Abstract
More people die every year from Mycobacterium tuberculosis infection than from infection by any other bacterial pathogen. Type VII secretion systems (T7SS) are used by both environmental and pathogenic mycobacteria to secrete proteins across their complex cell envelope. In the nonpathogen Mycobacterium smegmatis, the ESX-1 T7SS plays a role in conjugation, and the ESX-3 T7SS is involved in metal homeostasis. In M. tuberculosis, these secretion systems have taken on roles in virulence, and they also are targets of the host immune response. ESX-3 secretes a heterodimer composed of EsxG (TB9.8) and EsxH (TB10.4), which impairs phagosome maturation in macrophages and is essential for virulence in mice. Given the importance of EsxG and EsxH during infection, we examined their regulation. With M. tuberculosis, the secretion of EsxG and EsxH was regulated in response to iron and zinc, in accordance with the previously described transcriptional response of the esx-3 locus to these metals. While iron regulated the esx-3 expression in both M. tuberculosis and M. smegmatis, there is a significant difference in the dynamics of this regulation. In M. smegmatis, the esx-3 locus behaved like other iron-regulated genes such as mbtB. In M. tuberculosis, both iron and zinc modestly repressed esx-3 expression. Diminished secretion of EsxG and EsxH in response to these metals altered the interaction of M. tuberculosis with macrophages, leading to impaired intracellular M. tuberculosis survival. Our findings detail the regulatory differences of esx-3 in M. tuberculosis and M. smegmatis and demonstrate the importance of metal-dependent regulation of ESX-3 for virulence in M. tuberculosis.
INTRODUCTION
The intracellular pathogen Mycobacterium tuberculosis survives within phagocytic immune cells such as macrophages and dendritic cells (1). M. tuberculosis evades degradation by the endolysosomal pathway, growing in an early endosome-like compartment or escaping into the cytosol (2, 3). Acidified lysosomes are just one obstacle for the bacilli to overcome as the host also regulates metals such as iron, zinc, copper, and manganese to create an uninhabitable microenvironment (4). These metals are essential but at the same time can be toxic, so both host and pathogen tightly regulate them. For example, macrophages can increase zinc levels in M. tuberculosis-containing phagosomes to induce toxicity (5). Calprotectin, on the other hand, is released at sites of infection to bind and withhold zinc and manganese from bacteria (6). Similarly, host iron is bound to glycoproteins such as transferrin and lactoferrin, and during infection the host further limits available iron by reducing plasma iron levels via ferroportins (7–9). In addition, lipocalin 2-mediated sequestration of iron has been shown to be an important antimycobacterial innate immune response (10). To compete for iron, M. tuberculosis produces siderophores, mycobactin and carboxymycobactin, which are high-affinity iron chelators (11). Since iron is a strong redox catalyst that can be toxic to cells, M. tuberculosis stores excess iron in complex with ferritin (BfrB) (12). In addition to being a metabolic necessity, iron can also affect virulence through other means, such as ferric uptake regulator A (FurA)-modulated resistance to H2O2 and susceptibility to the antimicrobial drug isoniazid (13, 14). Thus, in order to survive over the course of an infection, M. tuberculosis has to appropriately respond to a range of concentrations of metals.
In mycobacteria, the iron-dependent regulator (IdeR), the zinc uptake repressor (Zur), and, as recently shown, manganese transport regulator (MntR) control gene expression in response to iron, zinc, and manganese, respectively. Analysis of the IdeR, Zur, and MntR regulons demonstrated that the esx-3 locus, which encodes the ESX-3 type 7 secretion system (T7SS), is transcriptionally regulated by iron, zinc, and manganese (15–17). ESX-3 is required for the utilization of iron from mycobactin in both nonpathogenic Mycobacterium smegmatis and in M. tuberculosis, although the mechanism by which ESX-3 promotes iron acquisition is not understood (18–20). In M. tuberculosis, ESX-3 is essential under standard laboratory conditions; growth can be restored to esx-3 mutants by the addition of exogenous mycobactin (20). M. smegmatis tolerates the deletion of esx-3, since it can also scavenge iron using the siderophore exochelin, which is absent in M. tuberculosis, as well as obtaining ferric iron through porins (21, 22). The transcriptional regulation of esx-3 also differs between M. smegmatis and M. tuberculosis. Although IdeR transcriptionally represses esx-3 in response to iron in both species, esx-3 is not transcriptionally regulated by zinc in M. smegmatis (18).
ESX-3 is required for secretion of EsxG, EsxH, and two pairs of PE-PPE proteins (18, 20). EsxG (Tb9.8) and EsxH (Tb10.4), which form a heterodimer (18, 23), are highly immunogenic and therefore investigated as vaccine candidates (24–26). A vaccine study utilizing an M. smegmatis strain that lacked the endogenous esx-3 locus and instead contained the M. tuberculosis esx-3 locus found that it protected against subsequent M. tuberculosis infection in mice (27). Tufariello et al. recently demonstrated that esxG and esxH are required for M. tuberculosis to grow in mice. Interestingly, esxG and esxH play a role in virulence beyond their requirement for iron acquisition (20). Consistent with these findings, we previously showed that the EsxG-EsxH complex from M. tuberculosis, but not that from the M. smegmatis EsxG-EsxH complex, targets the host hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs), a component of the endosomal sorting complexes required for transport (ESCRT) (28). M. tuberculosis EsxG-EsxH inhibits ESCRT, which is required for phagosome maturation. Accordingly, we found that an additional episomal copy of esxG-esxH introduced into wild-type M. tuberculosis enhances the ability of the bacilli to impair phagosome maturation. This suggested that the levels of EsxG and EsxH are important for host interactions and that the endogenous levels are limiting when the bacilli are grown in vitro.
We reasoned that iron and zinc might alter M. tuberculosis virulence by virtue of regulating expression of esxG and esxH. For example, if iron and zinc fully repress esx-3, then microenvironments within the host that are rich in iron and zinc might attenuate the bacilli. At the same time, since EsxG and EsxH are dominant T-cell antigens, diminished expression might allow individual bacilli to evade immune responses during the course of infection and also might compromise the efficacy of an EsxG- or EsxH-based vaccine. To better define the relative contribution of iron and zinc to the expression of the esx-3 locus, we directly examined how these metals influence transcription and secretion of EsxG and EsxH in M. tuberculosis compared to M. smegmatis. We then examined the role of metal-regulated expression of esx-3 in the intracellular survival of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type M. tuberculosis (H37Rv), M. tuberculosis ΔesxH (mc27846) (20), wild-type M. smegmatis (mc2155), M. smegmatis Δesx-3, and M. smegmatis Δesx-3::esx-3Mtb strains were grown in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC), 0.05% Tween 80, and 0.2% glycerol. The esxG-esxH complementing plasmid (pJP130), described below, was selected with 25 μg of kanamycin/ml. For iron and zinc starvation experiments, bacteria were grown in liquid Middlebrook 7H9 medium to logarithmic phase and then subcultured in chelated Sauton's (CS) medium (0.5 g of KH2PO4, 2 g of citric acid monohydrate, 4 g of asparagine, 1 g of MgSO4, 60 ml of glycerol, and 0.05% Tween 80 per liter). After the pH was adjusted to 7.4, Sauton's medium was chelated using 10 g of Chelex 100 (Sigma) over 2 days, after which 1 g of MgSO4 was added back. FeCl3 (50 μM) or ZnSO4 (3 μM) was added as a sterile solution prior to bacterial inoculation. M. tuberculosis strains grown in 7H9 were transferred to CS medium supplemented with iron and zinc as indicated at an optical density at 600 nm (OD600) of ∼0.2 and grown for 3 or 6 days, at which point they were washed twice with phosphate-buffered saline and subcultured into their respective growth media for one more day prior to sample collection. M. smegmatis strains grown in 7H9 were subcultured in CS medium supplemented with iron and zinc at an OD600 of ∼0.2 and grown for ∼9 h prior to sample collection.
Strain and plasmid construction.
The M. smegmatis Δesx-3::esx-3Mtb strain was generated by inserting pJM-TBesx3(Kan-L5), an integrated plasmid, with M. tuberculosis esx-3 (esx-3Mtb) into the chromosomal L5 integration site of the M. smegmatis Δesx-3 strain (18). In the first step, we used recombineering (29) to generate pJM-TBesx3, which contains the entire esx-3 locus, extending from 433 bp before the start codon of Rv0208 until the end of Rv0292. Escherichia coli was transformed with the cosmid clone 4-68, which contains the M. tuberculosis genomic regions of esx-3, and pKD119 (30), the arabinose-inducible recombinase plasmid. This strain was grown at 30°C, induced by arabinose, and prepared as electroporation-competent cells. PCR was performed using pJM1 (31) as the template with the primer pair TBEsx3JMF and TBEsx3JMR (see Table S1 in the supplemental material). The PCR product was digested with DpnI and transformed into the prepared competent cells and selected on 25 μg of chloramphenicol/ml at 30°C. pJM-TBesx3 was verified by sequencing the entire esx-3 locus. We then replaced the chloramphenicol acetyltransferase gene with L5 integrase-attP, together with aph, again by recombineering to form pJM-TBesx3(Kan-L5). PCR product Kan-L5 that contains L5 integrase-attP, together with aph, was amplified by using template plasmid pMC1s (32) and the primer pair Int-Ab-F and Int-Ab-R (see Table S1 in the supplemental material). The E. coli strain containing pKD119 and pJM-TBesx3 was grown at 30°C, induced by arabinose, transformed the DpnI-digested Kan-L5, and then selected on 50 μg of kanamycin/ml at 37°C. pJM-TBesx3(Kan-L5) was verified by sequencing. pJM-TBesx3(Kan-L5) was then transformed into the M. smegmatis Δesx-3 strain to form the M. smegmatis Δesx-3::esx-3Mtb strain.
The esxG-esxH complementing plasmid (pJP130) contains esxG-esxH-espG3. pJP130 was constructed by inserting espG3 (Rv0289) into pYUB1944, which contains esxG-esxH under the control of the hsp60 promoter (as described previously [20]). Rv0289 was amplified from M. tuberculosis genomic DNA by PCR using forward (TAGAAGCTTCTCGCGCTACATGGATGC) and reverse (CCAATCGATTTACGAGGATTGGGTGG) primers, digested with HindIII, and cloned into the HindIII site after Rv0288 in pYUB1944. The addition of Rv0289 to pYUB1944 resulted in enhanced secretion of EsxG and EsxH compared to the parent plasmid.
Bacterial protein preparation and Western blotting.
To prepare samples for Western blotting, bacteria were pelleted, and culture supernatants were filtered through a 0.22-μm-pore-size filter. Culture filtrate (CF) proteins were precipitated overnight at 4°C using 10% trichloroacetic acid. The protein precipitate was washed with acetone, air dried, and resuspended in sodium dodecyl sulfate (SDS) sample buffer. Bacterial pellets were lysed by bead beating in lysis buffer (50 mM Tris-HCl, 5 mM EDTA, 0.6% SDS, 10 mM NaH2PO4, 1× Halt protease inhibitor [Thermo Fisher Scientific]) with 0.1-mm zirconia/silica beads (BiosSpec Products, Inc.). Samples were boiled at 95°C for 5 min prior to SDS-PAGE. Protein extracts were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and probed with αEsxG-EsxH rabbit antisera (20) or αAg85 rabbit antisera (generously provided by the J. D. Ernst lab [33]). Proteins were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:10,000; Life Technologies) and visualized with the Amersham ECL reagent (GE Healthcare). Quantification of band intensities was performed using ImageJ software.
Recombinant EsxG-EsxH production and immunoblotting.
EsxGMt and EsxHMt were expressed in tandem as a single polypeptide separated by a linker (GLVPRGSTG) as previously described (28). To make EsxGMs-EsxHMs, Genewiz synthesized the full-length coding regions of EsxGMs and EsxHMs separated by a linker region that is identical to that in the EsxGMt-EsxHMt plasmid in pUC57. The EsxGMs-EsxHMs coding region was moved from pUC57 to pET22b on an NdeI-XhoI fragment. E. coli [BL21(DE3); Invitrogen] transformed with the EsxGMt-EsxHMt or EsxGMs-EsxHMs expression plasmid was grown in Luria broth at 37°C to an OD600 of 0.7 and then induced with 450 μM IPTG (isopropyl-β-d-thiogalactopyranoside; Promega) for 3 h, harvested by centrifugation at 4°C, and frozen at −80°C. Pellets were later resuspended in lysis buffer (300 mM NaCl, 50 mM NaH2PO4, 1× Halt protease inhibitor cocktail [Thermo Fisher Scientific] supplemented with 10 mM imidazole [Fisher BioReagents], 3 U of benzonase nuclease [Novagen]/ml, and 1 mg of lysozyme [Sigma-Aldrich]/ml at pH 8.0) and incubated at room temperature for 40 min, followed by centrifugation at 10,000 × g for 30 min at 4°C. The supernatant was incubated with nickel-nitrilotriacetic acid (Ni-NTA) Superflow resin (Qiagen) at 4°C for 1.5 h. The Ni-NTA resin was washed with lysis buffer containing up to 60 mM imidazole, followed by elution in lysis buffer containing 250 mM imidazole. Thrombin (Novagen) was added (1 U/ml) overnight at room temperature to cleave the linker connecting EsxG and EsxH. To remove thrombin, the samples were diluted in lysis buffer at a final concentration of 10 mM imidazole and rebound to Ni-NTA resin, washed, and eluted with 250 mM imidazole. The purity and the extent of thrombin cleavage were assessed by SDS-gel electrophoresis and Coomassie blue staining. Proteins were quantified with a NanoDrop 1000 using ultraviolet absorbance of 280 nm and/or by comparison to bovine serum albumin standards on a Coomassie gel.
Quantitative reverse transcription (RT)-PCR analysis.
For total RNA extraction, pellets were lysed by bead beating 5 OD600 units of each sample in 1 ml of TRIzol (Life Technologies) three times for 1 min. RNA was precipitated according to the manufacturer's instructions, resuspended in RNase-free water, and stored at −80°C. DNase-treated (DNase I, amplification grade; Life Technologies) RNA samples were used as the templates for cDNA synthesis (Superscript III reverse transcriptase; Life Technologies) and treated with RNase prior to amplification. The expression of mbtI, bfrB, esxG, and ppe4 were determined by using real-time PCR. Each sample was amplified using SYBR green (Roche Life Science) according to the manufacturer's protocol. Each cycle consisted of denaturation at 95°C for 15 s, annealing at 59.5°C for 5 s, and extension at 72°C for 15 s. 16S rRNA and dnaA were used for the normalization for M. tuberculosis and M. smegmatis, respectively. The primers used are listed in Table S1 in the supplemental material.
Intracellular bacterial growth assay.
Murine bone marrow-derived macrophages (BMDMs) were obtained and infected with a single cell suspension of M. tuberculosis at a multiplicity of infection of 3 as previously described (28). Extracellular bacteria were washed away at 4 h. At the indicated times, BMDMs were lysed with 0.2% Triton X-100, and serial dilutions were plated on Middlebrook 7H10 solid medium containing 200 ng of mycobactin J (Allied Monitor, Inc.)/ml.
Statistical analysis.
To assess statistical significance, differences between groups were analyzed by Student t test. We considered a P value below 0.05 to be statistically significant.
RESULTS
M. tuberculosis EsxG-EsxH secretion and iron regulation are impaired in the M. smegmatis Δesx-3::esx-3Mtb strain.
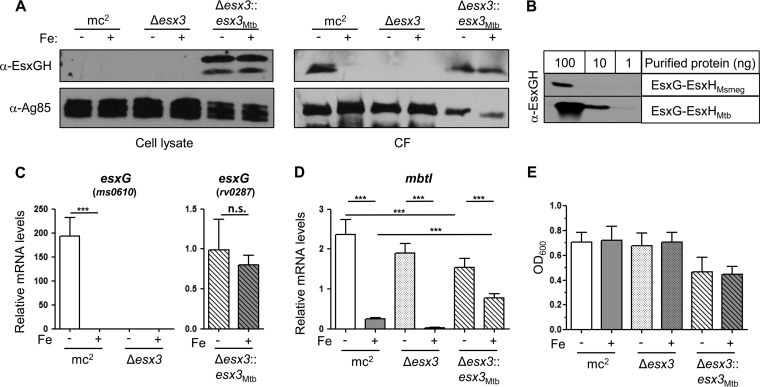
Previously, we found that M. tuberculosis EsxG and EsxH are not secreted when they are expressed in M. smegmatis (28). To examine whether M. tuberculosis EsxG and EsxH can be secreted by M. smegmatis if the entire M. tuberculosis esx-3 locus is provided, we compared secretion profiles from three strains: wild-type M. smegmatis (mc2155), M. smegmatis lacking esx-3 (Δesx-3), and M. smegmatis lacking esx-3 but containing M. tuberculosis esx-3 (Δesx-3::esx-3Mtb). This strain is similar to the published vaccine strain (27), so analyzing its EsxG-EsxH secretion profile was also of interest. We transferred mid-log-phase cultures grown in 7H9 medium into Sauton's medium with or without iron and analyzed secretion after 9 h of growth. We examined the secretion of EsxG and EsxH using antisera from rabbits immunized with purified recombinant M. tuberculosis EsxG-EsxH complex. In wild-type M. smegmatis, endogenous EsxG-EsxH was present in culture filtrate (CF) and undetectable in the pellet fraction. In contrast, we detected a large amount of M. tuberculosis EsxG-EsxH in the pellet fraction of the M. smegmatis Δesx-3::esx-3Mtb strain and only a minor fraction in the CF (Fig. 1A), suggesting an incompatibility between the M. tuberculosis ESX-3 machinery and M. smegmatis resulting in impaired secretion. Our antibody recognizes M. tuberculosis EsxG and EsxH much better than it recognizes M. smegmatis EsxG and EsxH, which are 70% identical to the M. tuberculosis homologues (Fig. 1B). Therefore, the amount of endogenous EsxG-EsxH secreted by mc2155 shown on the Western blot is >10-fold higher than the amount of M. tuberculosis EsxG-EsxH secreted from the Δesx-3::esx-3Mtb strain. In addition, the amount of M. smegmatis EsxG-EsxH in the CF of mc2155 noticeably decreased with the addition of iron, as expected. In contrast, we were surprised to find that the abundance of M. tuberculosis EsxG-EsxH in both the pellet and the CF from the Δesx-3::esx-3Mtb strain was unaffected by the addition of iron. In accordance with this finding, quantitative reverse transcription-PCR (qRT-PCR) analysis showed that whereas expression of the endogenous EsxG locus in M. smegmatis was induced 200-fold in the absence of iron, M. tuberculosis esxG in the Δesx-3::esx-3Mtb strain was unaffected by the addition of iron (Fig. 1C).
FIG 1.
Analysis of M. tuberculosis ESX-3 expressed in M. smegmatis. (A, C to E) Wild-type (mc2155), Δesx-3, and Δesx-3::esx-3Mtb M. smegmatis strains were subcultured from 7H9 into in CS medium with ZnSO4 and with or without iron as indicated for 9 h. (A) EsxG-EsxH and Ag85b were analyzed by Western blotting of cell lysates and CFs. (B) Anti-EsxG-EsxH Western blot of purified recombinant EsxG-EsxHMsmeg and EsxG-EsxHMtb. (C and D) The mRNA levels of esxG (C) and mbtI (D) were normalized to dnaA. In panel C, ms0610-specific primers were used for mc2155 and Δesx-3 strains, and rv0287-specific primers were used for the Δesx-3::esx-3Mtb strain. Data represent the means ± the standard errors of the mean (SEM) from three experiments with six technical replicates each. ***, P < 0.001 (unpaired Student t test). n.s., not significant. (E) OD600 measurements from two experiments after equal inocula were subcultured for 9 h as indicated. Data represent the means ± the SEM.
Because the Δesx-3::esx-3Mtb strain failed to repress esxG in response to iron, we examined its ability to repress mbtI. Interestingly, in contrast to the wild-type strain and the Δesx-3 mutant, the Δesx-3::esx-3Mtb strain failed to strongly repress mbtI in response to iron (Fig. 1D). This suggests that the Δesx-3::esx-3Mtb strain is defective in iron sensing, uptake, or utilization. In addition, although all strains were inoculated comparably, the endpoint ODs showed that the M. smegmatis strain containing M. tuberculosis ESX-3 grew less well in liquid medium (Fig. 1E), showing that the presence of M. tuberculosis ESX-3 and perhaps the accumulation of intracellular M. tuberculosis EsxG-EsxH impair iron regulation and is detrimental to the growth of M. smegmatis.
Iron and zinc modestly regulate the expression of the M. tuberculosis esx-3 locus.
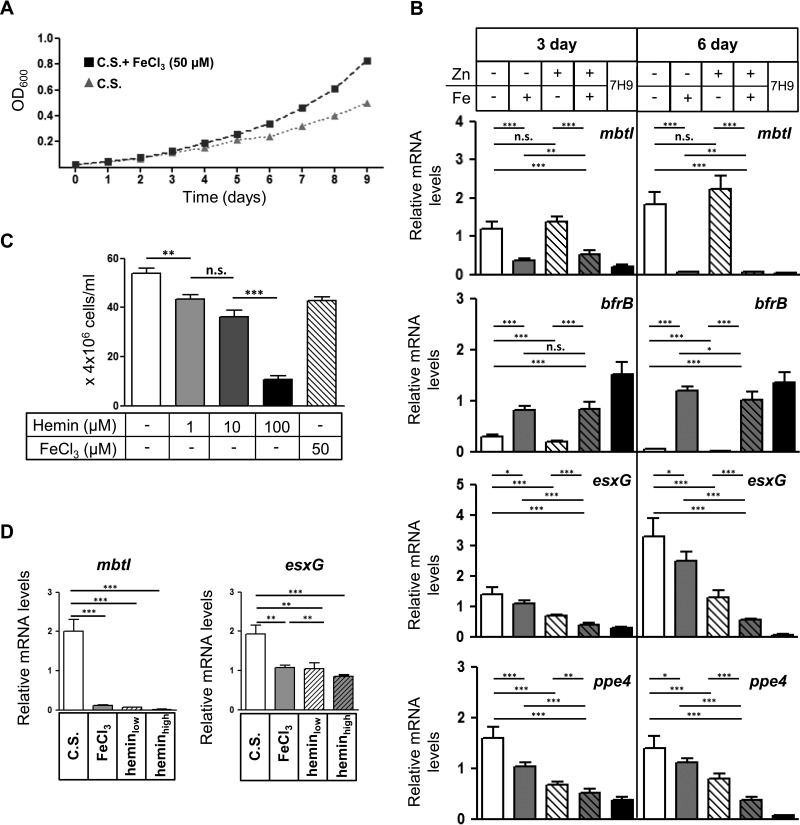
To examine the transcriptional response of the esx-3 locus to iron in M. tuberculosis itself, we first examined how long it took the growth of M. tuberculosis to slow down when mid-log-phase cultures were transferred from 7H9 into Sauton's medium without FeCl3. Since M. tuberculosis can store excess iron in ferritin, it was not surprising that for the first 3 days after transfer, M. tuberculosis grew equivalently irrespective of the presence of iron; growth diverged by the fourth day (Fig. 2A). To examine the transcriptional response of the esx-3 locus to iron and zinc, we harvested RNA for qRT-PCR analysis after 3 days of growth in CS medium with or without FeCl3 and ZnSO4 (50 and 3.67 μM, respectively). The esx-3 locus in M. tuberculosis extends from rv0282 to rv0292, with Zur and IdeR binding sites located upstream of eccA3 (rv0282) (34) and at least one additional internal promoter located upstream of esxG (rv0287) (16) (see Fig. S1 in the supplemental material). We analyzed expression of ppe4 (rv0286) and esxG, along with two other IdeR-regulated genes, mbtI and bfrB, which are encoded outside the esx-3 locus. mbtI encodes isochorismate synthase, a salicylate synthase required for mycobactin production that is repressed by iron-bound IdeR. On the other hand, bfrB, which encodes an M. tuberculosis ferritin that stores intracellular iron, belongs to a small subset of genes that are induced by IdeR (34, 35). As expected, the expression of mbtI was repressed 3-fold by the presence of iron and unaffected by zinc, whereas bfrB was induced 2.7-fold by iron and also unaffected by zinc (Fig. 2B). In contrast, esxG and ppe4 showed minimal response to iron. When zinc was absent, iron repressed ppe4 1.6-fold (P = 0.0002), whereas it repressed expression of esxG only 1.3-fold (P = 0.025). When zinc was included in the media, iron again only modestly repressed ppe4 and esxG, resulting in 1.3- and 1.7-fold reductions in transcript, respectively (Fig. 2B). The addition of zinc resulted in an ∼2-fold inhibition of esxG and ppe4 expression both in the presence and in the absence of iron. 7H9, a standard laboratory medium for M. tuberculosis that contains approximately 150 μM iron and 6 μM zinc, repressed esxG and ppe4 to a similar level as had CS medium supplemented with 50 μM iron and 3.67 μM zinc.
FIG 2.
Iron and zinc modestly repress expression of the M. tuberculosis esx-3 locus. (A) OD600 measurements of M. tuberculosis subcultured in chelated Sauton's medium (C.S.) with or without iron (3.67 μM ZnSO4 present in both conditions). (B) M. tuberculosis was grown in 7H9 or CS containing iron (FeCl3) or zinc (ZnSO4) as indicated for 3 or 6 days. The mRNA levels of mbtI, bfrB, esxG, and ppe4 were normalized to 16S rRNA and are shown as fold changes compared to the sample without iron or zinc. Data represent the means ± the SEM from three experiments with five technical replicates each. (C) M. tuberculosis was subcultured from 7H9 into CS medium without hemin or with increasing concentrations of hemin (1, 10, and 100 μM) or iron (50 μM) as indicated for 24 h, and bacterial CFU were enumerated. Data represent the means ± the SEM from one experiment with six technical replicates. (D) M. tuberculosis was grown in CS medium without iron supplementation or with hemin (low, 3 μM; high, 30 μM) or FeCl3 (50 μM) for 1 week. The mRNA levels of mbtI and esxG were normalized to 16S rRNA. Data represent the means ± the SEM from one experiment with five technical replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student t test). n.s., not significant.
To determine whether a longer duration of iron starvation would result in more dramatic changes in esx-3 expression, we compared cells grown in the presence or absence of iron for 6 days (Fig. 2B). mbtI and bfrB exhibited 25-fold repression and 22-fold induction, respectively, compared to the ∼3-fold differences seen after 3 days. This difference was largely attributable to more complete inhibition of expression in the repressing condition for both genes. For esxG and ppe4, iron and zinc individually again only yielded modest differences. Thus, the expression of esx-3 is only minimally responsive to iron, irrespective of the presence of zinc, in contrast to the dynamic regulation of other IdeR-regulated genes in M. tuberculosis. Likewise, zinc modestly repressed esxG and ppe4, with ∼2-fold repression at both the 3- and the 6-day time points (15). Overall, at both time points, the combined effect of iron and zinc together resulted in an approximately 3- to 6-fold decrease in expression of esx-3 relative to growth in deficient media. Notably, in the 6-day samples, esxG and ppe4 were dramatically more repressed in 7H9 than Sauton's medium containing both iron and zinc, which may reflect trace manganese in 7H9 (17). This suggests that when experiments are performed on bacteria grown in 7H9, they are likely to reflect relatively low levels of ESX-3-secreted effectors.
Because free iron had a limited effect on esx-3 expression, we tested heme, which M. tuberculosis can also utilize as an iron source (36). We found that when M. tuberculosis was transferred from 7H9 into Sauton's medium containing up to 100 μM hemin, this delivered an increasingly toxic dose of iron to M. tuberculosis (Fig. 2C). At sublethal doses, hemin strongly repressed mbtI ∼30-fold, but esxG showed just a 2-fold difference, similar to the response to FeCl3 (Fig. 2D). Even at toxic doses, where mbtI was repressed ∼100-fold, esxG showed just a 2-fold inhibition. Thus, although ESX-3 is required for the acquisition of iron from mycobactin, it is not completely repressed, even in toxic doses of iron, in contrast to what is observed for the mycobactin biosynthetic pathway. Instead, multiple metals individually appear to make a modest contribution to esx-3 regulation, as suggested by prior studies (19, 34, 37). This is quite distinct from the regulation of M. smegmatis esx-3, which displays a level of iron responsiveness comparable to that of the IdeR-regulated genes mbtI and bfrB (Fig. 3).
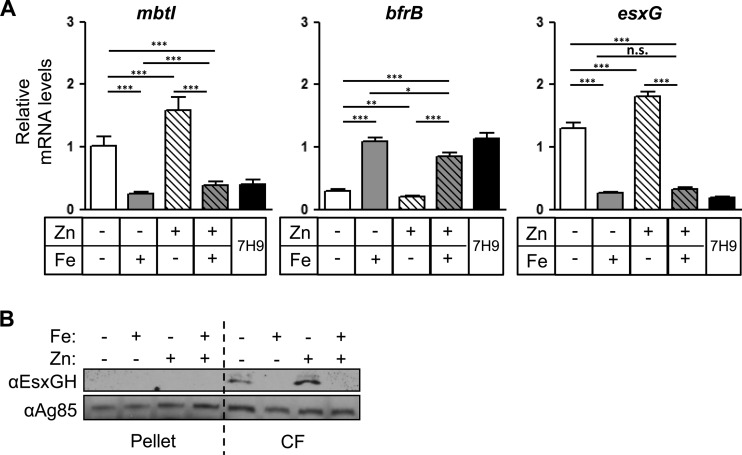
FIG 3.
Iron regulates expression and secretion of EsxG-EsxH in M. smegmatis. (A and B) M. smegmatis was grown in 7H9 or CS medium containing iron or zinc as indicated for 9 h. (A) The relative mRNA levels of mbtI, bfrB, and esxG were normalized to dnaA and are shown as fold changes compared to the sample without iron or zinc. Data represent the means ± the SEM from two experiments with six technical replicates each. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student t test). n.s., not significant. (B) EsxG-EsxH and Ag85 complex proteins were analyzed by Western blotting of cell lysates and CFs.
Secretion of M. tuberculosis ESX-3 substrates is regulated by iron and zinc.
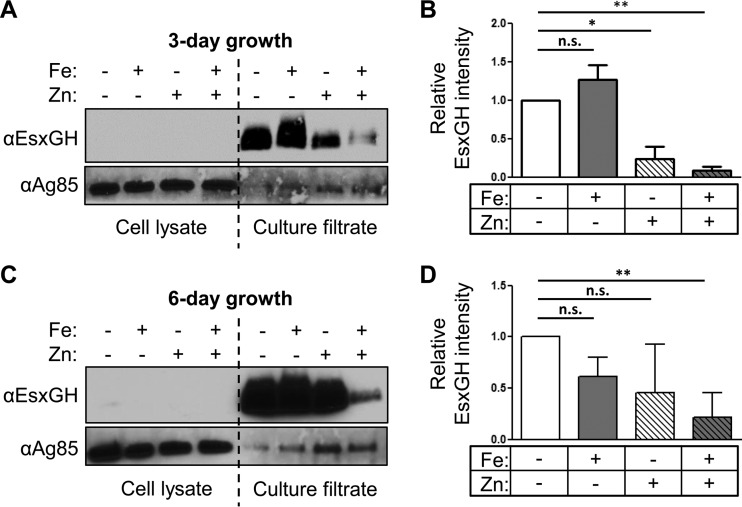
Siegrist et al. previously examined the effect of iron on the secretion of EsxH from M. smegmatis and BCG using EsxH fused to myc, which was expressed under the control of the hsp60 promoter (18). To determine whether the transcriptional changes we observed correlated with secretion differences, we examined the secretion of endogenous EsxG and EsxH from M. tuberculosis using the antibody raised against the EsxG-EsxH complex (20). Initially, we analyzed CF after 2 or 6 days of incubation in Sauton's medium with or without iron, and we did not detect an effect of iron on the level of secreted EsxG and EsxH. We reasoned that the initial amount of EsxG and EsxH secreted might mask differences that occur as the cells adapt over time, particularly given the modest transcriptional effects that we observed. Therefore, in subsequent experiments, after bacteria grew in Sauton's medium for either 3 or 6 days under iron replete or depleted conditions, they were subcultured again under their respective conditions for an additional 24 h and the proteins secreted into the CF during this 24-h period were analyzed by Western blotting (Fig. 4A). Using ImageJ software, we quantified the intensity of EsxG-EsxH on Western blots from three independent experiments and normalized to Ag85, which is secreted by the twin arginine translocator system and partitions in both the cell pellet and the CF (Fig. 4B) (38). We did not detect any EsxG-EsxH associated with the bacterial pellet, suggesting that M. tuberculosis secretes nearly all of the EsxG-EsxH that is produced. After 3 days of growth in media lacking iron and zinc, there was no difference in the EsxG-EsxH protein levels in CF compared to cells grown with iron. The addition of zinc alone caused an ∼4-fold decrease in secretion (P = 0.0148), which was further boosted to an ∼11-fold decrease by the addition of iron (P = 0.0009). When CF was analyzed from bacteria subcultured after 6 days of growth in distinct iron and zinc conditions, there was more experimental variability than seen after 3 days, but the overall trend was the same (Fig. 4C and D). Consistent with the expression data, EsxG-EsxH production was never completely suppressed. Overall, analysis of EsxG-EsxH protein secretion shows general concordance with the transcriptional analysis. This, along with the absence of an intracellular pool of EsxG-EsxH, suggests that iron and zinc do not also posttranscriptionally regulate EsxG-EsxH secretion. When we grew M. smegmatis in the presence or absence of iron and zinc for 9 h and collected proteins from both the bacterial pellet and the CF (Fig. 3B), even though we collected CF proteins after a single transfer into CS medium, we found that secreted EsxG-EsxH was clearly diminished by the addition of iron. Thus, as for the transcriptional response, the secretion of EsxG-EsxH in M. smegmatis is highly responsive to iron, whereas M. tuberculosis esx-3 modestly responds to both metals and is not fully repressed even in the presence of both iron and zinc.
FIG 4.
Iron and zinc inhibit secretion of M. tuberculosis EsxG-EsxH. (A to D) M. tuberculosis was grown in C.S. containing iron (FeCl3) or zinc (ZnSO4) as indicated. (A and C) EsxG-EsxH and Ag85 complex proteins were analyzed by Western blotting of cell lysates and CFs after 3-day (A) and 6-day (C) treatments. Long exposures of the CF are shown to demonstrate the presence of EsxG-EsxH in media containing both iron and zinc. (B and D) EsxG-EsxH protein levels in CF were quantified from three independent experiments using ImageJ software and normalized to Ag85. Data represent the means ± the SEM. *, P < 0.05; **, P < 0.01 (unpaired Student t test). n.s., not significant.
Iron- and zinc-mediated repression of esx-3 decreases M. tuberculosis survival in macrophages.
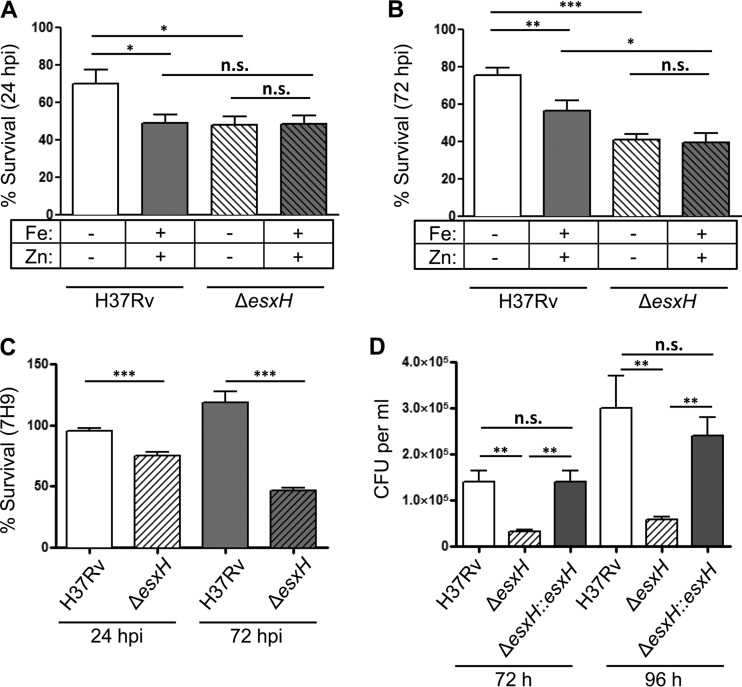
Over the course of infection, M. tuberculosis can exist in various environments and thus is exposed to a wide range of metals (39–41). In order to determine whether iron- and zinc-mediated regulation of EsxG and EsxH might influence virulence, we infected murine BMDMs with M. tuberculosis that was pregrown for 3 days in Sauton's medium with iron and zinc or without iron and zinc to test conditions that maximize the difference in EsxG-EsxH secretion. We anticipated that once inside cells, bacteria pregrown under both conditions would eventually achieve similar levels of expression, so we assessed the intracellular survival over the first 24 h of infection. We noticed that M. tuberculosis pregrown in Sauton's medium (either with or without iron and zinc) were impaired intracellularly relative to bacteria pregrown in 7H9 (compare Fig. 5A and B to Fig. 5C). In addition, we found that wild-type M. tuberculosis (H37Rv) grown in iron- and zinc-depleted Sauton's medium prior to infection displayed modestly higher survival in BMDMs than M. tuberculosis grown in iron- and zinc-replete Sauton's medium (Fig. 5A). These differences were still apparent at 72 h postinfection (hpi) (Fig. 5B). To distinguish whether the enhanced intracellular survival of M. tuberculosis grown in iron- and zinc-depleted conditions was mediated by EsxH, as opposed to an effect of the numerous other genes with altered expression or some physiological consequence of growing without iron and zinc, we examined an ΔesxH mutant, which fails to secrete both EsxG and EsxH (20). At 24 hpi, the ΔesxH mutant had impaired intracellular survival, similar to wild-type M. tuberculosis grown in the presence of iron and zinc, where little EsxG-EsxH is secreted. Moreover, iron and zinc did not affect the intracellular survival of the ΔesxH mutant at either 24 or 72 hpi (Fig. 5A and B). Overall, these findings are consistent with the idea that the secretion of EsxG-EsxH, which is maximal in low-iron and -zinc conditions, assists in bacterial survival during the initial interaction with macrophages. Thus, although growth of M. tuberculosis in vitro is improved by iron and zinc, the initial intracellular growth is impaired, which may be explained at least in part by the repression of esxG and esxH. Because the transcription of esx-3 was repressed to similar levels when M. tuberculosis strains were grown in 7H9 or CS medium with iron and zinc for 3 days, we also compared the intracellular survival rates of Rv and ΔesxH strains in BMDMs after pregrowth in 7H9. There was a mild difference in intracellular survival of the ΔesxH mutant compared to wild-type bacilli at 24 hpi; this difference became more pronounced by 72 hpi and could be complemented by a plasmid encoding esxG-esxH (Fig. 5C and D). Thus, although the secretion of EsxG-EsxH is likely to be relatively low in 7H9, it still contributes to the intracellular survival of M. tuberculosis.
FIG 5.
Iron- and zinc-mediated suppression of EsxH impair intracellular survival. BMDMs were infected with M. tuberculosis that had been pregrown for 3 days in CS medium containing iron or zinc as indicated (A and B) or in 7H9 (C and D) at a multiplicity of infection of 3. (A to C) CFU were enumerated at 4, 24, and 72 hpi, and data are expressed as the percent bacterial survival at 24 hpi (CFU at 24 hpi/CFU at 4 hpi) ± the SEM and 72 hpi (CFU at 72 hpi/CFU at 4 hpi) ± the SEM from three experiments, with five experimental replicates each. (D) BMDMs were infected with wild-type M. tuberculosis, ΔesxH, and ΔesxH::esxH strains, and the CFU were enumerated 72 and 96 hpi from five replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student t test). n.s., not significant.
DISCUSSION
Recent work has highlighted that M. tuberculosis ESX-3 and its secreted effectors, EsxG and EsxH, play a role in virulence beyond their previously recognized role in iron acquisition (20, 28). In addition, they are major immune targets of the host (24–26). Given the importance of these molecules during infection, we examined the relative contribution of iron and zinc to the expression and secretion of EsxG and EsxH. As expected, we found that both metals regulated the M. tuberculosis esx-3 locus. However, ppe4 and esxG exhibited only modest differences in expression in response to iron or zinc. In contrast, the dynamics of esx-3 expression in M. smegmatis mirrored the results seen with other iron-responsive genes. The differences in the amount of secreted EsxG and EsxH reflected the transcriptional regulation of the esx-3 locus. Since we performed these studies, manganese has also been shown to regulate expression of esx-3 (17). The additional repression of esx-3 seen after prolonged culturing in 7H9 compared to iron- and zinc-replete conditions may reflect the presence of trace manganese, although other differences in the media or growth rate may play a role as well.
We also monitored the regulation of M. tuberculosis ESX-3 when the locus was integrated into an M. smegmatis strain lacking its endogenous esx-3 locus. Analysis of EsxG and EsxH by Western blotting showed that the majority of the protein remained cell associated and was not secreted into the CF. One possibility is that the M. tuberculosis ESX-3 secretion apparatus does not assemble properly in M. smegmatis. Alternatively, there may be proteins that are required to assist in EsxG-EsxH recognition by ESX-3 that are encoded outside the locus. In addition, we found that expression of the M. tuberculosis locus was not iron-regulated when introduced into M. smegmatis. The IdeR binding sites differ between M. tuberculosis and M. smegmatis by two nucleotides (see Fig. S1 in the supplemental material). Thus, one possibility is that the M. smegmatis IdeR protein does not recognize the binding site in the M. tuberculosis promoter or that the spacing relative to the transcriptional start site, which differs in the two species, is not optimal. Alternatively, there may be an aberrant transcriptional start site at the ectopic locus of integration. However, a different possibility is raised by our observations that the presence of M. tuberculosis ESX-3 also affected iron-mediated repression of the endogenous mbtI gene. In wild-type M. smegmatis, mbtI was repressed 9.5-fold by the addition of iron, but in the M. smegmatis Δesx-3::esx-3Mtb strain mbtI expression was only repressed 2-fold (Fig. 1D). This difference reflects both lower expression in the absence of iron and diminished repression by iron in the Δesx-3::esx-3Mtb strain. Thus, one possibility is that the accumulation of Esx-3 substrates in the Δesx-3::esx-3Mtb strain impairs iron sensing or iron utilization or that the diminished iron responsiveness is a consequence of the attenuated growth in culture.
In order to determine whether iron- and zinc-mediated regulation of EsxG and EsxH affect bacterium-host interactions, we infected BMDMs with bacteria pregrown in media with or without iron and zinc. We found that bacterial survival was enhanced by pregrowth under conditions that maximized EsxG-EsxH secretion. Consistent with the idea that the effect of zinc and iron is mediated by differences in EsxG-EsxH secretion, these metals had no effect on the intracellular survival of the ΔesxH strain. Thus, the early interactions of M. tuberculosis with macrophages depend upon metals in the medium prior to infection, likely at least in part by virtue of altering EsxG and EsxH abundance, although it is also possible these effects are mediated by effectors that are cosecreted with EsxG and EsxH (20). Although it is not possible to measure the secretion of EsxG and EsxH by M. tuberculosis growing in standard 7H9, given the transcriptional repression that we observed of esx-3, we predict that there would be low levels of EsxG and EsxH secreted. This has important implications for studies of M. tuberculosis grown in vitro and subsequently used for infections. Although extremely low levels of EsxG and EsxH are sufficient to allow M. tuberculosis to utilize iron (20, 42), such restricted expression is unlikely to reflect the situation in vivo and may be limiting in terms of the interactions with macrophages. Moreover, these results suggest that the zinc and iron concentrations in distinct host microenvironments would create bacterial heterogeneity in ESX-3 function that might have important consequences in terms of pathogenesis and immune responses. Although decreased EsxH expression might be a way to evade prominent T cell-mediated immune responses, this would likely come at the cost of diminished fitness in vivo.
During the evolution of environmental mycobacteria to pathogenicity, the esx-3 locus acquired an upstream Zur binding site, resulting in zinc-dependent regulation that is lacking in the saprophytic relative. This adaptation presumably reflects the specific environments of M. tuberculosis in the host. For example, studies have shown very high levels of zinc inside mycobacterial phagosomes (43). In addition, ESX-3 has acquired additional functions in the obligate pathogen that are not present in M. smegmatis. We speculate that the altered transcriptional regulation of the locus in M. tuberculosis also reflects its importance in virulence. Recent studies, along with this one, demonstrate that the ESX-3 secretion system plays an important role in host-pathogen interaction due to its role in both metal homeostasis and virulence. Understanding how ESX-3 contributes to pathogenesis may enable novel strategies that can subvert this system and promote M. tuberculosis clearance.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the J. Philips, J. Ernst (NYU School of Medicine), and H. Darwin (NYU School of Medicine) laboratories for assistance, and we thank S. Koster (NYU), E. Rubin (HSPH), M. Rodriguez (Rutgers), and J. Tufariello (Albert Einstein College of Medicine) for helpful discussions and comments on the manuscript. We thank J. Tufariello and W. Jacobs, Jr. (Albert Einstein College of Medicine), for providing the esxH mutant.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00197-16.
REFERENCES
- 1.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 2.Philips JA, Ernst JD. 2012. Tuberculosis pathogenesis and immunity. Annu Rev Pathol 7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 3.Stanley SA, Cox JS. 2013. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol 374:211–241. doi: 10.1007/82_2013_332. [DOI] [PubMed] [Google Scholar]
- 4.Neyrolles O, Wolschendorf F, Mitra A, Niederweis M. 2015. Mycobacteria, metals, and the macrophage. Immunol Rev 264:249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A 110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, McVey Ward D, Ganz T, Kaplan J. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 9.Van Zandt KE, Sow FB, Florence WC, Zwilling BS, Satoskar AR, Schlesinger LS, Lafuse WP. 2008. The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J Leuk Biol 84:689–700. doi: 10.1189/jlb.1107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. 2008. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol 181:8521–8527. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- 11.Fang Z, Sampson SL, Warren RM, Gey van Pittius NC, Newton-Foot M. 2015. Iron acquisition strategies in mycobacteria. Tuberculosis 95:123–130. doi: 10.1016/j.tube.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Pandey R, Rodriguez GM. 2014. IdeR is required for iron homeostasis and virulence in Mycobacterium tuberculosis. Mol Microbiol 91:98–109. doi: 10.1111/mmi.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahrt TC, Song J, Siple J, Deretic V. 2001. Mycobacterial FurA is a negative regulator of catalase-peroxidase gene katG. Mol Microbiol 39:1174–1185. doi: 10.1111/j.1365-2958.2001.02321.x. [DOI] [PubMed] [Google Scholar]
- 14.Pym AS, Domenech P, Honore N, Song J, Deretic V, Cole ST. 2001. Regulation of catalase-peroxidase (KatG) expression, isoniazid sensitivity, and virulence by furA of Mycobacterium tuberculosis. Mol Microbiol 40:879–889. doi: 10.1046/j.1365-2958.2001.02427.x. [DOI] [PubMed] [Google Scholar]
- 15.Maciag A, Dainese E, Rodriguez GM, Milano A, Provvedi R, Pasca MR, Smith I, Palu G, Riccardi G, Manganelli R. 2007. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J Bacteriol 189:730–740. doi: 10.1128/JB.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maciag A, Piazza A, Riccardi G, Milano A. 2009. Transcriptional analysis of ESAT-6 cluster 3 in Mycobacterium smegmatis. BMC Microbiol 9:48. doi: 10.1186/1471-2180-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey R, Russo R, Ghanny S, Huang X, Helmann J, Rodriguez GM. 2015. MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol 98:1168–1183. doi: 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegrist MS, Unnikrishnan M, McConnell MJ, Borowsky M, Cheng TY, Siddiqi N, Fortune SM, Moody DB, Rubin EJ. 2009. Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc Natl Acad Sci U S A 106:18792–18797. doi: 10.1073/pnas.0900589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serafini A, Boldrin F, Palu G, Manganelli R. 2009. Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: essentiality and rescue by iron and zinc. J Bacteriol 191:6340–6344. doi: 10.1128/JB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tufariello JM, Chapman JR, Kerantzas CA, Wong KW, Vilchèze C, Jones CM, Cole LE, Tinaztepe E, Thompson V, Fenyö D, Niederweis M, Ueberheide B, Philips JA, Jacobs WR. 2016. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc Natl Acad Sci U S A 113:E348–E357. doi: 10.1073/pnas.1523321113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegrist MS, Steigedal M, Ahmad R, Mehra A, Dragset MS, Schuster BM, Philips JA, Carr SA, Rubin EJ. 2014. Mycobacterial Esx-3 requires multiple components for iron acquisition. mBio 5:e01073-14. doi: 10.1128/mBio.01073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CM, Niederweis M. 2010. Role of porins in iron uptake by Mycobacterium smegmatis. J Bacteriol 192:6411–6417. doi: 10.1128/JB.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilghari D, Lightbody KL, Veverka V, Waters LC, Muskett FW, Renshaw PS, Carr MD. 2011. Solution structure of the Mycobacterium tuberculosis EsxG-EsxH complex: functional implications and comparisons with other M. tuberculosis Esx family complexes. J Biol Chem 286:29993–30002. doi: 10.1074/jbc.M111.248732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hervas-Stubbs S, Majlessi L, Simsova M, Morova J, Rojas MJ, Nouze C, Brodin P, Sebo P, Leclerc C. 2006. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis Infection. Infect Immun 74:3396–3407. doi: 10.1128/IAI.02086-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skjøt RLV, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun 68:214–220. doi: 10.1128/IAI.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottenhoff THM, Kaufmann SHE. 2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog 8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, Chen M, Kim J, Lukose R, Chan J, Orme IM, Porcelli SA, Jacobs WR. 2011. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med 17:1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Köster S, Penberthy K, Kubota Y, Dricot A, Rogan D, Vidal M, Hill DE, Bean AJ, Philips JA. 2013. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog 9:e1003734. doi: 10.1371/journal.ppat.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copeland NG, Jenkins NA, Court DL. 2001. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murry JP, Pandey AK, Sassetti CM, Rubin EJ. 2009. Phthiocerol dimycocerosate transport is required for resisting interferon-gamma-independent immunity. J Infect Dis 200:774–782. doi: 10.1086/605128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo XV, Monteleone M, Klotzsche M, Kamionka A, Hillen W, Braunstein M, Ehrt S, Schnappinger D. 2007. Silencing Mycobacterium smegmatis by using tetracycline repressors. J Bacteriol 189:4614–4623. doi: 10.1128/JB.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava S, Ernst Joel D. 2014. Cell-to-cell transfer of Mycobacterium tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 15:741–752. doi: 10.1016/j.chom.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez GM, Voskuil MI, Gold B, Schoolnik GK, Smith I. 2002. ideR, an essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect Immun 70:3371–3381. doi: 10.1128/IAI.70.7.3371-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurthkoti K, Tare P, Paitchowdhury R, Gowthami VN, Garcia MJ, Colangeli R, Chatterji D, Nagaraja V, Rodriguez GM. 2015. The mycobacterial iron-dependent regulator IdeR induces ferritin (bfrB) by alleviating Lsr2 repression. Mol Microbiol 98:864–877. doi: 10.1111/mmi.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, McMath LM, Iniguez A, Kimmey JM, Sawaya MR, Whitelegge JP, Horwitz MA, Goulding CW. 2011. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc Natl Acad Sci U S A 108:5051–5056. doi: 10.1073/pnas.1009516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serafini A, Pisu D, Palù G, Rodriguez GM, Manganelli R. 2013. The ESX-3 secretion system is necessary for iron and zinc homeostasis in Mycobacterium tuberculosis. PLoS One 8:e78351. doi: 10.1371/journal.pone.0078351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boshoff HI, Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martín C, Cole ST. 2014. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog 10:e1004183. doi: 10.1371/journal.ppat.1004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerasi M, Ammendola S, Battistoni A. 2013. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol 3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcela Rodriguez G, Neyrolles O. 2014. Metallobiology of tuberculosis. Microbiol Spectrum 2:3. doi: 10.1128/microbiolspec.MGM2-0012-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegrist MS, Steigedal M, Ahmad R, Mehra A, Dragset MS, Schuster BM, Philips JA, Carr SA, Rubin EJ. 2014. Mycobacterial Esx-3 requires multiple components for iron acquisition. mBio 5:e01073-14. doi: 10.1128/mBio.01073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner D, Maser J, Lai B, Cai Z, Barry CE III, Honer Zu Bentrup K, Russell DG, Bermudez LE. 2005. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol 174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.