Abstract
It is unclear whether naturally acquired immunity to Plasmodium falciparum results from the acquisition of antibodies to multiple, diverse antigens or to fewer, highly conserved antigens. Moreover, the specific antibody functions required for malaria immunity are unknown, and hence informative immunological assays are urgently needed to address these knowledge gaps and guide vaccine development. In this study, we investigated whether merozoite-opsonizing antibodies are associated with protection from malaria in a strain-specific or strain-transcending manner by using a novel field isolate and an immune plasma-matched cohort from Papua New Guinea with our validated assay of merozoite phagocytosis. Highly correlated opsonization responses were observed across the 15 parasite strains tested, as were strong associations with protection (composite phagocytosis score across all strains in children uninfected at baseline: hazard ratio of 0.15, 95% confidence interval of 0.04 to 0.63). Opsonizing antibodies had a strong strain-transcending component, and the opsonization of transgenic parasites deficient for MSP3, MSP6, MSPDBL1, or P. falciparum MSP1-19 (PfMSP1-19) was similar to that of wild-type parasites. We have provided the first evidence that merozoite opsonization is predominantly strain transcending, and the highly consistent associations with protection against diverse parasite strains strongly supports the use of merozoite opsonization as a correlate of immunity for field studies and vaccine trials. These results demonstrate that conserved domains within merozoite antigens targeted by opsonization generate strain-transcending immune responses and represent promising vaccine candidates.
INTRODUCTION
Despite progress toward a reduced global burden of malaria, parasites of the genus Plasmodium continue to cause approximately 200 million clinical cases and 600,000 deaths annually (1). Although several malaria vaccines are currently in clinical trials, none has yet shown sufficient efficacy in settings of malaria endemicity to be a stand-alone vaccine against the highly complex, variant, and virulent Plasmodium falciparum parasite. Development of effective vaccines requires knowledge of the essential mechanisms for protective immunity, as well as robust assays to serve as correlates of immunity. The merozoite represents an attractive vaccine target since it is briefly exposed to the immune system prior to host cell invasion and since antibodies to numerous merozoite antigens have been associated with protective immunity in humans (2, 3).
Exactly which antibody functions are necessary to control parasitemia and clinical symptoms during natural infection remain unclear. Direct inhibition of parasite growth is commonly used in preclinical vaccine development; however, associations between growth-inhibitory antibodies measured in vitro and protective immunity have been inconsistent (4, 5). Recent evidence has strongly implicated antibodies that opsonize P. falciparum and elicit parasite killing through Fc receptor-dependent mechanisms as important for naturally acquired immunity (6–8). We previously developed a robust in vitro assay to measure opsonization-dependent merozoite phagocytosis (9, 10) and have demonstrated that phagocytic responses to the 3D7 P. falciparum laboratory strain are associated with protective immunity in a Papua New Guinea (PNG) cohort (6), findings that have been confirmed in African cohorts (11). However, currently it is not clear what impact antigenic diversity and the contribution of individual merozoite antigens have in generating an opsonizing antibody response, limiting the interpretive power of these assays for vaccine development.
Parasite genetic diversity is a significant hurdle to the development of effective subunit malaria vaccines, with many merozoite antigens displaying considerable sequence diversity (12). FMP2.1/AS02A and combination B vaccines that consist of a single allelic sequence of AMA-1 and MSP2, respectively, showed only partial protective efficacy that was restricted to infections with P. falciparum that expressed vaccine-like alleles (13, 14). Growth-inhibitory and trophozoite-agglutinating antibodies are acquired in a strain-specific manner (15–19); however, the impact of parasite diversity on opsonization remains unknown. More generally, it is unclear to what extent malaria immunity results from the accumulation of a broad repertoire of uniquely specific antibodies or from a narrow repertoire against conserved antigens. These questions are of critical importance as numerous single-antigen malaria vaccines are in development, and current attenuated whole-parasite vaccine strategies incorporate a single laboratory parasite strain.
Using a panel of common laboratory strains and parasites from PNG that we adapted to growth in vitro, we have comprehensively demonstrated that parasite diversity does not impact the ability of merozoite opsonization responses to predict immunity to malaria. Strikingly, similar opsonization responses were observed across all strains tested, whether the parasite line was from the same cohort as the plasma samples or not, and these responses were consistently associated with protective immunity. Through use of transgenic parasite lines, we report that the absence of prominent immunogenic merozoite surface antigen MSP3, MSP6, MSPDBL1, or MSP1-19 did not impact the overall level of merozoite phagocytosis. With the depletion of antibody reactivity to 3D7 merozoites, opsonization of merozoites from PNG strains also declined, indicating the presence of conserved antigenic targets across parasite strains. These findings demonstrate that merozoite-opsonizing antibodies in semi-immune children are cross-reactive and likely contribute to the broad protection against diverse strains that is a hallmark of naturally acquired immunity to malaria.
MATERIALS AND METHODS
Study population and ethics.
Plasma samples were obtained from a treatment-reinfection study of 198 children aged 5 to 14 years from Madang Province, PNG, and a full description of the cohort is available elsewhere (20). In brief, a plasma sample was collected at enrollment, following which all participants received 7 days of oral artesunate (4 mg/kg/day) monotherapy to clear parasitemia, in accordance with national guidelines at the time. Treatment failures were determined by MSP2 genotyping. The cohort was monitored for symptomatic illness and parasitemia for 6 months through fortnightly visits and presentation at the Mugil Health Centre, with parasitemia determined from finger-prick sampling by light microscopy (LM) and post-PCR ligase detection reaction-fluorescent microsphere assay (PCR). In this study, PCR detection of parasitemia was used to classify children as infected unless otherwise stated. Clinical malaria was defined as a measured fever (axillary temperature of ≥37.5°C) or history of febrile illness during the preceding 48 h, in conjunction with P. falciparum infection as measured by light microscopy. The study was approved by the Medical Research Advisory Committee of Papua New Guinea and by the Walter and Eliza Hall Institute Human Research Ethics Committee (HREC). Informed consent was obtained in writing from parents or guardians of all participants prior to enrollment.
Parasite strains and lab adaptation.
3D7, D10, K1, HB3, E8B, Palo Alto, and W2MEF strains were cultured as described previously (21). The D10PfMSP1-19 and D10PcMSP1-19 lines (where Pf is P. falciparum and Pc is Plasmodium chabaudi, respectively) were maintained with 24 ng/ml pyrimethamine (22), and 3D7ΔMSP3, 3D7ΔMSP6, and 3D7ΔMSPDBL1 lines were generated by transfection with constructs assembled in pCC1 (23). The PNG strain XHA_A has been reported previously (24), whereas all other PNG strains were adapted from erythrocytes cryopreserved in Glycerolyte 57 (Fenwal) from high-parasitemia baseline blood samples. Erythrocytes were thawed using a NaCl gradient and cultured in RPMI 1640 medium supplemented with 25 mg/ml HEPES, 50 μg/ml hypoxanthine, 2 mg/ml NaHC03, 5% (vol/vol) pooled AB human serum, and 0.25% (wt/vol) Albumax (Invitrogen) at 2% hematocrit for 2 weeks or until sufficiently expanded. All strains were confirmed to be single and unique strains by MSP2 genotyping (25), with XHA_C and XHA_K reduced to single clones by limiting dilution prior to use.
Phagocytosis assay.
Phagocytosis assays were performed as described previously (6, 9, 10). In brief, freshly prepared merozoites were generated by filtration of magnet-purified E64-treated schizonts (26), followed by hemozoin removal and staining with ethidium bromide (EtBr). Stained merozoites were then opsonized with diluted plasma samples (1 in 2,000) and then incubated for 40 min at 37°C with THP-1 monocytic cells. Phagocytosis by THP-1 cells was assessed by flow cytometry as the percentage of EtBr-positive cells with the test plasma minus the percentage of positive cells with a nonimmune Australian plasma pool. All cohort plasma samples were tested in a single experiment with a single batch of merozoites for each parasite line, and tests were run and analyzed blinded. For depletion experiments, 107 merozoites were added to plasma samples at a final dilution of 1/2,000 in 200 μl. Samples were incubated with gentle rolling for 1 h at room temperature (RT), followed by centrifugation (13,000 × g for 3 min) to pellet merozoites. A further 107 merozoites were used to repeat the depletion. Nondepleted plasma was handled similarly, except without the addition of merozoites. After depletion, plasma samples were used in phagocytosis assays as described above.
Statistical analysis.
Statistical analysis was performed using STATA, version 12 (STATA Corp.), and Prism, version 6.0 (GraphPad). Differences in median phagocytosis responses between categorical variables were assessed by a Wilcoxon rank sum test. For survival analyses, phagocytosis responses were categorized into equal-sized tertiles (low, medium, and high). Poisson regression for incidence rate ratios (IRR) of clinical malaria during follow-up and Cox proportional-hazard models for hazard ratios (HR) of time to first clinical episode included adjustment for age (≤9 years or >9 years) and location (≤1 km or >1 km) as described previously (20). Children that failed to clear infections following treatment at baseline (n = 12) were excluded from analysis. Tertile responses for each parasite strain were designated 0, 1, or 2 for a low, medium, or high response, respectively, and summed across strains to yield a reactivity score. These scores were then used to generate tertiles reflecting low, medium, and high reactivity scores and used in regression analysis as described above.
RESULTS
Opsonizing antibodies are influenced by P. falciparum infection and are higher in individuals protected from clinical malaria.
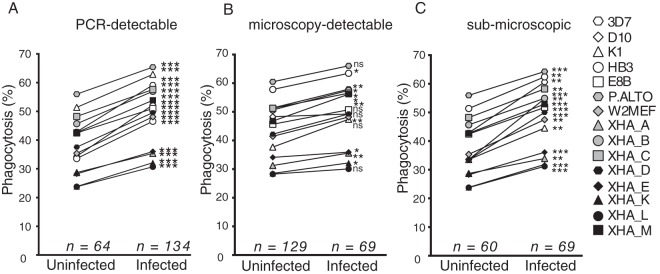
Merozoite-opsonizing antibodies were measured in 198 plasma samples against a panel of seven lab strains and eight strains from study participants that we adapted to in vitro culture. As the majority of children were parasitemic at the baseline time point, the influence of malaria infection on opsonization responses was assessed. Phagocytosis responses were higher in children with an infection at baseline measured by PCR than in uninfected children (Fig. 1A). When infections were determined by microscopy, the boosting effect of concurrent infection was inconsistent across strains (Fig. 1B). However, the moderate effect for microscopy-detected infections resulted from the influence of low-density infections as individuals with submicroscopic PCR-detected infection had significantly boosted opsonization against all parasite strains (Fig. 1C). Therefore, low-density P. falciparum infection was sufficient to elevate merozoite-opsonizing antibodies.
FIG 1.
Opsonizing antibodies are influenced by concurrent P. falciparum infection. Phagocytosis responses for each parasite strain were stratified by the density of P. falciparum infection at baseline. Infection was detected by PCR (A) or microscopy (B) or by PCR in children where infection was not detected by microscopy (C). Median values are shown, with differences assessed using a Wilcoxon rank sum test (*, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant).
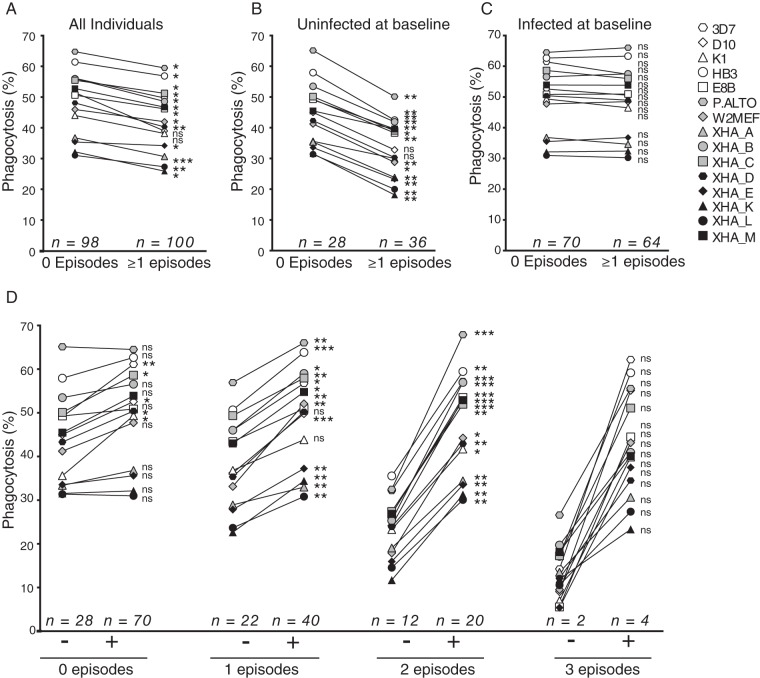
Following antimalarial drug treatment at baseline, all children were reinfected with P. falciparum during the 6-month follow-up period while half experienced at least one episode of clinical malaria (n = 100 of 198). Baseline opsonizing antibody levels were higher in children who did not experience clinical malaria during follow-up for all strains except D10 and K1 (Fig. 2A). The impact of baseline infection was investigated, and plasma from children uninfected at baseline and with no clinical malaria episodes during follow-up had significantly higher merozoite phagocytosis for all strains except D10 and K1 than plasma from children who developed clinical malaria (Fig. 2B). Boosted opsonizing antibodies present in plasma from children with baseline parasitemia were not different between children who did and those who did not experience a clinical episode during follow-up (Fig. 2C). Therefore, when opsonizing antibodies were not boosted by concurrent P. falciparum infection, higher phagocytosis responses across the majority of parasite lines were observed in children protected from clinical malaria, consistent with our previous observations with 3D7 merozoites (6). Children infected at baseline had a similar risk of reinfection and clinical disease as uninfected children during the 6 months of follow-up in this cohort (20), indicating that higher levels of opsonizing antibodies in children with baseline infections are not predictive of increased immunity to P. falciparum.
FIG 2.
Association of opsonizing antibodies, concurrent P. falciparum infection, and clinical malaria outcomes. Phagocytosis responses for each parasite strain were stratified by the presence of at least one episode of clinical malaria during follow-up for the entire cohort (A), for children uninfected at baseline (B), and for children with P. falciparum infection at baseline (C). (D) The cohort was stratified based on the number of clinical P. falciparum infections during follow-up, and phagocytosis responses were compared between individuals infected (+) and uninfected (−) at baseline. Median values are shown, with differences assessed using a Wilcoxon rank sum test (*, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant).
Children in the cohort experienced up to three episodes of clinical malaria during the 6 months of follow-up, and the effect of concurrent parasitemia on opsonizing antibodies differed across these clinical groups. Baseline infection had less effect in children with no clinical episodes during follow-up than in those that experienced one, two, or three episodes of clinical malaria (Fig. 2D). Significantly boosted responses were observed for 5 strains, 13 strains, and all strains in children that experienced no, one, and two clinical malaria episodes during follow-up, respectively. Although a striking trend for boosted antibodies was observed in children with three clinical episodes during follow-up, the small sample size did not enable the comparisons to reach statistical significance (P = 0.06 for all comparisons). Therefore, these data demonstrate that opsonizing antibody levels become more stable and less influenced by concurrent infections as immunity develops.
Broad opsonization responses are strongly associated with protection.
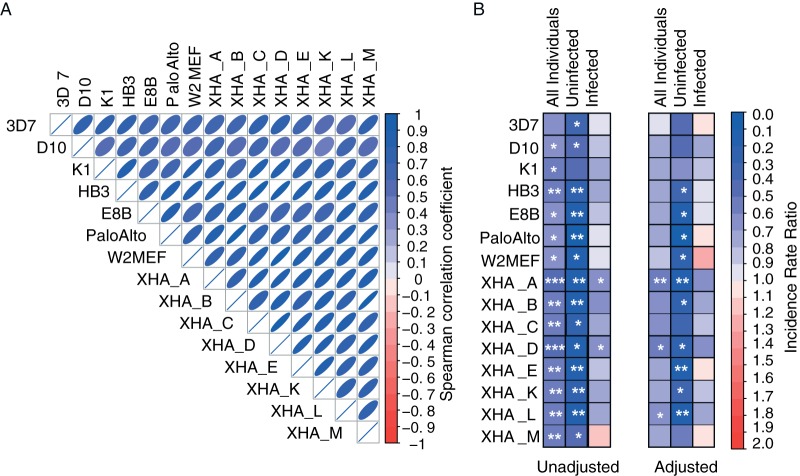
Phagocytosis responses were compared across the 15 parasite strains to determine how consistent opsonization responses were against diverse parasites. Responses were highly correlated between all parasite strains tested (Fig. 3A) and thus indicated that the acquired antibody repertoire could recognize a broad range of parasites. To investigate whether phagocytosis responses were associated with acquired immunity, children were stratified into low, medium, or high phagocytosis responders for each parasite strain and assessed for the incidence of clinical disease (Fig. 3B; see also Tables S1 to S3 in the supplemental material). High levels of phagocytosis were associated with a reduced incidence of clinical malaria for 14 out of 15 lines (IRR range across parasite strains, 0.45 to 0.67), and stronger associations with protection were observed in individuals without infection at baseline (IRR range across parasite strains, 0.07 to 0.49). Age and location were previously identified as confounding variables for antibody responses in this cohort (20), and after the Poisson regression model was adjusted for age and location, the majority of parasite strains remained significantly associated with immunity in individuals uninfected at baseline (IRR range across parasite strains, 0.08 to 0.62). In contrast, in individuals infected at baseline, phagocytosis responses to each strain were significantly associated with protection after adjustment (IRR range across parasite strains, 0.66 to 1.21). As the majority of children were infected at baseline, this likely accounts for the moderate protective associations measured for the whole cohort.
FIG 3.
Merozoite opsonization is highly correlated and associated with immunity across diverse parasite strains. (A) Phagocytosis responses from the entire cohort (n = 198) were correlated across 15 parasite strains by Spearman correlation. The strength of the correlation coefficient is indicated by the shape and color of the ellipses (all correlations, P < 0.0001). (B) Phagocytosis responses were stratified into three equal groups (tertiles) representing high, medium, and low opsonization levels, and incidence rate ratios (IRR) were calculated by Poisson regression to compare the incidence of clinical malaria over the 6-month follow-up period between the high- and low-phagocytosis tertiles. Results for the entire cohort and for uninfected and infected children at baseline are shown, and the strength of the IRR is indicated by the color scale (blue, protective, with an IRR of <0; red, increased incidence, with an IRR of >1). Poisson regression models were also adjusted for age and location (distance from coastline). Significant IRR are indicated as follows: *, P < 0.05; **, P < 0.01. See also Tables S1 to S3 in the supplemental material.
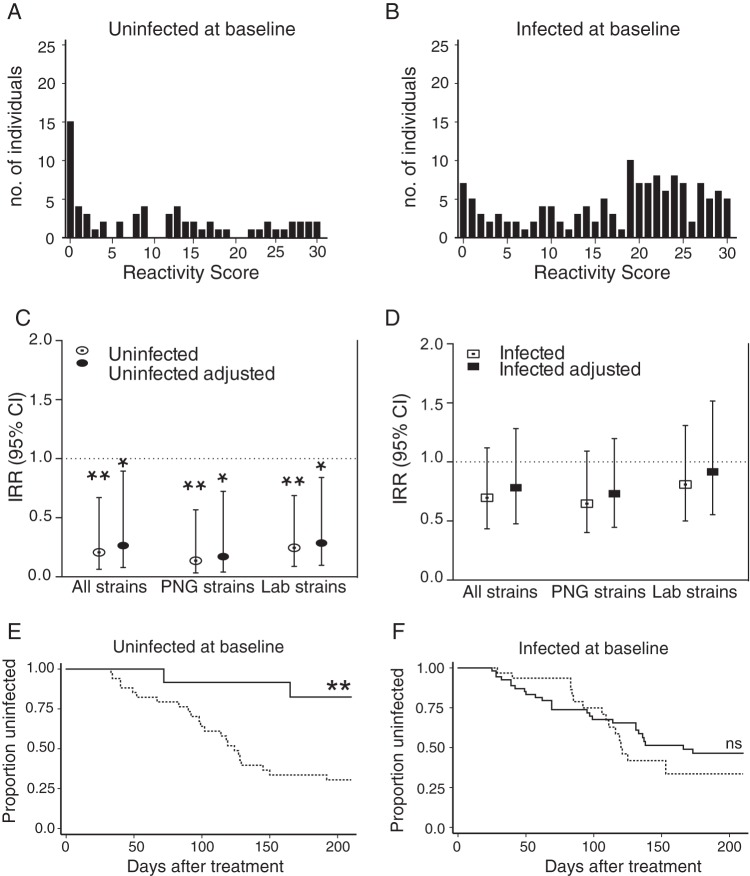
To further investigate the reactivity across multiple parasite strains, a reactivity score was generated in which tertile responses for each strain were assigned an arbitrary numerical value (low, 0; medium, 1; high, 2) and summed across strains. Children uninfected at baseline had a range of responses, and a large proportion of this group had low reactivity scores, reflective of low responses to all strains (Fig. 4A). A large proportion of children with baseline infections had high reactivity scores, indicating that infection-boosted responses recognized multiple strains (Fig. 4B). Nevertheless, in children uninfected at baseline, high reactivity scores were associated with a reduced incidence of clinical malaria compared to the incidence in those with low scores across lab strains, PNG strains, and all strains combined (Fig. 4C) (IRR of 0.24, P = 0.008; IRR of 0.14, P = 0.006; IRR of 0.21, P = 0.009, respectively). Reactivity scores were not associated with protection in children infected at baseline across lab strains, PNG strains, and all strains combined (Fig. 4D) (IRR of 0.92, P = 0.7; IRR of 0.73, P = 0.2; IRR of 0.78, P = 0.4, respectively). Similarly, strong associations with protective immunity were observed by analysis of time to clinical disease for uninfected individuals with high reactivity scores to all strains (Fig. 4E) (hazard ratio, 0.15; 95% confidence interval [CI], 0.04 to 0.64; P = 0.01). No significant reduction in risk of clinical malaria was observed for infected individuals (Fig. 4F) (hazard ratio, 0.83; 95% CI, 0.44 to 1.48; P = 0.49). Hence, consistent with the results for individual strains, opsonizing antibodies did not predict protection against subsequent clinical disease when boosted by concurrent infection. In contrast, high levels of merozoite opsonization across diverse parasite strains in plasma from uninfected children were associated with an 85% reduced risk of clinical malaria immunity.
FIG 4.
Opsonizing antibody responses recognize multiple strains and are associated with protective immunity. Reactivity scores were generated for all parasite strains, and the distribution of scores is depicted for children uninfected at baseline (n = 64) (A) and children infected at baseline (n = 134) (B). (C and D) Incidence rate ratios (IRR) of clinical malaria during follow-up were calculated for high- versus low-reactivity score tertiles across lab strains (n = 7), PNG strains (n = 8), and all strains (n = 15) for individuals uninfected at baseline (C) or infected at baseline (D). IRR and 95% confidence intervals are shown unadjusted and adjusted for age and location. (E and F) The risk of clinical malaria was compared by Cox regression analysis between high and low tertiles of reactivity scores against all parasite strains. Kaplan-Meier curves show the proportion of children that remained free of clinical malaria over time for high (solid line) versus low (dotted line) reactivity scores for children uninfected at baseline (E) or infected at baseline (F). Unadjusted curves are shown. Significance is indicated as follows: *, P < 0.05; **, P < 0.01; ns, not significant.
Variations in opsonization are not restricted to autologous parasite isolates.
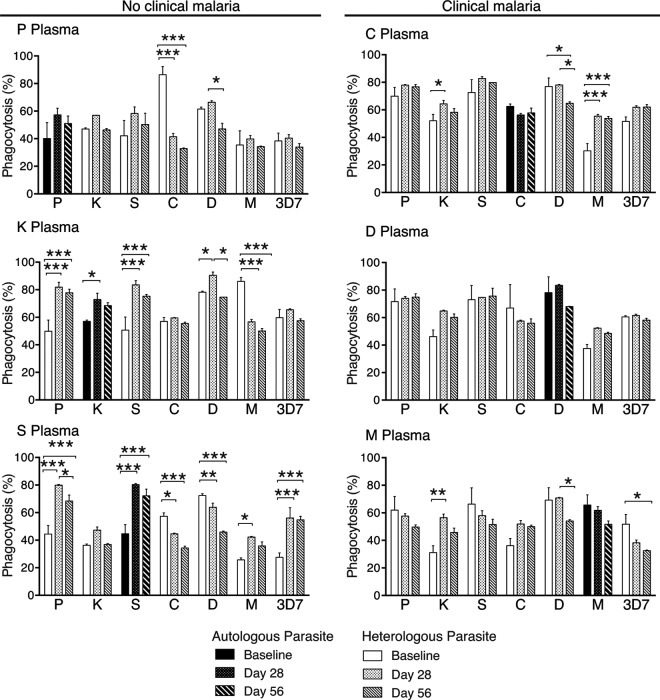
To assess whether opsonizing antibodies were acquired and boosted in a strain-specific manner, plasma samples from baseline, 28 days, and 56 days following antimalarial treatment were used to opsonize merozoites from six PNG strains and 3D7. To investigate the acquisition of antibodies without the influence of subsequent infections, analysis was restricted to the six individuals from which a parasite strain was successfully lab adapted and who were reinfected with P. falciparum after day 56. Of these children, three developed clinical malaria while three were asymptomatically infected. No consistent increase in strain-specific opsonizing antibodies was observed between baseline, day 28, and day 56 (Fig. 5). When variations in phagocytosis did occur, the changes were not restricted to the corresponding autologous parasite strain. Thus, these data do not support strain-specific acquisition of opsonizing antibodies but, rather, indicate that infection modulates opsonization responses that are reactive to multiple strains.
FIG 5.
Opsonization responses during follow-up against autologous and heterologous parasite strains. Phagocytosis responses to seven parasite strains were tested in triplicate for six study individuals, indicated by letters, with plasma collected at baseline, at 28 days, and at 56 days after antimalarial treatment. The autologous parasite strain corresponding to each individual is indicated by dark shading. Values are means ± standard deviations. Differences between means were determined by Sidak-corrected multiple comparison testing, and significance is indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Merozoite opsonization can transcend the diversity between parasite strains.
The consistent opsonization responses across the parasite strains in semi-immune individuals could have resulted from a broad antibody repertoire to strain-specific antigens, the recognition of conserved antigens, or a combination of both scenarios. To investigate whether opsonizing antibodies were cross-reactive across diverse parasite strains, plasma was preincubated with merozoites from the 3D7 lab strain or the PNG strain XHA_E to deplete strain-specific antibodies. When depleted plasma samples were used to opsonize PNG strain XHA_E and XHA_B merozoites, significant decreases in phagocytosis were observed for the majority of samples (Fig. 6). Although opsonization of XHA_E merozoites with XHA_E-depleted plasma demonstrated that complete depletion of XHA_E-specific antibodies was not achieved, phagocytosis of XHA_E and XHA_B merozoites was reduced with 3D7-depleted plasma. Hence, the decreased opsonization to XHA_B with XHA_E- and 3D7-depleted plasma demonstrates that shared opsonization targets exist between parasite strains.
FIG 6.
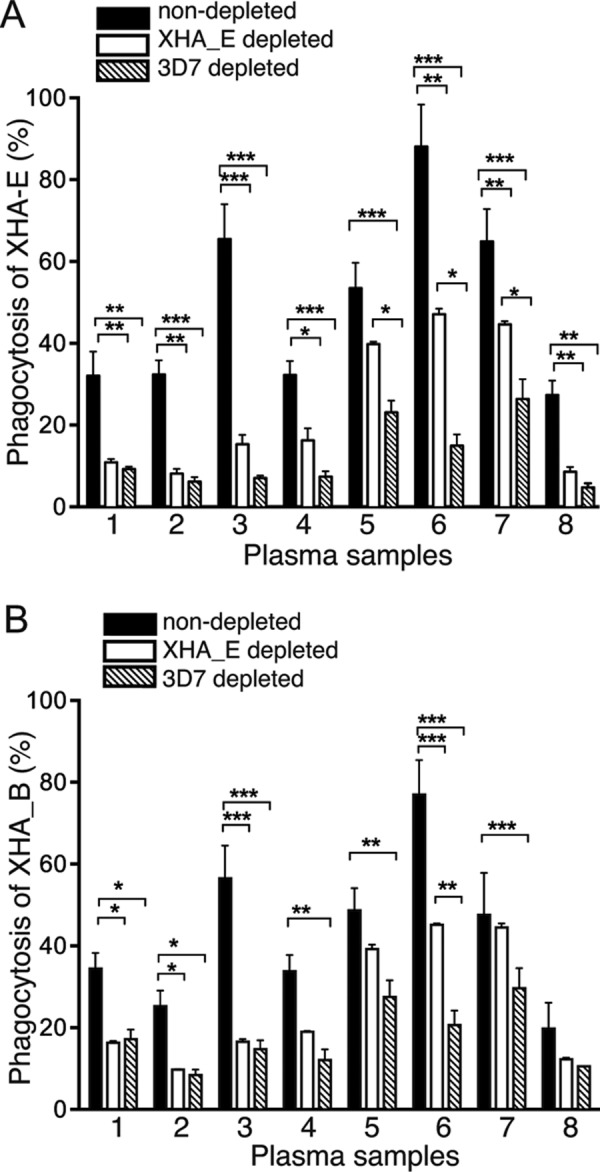
Depletion of opsonizing antibodies reduces responses to heterologous strains. Phagocytosis responses to nondepleted and XHA_E- and 3D7-depleted plasma samples were tested against XHA_E merozoites (A) and XHA_B merozoites (B). Plasma samples from eight study individuals were tested in triplicate. Values are means ± standard deviations. Differences between means were determined by Sidak-corrected multiple comparison testing, and significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Absence of MSP3, MSP6, MSPDBL1, or MSP1-19 does not affect opsonization responses.
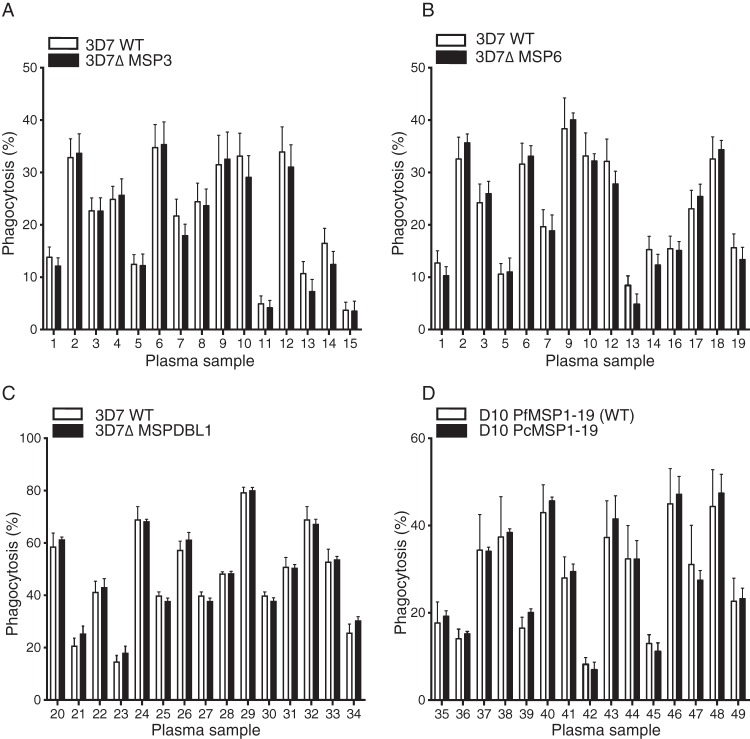
The MSP3 family and MSP1-19 have been implicated as targets of opsonizing antibodies in neutrophil respiratory burst assays (27), in ADCI assays (28), or, more recently, in merozoite phagocytosis assays (11, 29). Antibodies measured by enzyme-linked immunosorbent assay (ELISA) to the MSP3 family and MSP1-19 correlate with immunity from clinical malaria in this cohort (3, 30), and MSP1-19 antibodies were strongly correlated with phagocytosis responses to all strains (see Fig. S4 in the supplemental material). Therefore, it would be expected that phagocytosis of parasites deficient in MSP3, MSP6, or MSPDBL1 or containing the P. chabaudi MSP1-19 sequence would differ relative to wild-type parasites if these antigens were dominant targets of the phagocytic response. However, no differences in phagocytosis were observed for parasites deficient in any antigen tested (Fig. 7), indicating that there are multiple targets of opsonizing antibodies and that MSP3-, MSP6-, MSPDBL1-, and MSP1-19-specific sequences do not predominate as targets of the opsonization response.
FIG 7.
No impact of deletion of MSP3, MSP6, MSPDBL1, or MSP1-19 on opsonization responses. Phagocytosis of merozoites from 3D7ΔMSP3 (n = 6) (A), 3D7ΔMSP6 (n = 4) (B), 3D7ΔMSPDBL1 (n = 2) (C), and D10PcMSP1-19 (n = 2) (D) parasites and corresponding wild-type (WT) parasites was tested with cohort baseline plasma samples (n = 15). Values are means ± standard errors of the means.
DISCUSSION
A wealth of evidence has demonstrated that antibodies to merozoite antigens are important for immunity to P. falciparum; however, the mechanisms by which these antibodies provide protection from clinical disease remains less defined. We recently reported the first evidence for phagocytosis of opsonized merozoites as a correlate of protective immunity (6), which has now also been demonstrated in Kenyan cohorts (11). In this study, we have provided the first evidence that opsonic phagocytosis of P. falciparum merozoites is a robust correlate of acquired immunity using both laboratory parasite strains and plasma-matched isolates adapted from the field. Both growth inhibition and trophozoite functional antibodies have been shown to be largely strain specific (15–19), which limits their utility for assessing immunity in areas of endemicity with diverse parasite populations. In this study, high levels of opsonizing antibodies against all strains were associated with an 85% decrease in the risk of clinical malaria in individuals uninfected at baseline. These findings further validate merozoite opsonization as a robust functional assay of humoral immunity to malaria and provide strong support for merozoite opsonization and the phagocyte effector functions elicited by these antibodies as important immune mechanisms for reducing P. falciparum parasitemia and clinical disease.
Although baseline infection boosted opsonizing antibodies to multiple strains, the boosted responses were not associated with protection. Several studies have reported that antibodies to merozoite antigens decline quickly following infection in children, with reported antibody half-lives ranging from 5 to 52 days (31, 32). These values are consistent with both the intrinsic catabolic half-lives of 21 days for IgG1 and 7 days for IgG3 (33) and with the decay of short-lived plasma cells (34). These reported half-lives suggest that the boosted opsonizing antibodies measured from infected children would display rapid and substantial decay to potentially nonprotective levels. Thus, the presence of short-lived antibodies in plasma from infected individuals is likely to inflate the level of opsonization measured at baseline, resulting in phagocytosis responses that are not predictive of long-lived immunity. Interestingly, the boosting effect was most pronounced in those children susceptible to multiple clinical episodes during follow-up. This suggests that opsonizing antibodies stabilize once immunity has developed, which further indicates the ability of opsonizing antibody levels to predict immunity. Baseline infection per se did not alter risk of reinfection or of clinical disease during the 6 months of follow-up in this cohort (20). Nevertheless, infection strongly elevated levels of opsonizing antibodies as well as those of antibodies to numerous merozoite surface antigens in this cohort (3). The findings from this study demonstrate that a current P. falciparum infection strongly modulates merozoite antibodies, many of which are likely the result of a short-lived, extrafollicular plasma cell response. Thus, careful assessment of parasitemia should be incorporated into cohort designs that intend to measure merozoite humoral immunity in regions where malaria is endemic, especially as nonclinical submicroscopic infections were sufficient to strongly modulate opsonization responses, particularly in children with limited acquired immunity.
Following an infection at baseline, no consistent increases in opsonizing antibodies to autologous or heterologous parasite strains were observed on day 28 or 56 after antimalarial drug treatment. Where responses were variable over time, changes were not restricted to autologous parasite strains and thus were not strain specific. Additional evidence in support of conserved targets of opsonization was revealed by experiments in which plasma depleted of strain-specific antibodies displayed reduced phagocytosis of heterologous strains. Due to the moderate to high malaria transmission rate in the study site (entomologic inoculation rate of 37 infective bites/person/year) (20), study participants would have experienced numerous malaria episodes throughout childhood. Therefore, we hypothesize that following an initial malaria infection, opsonizing antibodies are generated to conserved and variant merozoite antigens, and during subsequent infections with heterogeneous parasites, antibodies targeted to conserved epitopes may be boosted incrementally to eventually predominate over responses to variant merozoite antigens. We have demonstrated that conserved targets constitute the majority of the opsonization repertoire and thus elucidated how opsonization responses transcend the antigenic variation between strains.
Antibody responses, as determined by ELISA, to recombinant MSP3, MSP6, MSPDBL1, and MSP1-19 have correlated with protective immunity in this cohort (3, 29, 30), and these antigens have also been described as targets of opsonizing antibodies (11, 29, 35). Through opsonization of transgenic parasites, these antigens proved dispensable for opsonization as a whole, which may indicate that multiple targets of merozoite opsonization exist. MSP1-19 was recently demonstrated to significantly contribute to the neutrophil respiratory burst response to P. falciparum merozoites (27), which suggests that the antigenic targets of these related immune mechanisms are different. Antibodies to the conserved C-terminal SPAM domain of the MSP3-family can cross-react across the MSP3 family (28), and antibodies to SPAM domains from MSPDBL1 and MSPDBL2 were associated with protective immunity in this cohort (29). Therefore, cross-reactive anti-SPAM antibodies could potentially opsonize merozoites deficient for a single MSP3 family member and may contribute to the observed strain-transcending response. Other merozoite antigens could also play important roles. Vaccination with conserved microneme and rhoptry proteins, such as P. falciparum Rh1 (PfRh1), PfRh2, PfRh4, EBA-175, and PfRh5, can elicit cross-reactive neutralizing antibodies against different P. falciparum strains (36–38). Whether these antigens or MSP3 family SPAM domains are important targets for merozoite opsonization remains to be determined.
Robust, reproducible, and well-validated assays are a priority to aid preclinical vaccine development and evaluate vaccine clinical trials (39). Using a novel combination of plasma samples and matched field isolates, we report similar phagocytosis responses across a large panel of laboratory strains and local isolates, further validating merozoite opsonization as a strong correlate of protective immunity in populations where malaria is endemic. The discovery of strain-transcending responses further supports the use of this assay to evaluate vaccine-induced immunity. Conserved antigens or their domains that elicit strain-transcending opsonization represent attractive vaccine candidates, and the identity of these epitopes warrants further exploration.
Supplementary Material
ACKNOWLEDGMENTS
We thank the study participants, Mugil Health Centre workers, and Papua New Guinea Institute of Medical Research staff for their support and assistance. Human erythrocytes and nonexposed plasma were provided by the Australian Red Cross blood service, Melbourne.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00145-16.
REFERENCES
- 1.World Health Organization. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/. [Google Scholar]
- 2.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K. 2014. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. 2013. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 191:795–809 doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg 83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 5.Dent AE, Bergmann-Leitner ES, Wilson DW, Tisch DJ, Kimmel R, Vulule J, Sumba PO, Beeson JG, Angov E, Moormann AM, Kazura JW. 2008. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One 3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill DL, Eriksson EM, Li Wai Suen CSN, Chiu CY, Ryg-Cornejo V, Robinson LJ, Siba PM, Mueller I, Hansen DS, Schofield L. 2013. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 8:e74627. doi: 10.1371/journal.pone.0074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B, Trape JF, Tall A, Longacre S, Perraut R. 2010. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One 5:e9871. doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiendrebeogo RW, Adu B, Singh SK, Dziegiel MH, Nebié I, Sirima SB, Christiansen M, Dodoo D, Theisen M. 2015. Antibody-dependent cellular inhibition is associated with reduced risk against febrile malaria in a longitudinal cohort study involving Ghanaian children. Open Forum Infect Dis 2:ofv044. doi: 10.1093/ofid/ofv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill DL, Eriksson EM, Carmagnac AB, Wilson DW, Cowman AF, Hansen DS, Schofield L. 2012. Efficient measurement of opsonising antibodies to Plasmodium falciparum merozoites. PLoS One 7:e51692. doi: 10.1371/journal.pone.0051692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill DL, Eriksson EM, Schofield L. High yield purification of Plasmodium falciparum merozoites for use in opsonizing antibody assays. J Vis Exp 89:51590. doi: 10.3791/51590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, Mccallum FJ, Reiling L, Jaworowski A, Anders RF, Marsh K, Beeson JG. 2014. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12:108. doi: 10.1186/1741-7015-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry AE, Schultz L, Buckee CO, Reeder JC. 2009. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One 4:e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck H-P, Alpers MP. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis 185:820–827. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 14.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois M-C, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner G, Plowe CV. 2011. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newbold CI, Pinches R, Roberts DJ, Marsh K. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol 75:281–292. doi: 10.1016/0014-4894(92)90213-T. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RJ, Phillips RS. 1976. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature 263:132–134. doi: 10.1038/263132a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown GV, Anders RF, Mitchell GF, Heywood PF. 1982. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature 297:591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- 18.Flyg BW, Perlmann H, Perlmann P, Esposito F, Berzins K. 1997. Wild isolates of Plasmodium falciparum malaria show decreased sensitivity to in vitro inhibition of parasite growth mediated by autologous host antibodies. Clin Exp Immunol 107:321–327. doi: 10.1111/j.1365-2249.1997.273-ce1163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolad A, Nebie I, Cuzin-Ouattara N, Traore A, Esposito F, Berzins K. 2003. Antibody-mediated in vitro growth inhibition of field isolates of Plasmodium falciparum from asymptomatic children in Burkina Faso. Am J Trop Med Hyg 68:728–733. [PubMed] [Google Scholar]
- 20.Michon P, Cole-Tobian JL, Dabod E, Schoepflin S, Igu J, Susapu M, Tarongka N, Zimmerman PA, Reeder JC, Beeson JG, Schofield L, King CL, Mueller I. 2007. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell RA, Saul A, Cowman AF, Crabb BS. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat Med 6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- 23.Lin CS, Uboldi AD, Marapana D, Czabotar PE, Epp C, Bujard H, Taylor NL, Perugini MA, Hodder AN, Cowman AF. 2014. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum. J Biol Chem 289:25655–25669. doi: 10.1074/jbc.M114.586495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drew DR, Hodder AN, Wilson DW, Foley M, Mueller I, Siba PM, Dent AE, Cowman AF, Beeson JG. 2012. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One 7:e51023. doi: 10.1371/journal.pone.0051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falk N, Maire N, Sama W, Owusu-Agyei S, Smith T, Beck HP, Felger I. 2006. Comparison of PCR-RFLP and Genescan-based genotyping for analyzing infection dynamics of Plasmodium falciparum. Am J Trop Med Hyg 74:944–950. [PubMed] [Google Scholar]
- 26.Boyle MJ, Wilson DW, Richards JS, Riglar DT, Tetteh KK, Conway DJ, Ralph SA, Baum J, Beeson JG. 2010. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci 107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joos C, Varela M-L, Mbengue B, Mansourou A, Marrama L, Sokhna C, Tall A, Trape J-F, Touré A, Mercereau-Puijalon O, Perraut R. 2015. Antibodies to Plasmodium falciparum merozoite surface protein-1p19 malaria vaccine candidate induce antibody-dependent respiratory burst in human neutrophils. Malar J 14:409. doi: 10.1186/s12936-015-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Soe S, Weisman S, Barnwell JW, Pérignon JL, Druilhe P. 2009. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4:e5410. doi: 10.1371/journal.pone.0005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu CY, Hodder AN, Lin CS, Hill DL, Li Wai Suen CS, Schofield L, Siba PM, Mueller I, Cowman AF, Hansen DS. 2015. Antibodies to the Plasmodium falciparum proteins MSPDBL1 and MSPDBL2 opsonize merozoites, inhibit parasite growth, and predict protection from clinical malaria. J Infect Dis 212:406–415. doi: 10.1093/infdis/jiv057. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DW, Fowkes FJI, Gilson PR, Elliott SR, Tavul L, Michon P, Dabod E, Siba PM, Mueller I, Crabb BS, Beeson JG. 2011. Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. PLoS One 6:e27705. doi: 10.1371/journal.pone.0027705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. 2008. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 76:1748–1755. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morell A, Terry WD, Waldmann TA. 1970. Metabolic properties of IgG subclasses in man. J Clin Invest 49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sze DM, Toellner KM, García de Vinuesa C, Taylor DR, MacLennan IC. 2000. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med 192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntosh RS, Shi J, Jennings RM, Chappel JC, de Koning-Ward TF, Smith T, Green J, van Egmond M, Leusen JHW, Lazarou M, van de Winkel J, Jones TS, Crabb BS, Holder AA, Pleass RJ. 2007. The importance of human FcγRI in mediating protection to malaria. PLoS Pathog 3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey AK, Reddy KS, Sahar T, Gupta S, Singh H, Reddy EJ, Asad M, Siddiqui FA, Gupta P, Singh B, More KR, Mohmmed A, Chitnis CE, Chauhan VS, Gaur D. 2013. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun 81:441–451. doi: 10.1128/IAI.01107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy KS, Pandey AK, Singh H, Sahar T, Emmanuel A, Chitnis CE, Chauhan VS, Gaur D. 2014. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect Immun 82:152–164. doi: 10.1128/IAI.00970-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, Diouf A, Miura K, Lambert LE, Ventocilla JA, Leiva KP, Milne KH, Illingworth JJ, Spencer AJ, Hjerrild KA, Alanine DG, Turner AV, Moorhead JT, Edgel KA, Wu Y, Long CA, Wright GJ, Lescano AG, Draper SJ. 2015. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in Aotus monkeys. Cell Host Microbe 17:130–139. doi: 10.1016/j.chom.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehy SH, Douglas AD, Draper SJ. 2013. Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum Vaccin Immunother 9:1831–1840. doi: 10.4161/hv.25383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.