Abstract
Blastocystis is one of the most common eukaryotic organisms found in humans and many types of animals. Several reports have identified its role in gastrointestinal disorders, although its pathogenicity is yet to be clarified. Blastocystis is transmitted via the fecal-to-oral route and colonizes the large intestines. Epithelial cells lining the intestine secrete antimicrobial peptides (AMPs), including beta-defensins and cathelicidin, as a response to infection. This study explores the effects of host colonic antimicrobial peptides, particularly LL-37, a fragment of cathelicidin, on different Blastocystis subtypes. Blastocystis is composed of several subtypes that have genetic, metabolic, and biological differences. These subtypes also have various outcomes in terms of drug treatment and immune response. In this study, Blastocystis isolates from three different subtypes were found to induce intestinal epithelial cells to secrete LL-37. We also show that among the antimicrobial peptides tested, only LL-37 has broad activity on all the subtypes. LL-37 causes membrane disruption and causes Blastocystis to change shape. Blastocystis subtype 7 (ST7), however, showed relative resistance to LL-37. An isolate, ST7 isolate B (ST7-B), from this subtype releases proteases that can degrade the peptide. It also makes the environment acidic, which causes attenuation of LL-37 activity. The Blastocystis ST7-B isolate was also observed to have a thicker surface coat, which may protect the parasite from direct killing by LL-37. This study determined the effects of LL-37 on different Blastocystis isolates and indicates that AMPs have significant roles in Blastocystis infections.
INTRODUCTION
Blastocystis is an intestinal protistan parasite commonly detected in humans and many types of animals (1, 2). This organism is classified under the stramenopiles, although it lacks chloroplasts or structures for locomotion, which are common features of many members of this group. Blastocystis is widely distributed throughout the world. Parasitological surveys usually place Blastocystis as the most common eukaryotic parasite detected (3, 4). This organism has a global distribution and has numerous animal hosts. There is little host specificity for this organism, and there are reports indicating the zoonotic potential of Blastocystis (5). This organism, in its cyst form, is transmitted via the fecal-to-oral route. It then colonizes the large intestine and is excysted to its various forms. These forms may appear vacuolar, multivacuolar, avacuolar, or granular under a light microscope. Blastocystis has been implicated in a number of intestinal disorders, although its pathogenesis is yet to be elucidated. There are few reports associating it with gastrointestinal disease, the most common symptoms of which are diarrhea, vomiting, nausea, and urticaria (1, 5–7). Blastocystis is a species complex that is comprised of up to 19 subtypes (STs), with ST1 to ST9 having been isolated from humans (8). Recently, a report found that ST12 and another possible novel ST also infect humans (9). Blastocystis STs may differ in size range, nuclear arrangement, growth rate, and morphology (8). There have been reports on the differences in host range, protease activity, and characteristics of the immune response triggered (10–12). There are also variations in drug sensitivities (13, 14). Some researchers have suggested that symptomatology is dictated by ST identity (15).
LL-37 is a 37-amino-acid fragment of human cathelicidin antimicrobial peptide (CAMP), which has a direct killing effect on prokaryotic and fungal organisms (16, 17). Because of its positive charge, it can bind to negatively charged surfaces, which is a feature of prokaryotic membranes (18). It can cause the formation of pores on the cell membrane and subsequently effect cell lysis. LL-37 is also a modulator of downstream immune responses such as the recruitment of other immune cells and the release of cytokines (19). Cathelicidin is produced by epithelial cells, including those lining the small and large intestines. It is processed by proteases, which results in the LL-37 fragment. There is basal secretion of LL-37 in the intestinal lumen. During infection, LL-37 becomes highly expressed, along with other antimicrobial peptides (AMPs). This leads to other immune responses such as the recruitment of inflammatory cells and the production of cytokines (20).
In this study, we explore the possible interactions between Blastocystis and human intestinal AMPs, particularly LL-37. We determined if the parasite can induce intestinal epithelial cells to secrete LL-37 using in vitro cell culture and a mouse model. We also tested several isolates of Blastocystis from three STs for susceptibility to three colonic AMPs, including LL-37. AMPs had variable effects on Blastocystis, and their effects on different Blastocystis STs were also not uniform. We identified possible parasite factors by which Blastocystis can possibly attenuate LL-37 activity.
MATERIALS AND METHODS
Parasite cultivation.
Nine previously axenized Blastocystis isolates (21–23) representing 3 STs (ST1, ST4, and ST7) were used for this study. Culture medium consisted of 9 ml prereduced Iscove's modified Dulbecco's medium (IMDM) (Thermo Scientific) supplemented with 10% horse serum (Gibco). Culture tubes were maintained inside 2.5-liter sealed anaerobic jars with an anaerobic gas pack (Oxoid) at 37°C. Human Blastocystis isolates were acquired from patients at the Singapore General Hospital in the early 1990s, before the Institutional Review Board was established at the National University of Singapore (NUS). Blastocystis isolates B, C, E, G, and H are maintained at a microbial collection at the Department of Microbiology and Immunology of the NUS. NUH2 and NUH9 were isolated in 2007 from stool samples submitted for routine heath screening. Permission from the National Healthcare Group Institutional Review Board was given before project commencement. All samples were anonymized. Blastocystis isolates WR1 and S1 were isolated from a Wistar rat and a Sprague-Dawley rat, respectively.
Subtyping of Blastocystis isolates.
The isolates were previously genotyped by using primers in a PCR assay based on the organism's small-subunit (SSU) rRNA (23, 24) and mitochondrion-like organelle (MLO) (25) genes. Total DNAs were extracted from 1 × 106 cells by using a Qiagen DNA stool kit (Qiagen) according to the manufacturer's instructions. PCR was performed by using Q5 High-Fidelity 2× master mix (New England BioLabs) with 500 ng of DNA sample. All PCR runs were completed by using a Bio-Rad iQ5 thermocycler. PCR products were sequenced and compared to the National Center for Biotechnology Information (NCBI) (USA) nucleotide sequence database to confirm subtype identities.
AMP susceptibility screening.
To determine the susceptibility of the 9 Blastocystis isolates to various human intestinal AMPs, a high-throughput viability assay developed previously by Mirza et al. (14) was applied. Briefly, flat-bottom 96-well plates (Greiner) were used, with each well containing 0.5 × 106 Blastocystis cells. The AMPs human beta-defensin 1 (hBD-1), hBD-2, and LL-37 were dissolved in complete medium at concentrations ranging between 0 and 50 μM. After 24 h of incubation, resazurin dye (Sigma-Aldrich) at a 5% final dilution was added and incubated for another 3 h. Reading of fluorescence was done at 550-nm excitation and 570-nm emission wavelengths by using a Tecan Infinite F200 microplate reader. Half-maximal inhibitory concentration (IC50) values were calculated by using GraphPad Prism software. Synthetic AMPs at 95% purity were obtained from Singapore Advanced Biologics.
Exposure of mouse intestinal explants to Blastocystis.
C57BL/6 mice were obtained from the NUS and housed in an animal biosafety level 2 clean animal facility. Mice (7 to 9 weeks old) were euthanized by CO2 inhalation. The intestinal tract was dissected and placed into 50-ml tubes with complete medium comprised of Dulbecco's modified Eagle's medium (DMEM) (HyClone) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 1% each sodium pyruvate (Gibco) and nonessential amino acids (Gibco), and 2,000 U/ml penicillin-streptomycin (Gibco). The tissue was then dissected into segments of distal colon, proximal colon, cecum, and terminal ileum and opened along the mesenteric edge, and intestinal contents were removed. The segments were washed gently in cold penicillin-streptomycin and cut into bits measuring 1.5 cm by 1 cm. The explants were affixed onto 2% agarose layers in 6-well plates with the serosal surface facing down in prewarmed complete medium with penicillin-streptomycin (Gibco). Explants were incubated with 5 × 107 live Blastocystis ST7 isolate B (ST7-B) parasites for 1 h at 37°C. For all assays, more than one litter was used in order to avoid any litter-specific effects. After incubation, the explants were frozen in TRIzol (Invitrogen) at −80°C until further processing. The animal experiments were performed in accordance with Singapore National Advisory Committee for Laboratory Animal Research guidelines. The protocol (R13-5890) was reviewed and approved by the NUS Institutional Animal Care and Use Committee.
Coculture of Blastocystis with HT-29 intestinal epithelial cells.
HT-29 epithelial cells were used to study whether Blastocystis parasites affect the expression of LL-37 in host cells. Cells were maintained in T-75 flasks (Corning) in a humidified incubator with 5% CO2 at 37°C. Complete culture medium consisted of 10% heat-inactivated FBS (Gibco) and 1% each sodium pyruvate (Gibco), nonessential amino acids (Gibco), and penicillin-streptomycin in DMEM (Thermo Scientific). Cells were seeded onto 6-well plates (Greiner) by using complete medium. After reaching confluence, HT-29 cells were incubated for 48 h with serum-free medium supplemented with 3 mM sodium butyrate (Sigma-Aldrich). HT-29 cells were then cocultured with Blastocystis isolates NUH9 (ST1-NUH9), WR1 (ST4-WR1), and ST7-B at an MOI (multiplicity of infection) of 10 for 1 h. Culture supernatants were collected and set aside for determination of LL-37 secretion. The viability of HT-29 cells after coculture was determined by using a trypan blue exclusion assay.
Quantitative real-time PCR.
Induction of cathelicidin-related antimicrobial peptide (CRAMP) gene expression on mouse intestinal explants and CAMP gene expression on HT-29 cells was determined after exposure to Blastocystis. Mouse explants in TRIzol were homogenized with zirconium oxide beads in a tissue homogenizer (WisBioMed), and RNA was extracted from homogenates by using the TRIzol method, followed by further purification with the NucleoSpin RNA kit (Macherey-Nagel). HT-29 cells were homogenized and lysed with RNAzol (Molecular Research Center). Total RNA was extracted according to the manufacturer's protocol. Synthesis of cDNA was done by using an iScript cDNA synthesis kit (Bio-Rad) with 1 μg of RNA sample in an iCycler thermocycler (Bio-Rad) according to the suggested protocol. Quantitative real-time PCR (qRT-PCR) was then performed by using an iTaq Universal SYBR green Supermix kit (Bio-Rad), 1 μl of cDNA, and 500 nM (each) the following primers (Sigma-Aldrich): CRAMP-F (5′-TTT TGA CAT CAG CTG TAA CG-3′) and CRAMP-R (5′-GCT TTT CAC CAA TCT TCT CC-3′) for mouse samples and CAMP-F (5′-AGT GAA GGA GAC TGT ATG TG-3′) and CAMP-R (5′-ATT TTC TTG AAC CGA AAG GG-3′) for HT-29 samples. Mouse beta-actin (mBA-F [5′-GAT GTA TGA AGG CTT TGG TC-3′] and mBA-R [5′-TGT GCA CTT TTA TTG GTC TC-3′]) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (hGAPdH-F [5′-CTT TTG CGT CGC CAG-3′] and hGAPdH-R [5′-TTG ATG GCA ACA ATA TCC AC-3′]) genes were used as endogenous controls. Gene expression analysis was done by using iQ5 v2.1 software (Bio-Rad), which uses the method.
LL-37 secretion assay.
To quantitatively determine LL-37 secretion in HT-29 cells, supernatants (100 μl) from cocultures with Blastocystis were used to coat flat-bottom 96-well MaxiSorp plates (Nunc) for 18 h at 4°C. The excess supernatant was disposed of, and the LL-37 peptide concentration was determined by using an enzyme-linked immunosorbent assay (ELISA). The plates were washed 3 times by using wash buffer containing 1× phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBS-T) and blocked with 1% bovine serum albumin (BSA) (Santa Cruz Biotechnology) for 2 h at 25°C. LL-37 was probed with a 1:5,000 dilution of rabbit polyclonal anti-LL-37 human IgG antibody (Abcam) in blocking solution. After 1 h, the wells were washed 4 times with PBS-T. A 1:1,000 dilution of goat polyclonal secondary antibody to rabbit IgG horseradish peroxidase (HRP)-conjugated antibody (Abcam) in blocking solution was added, and the mixture was incubated at 25°C. After 1 h, the wells were washed 4 times with PBS-T. The wells were then incubated with the substrate 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich) for 5 to 10 min. The absorbance was read at 492 nm by using a Tecan Infinite F200 microplate reader.
Fluorescence microscopy.
To visualize the expression of LL-37 in HT-29 cells, a fluorescence microscopy assay was used. HT-29 cells were grown to confluence in 24-well cell culture plates (Greiner) with poly-l-lysine-treated glass coverslips. Complete medium was then replaced with 3 mM sodium butyrate in serum-free medium. After 48 h, cocultivation with Blastocystis isolates ST1-NUH9, ST4-WR1, and ST7-B at an MOI of 10 for 1 h was done. HT-29 cells were then washed with PBS and fixed with absolute methanol for 15 min at −20°C. The cells were then washed with PBS and blocked with 5% normal goat serum in PBS for 2 h. LL-37 was probed by using a 1:500 dilution of rabbit polyclonal anti-LL-37 human IgG antibody (Abcam) in blocking solution overnight at 4°C. After the cells were washed 3 times with PBS-T for 5 min each, incubation was done with a 1:1,000 dilution of goat polyclonal secondary antibody to rabbit IgG fluorescein isothiocyanate (FITC)-conjugated antibody (Abcam) in blocking solution for 1 h at 25°C. The cells were then washed 3 times. The glass coverslips were then mounted by using Vectashield mounting medium and examined by using an Olympus BX60 fluorescence microscope. Total cell fluorescence was quantified by using ImageJ software version 1.48 (NIH).
Viability staining and flow cytometry.
Propidium iodide (PI) (BioVision) was used to stain Blastocystis cells to assess viability after LL-37 treatment. Blastocystis isolates ST1-NUH9, ST4-WR1, and ST7-B (1 × 107 cells/ml) were treated with LL-37 at concentrations of 0, 10, and 100 μg/ml for 1 h. A necrotic control was added as a positive control. This was achieved by heating the cells at 95°C for 15 min. PI was then added to the cell suspension according to the manufacturer's instructions. Cells were then analyzed by using an LSRFortessa instrument (BD Biosciences). Data analysis was done by using Summit software version 4.3 (Dako).
Scanning electron microscopy.
The effects of the LL-37 peptide on the Blastocystis membrane and surface coat were visualized by using a scanning electron microscope. Blastocystis cells with and without LL-37 treatment were fixed overnight with 4% glutaraldehyde in PBS. The cells were then washed and attached to 0.1% poly-l-lysine-treated coverslips for 30 min. The cells were dehydrated with increasing concentrations of ethanol. The coverslips with attached cells were placed into a critical-point dryer (CPD 030; Balzers) for carbon dioxide infiltration. After drying, the coverslips were coated with 5- to 10-nm gold particles with a sputter coater. The cells were visualized with a JEOL JSM-6701F scanning electron microscope.
Imaging flow cytometry.
Morphological changes of Blastocystis cells caused by LL-37 were observed by using an imaging flow cytometer. Blastocystis cultures were harvested and washed twice with PBS. Cell suspensions with 1 × 107 cells/ml were stained with 1 μg/ml PI (BioVision), 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies), and 1 μg/ml Hoechst 33342 (Life Technologies) for 15 min. The stained cells were analyzed by using an Amnis ImageStream MkII imaging flow cytometer (Merck Millipore) with a 4-laser attachment (375, 488, 561, and 642 nm). At least 2,000 events per run were acquired at low flow speed and a ×60 magnification. The gating strategy included selection for focused cells and then single cells. Histograms based on circularity indices were generated. Data analysis was done by using IDEAS software version 6.1.
LL-37 bound to the surfaces of Blastocystis cells was also visualized by using imaging flow cytometry. Before the samples were run, 1 × 107 cells were treated with 50 μg/ml LL-37 at room temperature. PI was added, and the cells were fixed by using 4% formaldehyde for 30 min. The cells were washed twice and suspended in PBS. LL-37 bound to the Blastocystis cell surface was probed with a 1:1,000 dilution of rabbit polyclonal anti-LL-37 human IgG antibody (Abcam) for 30 min. Goat polyclonal secondary antibody to rabbit IgG FITC-conjugated antibody (Abcam) at a 1:500 concentration was then added, and the mixture was incubated for 30 min. All the reactions were done at 25°C.
LL-37 degradation and viability assays.
Blastocystis ST7-B and ST4-WR1 excretory-secretory products (ESPs) were harvested from cultures grown for 24 h. The cultures were washed twice in serum-free IMDM. The cells were then incubated for 2 h in prereduced IMDM at 37°C under anaerobic conditions at 1 × 108 cells per ml of IMDM. Culture supernatants were then collected by centrifugation at 1,000 × g for 10 min and filtered twice by using 0.2-μm-pore-size filters (Millipore). Blastocystis secreted products were stored at −80°C until use. The effect of Blastocystis ESPs on peptide integrity was quantitatively determined by an enzyme-linked immunosorbent assay (ELISA). Briefly, each well of flat-bottom 96-well Nunc MaxiSorp plates was coated with 50 μl of LL-37 at a concentration of 2 μg/ml for 18 h at 4°C. Plates coated with LL-37 were exposed for 2 h to Blastocystis ESPs alone or ESPs previously incubated for 6 h with 1× Halt protease inhibitor cocktail (Bio-Rad) at 37°C. The wells were then washed with PBS, and the concentration of intact LL-37 was determined by using an ELISA as outlined above. LL-37 peptide solutions previously incubated with Blastocystis ESPs were also used for the treatment of Blastocystis cultures. Viability was determined by using flow cytometry and PI staining after treatment. Denatured ESPs were also used in this assay, which entailed heating of the ESPs for 15 min at 95°C.
Transmission electron microscopy (TEM).
Cultures of Blastocystis cells (1 × 107 cells) grown for 24 h were harvested, washed, and treated with 100 μg/ml cationized ferritin (Sigma-Aldrich) for 2 h. Cationized ferritin binds to negatively charged surfaces (26). We found that it was useful to visualize the surface coat of Blastocystis by providing better contrast and maintaining its integrity during the preparation of cells for electron microscopy. The cells were then fixed overnight at 4°C with 8% glutaraldehyde. The cells were then washed 3 times with PBS. Postfixation included incubation of cells with 1% osmium tetroxide and 1% potassium ferrocyanide in PBS for 2 h. The cells were then washed 3 times. The cells were dehydrated with absolute ethanol 5 times. Embedding was done for 48 h by using London resin white. The solidified blocks were sectioned, mounted, and viewed by using a transmission electron microscope (JEM-1010; JEOL).
Statistical analysis.
Tests for significant differences were done by using analysis of variance (ANOVA) and Student's t test. Error bars represent standard errors from at least 3 independent experiments. Calculations and generation of graphs were done with GraphPad Prism 5.0.
RESULTS
Blastocystis STs are inhibited by LL-37.
Nine Blastocystis isolates representing 3 STs were tested for susceptibility to AMP treatment (Table 1). All isolates from the three subtypes were considered susceptible to LL-37, although ST7 isolates showed higher IC50s. Only ST4 isolates were susceptible to hBD-2, while treatment of all isolates with hBD-1 did not cause a loss of Blastocystis viability.
TABLE 1.
IC50s of hBD-1, hBD-2, and LL-37 for Blastocystis STs
| Subtype | Isolate | IC50 (μM) |
||
|---|---|---|---|---|
| HBD-1 | HBD-2 | LL-37 | ||
| ST1 | NUH2 | >50 | >50 | 6.0 |
| NUH9 | >50 | >50 | 4.7 | |
| ST4 | WR1 | >50 | 11.4 | 3.2 |
| S1 | >50 | 5.0 | 5.4 | |
| ST7 | B | >50 | >50 | 42.6 |
| C | >50 | >50 | 23.4 | |
| E | >50 | >50 | 23.2 | |
| G | >50 | >50 | 27.7 | |
| H | >50 | >50 | 28.7 | |
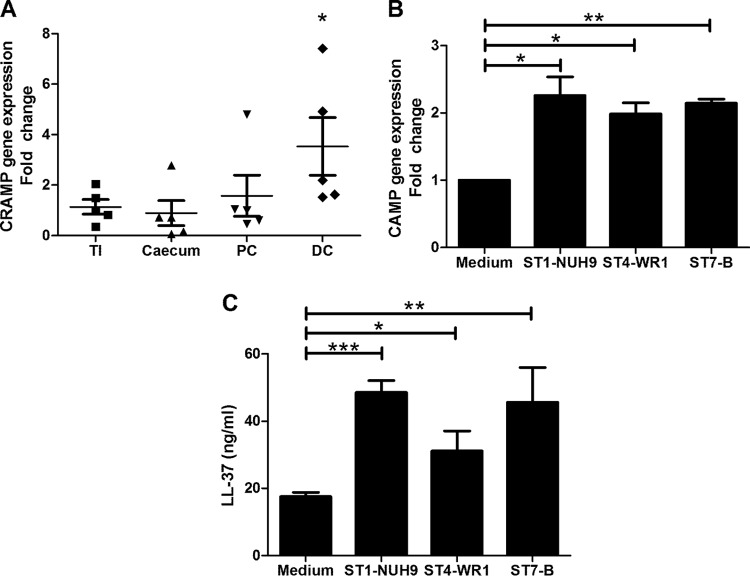
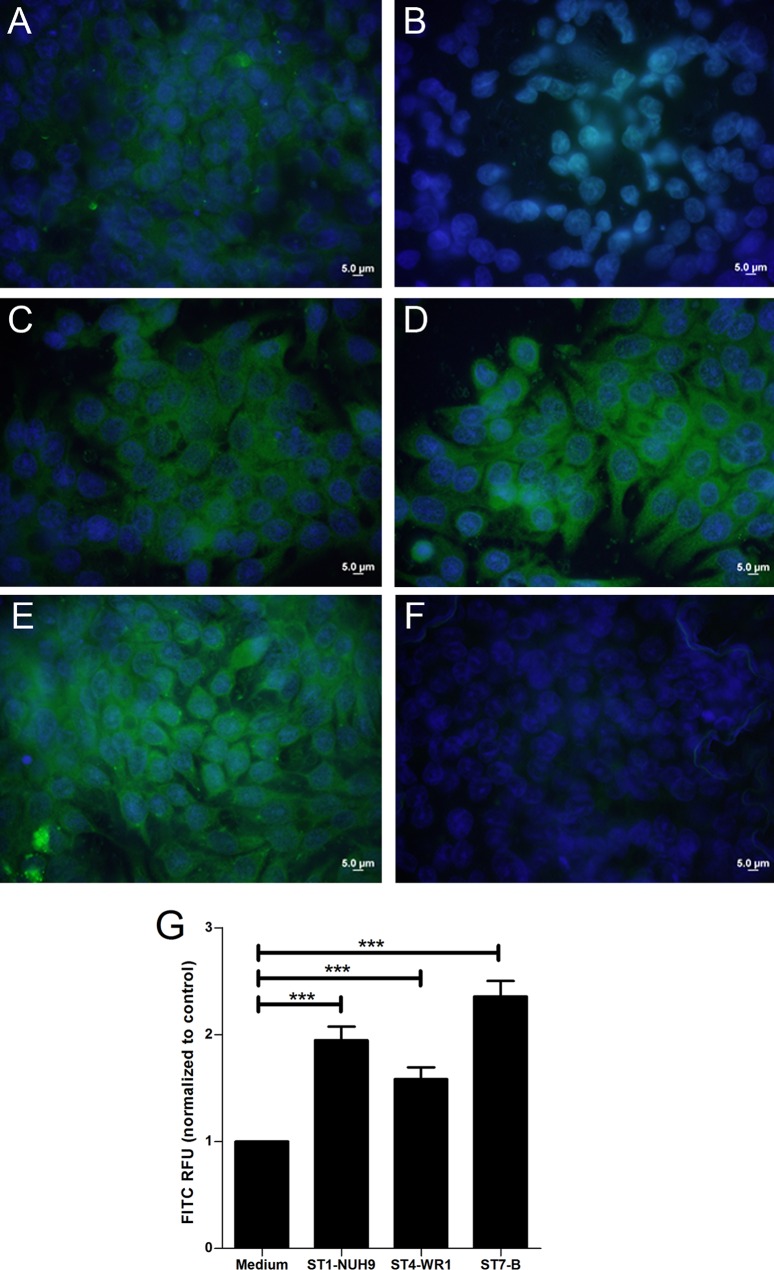
Blastocystis induces cathelicidin expression in mouse intestinal explants and human intestinal epithelial cells. Mouse intestinal samples were sectioned into terminal ileum, cecum, proximal colon, and distal colon sections and subsequently inoculated with Blastocystis ST7-B cells. qRT-PCR analysis showed that mouse distal colon explants showed a net upregulation of the CRAMP gene when exposed to the parasite (Fig. 1A). HT-29 cells also showed an upregulation of cathelicidin gene expression when coincubated with the Blastocystis ST1-NUH9, ST4-WR1, and ST7-B isolates (Fig. 1B). ELISA results showed an increase in the cathelicidin concentration in the HT-29 culture supernatant when incubated with Blastocystis (Fig. 1C). At equal MOIs, ST1-NUH9 showed the highest level of induction, followed by the ST7-B and ST4-WR1 isolates. HT-29 cells showed 95% viability in all the wells, and no significant difference in viability was found between cells with parasites and those without (see Fig. S1 in the supplemental material). Fluorescence microscopy also showed an increase in FITC staining of HT-29 cells incubated with Blastocystis (Fig. 2).
FIG 1.
Blastocystis induces LL-37 expression in mouse distal colon explants and HT-29 colonic epithelial cells. (A) Intestines from five mice were externalized and partitioned into terminal ileum (TI), cecum, proximal colon (PC), and distal colon (DC). These segments were exposed to Blastocystis ST7-B cells for 1 h. CRAMP gene expression was determined by using qRT-PCR. Distal colon explants show significant upregulation of the CRAMP gene. (B) Three Blastocystis isolates (NUH9, WR1, and B) representing three STs (ST1, -4, and -7, respectively) also induced CAMP gene expression in confluent HT-29 cells after 30 min of coincubation at an MOI of 10. (C) The supernatant from the coculture shows a significant increase in the LL-37 content after 1 h of coincubation, as determined by an ELISA. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
FIG 2.
Confluent HT-29 cells in monolayers increase the production of cathelicidin after incubation with Blastocystis. (A and B) Immunofluorescence assay images show a basal level of cathelicidin production in differentiated HT-29 cells (A), which was absent in undifferentiated cells (B). (B to E) Differentiated HT-29 cells incubated with Blastocystis ST1-NUH9 (C), ST4-WR1 (D), and ST7-B (E) show brighter fluorescence after 1 h of coincubation at an MOI of 10. (F) Negative control. (G) Total fluorescence units were quantified by using ImageJ software. RFU, relative fluorescence units. ***, P < 0.0001.
LL-37 disrupts the Blastocystis cell membrane.
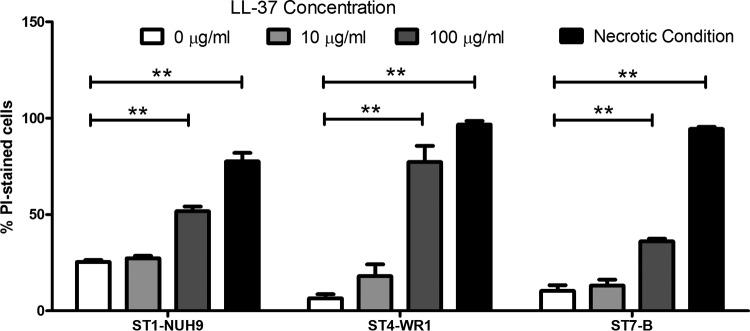
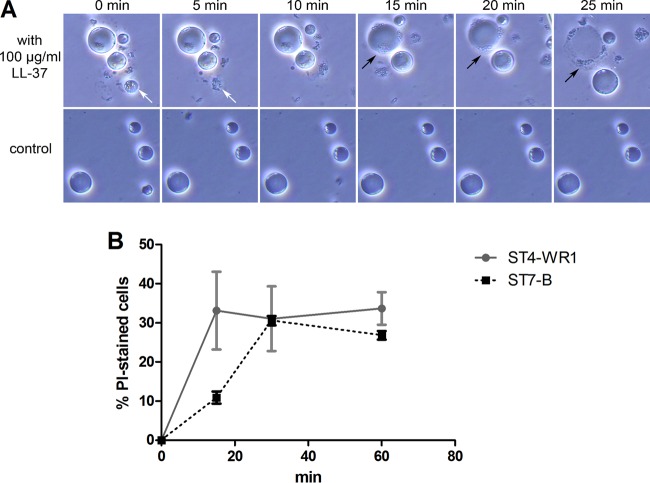
Three isolates (ST1-NUH9, ST4-WR1, and ST7-B), each representing a particular subtype, were incubated with 0, 10, and 100 μg/ml LL-37 for 1 h, and membrane disruption was analyzed by staining with PI. The proportion of cells with PI staining was determined by flow cytometry. All three isolates showed increases in the proportions of permeabilized cells as the concentration of LL-37 increased. At 100 μg/ml LL-37, >50% of ST1-NUH9 and ST4-WR1 cells had PI staining, while only 36% of ST7-B cells showed PI staining (Fig. 3). Time-lapse micrographs show that by as early as 5 min of incubation of the Blastocystis ST7-B isolate with the peptide, smaller cells were already undergoing lysis. Larger cells were observed being lysed after 20 min. Before complete lysis, vesicles were seen forming in a few sections of the cell membrane (Fig. 4A). The cells appeared to flatten as well. In addition, the membrane-disruptive effect of LL-37 on a “resistant” isolate (ST7-B) was slower than that on a “sensitive” isolate (ST4-WR1). Flow cytometry analysis showed that >30% of ST4-WR1 cells had PI staining after 15 min of treatment with LL-37, while it took 30 min of treatment for ST7-B cells to reach this proportion (Fig. 4B).
FIG 3.
LL-37 causes disruption of the Blastocystis cell membrane. PI staining and flow cytometry were used to detect cells with permeabilized membranes. Graphs show proportions of PI-stained cells in ST1-NUH9, ST4-WR1, and ST7-B populations after incubation with 0, 10, and 100 μg/ml LL-37 for 1 h. Necrotic conditions were used as a positive control for PI staining. This was attained by heating Blastocystis cells for 15 min at 80°C. There is an increase in the proportion of permeabilized Blastocystis cells at higher concentrations of LL-37. The ST-B isolate showed relative resistance to LL-37 compared to both the ST1-NUH9 and ST4-WR1 isolates. **, P < 0.001.
FIG 4.
(A) Time lapse images of Blastocystis ST7-B cells treated with 100 μg/ml LL-37 (top) and controls (bottom). Images were taken every 5 min. Arrows point to cells undergoing lysis. Cells are lysed as early as 5 min after the addition of LL-37 (white arrow). (B) Time course lysis experiments using PI staining and flow cytometry were also done. Graphs show that the membrane-disrupting effect of LL-37 is faster among ST4-WR1 than among ST7-B cells.
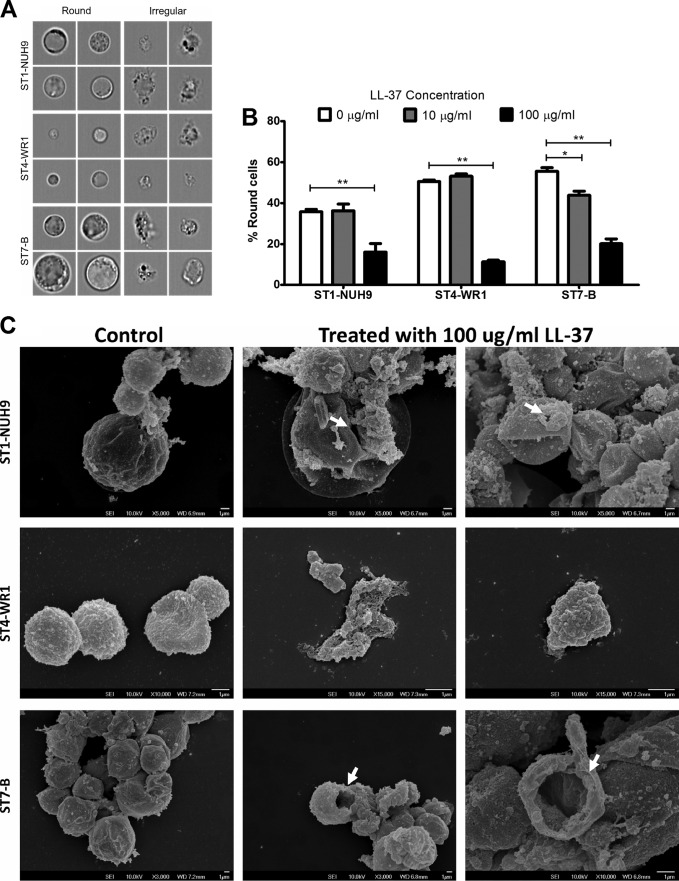
LL-37 causes morphological changes in Blastocystis. An imaging flow cytometer was used to analyze the effect of LL-37 on the morphology of parasites (Fig. 5A). On average, Blastocystis cells appear round, with the cytoplasm being pushed to the edge by a large central vacuole. Irregularly shaped cells in culture are an indication of poor viability. All three of the isolates studied showed a decrease in the proportion of round cells when LL-37 was added to the cultures. At 10 μg/ml LL-37, the change in the percentage of round cells was minimal. At 100 µg/ml LL-37, the proportion of round cells is lower by at least half than for untreated cultures in all the ST populations (Fig. 5B). Scanning electron microscopy analysis showed the possible phenotypic features of affected Blastocystis cells. LL-37-treated cells exhibited membrane pores and compressed cells, which may be due to cytoplasmic leakage. ST4-WR1 cells also showed fragmentation when treated with LL-37 (Fig. 5C).
FIG 5.
LL-37 causes morphological changes in Blastocystis isolates. Blastocystis cells were incubated with LL-37 for 1 h and analyzed by using an imaging flow cytometer. (A) Images show round and irregular cells, as determined by the circularity index generated by the software. (B) Bar graphs show that treatment of Blastocystis STs with LL-37 decreases the proportion of round cells. (C) Scanning electron micrographs also show that these cells have irregular shapes when treated with LL-37. The white arrows point to possible large membrane pores that could lead to cytoplasmic leakage. LL-37-treated Blastocystis ST4-WR1 cells also became highly fragmented. *, P < 0.05; **, P < 0.001.
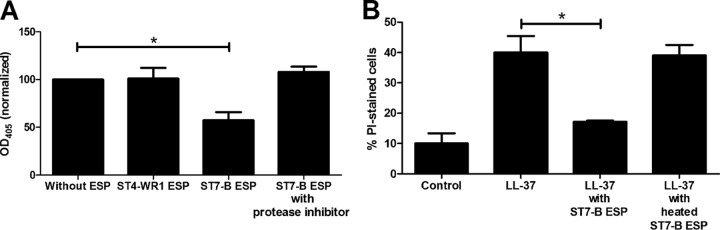
Blastocystis ST7-B, but not ST4-WR1, secretes proteases that can degrade LL-37. A modified ELISA was used to determine if Blastocystis ESPs can degrade LL-37. We used ESPs from ST7-B and ST4-WR1 cultures to represent LL-37-resistant and LL-37-susceptible isolates, respectively. The absorbance of wells after incubation with ESPs of the ST7-B isolate was lower than that of wells after incubation with ESPs of ST4-WR1 (Fig. 6A). This indicates that ST7-B isolates produce proteases that can degrade LL-37. This degradation was inhibited when ESPs were incubated with a protease cocktail inhibitor prior to the degradation assay. In addition, PI staining was also used to determine the viability of Blastocystis cells after treatment with LL-37 previously incubated with the ST7-B ESPs (Fig. 6B). The secreted proteases in the ST7-B ESPs inactivated LL-37 activity on Blastocystis cells. This, however, was reversed when ESPs were previously heat denatured before incubation with LL-37.
FIG 6.
(A) Blastocystis isolate ST7-B (but not ST4-WR1) excreted-secreted products (ESPs) degrade LL-37, as shown by ELISA results. ELISA plates were coated with the LL-37 peptide, and the plates were incubated with Blastocystis ESPs for 2 h. Degradation was prevented when ST7-B ESPs were incubated with a protease inhibitor cocktail prior to incubation with the peptide. OD405, optical density at 405 nm. (B) Viability experiments were also done by using LL-37 previously incubated with ST7-B ESPs. The graph shows that ST7-B ESPs attenuated LL-37 activity. This was reversed when the ESP was first heated to denature proteins. *, P < 0.05.
The Blastocystis ST7-B isolate decreases the pH in culture and attenuates LL-37 activity. The pH of complete medium for Blastocystis was adjusted and used to determine if it has an effect on the activity of LL-37 on Blastocystis. At pH 6.6, LL-37 had a minimal effect on the Blastocystis ST7-B and ST4-WR1 isolates, with cell counts at 90% and 88%, respectively, compared to that with untreated cultures. At pH 7.1, the relative resistance of ST7-B was observed and compared to that of ST4-WR1. Cell counts at this pH with LL-37 were 76% and 41% for the ST7-B and ST4-WR1 isolates, respectively, compared to untreated cultures. At pH 8.0, ST7-B and ST4-WR1 cell counts were 44% and 38%, respectively, of those of untreated cultures. At this pH, the highest level of activity of LL-37 was observed, which negated the relative resistance of the ST7-B isolate (Fig. 7A). We also determined the pH of Blastocystis culture medium after 24 h. The average pH of the Blastocystis ST7-B culture was 6.8, while that of the ST-WR1 culture was 7.0 (Fig. 7B).
FIG 7.
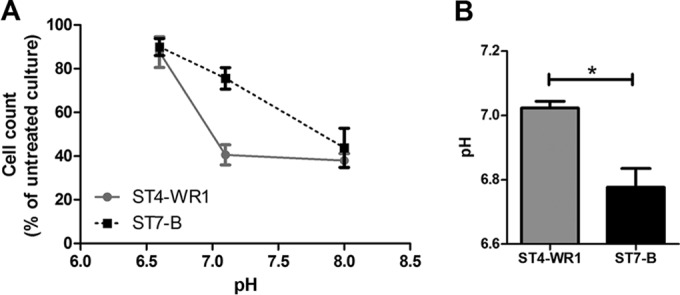
pH affects the activity of LL-37 on Blastocystis. (A) At acidic pH, LL-37 does not inhibit Blastocystis, while at neutral and alkaline pHs, both ST4-WR1 and ST7-B are inhibited by the AMP. (B) Blastocystis ST7-B (but not ST4-WR1) cultures cause the medium to be acidic after 24 h. *, P < 0.05.
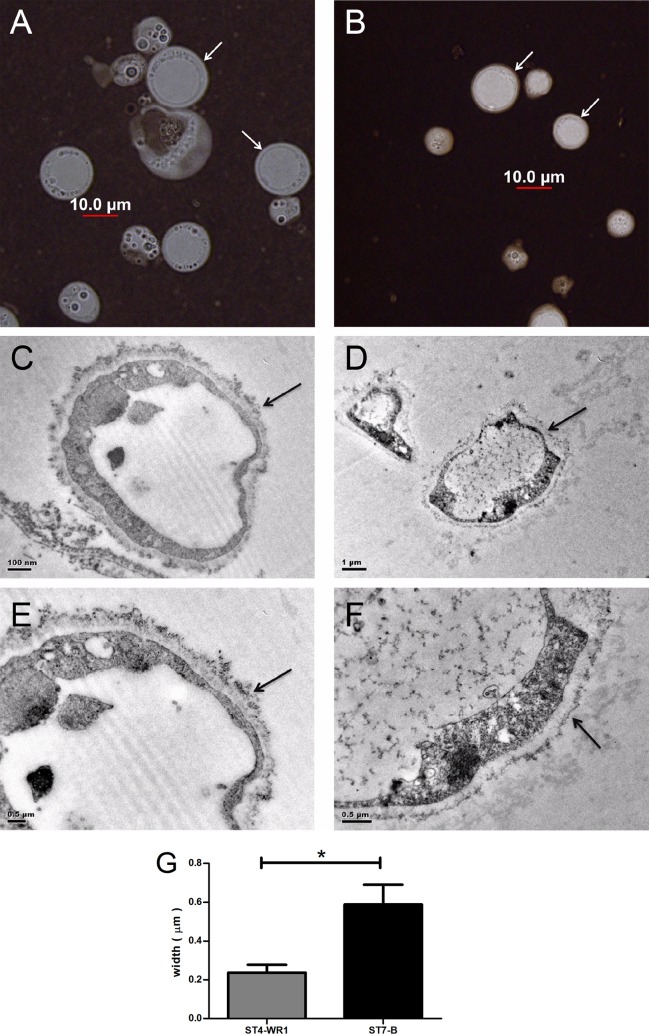
The Blastocystis ST7-B isolate has a thick surface coat compared to that of ST4-WR1. India ink was used as negative stain to visualize the surface coat of Blastocystis cells. Most cells from ST7-B cultures showed thicker surface coats (Fig. 8A and B). To observe the surface closely, we used transmission electron microscopy (TEM). Cationized ferritin was used to label negatively charged elements on the cell surface. Again, TEM showed a thicker surface coat for ST7-B cells than for ST4-WR1 cells (Fig. 8C to F). The coat's thickness in ST7-B cells was almost three times that of the coats in ST4-WR1 cells (Fig. 8G). Furthermore, ST7-B surface coats also appeared to be denser.
FIG 8.
Blastocystis ST7-B cells (A, C, and E) have thicker and denser surface coats (arrows) than do ST4-WR1 cells (B, D, and F), as shown by using India ink as a negative stain for bright microscopy (A and B) and transmission electron microscopy (C, D, E, and F). (G) Images from TEM were used to measure the thickness of the surface coat. The surface coat of the ST7-B isolate is more than double the thickness of that of the ST4-WR1 isolate. *, P < 0.05.
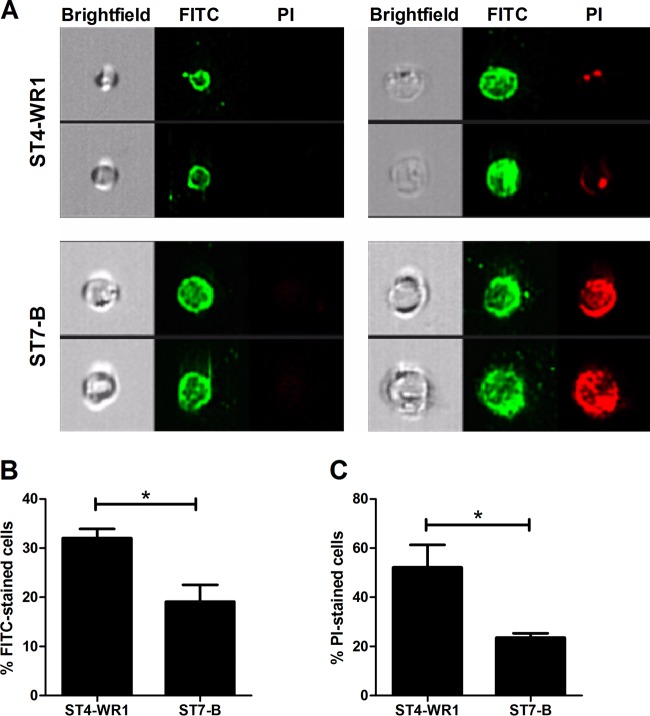
LL-37 binds more to Blastocystis ST4-WR1 cell surfaces than to ST7-B cell surfaces. Flow cytometry imaging was used to visualize LL-37 bound to Blastocystis cells (Fig. 9A). The proportion of cells with bound LL-37 was higher for ST4-WR1 than for ST7-B (Fig. 9B). Gating out for those cells with bound LL-37, we found that a high percentage of ST7-B cells was viable compared to the percentage of viable ST4-WR1 cells (Fig. 9C). The specificity of the primary antibody used was demonstrated by using LL-37-treated and untreated Blastocystis cells with or without the primary or secondary antibody.
FIG 9.
LL-37 binds to the cell surface of Blastocystis. (A) Images from the bright-field, FITC, and PI channels are displayed, showing both viable and nonviable cells with bound LL-37. LL-37 was detected by using FITC-conjugated antibodies. Viability was determined by using PI staining. (B) Blastocystis ST4-WR1 had a higher proportion of cells with bound LL-37 peptide. (C) Among the cells with bound LL-37, Blastocystis ST4-WR1 had a lower level of viability. *, P < 0.05.
DISCUSSION
Blastocystis causes large-bowel infections among humans, where it may cause diarrhea, abdominal pain, nausea, and other related symptoms. The organism is transmitted by the fecal-oral route by the means of the cyst form. Upon reaching the large intestine, it transforms into its various vegetative forms. Pathogenic events include epithelial barrier disruption, adhesion, modulation, and evasion of host immune responses (5). Since most infections are self-limiting, it may be presumed that in these cases, Blastocystis is eradicated before it can colonize and penetrate the intestinal barrier. Events surrounding the removal of this parasite need to be elucidated. AMPs may be involved in these events, and therefore their possible roles in limiting Blastocystis-related pathologies need to be determined.
Host AMPs are important elements of innate immunity. AMP activities include membrane pore formation, which may cause cell death directly or allow other molecules to pass through the membrane and bind to intracellular targets (27). Some of these molecules are expressed by epithelial cells and provide an initial defense against invading pathogens. In this study, we explored the role of these peptides in human Blastocystis infections. We focused on the activity of LL-37 on Blastocystis after determining that this peptide has broad activity against all the Blastocystis STs that we tested using a screening assay (Table 1). There have been numerous studies showing the effectiveness of AMPs against bacterial, fungal, and even viral pathogens. There are, however, few studies on the action of AMPs on protistan parasites. A review (17) on the effect of AMPs on two parasites that cause the tropical diseases leishmaniasis and malaria outlined both positive and negative aspects of the use of AMPs against protists. Studies have shown that AMPs can also disrupt cellular processes in parasites (17). This makes AMPs promising candidates for drug development. Regarding the use of AMPs in human Blastocystis infections, one study found that analogs of an anuran skin peptide called magainin were effective against Blastocystis hominis, Trypanosoma cruzi, and Entamoeba histolytica (28). This 2-decade-old study showed that these AMPs can disrupt the cell membrane, leading to leakage of cell contents and, eventually, death.
Blastocystis is an enteric parasite, and it is expected to stimulate host epithelial cells to mount immune responses. For example, Blastocystis was found to induce nitric oxide production in epithelial cells as part of the host defense (14). In addition, Blastocystis could also induce epithelial cells to express antimicrobial peptides as part of the host's innate immune response. This is seen in other gastrointestinal infections by protistan parasites such as Entamoeba (29) and Cryptosporidium (30). In this study, we have also found that all 3 represented Blastocystis STs are capable of inducing the expression and secretion of LL-37 by intestinal epithelial cells (Fig. 1B and C and Fig. 2). Blastocystis ST7-B was also able to induce LL-37 gene expression in mouse distal colon explants (Fig. 1A). This could indicate that LL-37 plays a role when Blastocystis tries to colonize the intestine. It is possible that LL-37 is among the first host defense peptides that are secreted in order to prevent parasite colonization and invasion. Furthermore, the differences in the abilities of the STs to induce the expression of LL-37 could also be relevant. Blastocystis ST1-NUH9 is relatively sensitive to LL-37 and was found to induce the highest level of secretion of LL-37 in HT-29 cells. This suggests that the pathogenic potential of ST1-NUH9 would be weaker than that of the ST7-B isolate.
As with AMP studies done on other protistan parasites, Blastocystis is likely to be susceptible to the direct killing effect of AMPs. As mentioned above, Blastocystis is composed of many subtypes showing various biological properties, and it is probable that a few STs will be resistant. Not all AMPs, however, may be effective against Blastocystis, although its mechanism of resistance may be different from that of prokaryotic organisms. For example, the membrane surface of Blastocystis, a eukaryotic organism, has a structure different from that of prokaryotes, and elements of this parasite's membrane may shield the negative charges. This difference may a factor in the relative resistance of the parasite to antimicrobial peptide treatment. Some bacteria produce proteases that can inactivate AMPs (31–33). This may also be the case in Blastocystis.
In this study, resazurin-based viability assays showed that among the intestinal AMPs, only hBD-2 and LL-37 can inhibit Blastocystis. The effectiveness of hBD-2, however, is limited only to ST4. hBD-1 is constitutively expressed in colonic epithelial cells. It was found to kill a number of bacterial species, whether Gram negative or Gram positive (34). We did not find any inhibition when this peptide was added to Blastocystis cultures. hBD-1 and hBD-2 share very similar structures. A difference is found only on the tri-/disulfide motifs (35). Both motifs have been found to kill Staphylococcus aureus and Escherichia coli. It is interesting to note, therefore, that only hBD-2 can inhibit Blastocystis.
On the other hand, LL-37 has been found to be effective against all Blastocystis STs. Cathelicidins are characterized by a conserved N-terminal domain that is proteolytically cleaved to generate the mature, active peptide contained within the C terminus (36). These molecules are expressed in various immune cells, in salivary glands, and in epithelia of respiratory, digestive, and reproductive tracts, while keratinocytes and intestinal cells can be induced to enhance expression. Its main effect is pore formation in bacterial membranes. It was also found to be capable of modulating toxic effects due to bacterial infection (37). LL-37's other activities include a chemotactic effect on blood cells, activation of histamine release from mast cells, or induction of angiogenesis (38). The absence of LL-37 has been associated with chronic periodontal disease (36). The only cathelicidin identified in humans is termed LL-37, indicating the 37-amino-acid sequence. It has been found to inhibit a number of Gram-positive, Gram-negative, and Gram-indeterminate bacterial species (39). It has also been found to cause membrane disruption with vacuolar enlargement in Candida albicans within 5 min (40). We have found the same effect on all representative isolates of Blastocystis ST1, ST4, and ST7. LL-37 causes a decrease in viability (Table 1) and membrane permeabilization, as observed by using PI staining (Fig. 5). We also observed the rapidity of LL-37 action. Blastocystis cells were lysed within 5 min of treatment with LL-37 (Fig. 4). The change in the morphology of cells from round to irregular was also observed by using imaging flow cytometry as well as scanning electron microscopy (Fig. 5). Our previous observation has been that irregularly shaped Blastocystis cells are an indication of poor health (41). LL-37, as it causes pore formation, may be responsible for the shape change in Blastocystis cells.
However, as determined by viability assays, the effects of LL-37 are not similar for all the isolates tested, as ST7 isolates have higher IC50s (Table 1). PI staining (Fig. 3) and observed morphological changes (Fig. 5) also confirm this finding. The various effects of LL-37 on different Blastocystis STs suggest that its effectiveness is subtype dependent. Blastocystis ST7 isolates showed relative resistance compared to ST4 and ST1 isolates. We therefore attempted to identify the factors in Blastocystis ST7 isolates that may be responsible for their relative resistance to LL-37.
Blastocystis is known to release proteases (42, 43). Some of these proteases have been found to directly protect the parasite, such as those that can degrade antibodies (43). In this study, we have observed that the excretory-secretory products of Blastocystis contain proteases that can degrade LL-37. This effect was seen only in an ST7 isolate. In a sensitive strain such as ST4-WR1, this finding was not observed (Fig. 6). A study on the effect of LL-37 on an intestinal parasite, Entamoeba histolytica, also revealed the same outcome (29). The resistance of E. histolytica to LL-37 was partly due to its ability to release cysteine proteases that could degrade LL-37.
The attenuation of LL-37 by pH has been reported in previous bacterial studies. An acidic pH decreased the ability of LL-37 to kill Staphylococcus aureus and Pseudomonas aeruginosa (44). That study found that decreasing the pH from 8.0 to 6.8 can attenuate LL-37 activity. In this study, we also observed that at pH 6.5, both Blastocystis ST4-WR1 and ST7-B were not affected by LL-37. However, at alkaline pH (pH 8.0), both isolates became susceptible to LL-37 killing (Fig. 7A). Furthermore, we have determined that the Blastocystis ST7-B isolate is able to cause a change in the pH after 24 h. We have observed that the pH adjustment can go as low as pH 6.5 (Fig. 7B). This is enough to decrease the activity of LL-37 and protect the parasite from direct killing. In contrast, this pH adjustment was not seen in ST4-WR1 cultures and therefore may be a reason why this isolate is more susceptible to LL-37 attack.
Blastocystis cells feature an outer surface coat (45, 46) that has been proposed to confer protection to the parasite (5). We have observed that the characteristics of this surface can vary from one isolate to another. Using a negative stain (India ink), we observed that the thickness of the surface coat is greater in Blastocystis ST7-B than in Blastocystis ST4-WR1 cells (Fig. 8A and B). Using transmission electron microscopy, the former also showed a denser structure than the latter (Fig. 8C to F). Recently, a study found LL-37 sequestration by a membrane-associated protein in a virulent bacterium (47). This protects the bacterium by neutralizing the activity of LL-37. It is also possible that some elements in the Blastocystis surface coat have the same function. LL-37 was seen to bind to the surface of the cell in the two isolates tested (ST7-B and ST4-WR1), but isolate ST7-B was less affected than ST4-WR1, as observed from the proportion of PI-stained cells (Fig. 9). It is possible that due to the thickness and density of the surface coat of the ST7-B isolate, LL-37 molecules may become trapped and may not have access to the parasite's cell membrane. This would then protect the cell from lysis.
Blastocystis ST1, along with ST3, is the most frequently detected ST in parasitological surveys in the world (8). ST4 is commonly detected in Europe. These particular STs are highly sensitive to LL-37 (Table 1 and Fig. 3 and 5). This could be one of the reasons why most Blastocystis infections are asymptomatic and self-limiting, as reported previously (5, 13). On the other hand, ST7-B is relatively resistant to LL-37 and is also rare in terms of being detected in human populations. This ST is also resistant to a number of drugs, including metronidazole (13, 48), and is capable of downregulating the expression of nitric oxide synthase (14), which is an essential element in the innate immune response. Isolates of ST7 have also been found to cause epithelial barrier disruption and have more adhesive properties in cell cultures (49), thereby making this ST potentially more pathogenic than the rest of the STs. Increases in prevalence rates of Blastocystis ST7 isolates therefore would pose greater risks to the population.
In conclusion, this study explored the interactions between Blastocystis and the antimicrobial peptide LL-37 (Fig. 10). This is the first study to determine the effects of an important element in innate immunity to Blastocystis, a common eukaryote found in the human colon. Our ex vivo and in vitro culture experiments indicate that LL-37 is a significant molecule secreted by the host during Blastocystis infections. We have also shown for the first time that Blastocystis isolates are susceptible to the effects of LL-37. The ST7-B isolate is able to alleviate the detrimental effects of this AMP by various modes: first by the secretion of proteases that degrade LL-37 and second by making its environment acidic, which is enough to attenuate LL-37 activity. We also speculate on the protective function of the parasite's surface coat against AMPs. This study further differentiates the subtypes of Blastocystis, particularly regarding host immune responses and the parasite's ability to evade these responses.
FIG 10.
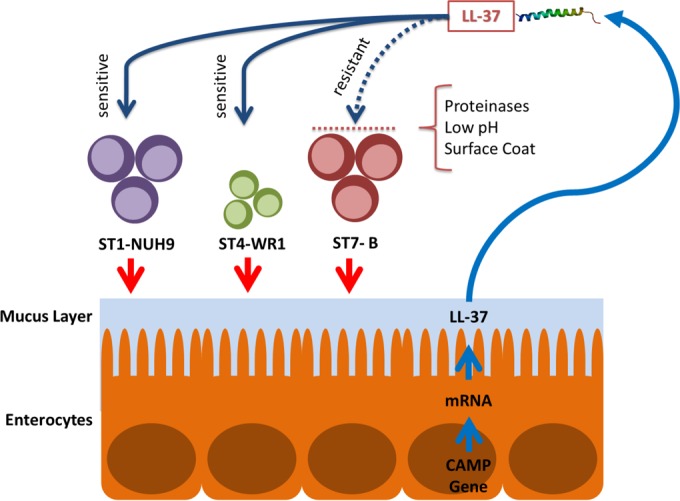
Interactions between Blastocystis subtypes and LL-37. Blastocystis STs induce epithelial cells to secrete LL-37. ST1 and ST4 are susceptible to LL-37 attack, while ST7 is relatively resistant. An isolate of ST7 (B isolate) is found to secrete proteinases that degrade LL-37. It also decreases the pH, which decreases LL-37 activity. It also has a thicker surface coat that could possibly protect the parasite against LL-37 binding. (The LL-37 structure was obtained from RCSB Protein Data Bank [PDB] accession number 2K6O [50].)
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Geok Choo Ng, Josephine Howe, Zhaona Wu, and Wan Ni Chia for technical assistance.
J.A.Y. acknowledges the generous Graduate Research Scholarship grant from the National University of Singapore.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00339-16.
REFERENCES
- 1.Clark CG, van der Giezen M, Alfellani MA, Stensvold CR. 2013. Recent developments in Blastocystis research. Adv Parasitol 82:1–32. doi: 10.1016/B978-0-12-407706-5.00001-0. [DOI] [PubMed] [Google Scholar]
- 2.Scanlan PD, Stensvold CR, Rajilić-Stojanović M, Heilig HGHJ, De Vos WM, O'Toole PW, Cotter PD. 2014. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol 90:326–330. doi: 10.1111/1574-6941.12396. [DOI] [PubMed] [Google Scholar]
- 3.Bart A, Wentink-Bonnema EM, Gilis H, Verhaar N, Wassenaar CJ, van Vugt M, Goorhuis A, van Gool T. 2013. Diagnosis and subtype analysis of Blastocystis sp. in 442 patients in a hospital setting in the Netherlands. BMC Infect Dis 13:389. doi: 10.1186/1471-2334-13-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stensvold CR, Suresh GK, Tan KSW, Thompson RCA, Traub RJ, Viscogliosi E, Yoshikawa H, Clark CG. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol 23:93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Tan KSW. 2008. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21:639–665. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle CM, Varughese J, Weiss LM, Tanowitz HB. 2012. Blastocystis: to treat or not to treat. Clin Infect Dis 54:105–110. doi: 10.1093/cid/cir810. [DOI] [PubMed] [Google Scholar]
- 7.Poirier P, Wawrzyniak I, Vivarès CP, Delbac F, El Alaoui H. 2012. New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog 8:e1002545. doi: 10.1371/journal.ppat.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfellani MA, Stensvold CR, Vidal-Lapiedra A, Onuoha ESU, Fagbenro-Beyioku AF, Clark CG. 2013. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop 126:11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Ramírez JD, Sánchez A, Hernández C, Flórez C, Bernal MC, Giraldo JC, Reyes P, López MC, García L, Cooper PJ, Vicuña Y, Mongi F, Casero RD. 2016. Geographic distribution of human Blastocystis subtypes in South America. Infect Genet Evol 41:32–35. doi: 10.1016/j.meegid.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Mirza H, Tan KSW. 2009. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol Res 104:355–361. doi: 10.1007/s00436-008-1203-1. [DOI] [PubMed] [Google Scholar]
- 11.Ramírez JD, Sánchez LV, Bautista DC, Corredor AF, Flórez AC, Stensvold CR. 2014. Blastocystis subtypes detected in humans and animals from Colombia. Infect Genet Evol 22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Roberts T, Stark D, Harkness J, Ellis J. 2013. Subtype distribution of Blastocystis isolates from a variety of animals from New South Wales, Australia. Vet Parasitol 196:85–89. doi: 10.1016/j.vetpar.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Roberts T, Stark D, Harkness J, Ellis J. 2014. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog 6:17. doi: 10.1186/1757-4749-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza H, Wu Z, Kidwai F, Tan KSW. 2011. A metronidazole-resistant isolate of Blastocystis spp. is susceptible to nitric oxide and downregulates intestinal epithelial inducible nitric oxide synthase by a novel parasite survival mechanism. Infect Immun 79:5019–5026. doi: 10.1128/IAI.05632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safadi DE, Meloni D, Poirier P, Osman M, Cian A, Gaayeb L, Wawrzyniak I, Delbac F, Alaoui HE, Delhaes L, Dei-Cas E, Mallat H, Dabboussi F, Hamze M, Viscogliosi E. 2013. Molecular epidemiology of Blastocystis in Lebanon and correlation between subtype 1 and gastrointestinal symptoms. Am J Trop Med Hyg 88:1203–1206. doi: 10.4269/ajtmh.12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanetti M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75:39–48. [DOI] [PubMed] [Google Scholar]
- 17.Torrent M, Pulido D, Rivas L, Andreu D. 2012. Antimicrobial peptide action on parasites. Curr Drug Targets 13:1138–1147. doi: 10.2174/138945012802002393. [DOI] [PubMed] [Google Scholar]
- 18.Yin LM, Edwards MA, Li J, Yip CM, Deber CM. 2012. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J Biol Chem 287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahlenberg JM, Kaplan MJ. 2013. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevins CL, Salzman NH. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 21.Chen XQ, Singh M, Ho LC, Tan SW, Ng GC, Moe KT, Yap EH. 1997. Description of a Blastocystis species from Rattus norvegicus. Parasitol Res 83:313–318. doi: 10.1007/s004360050255. [DOI] [PubMed] [Google Scholar]
- 22.Ho LC, Singh M, Suresh G, Ng GC, Yap EH. 1993. Axenic culture of Blastocystis hominis in Iscove's modified Dulbecco's medium. Parasitol Res 79:614–616. doi: 10.1007/BF00932249. [DOI] [PubMed] [Google Scholar]
- 23.Wong KHS, Ng GC, Lin RTP, Yoshikawa H, Taylor MB, Tan KSW. 2008. Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res 102:663–670. doi: 10.1007/s00436-007-0808-0. [DOI] [PubMed] [Google Scholar]
- 24.Noël C, Dufernez F, Gerbod D, Edgcomb VP, Delgado-Viscogliosi P, Ho L-C, Singh M, Wintjens R, Sogin ML, Capron M, Pierce R, Zenner L, Viscogliosi E. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J Clin Microbiol 43:348–355. doi: 10.1128/JCM.43.1.348-355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirier P, Meloni D, Nourrisson C, Wawrzyniak I, Viscogliosi E, Livrelli V, Delbac F. 2014. Molecular subtyping of Blastocystis spp. using a new rDNA marker from the mitochondria-like organelle genome. Parasitology 141:670–681. doi: 10.1017/S0031182013001996. [DOI] [PubMed] [Google Scholar]
- 26.Souza WD, Arguello C, Martinez-Palomo A, Trissl D, Gonzáles-Robles A, Chiari E. 1977. Surface charge of Trypanosoma cruzi. Binding of cationized ferritin and measurement of cellular electrophoretic mobility. J Eukaryot Microbiol 24:411–415. [DOI] [PubMed] [Google Scholar]
- 27.Peters BM, Shirtliff ME, Jabra-Rizk MA. 2010. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CM, Chen HC, Zierdt CH. 1990. Magainin analogs effective against pathogenic protozoa. Antimicrob Agents Chemother 34:1824–1826. doi: 10.1128/AAC.34.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobo ER, He C, Hirata K, Hwang G, Tran U, Eckmann L, Gallo RL, Reed SL. 2012. Entamoeba histolytica induces intestinal cathelicidins but is resistant to cathelicidin-mediated killing. Infect Immun 80:143–149. doi: 10.1128/IAI.05029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacometti A, Cirioni O, Del Prete MS, Skerlavaj B, Circo R, Zanetti M, Scalise G. 2003. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J Antimicrob Chemother 51:843–847. doi: 10.1093/jac/dkg149. [DOI] [PubMed] [Google Scholar]
- 31.Schmidtchen A, Frick I-M, Andersson E, Tapper H, Björck L. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol 46:157–168. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 32.Thwaite JE, Hibbs S, Titball RW, Atkins TP. 2006. Proteolytic degradation of human antimicrobial peptide LL-37 by Bacillus anthracis may contribute to virulence. Antimicrob Agents Chemother 50:2316–2322. doi: 10.1128/AAC.01488-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho S, Pothoulakis C, Koon HW. 2013. Antimicrobial peptides and colitis. Curr Pharm Des 19:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selsted ME, Tang YQ, Morris WL, McGuire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS. 1993. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem 268:6641–6648. [PubMed] [Google Scholar]
- 36.Wiesner J, Vilcinskas A. 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 37.Bucki R, Leszczyńska K, Namiot A, Sokołowski W. 2010. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz) 58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 38.Jäger S, Stange EF, Wehkamp J. 2013. Antimicrobial peptides and inflammatory bowel disease, p 255–273. In Hiemstra PS, Zaat SAJ (ed), Antimicrobial peptides and innate immunity. Springer, Basel, Switzerland. [Google Scholar]
- 39.Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Ordonez SR, Amarullah IH, Wubbolts RW, Veldhuizen EJA, Haagsman HP. 2014. Fungicidal mechanisms of cathelicidins LL-37 and CATH-2 revealed by live-cell imaging. Antimicrob Agents Chemother 58:2240–2248. doi: 10.1128/AAC.01670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yason JA, Tan KSW. 2015. Seeing the whole elephant: imaging flow cytometry reveals extensive morphological diversity within Blastocystis isolates. PLoS One 10:e0143974. doi: 10.1371/journal.pone.0143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sio SWS, Puthia MK, Lee ASY, Lu J, Tan KSW. 2006. Protease activity of Blastocystis hominis. Parasitol Res 99:126–130. doi: 10.1007/s00436-006-0131-1. [DOI] [PubMed] [Google Scholar]
- 43.Puthia MK, Vaithilingam A, Lu J, Tan KSW. 2005. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol Res 97:386–389. doi: 10.1007/s00436-005-1461-0. [DOI] [PubMed] [Google Scholar]
- 44.Alaiwa MHA, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. 2014. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci U S A 111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaman V, Howe J, Ng M, Goh TK. 1999. Scanning electron microscopy of the surface coat of Blastocystis hominis. Parasitol Res 85:974–976. doi: 10.1007/s004360050668. [DOI] [PubMed] [Google Scholar]
- 46.Lanuza MD, Carbajal JA, Borrás R. 1996. Identification of surface coat carbohydrates in Blastocystis hominis by lectin probes. Int J Parasitol 26:527–532. doi: 10.1016/0020-7519(96)00010-0. [DOI] [PubMed] [Google Scholar]
- 47.LaRock CN, Döhrmann S, Todd J, Corriden R, Olson J, Johannssen T, Lepenies B, Gallo RL, Ghosh P, Nizet V. 2015. Group A streptococcal M1 protein sequesters cathelicidin to evade innate immune killing. Cell Host Microbe 18:471–477. doi: 10.1016/j.chom.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirza H, Teo JDW, Upcroft J, Tan KSW. 2011. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob Agents Chemother 55:637–648. doi: 10.1128/AAC.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Z, Mirza H, Tan KSW. 2014. Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7. PLoS Negl Trop Dis 8:e2885. doi: 10.1371/journal.pntd.0002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G. 2008. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283:32637–32643. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.