Abstract
Caffeic acid phenethyl ester (CAPE) is a bioactive component derived from honeybee hive propolis. CAPE has been shown to have anti-mitogenic, anti-carcinogenic, and other beneficial medicinal properties. Many of its effects have been shown to be mediated through its inhibition of NF-κB signaling pathways. We took a systematic approach to uncover CAPE’s effects from hours to days on the signaling networks in human prostate cancer cells. We observed that CAPE dosage-dependently suppressed the proliferation of LNCaP, DU-145, and PC-3 human prostate cancer cells. Administration of CAPE by gavage significantly inhibited the tumor growth of LNCaP xenografts in nude mice. Using LNCaP cells as a model system, we examined CAPE’s effect on gene expression, protein signaling, and transcriptional regulatory networks using Micro-Western Arrays and PCR arrays. We built a model of CAPE’s impact on cell signaling which suggested that it acted through inhibition of Akt-related protein signaling networks. Over-expression of Akt1 or cMyc, a downstream target of Akt signaling, significantly blocked the anti-proliferative effects of CAPE. In summary, our results suggest that CAPE administration may be useful as an adjuvant therapy for prostate and potentially other types of cancers that are driven by the AMPK and Akt signaling networks.
Introduction
Prostate cancer is the most common non-cutaneous carcinoma of men in the United States. Androgen ablation therapy is the primary and standard treatment for metastatic prostate cancer. However, most prostate cancer patients receiving androgen ablation therapy ultimately develop recurrent castration-resistant tumors within 12–33 months after treatment with a mean survival of 2–3 years. Adjuvant therapies are therefore critically needed for improving the outcome of prostate cancer patients. Phosphatase and tensin homolog (PTEN) is frequently deleted in prostate cancer, resulting in activation of PI3K/Akt signaling (1). PI3K/Akt signaling plays an important role in survival and progression of prostate cancer cells (1). Akt is a serine/threonine protein kinase regulating a variety of cellular responses, including inhibition of apoptosis and stimulation of cell proliferation. Up-regulation of tumor PI3K/Akt activity is associated with a poor clinical outcome of patients with prostate cancer (2). Therefore, small molecule inhibitors that can suppress PI3K/Akt signaling with minimal side effects are potential candidates for prostate cancer treatment. Caffeic acid phenethyl ester (CAPE) is a bioactive component extracted from honeybee hive propolis. CAPE is a known inhibitor of NF-κB (3) that has strong antioxidant (4) properties. CAPE is marketed over-the-counter as a health foods supplement that does not typically have substantial side effects. CAPE has been shown to suppress Akt signaling and cause growth inhibition in human CD4+ T cells (5) and human coronary smooth muscle cells (6). CAPE treatment has been shown to induce apoptosis through activation of p53-regulated Bax (7, 8), c-Jun N-terminal kinase (JNK) (7), and p38 mitogen-activated protein kinase (p38 MAPK) (7), as well as through suppression of NF-κB activity (7, 9), and reduction of Bcl-2, cIAP-1, cIAP-2, and XIAP (9, 10) expression in several human cancer cell lines. In addition, CAPE has been shown to induce cell cycle arrest in cancer cells through suppression of cyclin D1 (10, 11), cyclin E (10), and c-Myc expression (12), as well as via induction of the cyclin dependent kinase inhibitors p21waf1/cip1 (10), p27Kip1 (10), and p16INK4A (10). We reasoned that CAPE might be a potentially useful therapeutic for prostate cancer treatment. We thus applied several systems-level approaches to examine the molecular mechanisms by which CAPE inhibited the growth of prostate cancer cells.
Material and Methods
Chemicals
Chemicals were purchased from Sigma (St. Louis, MO).
Cell Culture
LNCaP 104-S, DU-145, and PC-3 cells were passaged and maintained as previously described (13–20).
Cell Proliferation Assay
Relative cell number was analyzed by measuring DNA content of cell lysates with the fluorescent dye Hoechst 33258 (Sigma) as described previously (13, 14, 17–20).
Soft Agar Colony Formation Assay
8000 cells were suspended in 0.3% low melting agarose (Lonza) with 10% fetal bovine serum in DMEM medium and then layered on top of 3 ml of 0.5% low melting agarose plus 10% fetal bovine serum in DMEM medium in 6 cm dishes. Cells were allowed to grow at 37 °C with 5% CO2 for 14 days. The plates were stained with 0.005% crystal violet in 30% ethanol for 6 h.
Flow Cytometric Analysis
After 96 h of culture in the presence of different concentrations of CAPE, cells were processed, cell cycle profiles were determined by flow cytometric analysis using a BD Facscan flow cytometer (BD Biosciences, San Jose, CA), and data was analyzed using ModFit LT software (Verity Software House, Topsham, ME) as described (17–20).
Western Blotting Analysis
Cells were lysed in SDS lysis buffer (240 mM Tris-acetate, 1% SDS, 1% glycerol, 5mM EDTA pH 8.0) with DTT, protease inhibitors, and a cocktail of phosphatase inhibitors. Expression of proteins including Akt1/2/3 (with an antibody that did not discriminate between the proteins), Akt1, Akt2, Akt3, Skp2, α-tubulin, cyclin A, phospho-Cdk2 (T160), phospho-GSK3α (S21), phospho-GSK3β (S9), GAPDH, phospho-Rb (S807/811), phospho-P38 MAPK (T180/Y182), phospho-Akt (S473), phospho-Akt (T308), and phospho-P90 RSK(S380) were detected by antibodies from Cell Signaling Technology (Danvers, Massachusetts). Anti-rabbit and anti-mouse IgG secondary antibodies were from Invitrogen (Carlsbad, California) and LI-COR BioSciences (Lincoln, Nebraska). p21waf1/cip1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), and p27Kip1 antibody was from BD BioSciences (San Jose, CA). Blots were scanned and quantified using a LI-COR Odyssey near-infrared imaging system. α-tubulin, β-actin, and GAPDH were used as loading controls.
Micro-Western Arrays
104-S cells were treated with vehicle (ethanol) or 10 μM CAPE by replacing medium with new medium (DMEM plus 8% FBS plus 1 nM dihydrotestosterone (DHT)) and the treatment lasted for 0, 30, 60, 120, 240, and 480 min. Cells were lysed at the indicated time points, and micro-western arrays were performed to measure protein expression and modification as previously described (21, 22).
Protein over-expression
Ectopic expression of Akt1 and c-Myc was achieved by infecting LNCaP 104-S cells with pSRα or pBabe retroviruses carrying the cDNA of the indicated proteins, respectively. Antibiotic-resistant (G418 and puromycin) colonies were expanded and screened for increased target protein expression by western blot analysis. Cells infected with retrovirus carrying empty vectors were used as controls.
PCR Arrays
LNCaP 104-S cells were treated with 0 or 10 μM CAPE for 48 h. Total RNA was isolated with the TRIZOL Reagent (Invitrogen, Carlsbad, CA) and contaminating DNA was removed using DNase I (DNA-free, Ambion, Austin, TX). cDNA was synthesized from total RNA using SMARTScribe Reverse Transcriptase (Clontech, Mountain View, CA). Expression of mRNA of selected genes was assayed using pre-coated 96-well SYBR Green real-time PCR arrays (SABiosciences, Frederick, MD), including the human Cancer Pathway Finder PCR Array (PAHS-033) and the human PI3K-AKT Signaling Pathway PCR Array (PAHS-058), with RT2 qPCR Master Mixes (SABiosciences, Frederick, MD) and StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Experiments were performed with 4 biological replicates and 1–2 technical replicates per each sample. Five housekeeping genes (B2M, HPRT1m, RPL13A, GAPDH, and ACTB) were included in each array as RNA content controls. Gene expression was determined using the 2−ΔΔCt method and fold changes were calculated as the difference in gene expression between the CAPE-treated and control groups. Data were analyzed using the RT2 Profiler PCR Array Data Analysis website (http://www.sabiosciences.com/pcr/arrayanalysis.php). Functional annotation enrichment for both up- and down-regulated genes after CAPE treatment was performed using Database for Annotation, Visualization and Integrated Discovery (DAVID) with the entire set of genes on both RT-PCR arrays as background. P values represent a minus log transformation of the geometric mean of all of the enrichment p-values (EASE scores) for each annotation term associated with the gene members in the group.
Xenografts in Athymic Mice
Experiments involving mice were approved by the University of Chicago Institutional Animal Care and Use Committee. 6–8 week old male Balb/c nu/nu mice (NCI Frederick, MD) were injected subcutaneously in both flanks with 1 × 106 LNCaP 104-S cells suspended in 50 μl DMEM mixed with 50 μl of Matrigel (BD Bioscience, Franklin Lakes, NJ). CAPE (10 mg/kg/day in 0.2 ml sesame oil) or vehicle (sesame oil) was administered to mice by gavage starting one week after cancer cell injection. Tumors were measured weekly using the formula: volume = length × width × height × 0.52 (14–16, 20). The CAPE treatment group comprised 10 mice with 17 tumors while the vehicle control group comprised 9 mice with 16 tumors. CAPE and vehicle treatment stopped at the 6th week and tumors were allowed to grow for another two weeks.
NF-κB reporter assays
For measuring NF-κB activity, cells were seeded 3 × 104 cells/well in 48-well plate for 24 h, then transfected with phRL-CMV-Renilla luciferase plasmid (1 ng/well), 4X NF-κB (50 ng/well), and Bluescript SKII + (750 ng/well) using calcium phosphate co-precipitation method (13). 24 h after transfection, cells were treated with increasing concentrations of CAPE for 24 h and were lysed in 50 μL passive lysis buffer (Promega, Madison, WI). Luciferase activity was measured using a Dual-Luciferase kit (Promega) in a Monolight luminometer (BD Biosciences).
Results and Discussion
CAPE treatment suppressed the proliferation of human prostate cancer cells
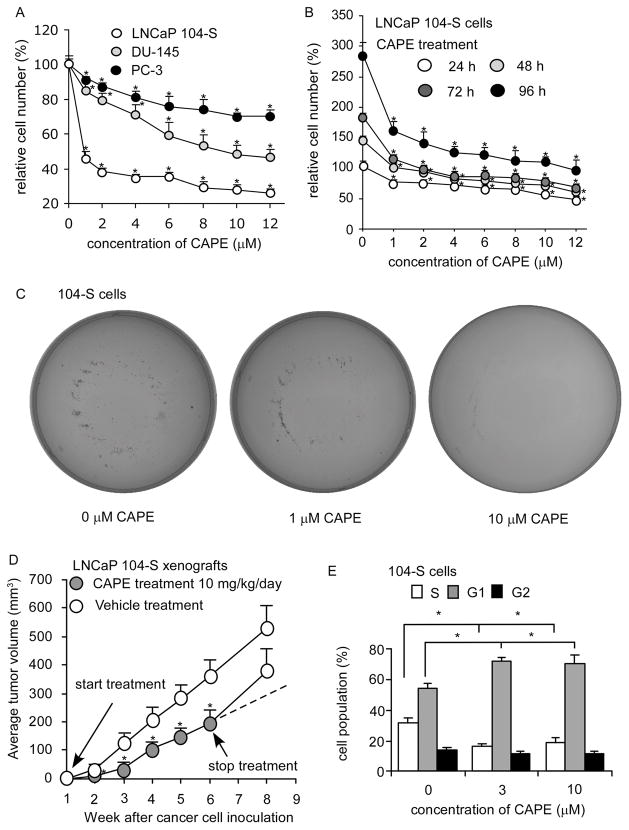
The proliferation of LNCaP 104-S, DU-145 and PC-3 human prostate cancer cells was dosage-dependently suppressed by CAPE treatment (Fig. 1A) with an IC50 of 0.68 μM, 9.54 μM, and 18.65 μM, respectively. We thus chose LNCaP 104-S cells for further investigation. The growth inhibitory effect of CAPE was evident within 24 hours of treatment but the suppressive effect accumulated over time (Fig. 1B). A colony formation assay revealed that treatment of 1 μM CAPE reduced colony formation by 50% while treatment with 10 μM CAPE completely blocked the formation of LNCaP 104-S colonies (Fig. 1C), confirming the anti-cancer effect of CAPE in prostate cancer cells.
Figure 1. CAPE suppressed proliferation of prostate cancer cells in vitro and in vivo.
(A) LNCaP, PC-3, and DU-145 cells were treated with increasing concentrations of CAPE for 96 h to determine suppressive effect of CAPE on prostate cancer cell lines. (B) LNCaP 104-S cells were treated with increasing concentrations of CAPE for 24, 48, 72, 96 h to investigate the suppressive effects of CAPE. Relative cell number was normalized to cell number of control (no treatment) at 24 h. (*) represented that cell number difference was statistically significant (p < 0.05) compared to that of control (no treatment) at the same time period. (C) Anticancer effect of CAPE was determined by a colony formation assay of LNCaP 104-S cells treated with 0, 1, 10 μM for 14 days. Image is representative of three biological replicates. (D) LNCaP 104-S cells were injected subcutaneously into athymic mice to form tumors. CAPE (10 mg/kg/day in sesame oil) or vehicle (sesame oil) was administered by gavage starting one week after cancer cell injection. Tumor volume of mice carrying 104-S xenografts was measured weekly. CAPE and vehicle treatment were stopped at 6th week and tumors were allowed to grow for another two weeks. Dashed line represents expected tumor growth if CAPE treatment continued. Tumor volume was shown as volume plus standard error (SE). (E) LNCaP 104-S cells were treated with CAPE for 96 h, harvested, and stained with propidium iodide dye for flow cytometric analysis of cell cycle distribution. (*) represents statistically significant difference (p < 0.05) between the two group of cells being compared.
CAPE caused retardation of xenograft growth and G1 cell cycle arrest in LNCaP cells
Administration of CAPE by gavage (10 mg/kg body weight per day) for six weeks resulted in a 50% reduction of tumor volume (p = 0.0008) (Fig. 1D). CAPE treatment did not affect the body weight of the mice (data not shown), suggesting that the dosage used was not overtly toxic. Termination of CAPE treatment at the 6th week resulted in the return of 104-S tumors growth to a rate comparable to the control group, indicating that CAPE inhibited tumor growth but did not completely eliminate the xenografted tumor cells. Following 96 h of treatment with at least 3 μM CAPE, flow cytometric analysis revealed a significant reduction of cells in the S phase and an increase of cells in either the G0 or G1 population phases (Fig. 1E). We did not observe any sub-G1 population. Annexin V staining (data not shown) confirmed that apoptosis was not induced in 104-S cells by CAPE indicating that CAPE treatment inhibited LNCaP cell proliferation through induction of either a G1 cell cycle arrest or a return of cells to a quiescent G0 state.
CAPE treatment affected expression of genes related to Akt signaling and cell cycle regulation
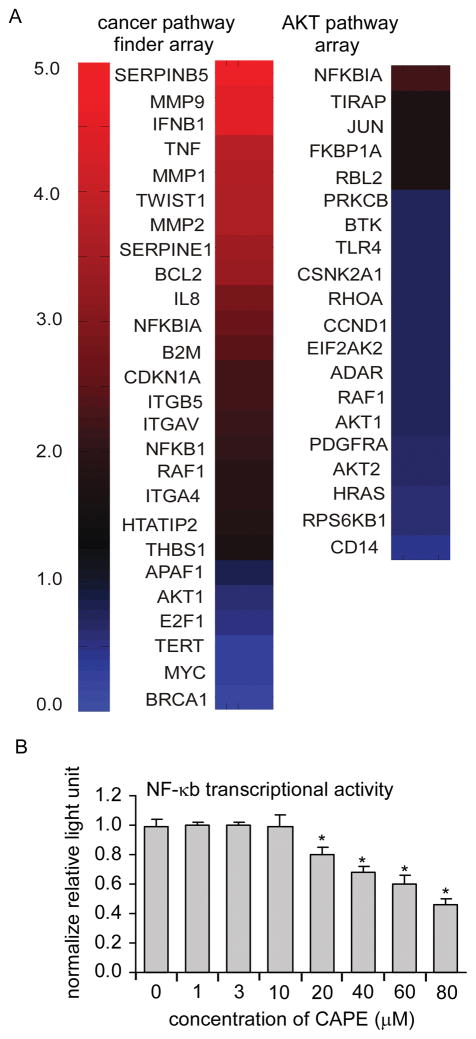
As PI3K/Akt signaling plays an important role in survival and progression of prostate cancer cells (1, 23), we used PCR arrays to quantitatively monitor the expression of many genes with known involvement in cancer- and Akt-related signaling pathways (Supp. Table 1). Of the 162 total genes examined, 20 (12.3%) were significantly down-regulated, and 23 (14.2%) significantly up-regulated after CAPE treatment (p < 0.05) (Fig. 2A). In the Akt signaling array, 95% (19 out of 20) of the genes affected by CAPE treatment were significantly down-regulated, indicating that CAPE suppressed Akt-related transcriptional activity. Many genes important for cell proliferation were down-regulated following CAPE treatment, including BRCA1, AKT1, AKT2, MYC, E2F1, TERT, HRAS, RPS6KB1, and RHOA (Fig. 2A). Simultaneously, the cell cycle inhibitory gene p21 (CDKN1A) was up-regulated. SERPINB5 (also known as maspin) expression was up-regulated by 5-fold. Maspin is a tumor suppressor protein and an MMP-inhibitor like protein, whose expression has been linked to repression of prostate cancer metastasis (24). Recently, expression of maspin by stable transfection was shown to decrease the survival of lung cancer tumors in nude mice through reduction of Akt phosphorylation (25). We tested the −2000 bp to +200 bp regions of all 43 differentially expressed genes for enrichment of any known transcription factor binding sites via position weight matrices from Jasper and Transfac 6.0 using TFM-Explorer (26) relative to all mRNAs on the RT-PCR arrays (p < 0.05). Enriched binding site motifs included OCT1, MEF2, TCF11, MEIS1A/HOXA9, BRN2, and VMAF. Many previous studies have reported that CAPE suppresses NF-κB activity in the time-frame of minutes to hours (3). However, we observed that treatment of LNCaP cells with 10 μM CAPE for 48 h caused up-regulation of genes that were significantly enriched for known NF-κB targets according to a database of known NF-κB targets (p value = 9 × 10−6, permutation analysis), http://bioinfo.lifl.fr/NF-KB/ (27) and Transcriptional Regulatory Element Database (28). Our analysis demonstrated that 16 of 23 (69.6%) up-regulated genes were known NF-κB target genes versus 46/162 (28.4%) NF-κB target genes examined in the analysis. Up-regulated NF-κB target genes included the pro-inflammatory cytokines (IFNB1, TNF, and IL8), the matrix metalloproteases (MMP1, MMP2, and MMP9), regulator of morphogenesis and metastasis (TWIST) (29), and the cell cycle inhibitor p21Cip (Fig. 2A). To further address whether CAPE treatment caused activation of NF-κB, we examined the transcriptional output of an NF-κB driven luciferase reporter construct transfected into LNCaP104-S cells (Fig. 2B). Treatment with 1–10 μM CAPE did not cause a significant change in luciferase activity. Therefore, CAPE appeared to cause the up-regulation of many known NF-κB target genes without activation of NF-κB activity.
Figure 2. CAPE caused reduction in expression of genes involved in cell proliferation and Akt signaling in LNCaP cells.
104-S cells were treated with 0 or 10 μM CAPE for 48 h and mRNA was extracted and analyzed by PCR arrays for quantitative analysis of mRNA expression of genes related to cancer and the Akt pathway. Heatmap indicates the fold change of mRNA of CAPE treated LNCaP 104-S cells compared to control 104-S cells after 96 hr. A value of 1.0 means no change. Values less than 1.0 indicate a decrease in expression while values greater than 1.0 indicate increased expression. Experiments were performed with 4 biological replicates and 1–2 technical replicates per each sample. The left column displays the significantly changed genes from the Cancer Pathway Finder PCR Array while the second column displays genes significantly changed from the PI3K/Akt signaling PCRArray. Data for this figure can be found in Supp. Table 1.
CAPE squelched early phosphorylation events following replenishment of cell medium in LNCaP 104-S cells
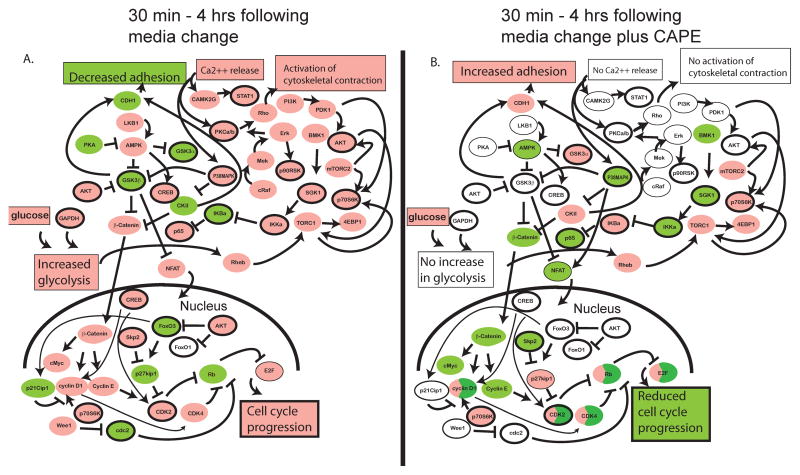
To determine the global short-term effects of CAPE on the changes in cellular signaling proteins related to Akt signaling, cell stress, and cell proliferation in LNCaP 104-S cells, we performed Micro-Western Array analysis (MWA) (21, 22, 30, 31) of 28 protein abundances and modifications in LNCaP 104-S cells at six time points from 0 to 480 min following replenishment of serum- and androgen-containing medium in the absence or presence of 10 μM CAPE (Fig. 3; Supp. Fig 1). In the absence of CAPE, replenishment of growth medium resulted in the phosphorylation of cyclic AMP response element binding protein Creb(S133) and glycogen synthase kinase 3 beta Gsk3β(S9) within 30 minutes (Fig. 3). The phosphorylation of Creb returned to baseline levels at 4h consistent with the known burst-attenuation kinetics of cAMP (32). In the time between 1h and 4h, the phosphorylation of serum and glucocorticoid-inducible kinase SGK(S78), FoxO3a(T32), Gsk3α(S21), Akt(S473), and Akt(T308) were also subtly increased by about one fold. SGK, like Akt, promotes proliferation through phosphorylation-mediated inactivation of the forkhead transcription factor FoxO3, thus preventing its continuous induction of cell cycle arrest genes (33). Additionally, the abundance of p65/RelA and the phosphorylation of I Kappa B Kinase (IKK)(S176) were subtly increased, consistent with a role for NF-κB in driving a proliferative response following replenishment of growth medium (Fig. 4). Following CAPE treatment, almost all of the previous changes in protein abundance and modification were lost or substantially delayed (Fig. 3). Not only did we observe an absence of up-regulation of p-Creb, p-Gsk3β, p65/RelA, and other proteins, we observed subtle decreases in the levels of Stat1, p-Stat1, p65/RelA, and p-SGK following CAPE treatment. S-phase kinase-associated protein 2 (Skp2), a member of the F-box protein family which is responsible for ubiquitination and down-regulation of p27Kip cell cycle inhibitors, was subtly down-regulated. These data helped to provide mechanistic understanding for the observed cell cycle arrest. Our results suggested that CAPE treatment altered cell signaling networks in a manner that resulted in a lack of cell proliferation in response to the availability of nutrition. Notably, the effect of CAPE on cell signaling was observed at 30 min, the earliest measured time point. The identity of the targets effected at 30 min included p-Creb(S133) and p-Gsk3β(S9), suggesting that CAPE acted at early time points through inhibition of 5′ AMP-activated protein kinase (AMPK) rather than Akt which was phosphorylated at much later time points than Creb or Gsk3β in response to medium addition (Fig. 3). We constructed a model of protein influences based on the literature and based on the timing of changes that we observed (Fig. 4). Medium addition was suggested to cause the activation of many pathways, consistent with up-regulation of cell-proliferation including AMPK, SGK1, Akt, Torc1, Torc2, p65/RelA, and β-catenin pathways. Following CAPE treatment, AMPK, SGK1, and NF-κB pathways were down-regulated. This down-regulation likely resulted in the decrease of β-catenin and Creb signaling, cyclin D1 and cyclin E1 expression, Cdk2 and Cdk4 activity, as well as increase of p27Kip1 expression.
Figure 3. Heatmap of protein abundance and phosphorylation fold changes measured by Micro-Western Arrays following replenishment of androgen-containing growth medium in the absence (left) or presence (right) of 10 μM CAPE treatment in LNCaP 104-S cells.
Medium of 104-S cells was replaced with fresh medium containing 8% FBS DMEM plus 1 nM dihydrotestosterone (DHT) in the absence (left) or presence (right) of 10 μM CAPE. Cell lysates were collected prior to treatment (0 min), and following 30, 60, 120, 240, and 480 min. Micro-Western Arrays were performed to measure the changes in abundance and modification of indicated proteins. Proteins were organized in the y-axis of the heatmap based on time of maximal fold change amplitude. Time course graphs depicting signal to noise ratio and standard deviation of three technical replicates for each measurement are shown in Supp. Fig 1, and Supp. Table 2.
Figure 4. Putative model of CAPE describing the cause-effect relationship of the activity of 19 measured and 27 inferred proteins in LNCaP 104-S cells following addition of fresh serum-containing growth medium in the absence (A) or presence (B) of 10 μM CAPE in the time between 0 minutes and 4 hours.
Protein nodes measured in the Micro-Western Array experiment were arranged in the figure along with unmeasured protein nodes based on relationships suggested from the literature. Nodes with measured or inferred up-regulation in activity are colored pink. Those with a measured or inferred down-regulation in activity are colored green. Those with no measured or inferred change are colored white. Those proteins downstream of opposing influences are colored half green and half pink. Measured nodes have a deep black outline while inferred (unmeasured) nodes have no black outline (in the case of colored nodes) or a thin black outline (in the case of non-colored nodes). Protein nodes are depicted in small ovals while cellular behaviors are depicted in large boxes.
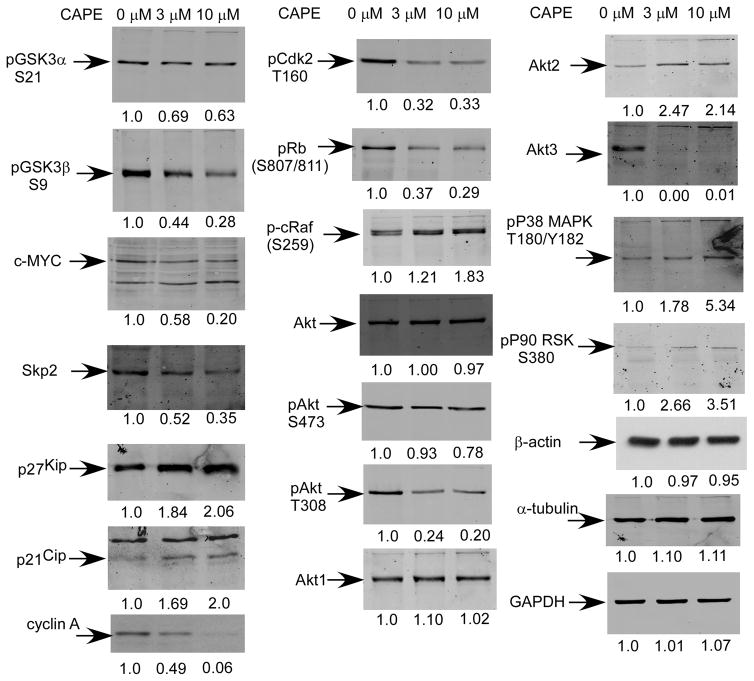
CAPE caused a reduction in abundance and activity of cell cycle promoting proteins and AKT signaling pathway proteins
We used traditional Western blotting to further examine the effects of CAPE on cells following longer-term treatment. Following 96 h treatment, changes in protein expression and modification induced by CAPE were similar to those observed following short-term treatment but effects on the AKT pathway were more evident. GSK3 phosphorylation was reduced and the abundance and modification of components of the cell cycle changed in a manner consistent with a reduction in cell proliferation. 10 μM CAPE treatment caused a modest reduction in the phosphorylation of glycogen synthase kinase 3 alpha (Gsk3α)(S21) and a major (70%) reduction in the phosphorylation of Gsk3β(S9) (Fig. 5). The reduction in phosphorylation of Gsk3β would be expected to result in an increase in its activity (34), which would result in phosphorylation and targeted-destruction of cell cycle stimulatory proteins such as β-catenin, cyclin D1, cyclin E, and c-Myc (35–37). The abundance of c-Myc and Skp2 decreased by several fold (Fig. 5; Supp. Fig. 2) while the abundance of cell cycle inhibitory proteins p27Kip1 and p21Cip1 (38) increased by two-fold. Furthermore, CAPE treatment resulted in a dramatic reduction in the abundance of cyclin A and phospho-Cdk2(T160). Cyclin A binds to Cdk2 and is required for cells to progress through the S phase. The cyclin A/Cdk2 complex is inhibited by cell cycle inhibitor p21Cip. Phosphorylation of Cdk2 on T160 is necessary for its activation (39) and is required for traversing the G1/S checkpoint through phosphorylation of pRb. Consistent with inactivation of Cdk2, phospho-pRb(S807/811) was decreased by 70%. CAPE treatment led to increased levels of phospho-c-Raf(S259), (Fig. 5). Phosphorylation of c-Raf on S259 and S621 creates 14-3-3 binding sites which are thought to maintain it in an auto-inhibited state (40). Down-regulation of cyclin A, c-Myc, Skp2, phosphorylated Rb, and Cdk2, coupled with increased phospho-cRaf(S259), p21Cip and p27Kip1 abundance likely contributed to the sustained induction of G1 cell cycle arrest following 96 h CAPE treatment.
Figure 5. CAPE treatment resulted in decreased abundance and phosphorylation of proteins in cell cycle regulation and AKT signaling in LNCaP 104-S cells following 96 h treatment.
Protein expression of phospho-GSK3α S21, phospho-GSK3β S9, c-Myc, Skp2, p27Kip1, p21Cip1, cyclin A, pCdk2 T160, phospho-Rb (S807/811), phospho-c-Raf S259, total Akt, phospho-Akt S473, phospho-Akt T308, Akt1, Akt2, Akt3, phospho-P38 MAPK T180/Y182, phospho-P90 RSK S380, β-actin, α-tubulin, and GAPDH in 104-S cells treated with 0, 3, and 10 μM CAPE for 96 h were assayed by Western blotting. Graphical representations of mean and standard deviation are presented in Supplemental Figure 2.
However, the long-term signaling data highlighted changes in Akt and p38 mitogen activated protein kinase signaling that were not apparent following short term treatment. While CAPE did not affect total Akt levels, it caused a 20% reduction in phospho-Akt(S473) and an 80% reduction in phospho-Akt(T308) levels (Fig. 5). While S473 is a known target of mTor, T308 is a known target of PDK1. CAPE treatment led to increased Akt2 levels and caused a complete loss of Akt3 protein expression. CAPE treatment caused an increase in phospho-p38 MAPK(T180/Y182) and phospho-p90 ribosomal S6 kinase (p90RSK)(S380). p38 MAPK activity can negatively regulate cell cycle progression both at the G1/S and the G2/M transitions in part through phosphorylation and stabilization of cell cycle inhibitor p21Cip1 (41–43). Increased phosphorylation of p90RSK(S380) could indicate either increased ERK or JNK activity. However, given the role of JNK in responding to cell stress, we speculate that increased JNK activity was likely responsible for this phosphorylation.
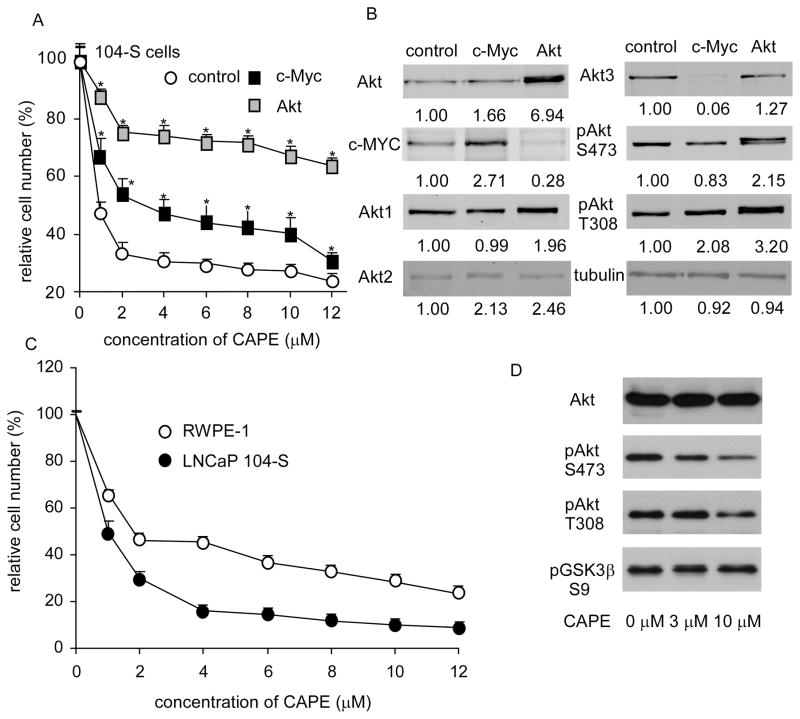
Overexpression of Akt1 or c-Myc blocked the suppressive effect of CAPE on cell proliferation
To confirm that CAPE suppressed cell proliferation through suppression of Akt, we overexpressed Akt1 and c-Myc in LNCap 104-S. Akt over-expression would be predicted to repress Gsk3β resulting in increased β-catenin and NFAT activity while over-expression of c-Myc would be expected to have less effect as many parallel pathways would still down-regulated in response to increased Gsk3β activity (44). As expected, the anti-proliferative effect of CAPE was significantly reduced in 104-S cells transfected with Akt1. c-Myc over-expression was not as effective as AKT in rescuing CAPE’s anti-proliferative effect (Fig. 6A). Somewhat surprisingly, overexpression of c-Myc caused a complete loss in Akt3 expression (Fig. 6B, Supp. Fig. 3). Similarly, Akt1 over-expression caused a dramatic reduction in c-Myc expression (Fig. 6B, Supp. Fig. 3) indicating that the expression of Akt isoforms and cMyc proteins were coordinately regulated and that each Akt protein isoform was differentially regulated in LNCaP cells.
Figure 6. Over-expression of Akt and c-Myc blocked the suppressive effect of CAPE on proliferation and CAPE blocked AR transcriptional activity.
(A) 104-S cells overexpressing Akt1 or c-Myc were treated with increasing concentrations of CAPE for 96 hr and analyzed by 96-well proliferation assay. (B) Protein expression of Akt, c-Myc, Akt1, Akt2, Akt3, phospho-Akt S473, phospho-Akt T308, and α-tubulin were assayed by Western blotting of 104-S cell lines overexpressing c-Myc or Akt1 prior to CAPE treatment. (C) RWPE-1 cells were treated with increasing concentrations of CAPE for 96 h to determine suppressive effect of CAPE on normal prostate epithelial cells. (D) Protein expression of total Akt, phospho-Akt S473, phospho-Akt T308, phospho-GSK3β S9, and β-actin in RWPE-1 cells treated with 0, 3, and 10 μM CAPE for 96 h was assayed by Western blotting.
Finally, we sought to determine the effect of CAPE on the proliferation of normal prostate cells. We found that RWPE-1 cells, a model of normal prostate cells were more resistant than LNCP 104-S cells to the anti-proliferative effects of CAPE. The IC50 of RWPE-1 cells (4.83 μM) was 8 fold higher than that of 104-S cells (Fig. 6C). CAPE treatment of RWPE-1 cells for 96 hr led to substantial reduction in Akt(S473) levels but only a modest reduction in Akt(T308) and GSK3β(S9) (Fig. 6D). While RWPE-1 is a model for normal prostate epithelial cells, it has been transfected with a single copy of the human papilloma virus 18 (HPV-18) (ATCC CRL-11609). It is not clear if the HPV-18 virus renders the cells more or less vulnerable to CAPE treatment. However, as normal prostate epithelial cells usually do not proliferate, we believe that normal prostate epithelial cells in patients will be more resistant to CAPE treatment, especially given that the growth of xenografted prostate tumors was inhibited by CAPE without an apparent drop in body weight or appetite of the mice harboring the tumors (data not shown).
CAPE has been shown to inhibit RANKL-induced osteoclastogenesis of precursor cells of the monocyte-macrophage lineage when added in the early stages of development (45). This effect was shown to occur concurrently with suppression of NF-κB and NFAT transcriptional activity consistent with previous findings of Marquez et al (46). Very recent work has shown that Akt and GSK3β signaling are required for RANKL-induced activation of NFATc1 during osteoclast differentiation (47). Taken together with our own data, these results are consistent with a model whereby CAPE blocks the activity of AMPK and/or other upstream kinases, resulting in reduced GSK3β phosphorylation and increased GSK3β activity. Following short-term CAPE treatment, these changes may result in phosphorylation, nuclear export, and decreased transcriptional activity of NFATc1. However, following long term treatment, our results demonstrated dramatic down regulation of AKT concurrent with up-regulation in p38 kinase phosphorylation and inferred activity. p38 activity has been shown to be necessary for induction of NFAT in response RANKL (48). Our data support a model whereby long-term CAPE treatment leads to suppressed Akt activity and increased p38 activity which may induce NFAT activity leading to the up-regulation of many NF-κB target genes (Fig. 2). Both NF-κB and NFAT family proteins bind DNA through Rel homology domains and our data indicated that transcription from an NF-κB reporter was not up-regulated. Our results provided systems-level insight that into the molecular mechanism of CAPE’s anti-proliferative effect in prostate cancer cells. As the achievable concentration of CAPE in human serum is around 5.0 μg/ml (17 μM) (49) and our study indicated that CAPE treatment at 10 μM could effectively suppress different prostate cancer cell lines (Fig. 1A), we believe that CAPE is a promising adjuvant therapeutic candidate for prostate cancer.
Supplementary Material
Acknowledgments
This work was supported by grant from The Cancer Research Foundation; the Illinois Division of the American Cancer Society; and an internal research grant from the American Cancer Society to RBJ; National Institutes of Health (NIH) grant CA58073 to SL; CS-101-PP-14 (National Health Research Institutes), DOH100-TD-C-111-004 (Department of Health), and NSC 99-2320-B-400-015-MY3 (National Science Council) grants in Taiwan to CPC. RJH was supported by NIH Training Grant T32 GM007197. MFC was supported by a NIH Systems Biology of Oxygen training grant. We thank Dr. Kay Macleod for critical reading of the manuscript.
References
- 1.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 2.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–6. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 3.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhimani RS, Troll W, Grunberger D, Frenkel K. Inhibition of oxidative stress in HeLa cells by chemopreventive agents. Cancer Res. 1993;53:4528–33. [PubMed] [Google Scholar]
- 5.Wang LC, Chu KH, Liang YC, Lin YL, Chiang BL. Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin Exp Immunol. 2010;160:223–32. doi: 10.1111/j.1365-2249.2009.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho HC, Hsu SL, Ting CT, Kuo CY, Yang VC. Caffeic acid phenethyl ester inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using a local delivery system. Cell Mol Biol (Noisy-le-grand) 2009;55(Suppl):OL1161–7. [PubMed] [Google Scholar]
- 7.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–26. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143–9. doi: 10.1097/00001813-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 9.McEleny K, Coffey R, Morrissey C, Fitzpatrick JM, Watson RW. Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int. 2004;94:402–6. doi: 10.1111/j.1464-410X.2004.04936.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HC, Kuo WH, Lee YJ, Lin WL, Chou FP, Tseng TH. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006;234:199–208. doi: 10.1016/j.canlet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 11.He YJ, Liu BH, Xiang DB, Qiao ZY, Fu T, He YH. Inhibitory effect of caffeic acid phenethyl ester on the growth of SW480 colorectal tumor cells involves beta-catenin associated signaling pathway down-regulation. World J Gastroenterol. 2006;12:4981–5. doi: 10.3748/wjg.v12.i31.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaoka T, Banskota AH, Tezuka Y, Harimaya Y, Koizumi K, Saiki I, et al. Inhibitory effects of caffeic acid phenethyl ester analogues on experimental lung metastasis of murine colon 26-L5 carcinoma cells. Biol Pharm Bull. 2003;26:638–41. doi: 10.1248/bpb.26.638. [DOI] [PubMed] [Google Scholar]
- 13.Chuu CP, Chen RY, Hiipakka RA, Kokontis JM, Warner KV, Xiang J, et al. The liver X receptor agonist T0901317 acts as androgen receptor antagonist in human prostate cancer cells. Biochem Biophys Res Commun. 2007;357:341–6. doi: 10.1016/j.bbrc.2007.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (−)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009;275:86–92. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082–4. doi: 10.1158/0008-5472.CAN-04-3992. [DOI] [PubMed] [Google Scholar]
- 16.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66:6482–6. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 17.Chuu CP, Kokontis JM, Hiipakka RA, Fukuchi J, Lin HP, Lin CY, et al. Androgen suppresses proliferation of castration-resistant LNCaP 104-R2 prostate cancer cells through androgen receptor, Skp2, and c-Myc. Cancer science. 2011;102:2022–8. doi: 10.1111/j.1349-7006.2011.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643–8. [PubMed] [Google Scholar]
- 19.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–9. doi: 10.1158/0008-5472.CAN-04-2332. [DOI] [PubMed] [Google Scholar]
- 20.Kokontis JM, Hsu S, Chuu CP, Dang M, Fukuchi J, Hiipakka RA, et al. Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity. Prostate. 2005;65:287–98. doi: 10.1002/pros.20285. [DOI] [PubMed] [Google Scholar]
- 21.Ciaccio MF, Wagner JP, Chuu CP, Lauffenburger DA, Jones RB. Systems analysis of EGF receptor signaling dynamics with microwestern arrays. Nat Methods. 2010;7:148–55. doi: 10.1038/nmeth.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Kuo WL, Seiwert TY, Lingen M, Ciaccio MF, Jones RB, et al. Effect of complementary pathway blockade on efficacy of combination enzastaurin and rapamycin. Head Neck. 2011 doi: 10.1002/hed.21701. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–7. [PubMed] [Google Scholar]
- 24.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–4. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 25.Nam E, Park C. Maspin suppresses survival of lung cancer cells through modulation of Akt pathway. Cancer Res Treat. 2010;42:42–7. doi: 10.4143/crt.2010.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonon L, Touzet H, Varre JS. TFM-Explorer: mining cis-regulatory regions in genomes. Nucleic Acids Res. 2010;38(Suppl):W286–92. doi: 10.1093/nar/gkq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 28.Zhao F, Xuan Z, Liu L, Zhang MQ. TRED: a Transcriptional Regulatory Element Database and a platform for in silico gene regulation studies. Nucleic Acids Res. 2005;33:D103–7. doi: 10.1093/nar/gki004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Chevrier N, Mertins P, Artyomov MN, Shalek AK, Iannacone M, Ciaccio MF, et al. Systematic Discovery of TLR Signaling Components Delineates Viral-Sensing Circuits. Cell. 2011;147:853–67. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hause RJ, Kim HD, Leung KK, Jones RB. Targeted protein-omic methods are bridging the gap between proteomic and hypothesis-driven protein analysis approaches. Expert Rev Proteomics. 2011;8:565–75. doi: 10.1586/epr.11.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki K, Cripe TP, Koch SR, Andreone TL, Petersen DD, Beale EG, et al. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984;259:15242–51. [PubMed] [Google Scholar]
- 33.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–65. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–63. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 36.Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, Gurien-West M, et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12:381–92. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 37.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–52. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 39.Gu Y, Rosenblatt J, Morgan DO. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison DK, Heidecker G, Rapp UR, Copeland TD. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–16. [PubMed] [Google Scholar]
- 41.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–16. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 43.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 44.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. The Journal of biological chemistry. 2003;278:51606–12. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 45.Ang ES, Pavlos NJ, Chai LY, Qi M, Cheng TS, Steer JH, et al. Caffeic acid phenethyl ester, an active component of honeybee propolis attenuates osteoclastogenesis and bone resorption via the suppression of RANKL-induced NF-kappaB and NFAT activity. Journal of cellular physiology. 2009;221:642–9. doi: 10.1002/jcp.21898. [DOI] [PubMed] [Google Scholar]
- 46.Marquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Munoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. The Journal of pharmacology and experimental therapeutics. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]
- 47.Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, et al. Akt Induces Osteoclast Differentiation through Regulating the GSK3beta/NFATc1 Signaling Cascade. Journal of immunology. 2011 doi: 10.4049/jimmunol.1101254. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) The Journal of biological chemistry. 2000;275:31155–61. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 49.Celli N, Dragani LK, Murzilli S, Pagliani T, Poggi A. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J Agric Food Chem. 2007;55:3398–407. doi: 10.1021/jf063477o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.