyl)-2-oxo-1,2-dihydropyridine-3-carboxamido)butyl)-6-oxo-1,6-dihydropyridine-2-carboxa-mide (7)
A solution of 1-(benzyloxy)-6-oxo-1,6-dihydropyr-idine-2-carbonyl chloride (0.789g, 3 mmol) in methylene chloride (15 mL) was added dropwise to a stirred solution of triethylamine (0.835 mL, 6 mmol), 6 (0.351g, 1 mmol), and DMAP (0.006g, 0.05 mmol) in dry methylene chloride (10 mL) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 24 h. The reaction mixture was washed with 10% NaHCO3 solution, followed by water. The organic phase was dried over anhydrous Na2SO4 and was then removed with a rotary evaporator. The crude product was purified through column chromatography over silica gel using 2–4% methanol in dichloromethane eluent producing 5 as brown foam (yield 56%).
1H NMR (600 MHz, CDCl3): (mixture of rotamers) δ 8.16–7.25 (m, 20H), 7.19–4.86 (m, 16H), 3.82–2.06 (m, 25H), 1.84–0.63 (m, 8H); 13C NMR (600 MHz, CDCl3): (mixture of rotamers) δ 1420.16, 142.14, 139.12, 139.05, 139.0, 138.97, 138.87, 138.82, 138.78, 138.70, 138.66, 138.59, 138.55, 138.50, 133.16, 133.13, 133.06, 130.86, 130.80, 130.72, 130.66, 130.45, 135.41, 135.37, 130.34, 130.32, 130.29, 130.26, 130.23, 129.79, 129.76, 129.73, 129.70, 129.65, 129.62, 129.56, 123.97, 123.92, 123.83, 123.76, 123.74, 123.36, 122.79, 122.65, 122.57, 122.53, 122.52, 122.48, 104.07, 103.62, 103.54, 103.49, 103.40, 103.34, 79.66, 46.97, 46.08, 46.02, 41.99, 40.64, 36.95, 36.93, 36.89, 36.81, 34.61, 33.39, 33.23, 33.11, 26.09, 26.88, 25.52, 25.49, 25.32, 25.18, 25.11, 24.84, 24.80, 24.53, 24.17, 24.13, 23.98; HRMS calculated for C70H69N9O14 ([M + H]+), 1260.5042, found 1260.5038.
N-(4-(N-(3-(N-(4-Aminophenethyl)-1-(benzyloxy)-2-oxo-1,2-dihydropyridine-3-carboxamido)propyl)-1-(benzyloxy)-2-oxo-1,2-dihydropyridine-3-carboxamido)butyl)-1-(benzyl-oxy)-N-(3-(1-(benzyloxy)-6-oxo-1,6-dihydropyridine-2-carboxamido)propyl)-6-oxo-1,6-dihydropyridine-2-carboxa-mide (8)
To a suspension of Raney nickel in (1:1) MeOH:THF (10 mL), 7 (0.2 g, 0.157 mmol) was added and hydrogenated at balloon H2 pressure at room temperature for 3 h. The catalyst was filtered through a Celite pad under inert atmosphere. The filtrate was evaporated under reduced pressure to obtain the crude amine. This crude product was used directly in the next step without further workup. For characterization purposes, some of this product was HPLC purified as a cream colored solid.
1H NMR (600 MHz, DMSO-d6): (mixture of rotamers) δ 8.70–8.64 (m, 1H), 7.44–7.33 (m, 27H), 6.93–6.89 (m, 1H), 6.89–6.80 (m, 3H), 6.64–6.62 (m, 4H), 6.31–6.10 (m, 4H), 5.38–5.26 (m, 5H), 5.04–4.99 (m, 3H), 3.60–3.55 (m, 18H), 3.16–3.13 (m, 14H), 1.76–1.21 (m, 10H); 13C NMR (600 MHz, DMSO-d6): (mixture of rotamers) δ 158.77, 158.70, 158.28, 158.25, 158.18, 158.14, 155.63, 155.30, 155.27, 155.09, 155.06, 155.03, 141.89, 141.63, 141.60, 140.70, 140.59, 140.51, 136.82, 136.77, 136.64, 131.65, 161.41, 131.38, 131.35, 127.49, 127.44, 127.38, 127.29, 126.97, 126.87, 126.27, 126.23, 126.20, 120.31, 120.35, 119.75, 117.81, 115.46, 113.1, 101.62, 100.26, 100.1, 76.16, 76.10, 59.81, 52.71, 45.56, 45.49, 43.71, 43.47, 43.31, 41.3, 39.83, 39.65, 34.51, 34.45, 34.18, 34.11, 31.09, 29.71, 25.62, 24.36, 23.29, 22.73, 22.54, 22.43, 21.75, 21.38, 21.29; HRMS calculated for C70H72N9O12 ([M + H]+), 1230.5300, found 1230.5299.
4-(11,15-Bis(1-hydroxy-2-oxo-1,2-dihydropyridine-3-car-bonyl)-1-(1-hydroxy-6-oxo-1,6-dihydropyridin-2-yl)-6-(1-hy-droxy-6-oxo-1,6-dihydropyridine-2-carbonyl)-1-oxo-2,6,11,15-tetraazaheptadecan-17-yl)benzenaminium chloride (9)
Crude 8 was dissolved in a 1:1 mixture of acetic acid and concentrated HCl (6 mL) at room temperature and heated to 45–50 °C for 18 h. The reaction progress was monitored by LC-MS. The crude product was dried and redissolved in water/acetonitrile and purified by HPLC on a preparative C18 column (Waters Symmetry C18 Prep Column, 100 Å, 5 μm, 19 mm × 100 mm) at 17.059 mL/min using a gradient of 10–23% MeCN in water (both containing 0.1% TFA) with an initial hold at 10% MeCN for 1.33 min and then a ramp to 23% MeCN over 20 min. The product peak was collected from 6.45 to 8.26 min and the eluted solution was lyophilized to recover the product as an off-white solid. The purified ligand was collected in multiple small batches with an approximate combined yield of 70%.
The purified sample was confirmed by HPLC on an analytical C18 column (Waters Symmetry C18 Analytical Column, 100 Å, 5 μm, 4.6 mm × 100 mm) at 1 mL/min using a gradient of 10–30% MeCN in water (both containing 0.1% TFA) with an initial hold at 10% MeCN for 1.33 min and then a ramp to 30% MeCN over 30 min followed by a ramp to 95% MeCN over 1 min and an isocratic hold at 95% MeCN for 5.66 min.
1H NMR (500 MHz, CDCl3): (mixture of rotamers) δ 7.44–7.28 (m, 5H), 7.20–7.16 (dd, J1, J2 = 6 Hz, 1H), 7.08–7.01 (m, 2H), 6.54–6.53 (m, 4H), 6.33–6.32 (m, 3H), 6.18–6.21 (m, 0.5H), 5.74–5.67 (m, 0.5H), 3.65–3.51 (m, 4H), 3.51–3.16 (m, 8H), 3.12–2.70 (m, 11H), 1.91–1.38 (m, 10H) ; 13C NMR (500 MHz, CDCl3): (mixture of rotamers) δ 158.8, 158.6, 158.4, 158.1, 157.9, 157.8, 157.77, 157.73, 142.5, 142.49, 142.45, 142.41, 142.32, 142.24, 142.04, 138.10, 138.0, 137.59, 137.51, 130.36, 130.31, 130.18, 130.08, 119.76, 119.45, 117.5, 115.6, 104.3, 102.5, 102.47, 102.41, 50.01, 48.12, 47.9, 46.0, 46.0, 43.7, 42.3, 37.2, 37.0, 36.97, 33.80, 32.65, 32.49, 28.16, 26.90, 26.23, 25.4, 25.4, 25.2, 25.1, 24.4, 24.3, 24.3, 24.2; HRMS calculated for C42H47N9O12 ([M + H]+), 870.3422, found 870.3420.
1-Hydroxy-N-(3-(1-hydroxy-6-oxo-1,6-dihydropyridine-2-carboxamido)propyl)-N-(4-(1-hydroxy-N-(3-(1-hydroxy-N-(4-isothiocyanatophenethyl)-2-oxo-1,2-dihydropyridine-3-carboxamido)propyl)-2-oxo-1,2-dihydropyridine-3-carboxamido)butyl)-6-oxo-1,6-dihydropyridine-2-carboxa-mide (p-SCN-Bn-HOPO, 10)
The benzylamine was transformed into a benzyl isothiocyanate according to Maingot et al.45 NEt3 (0.0012 g, 0.012 mmol) was added to a solution of 8 (0.01 g, 0.011 mmol) in (8:2) acetonitrile and water (1 mL). Next, di-2-pyridyl thionocarbonate (0.011 g, 0.05 mmol) was added at room temperature and stirred vigorously for 1 h. The crude reaction solution was directly purified by HPLC on a preparative C18 column (Waters Symmetry C18 Prep Column, 100 Å, 5 μm, 19 mm × 100 mm) at 17.059 mL/min using a gradient of 5–75% MeCN in water (both containing 0.1% TFA) with an initial hold at 5% MeCN for 1.33 min and then a ramp to 75% MeCN over 30 min. The product peak was collected from 15.01 to 15.5 min and the eluted solution was lyophilized to recover the product as a white solid. The purified ligand was collected in multiple small batches with an approximate combined yield of 32%.
The purified sample was confirmed by HPLC on an analytical C18 column (Waters Symmetry C18 Analytical Column, 100 Å, 5 μm, 4.6 mm × 100 mm) at 1 mL/min using a gradient of 5–75% MeCN in water (both containing 0.1% TFA) with an initial hold at 5% MeCN for 1.33 min and then a ramp to 75% MeCN over 30 min followed by a ramp to 95% MeCN over 1 min and an isocratic hold at 95% MeCN for 5.66 min.
1H NMR (500 MHz, CDCl3): (mixture of rotamers) δ 7.37–7.31 (m, 7H), 7.12–7.08 (m, 1H), 6.58–6.52 (m, 4H), 6.34–5.81 (m, 4H), 3.62–3.54 (m, 12H), 3.13–2.83 (m, 11H), 2.01–1.24 (m, 11H) ; 13C NMR (500 MHz, CDCl3): (mixture of rotamers) δ 161.78, 161.71, 161.54, 161.4, 160.65, 160.61, 157.89, 157.83, 157.78, 157.73, 142.59, 142.48, 142.41, 142.33, 142.31, 142.21, 139.58, 138.49, 138.90, 138.81, 138.17, 138.0, 137.57, 137.50, 134.07, 130.76, 130.74, 130.59, 130.52, 128.89, 128.66, 126.41, 126.32, 119.74, 119.53, 119.43,104.33, 102.50, 102.39, 49.62, 48.11, 47.96, 46.49, 46.01, 45.70, 37.24, 37.01, 36.96, 34.04, 32.83, 32.71, 28.15, 27.12, 26.85, 26.22, 25.46, 25.26, 25.17, 24.36, 24.18; HRMS calculated for C43H45N9O12S ([M + H]+), 912.2987, found 912.2987.
Ligand–Antibody Conjugation
Trastuzumab (purchased commercially as Herceptin, Genentech, San Francisco, CA) was purified using prepacked size exclusion chromatography (SEC) columns (Sephadex G-25 M, PD-10 Desalting Columns, 50 kDa, GE Healthcare) and centrifugal filter units with a 50 000 molecular weight cutoff (Amicon Ultra 4 Centrifugal Filtration Units, Millipore Corp., Billerica, MA) and phosphate buffered saline (PBS, pH 7.4) to remove α_α-trehalose dihydrate, L-histidine, and polysorbate 20 additives. After purification, the antibody was taken up in PBS at pH 7.4. Subsequently, ~60 μL of antibody solution (~13 nmol) were diluted to 1 mL with PBS at pH 7.4. The pH of the antibody solution was raised to 8.8–9.0 with 0.1 M Na2CO3 before the slow addition of 5 equiv of p-SCN-Bn-HOPO or p-SCN-Bn-HOPO in ~12 μL of DMSO. The reaction was incubated at 37 °C for 1 h and shaken at 300 rpm, followed by SEC and centrifugal filtration to purify the ligand–antibody conjugate. The final bioconjugates were stored in PBS pH 7.4 at 4 °C.
Chelate number was determined using an isotopic dilution assay. A stock solution of 1 mM ZrCl4 was made up in 1 M oxalic acid. Approximately ~200 μCi of 89Zr oxalate solution in 1 M oxalic acid was added to 100 μL of the stock solution. This mixture was then neutralized to pH 7 with 1 M Na2CO3 to create a ~200 μL Zr working solution of ~500 μM Zr4+. Triplicate solutions of DFO-Trastuzumab and HOPO-Trastuzumab were prepared containing 300–400 pmol of antibody in 30 μL of PBS. Aliquots of 20, 25, and 30 μL of the Zr working solution were added to the three ligand–antibody samples for each ligand. The solutions were incubated at room temperature with gentle mixing overnight. The next day a volume of 50 mM EDTA equal to 1/9 of the volume of the reaction mixture was added to each sample and left to incubate for 15 min in order to scavenge any nonspecifically bound Zr4+. Samples were then analyzed using radio-TLC to determine the extent of radiolabeling. The average number of chelates per antibody was calculated as the ratio of bound vs unbound radioactive 89Zr × the moles of Zr4+ ÷ the moles of antibody. The experiment was run in duplicate using the same batch of ligand-Trastuzumab conjugate as the in vivo studies.
Radiolabeling Studies
89Zr was received after target processing as 89Zr-oxalate in 1.0 M oxalic acid. This solution is then neutralized with 1.0 M sodium carbonate to reach pH 6.8–7.2. Both the DFO and HOPO ligands as well as their respective antibody complexes were labeled at various concentrations in water or saline with the neutralized 89Zr solution at room temperature for varying lengths of time, typically 10–180 min. Reactions were monitored via radio-TLC with different stationary phases depending on the nature of the reaction. 89Zr-ligand complexes required Varian ITLC-SA strips (Agilent Technologies) whereas 89Zr-ligand-trastuzumab complexes employed Varian ITLC-SG strips (Agilent Technologies), but both analysis methods used 50 mM EDTA at pH 5 as the mobile phase. 89Zr complexes remained at the origin, while free 89Zr was taken up by EDTA in the mobile phase and migrated along the ITLC strip.
Serum Stability Studies
89Zr-ligand and 89Zr-ligand-antibody complexes were prepared according to the radio-labeling protocol as described above. For each 89Zr complex, samples were made consisting of 900 μL human serum and 100 μL of the 89Zr species and were placed in a heat block at 37 °C with agitation. Samples were monitored using radio-TLC before being added to the serum and then after 1 week of incubation. The stability of the complexes was measured as the percentage of 89Zr that was retained at the origin of the ITLC strip and therefore still intact.
Immunoreactivity Assay
The immunoreactivity of the 89Zr-DFO-trastuzumab and 89Zr-HOPO-trastuzumab bioconjugates was determined using specific radioactive cellular-binding assays following procedures derived from Lindmo et al.46,47 To this end, BT474 cells were suspended in microcentrifuge tubes at concentrations of 2.5, 2.0, 1.5, 1.25, 1.0, 0.75, and 0.25 × 106 cells/mL in 500 μL PBS (pH 7.4). Aliquots of either 89Zr-DFO-trastuzumab or 89Zr-HOPO-trastuzumab (50 μL of a stock solution of ~10 μCi in 10 mL of 1% bovine serum albumin in PBS pH 7.4) were added to each tube (n = 3; final volume: 550 μL), and the samples were incubated on a mixer for 60 min at room temperature. The treated cells were then pelleted via centrifugation (600 G for 2 min), aspirated, and washed twice with cold PBS before removing the supernatant and counting the activity associated with the cell pellet. The activity data were background-corrected and compared with the total number of counts in appropriate control samples. Immunoreactive fractions were determined by linear regression analysis of a plot of (total/bound) activity against (1/[normalized cell concentration]). No weighting was applied to the data, and data were obtained as n = 3.
PET Imaging
PET imaging experiments were conducted on a microPET Focus 120. Female athymic nude mice with BT474 xenografts on their right shoulders were administered 89Zr-HOPO-trastuzumab (9.25–9.99 MBq [250–270 μCi] in 200 μL 0.9% sterile saline) or 89Zr-DFO-trastuzumab (9.25–9.99 MBq [250–270 μCi] in 200 μL 0.9% sterile saline) via intravenous tail vein injection (t = 0). Approximately 5 min prior to the acquisition of PET images, mice were anesthetized by inhalation of 2% isoflurane (Baxter Healthcare, Deerfield, IL)/oxygen gas mixture and placed on the scanner bed; anesthesia was maintained using 1% isoflurane/gas mixture. PET data for each mouse were recorded via static scans at various time points (n = 4) between 6 h and 9 d. An energy window of 350–700 keV and a coincidence timing window of 6 ns were used. Data were sorted into 2D histograms by Fourier rebinning, and transverse images were reconstructed by filtered back-projection (FBP) into a 128 × 128 × 63 (0.72 × 0.72 × 1.3 mm3) matrix. The image data were normalized to correct for nonuniformity of response of the PET, dead-time count losses, positron branching ratio, and physical decay to the time of injection, but no attenuation, scatter, or partial-volume averaging correction was applied. The counting rates in the reconstructed images were converted to activity concentrations (percentage injected dose per gram of tissue, %ID/g) by use of a system calibration factor derived from the imaging of a mouse-sized water-equivalent phantom containing 89Zr. Images were analyzed using ASIPro VM software (Concorde Microsystems).
Biodistribution
Acute in vivo biodistribution studies were performed in order to compare the uptake of 89Zr-HOPO-trastuzumab and 89Zr-DFO-trastuzumab in BT474 tumor-bearing female athymic nude mice. Mice were warmed gently with a heat lamp for 5 min before administration of 89Zr-HOPO-trastuzumab (0.59–0.67 MBq [16–18 μCi] in 200 μL 0.9% sterile saline) or 89Zr-DFO-trastuzumab (0.67–0.74 MBq [18–20 μCi] in 200 μL 0.9% sterile saline) via intravenous tail vein injection (t = 0). Animals (n = 4 per group) were euthanized by CO2(g) asphyxiation at 1, 3, 5, 7, 9, and 14 d. After asphyxiation, 14 organs were removed, rinsed in water, dried in air for 5 min, weighed, and assayed for radioactivity on a gamma counter calibrated for 89Zr. Counts were converted into activity using a calibration curve generated from known standards. Count data were background- and decay-corrected to the time of injection, and the percent injected dose per gram (%ID/g) for each tissue sample was calculated by normalization to the total activity injected. The full data set of organs is also included in the Supporting Information with values represented in %ID without normalization (Table S.5).
Supplementary Material
Acknowledgments
The authors thank Kuntal Sevak, Kathryn Carnazza, and Kim Edwards for skillful tail-vein injections. Services provided by the MSKCC Radiochemistry and Molecular Imaging Probe Core and the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH grant P30 CA08748. C.H. would like to thank the support from the Molecular Design Institute at the Department of Chemistry of New York University for purchasing the single crystal diffractometer. The authors gratefully acknowledge the CTSC grant TR000457 of the National Center for Advancing Translational Sciences of the National Institutes of Health. The authors also thank the NIH (award 1F31CA180360-01, M.A.D.), the NSF (award IGERT DGE 0965983, L.C.F.), and the DOE (awards FG02-09ER16097, L.C.F. and DE-SC0002184, J.S.L.) for their generous funding. Research Infrastructure at Hunter College is partially supported by a Research Centers in Minority Institutions Program grant from the National Institute on Minority Health and Health Disparities (MD007599) of the National Institutes of Health.
ABBREVIATIONS
- PET
positron emission tomography
- DFO
desferrioxamine B
- HOPO
3,4,3-(LI-1,2-HOPO)
- radio-TLC
radioactive thin layer chromatography
- DFT
density functional theory
Footnotes
Author Contributions
All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
- Detailed data and figures from crystal structure analysis, bifunctional ligand synthesis and characterization, and biodistribution studies (PDF)
- Crysallographic data (CIF)
References
- 1.Wu AM, Olafsen T. Antibodies for Molecular Imaging of Cancer. Cancer J. 2008;14:191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 2.Borjesson PKE, Jauw YWS, de Bree R, Roos JC, Castelijns JA, Leemans CR, van Dongen GAMS, Boellaard R. Radiation Dosimetry of Zr-89-Labeled Chimeric Monoclonal Antibody U36 as Used for Immuno-PET in Head and Neck Cancer Patients. J Nucl Med. 2009;50:1828–1836. doi: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- 3.Borjesson PK, Jauw YW, Boellaard R, de Bree R, Comans EF, Roos JC, Castelijns JA, Vosjan MJ, Kummer JA, Leemans CR, Lammertsma AA, van Dongen GA. Performance of Immuno-Positron Emission Tomography with Zirconium-89-Labeled Chimeric Monoclonal Antibody U36 in the Detection of Lymph Node Metastases in Head and Neck Cancer Patients. Clin Cancer Res. 2006;12:2133–40. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- 4.Dijkers EC, Munnink THO, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of Zr-89-Trastuzumab and PET Imaging of HER2-Positive Lesions in Patients with Metastatic Breast Cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 5.Perk LR, Vosjan MJWD, Visser GWM, Budde M, Jurek P, Kiefer GE, van Dongen GAMS. P-Isothiocyanatobenzyl-Desferrioxamine: A New Bifunctional Chelate for Facile Radiolabeling of Monoclonal Antibodies with Zirconium-89 for Immuno-PET Imaging. Eur J Nucl Med Mol Imaging. 2010;37:250–259. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi SNF, Visser OJ, Vosjan MJWD, van Lingen A, Hoekstra OS, Zijlstra JM, Huijgens PC, van Dongen GAMS, Lubberink M. Biodistribution, Radiation Dosimetry and Scouting of Y-90-Ibritumomab Tiuxetan Therapy in Patients with Relapsed B-Cell Non-Hodgkin’s Lymphoma Using Zr-89-Ibritumomab Tiuxetan and PET. Eur J Nucl Med Mol Imaging. 2012;39:512–520. doi: 10.1007/s00259-011-2008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET Imaging with 89Zr: From Radiochemistry to the Clinic. Nucl Med Biol. 2013;40:3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vugts DJ, van Dongen GAMS. 89Zr-Labeled Compounds for PET Imaging Guided Personalized Therapy. Drug Discovery Today: Technol. 2011;8:e53–e61. doi: 10.1016/j.ddtec.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Hong H, Severin GW, Yang Y, Engle JW, Zhang Y, Barnhart TE, Liu G, Leigh BR, Nickles RJ, Cai W. Positron Emission Tomography Imaging of Cd105 Expression with Zr-89-Df-Trc105. Eur J Nucl Med Mol Imaging. 2012;39:138–148. doi: 10.1007/s00259-011-1930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohn A, Zimmermann K, Schaub E, Hirzel W, Schubiger PA, Schibli R. Production and Separation of “Non-Standard” PET Nuclides at a Large Cyclotron Facility: The Experiences at the Paul Scherrer Institute in Switzerland. Q J Nucl Med Mol Imaging. 2008;52:145–150. [PubMed] [Google Scholar]
- 11.Zhang Y, Hong H, Cai W. PET Tracers Based on Zirconium-89. Curr Radiopharm. 2011;4:131–9. doi: 10.2174/1874471011104020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice SL, Roney CA, Daumar P, Lewis JS. The Next Generation of Positron Emission Tomography Radiopharmaceuticals in Oncology. Semin Nucl Med. 2011;41:265–282. doi: 10.1053/j.semnuclmed.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikotun OF, Lapi SE. The Rise of Metal Radionuclides in Medical Imaging: Copper-64, Zirconium-89 and Yttrium-86. Future Med Chem. 2011;3:599–621. doi: 10.4155/fmc.11.14. [DOI] [PubMed] [Google Scholar]
- 14.Zeglis BM, Lewis JS. A Practical Guide to the Construction of Radiometallated Bioconjugates for Positron Emission Tomography. Dalton T. 2011;40:6168–6195. doi: 10.1039/c0dt01595d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulmert D, Evans MJ, Holland JP, Rice SL, Wongvipat J, Pettersson K, Abrahamsson PA, Scardino PT, Larson SM, Lilja H, Lewis JS, Sawyers CL. Imaging Androgen Receptor Signaling with a Radiotracer Targeting Free Prostate-Specific Antigen. Cancer Discovery. 2012;2:320–327. doi: 10.1158/2159-8290.CD-11-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. Zr-89-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression in Vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munnink THO, Arjaans MEA, Timmer-Bosscha H, Schroder CP, Hesselink JW, Vedelaar SR, Walenkamp AME, Reiss M, Gregory RC, Lub-de Hooge MN, de Vries EGE. PET with the Zr-89-Labeled Transforming Growth Factor-Beta Antibody Fresolimumab in Tumor Models. J Nucl Med. 2011;52:2001–2008. doi: 10.2967/jnumed.111.092809. [DOI] [PubMed] [Google Scholar]
- 18.Nagengast WB, de Korte MA, Munnink THO, Timmer-Bosscha H, den Dunnen WF, Hollema H, de Jong JR, Jensen MR, Quadt C, Garcia-Echeverria C, van Dongen GAMS, Lub-de Hooge MN, Schroder CP, de Vries EGE. (89)Zr-Bevacizumab PET of Early Antiangiogenic Tumor Response to Treatment with HSP90 Inhibitor Nvp-Auy922. J Nucl Med. 2010;51:761–767. doi: 10.2967/jnumed.109.071043. [DOI] [PubMed] [Google Scholar]
- 19.Perk LR, Stigter-van Walsum M, Visser GWM, Kloet RW, Vosjan MJWD, Leemans CR, Giaccone G, Albano R, Comoglio PM, van Dongen GAMS. Quantitative PET Imaging of Met-Expressing Human Cancer Xenografts with Zr-89-Labelled Monoclonal Antibody Dn30. Eur J Nucl Med Mol Imaging. 2008;35:1857–1867. doi: 10.1007/s00259-008-0774-5. [DOI] [PubMed] [Google Scholar]
- 20.Verel I, Visser GW, Boellaard R, Boerman OC, van Eerd J, Snow GB, Lammertsma AA, van Dongen GA. Quantitative 89Zr Immuno-PET for in Vivo Scouting of 90Y-Labeled Monoclonal Antibodies in Xenograft-Bearing Nude Mice. J Nucl Med. 2003;44:1663–70. [PubMed] [Google Scholar]
- 21.Viola-Villegas NT, Rice SL, Carlin S, Wu X, Evans MJ, Sevak KK, Drobjnak M, Ragupathi G, Sawada R, Scholz WW, Livingston PO, Lewis JS. Applying PET to Broaden the Diagnostic Utility of the Clinically Validated CA19.9 Serum Biomarker for Oncology. J Nucl Med. 2013;54:1876–1882. doi: 10.2967/jnumed.113.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perk LR, Visser OJ, Stigter-van Walsum M, Vosjan MJWD, Visser GWM, Zijlstra JM, Huijgens PC, van Dongen GAMS. Preparation and Evaluation of Zr-89-Zevalin for Monitoring of Y-90-Zevalin Biodistribution with Positron Emission Tomography. Eur J Nucl Med Mol Imaging. 2006;33:1337–1345. doi: 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- 23.Dijkers ECF, Kosterink JGW, Rademaker AP, Perk LR, van Dongen GAMS, Bart J, de Jong JR, de Vries EGE, Lub-de Hooge MN. Development and Characterization of Clinical-Grade Zr-89-Trastuzumab for Her2/Neu ImmunoPET Imaging. J Nucl Med. 2009;50:974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Kim YS, Chakraborty S, Zhou Y, Wang F, Liu S. Impact of Bifunctional Chelators on Biological Properties of 111In-Labeled Cyclic Peptide RGD Dimers. Amino Acids. 2011;41:1059–1070. doi: 10.1007/s00726-009-0439-0. [DOI] [PubMed] [Google Scholar]
- 25. [October 28, 2013]; Http://Clinicaltrials.Gov/Ct2/Results?Term=89Zr&Cntry1=Na%3aus.
- 26.Meijs WE, Herscheid JDM, Haisma HJ, Pinedo HM. Evaluation of Desferal as a Bifunctional Chelating Agent for Labeling Antibodies with Zr-89. Appl Radiat Isot. 1992;43:1443–1447. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- 27.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of Metastatic Peritoneal and Pulmonary Colorectal Cancer in Mice with Human Epidermal Growth Factor Receptor 1-Targeted Zr-89-Labeled Panitumumab. J Nucl Med. 2012;53:113–120. doi: 10.2967/jnumed.111.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abou DS, Ku T, Smith-Jones PM. In Vivo Biodistribution and Accumulation of 89Zr in Mice. Nucl Med Biol. 2011;38:675–81. doi: 10.1016/j.nucmedbio.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijs WE, Haisma HJ, Klok RP, vanGog FB, Kievit E, Pinedo HM, Herscheid JDM. Zirconium-Labeled Monoclonal Antibodies and Their Distribution in Tumor-Bearing Nude Mice. J Nucl Med. 1997;38:112–118. [PubMed] [Google Scholar]
- 30.Pandit-Taskar N, O’Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, Lefkowitz RA, Carrasquillo JA, Martinez DF, Fung AM, Solomon SB, Gonen M, Heller G, Loda M, Nanus DM, Tagawa ST, Feldman JL, Osborne J, Lewis JS, Reuter V, Weber WA, Bander NH, Scher HI, Larson SM, Morris MJ. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin Cancer Res. 2015:552. doi: 10.1158/1078-0432.CCR-15-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price EW, Zeglis BM, Lewis JS, Adam MJ, Orvig C. H6phospa-Trastuzumab: Bifunctional Methylenephosphonate-Based Chelator with 89Zr, 111In and 177Lu. Dalton T. 2014;43:119–131. doi: 10.1039/c3dt51940f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma MT, Meszaros LK, Paterson BM, Berry DJ, Cooper MS, Ma Y, Hider RC, Blower PJ. Tripodal Tris(Hydroxypyridinone) Ligands for Immunoconjugate PET Imaging with 89Zr4+: Comparison with Desferrioxamine-B. Dalton T. 2015;44:4884–4900. doi: 10.1039/c4dt02978j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guérard F, Lee YS, Brechbiel MW. Rational Design, Synthesis, and Evaluation of Tetrahydroxamic Acid Chelators for Stable Complexation of Zirconium(IV) Chem - Eur J. 2014;20:5584–5591. doi: 10.1002/chem.201304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deri MA, Ponnala S, Zeglis BM, Pohl G, Dannenberg JJ, Lewis JS, Francesconi LC. Alternative Chelator for 89Zr Radiopharmaceuticals: Radiolabeling and Evaluation of 3,4,3-(LI-1,2-HOPO) J Med Chem. 2014;57:4849–4860. doi: 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patra M, Bauman A, Mari C, Fischer CA, Blacque O, Haussinger D, Gasser G, Mindt TL. An Octadentate Bifunctional Chelating Agent for the Development of Stable Zirconium-89 Based Molecular Imaging Probes. Chem Commun. 2014;50:11523–11525. doi: 10.1039/c4cc05558f. [DOI] [PubMed] [Google Scholar]
- 36.Pandya DN, Pailloux S, Tatum D, Magda D, Wadas TJ. Di-Macrocyclic Terephthalamide Ligands as Chelators for the PET Radionuclide Zirconium-89. Chem Commun. 2015;51:2301–2303. doi: 10.1039/c4cc09256b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai C, Summer D, Rangger C, Franssen GM, Laverman P, Haas H, Petrik M, Haubner R, Decristoforo C. Novel Bifunctional Cyclic Chelator for 89Zr Labeling–Radiolabeling and Targeting Properties of RGD Conjugates. Mol Pharmaceutics. 2015;12:2142–2150. doi: 10.1021/acs.molpharmaceut.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerard F, Lee YS, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Investigation of Zr(IV) and 89Zr(IV) Complexation with Hydroxamates: Progress Towards Designing a Better Chelator Than Desferrioxamine B for Immuno-PET Imaging. Chem Commun. 2013;49:1002–1004. doi: 10.1039/c2cc37549d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorden AEV, Xu J, Raymond KN, Durbin P. Rational Design of Sequestering Agents for Plutonium and Other Actinides. Chem Rev. 2003;103:4207–4282. doi: 10.1021/cr990114x. [DOI] [PubMed] [Google Scholar]
- 40.Sturzbecher-Hoehne M, Choi TA, Abergel RJ. Hydroxypyridinonate Complex Stability of Group (IV) Metals and Tetravalent F-Block Elements: The Key to the Next Generation of Chelating Agents for Radiopharmaceuticals. Inorg Chem. 2015;54:3462–3468. doi: 10.1021/acs.inorgchem.5b00033. [DOI] [PubMed] [Google Scholar]
- 41.Daumann LJ, Tatum DS, Snyder BER, Ni C, Law G-l, Solomon EI, Raymond KN. New Insights into Structure and Luminescence of EuIII and SmIII Complexes of the 3,4,3-LI(1,2-HOPO) Ligand. J Am Chem Soc. 2015;137:2816–2819. doi: 10.1021/ja5116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.APEX2. Bruker AXS Inc; Madison, WI: 2015. 2014.11. [Google Scholar]
- 43.Sheldrick GM. SHELX, 2014/7. Universität Göttingen; Germany: 2014. [Google Scholar]
- 44.Geall AJ, Taylor RJ, Earll ME, Eaton MAW, Blagbrough IS. Synthesis of Cholesteryl Polyamine Carbamates: Pka Studies and Condensation of Calf Thymus DNA. Bioconjugate Chem. 2000;11:314–326. doi: 10.1021/bc990115w. [DOI] [PubMed] [Google Scholar]
- 45.Maingot L, Elbakali J, Dumont J, Bosc D, Cousaert N, Urban A, Deglane G, Villoutreix B, Nagase H, Sperandio O, Leroux F, Deprez B, Deprez-Poulain R. Aggrecanase-2 Inhibitors Based on the Acylthiosemicarbazide Zinc-Binding Group. Eur J Med Chem. 2013;69:244–261. doi: 10.1016/j.ejmech.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr Determination of the Immunoreactive Function of Radiolabeled Monoclonal Antibodies by Linear Extrapolation to Binding at Infinite Antigen Excess. J Immunol Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 47.Lindmo T, Bunn PA., Jr . Determination of the True Immunoreactive Fraction of Monoclonal Antibodies after Radiolabeling. In: Langone JJ, Vunakis HV, editors. Methods in Enzymology. Vol. 121. Academic Press; 1986. pp. 678–691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.