Abstract
Background/Objectives
Exposure to environmental chemicals could be one of the contributors to the increasing obesity epidemic. Very little is known about the association of phthalates, ubiquitous chemicals widely used in consumer products, with obesity and lipid metabolism. This study investigated the association of urinary phthalate metabolites and, for the first time, the ratios of the major metabolites of the most common phthalate, di-2-ethylhexyl phthalate (DEHP), with body mass index (BMI), waist circumference, and serum lipid levels in the U.S. female population.
Methods
This cross-sectional study used the data from the National Health and Nutrition Examination Survey, 1999–2004 and was restricted to women aged ≥18 years, who were not pregnant and had no history of diabetes. Using multivariate ordered logistic regression, we examined associations of seven urinary phthalate metabolites and their metabolic ratios with the BMI, waist circumferences, total cholesterol, triglycerides, and high-density and low-density lipoprotein cholesterol.
Results
BMI was positively associated with monobutyl phthalate (MBP) and mono-2-ethylhexyl phthalate (MEHP) (OR=1.13, 95% CI, 1.03-1.23 and OR=1.12, 95% CI, 1.03-1.23, respectively). Waist circumference was positively associated with MBP (OR=1.13, 95% CI, 1.03-1.24). A higher ratio of MEHP to mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) was positively associated with both BMI (OR=1.21, 95% CI, 1.09-1.34) and waist circumference (OR=1.20, 95% CI, 1.10-1.31). There were no other significant associations.
Conclusions
A higher metabolic ratio of MEHP to MEHHP, reflective of slower oxidative conversion of MEHP, is associated with greater BMI and waist circumference.
Introduction
Phthalates are ubiquitous chemicals that are widely used in numerous consumer products and detectable in wild life and humans (1). Secondary phthalate metabolites are detected in 100% of the samples from the general U.S. population (2–4). Recent research suggests that the exposure to environmental chemicals could be one of the relevant contributors to the recent dramatic rise in obesity in developed countries (5–7). Several endocrine disruptors were shown to interfere with the body's adipose tissue biology, endocrine hormone systems or central hypothalamic-pituitary-adrenal axis via different mechanisms important to weight control (8). Previous studies in animals suggested that phthalates can significantly alter normal metabolism in liver and several other tissues and affect blood lipid levels (9). It was suggested that the effect of phthalates on lipid metabolism could be mediated through the peroxisome proliferator activated receptors (PPARs) leading to either hypolipidemic and anti-adipogenic effects as the result of PPARα activation or pro-adipogenic effects due to the activation of PPARγ (10, 11). While PPARα-dependent stimulation of fatty acid oxidation requires continuous mode of exposure, PPARγ effects could occur even with a single or episotic exposure and lead to permanent changes in adipocyte differentiation and increased cell number (5).
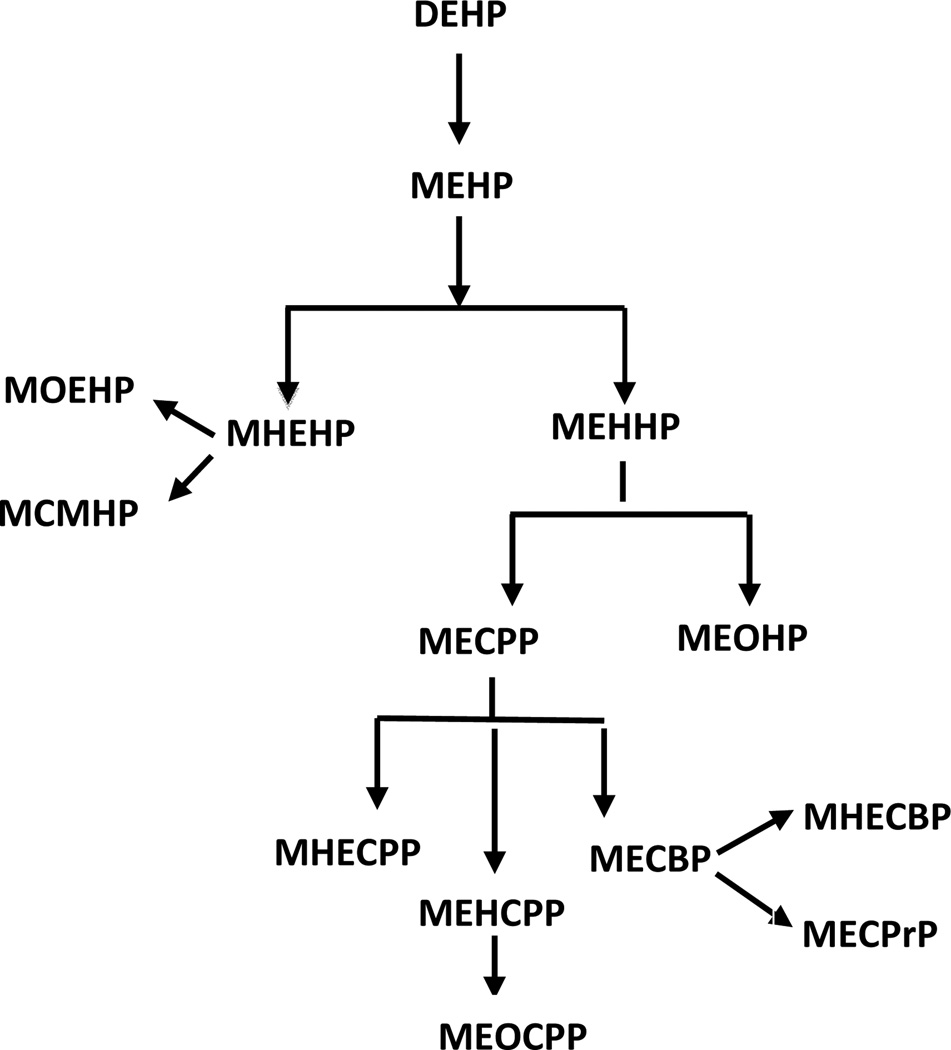
The evidence on the association of phthalates with obesity and lipid metabolism is very limited and inconsistent (12). Furthermore, the majority of the previous studies focusing on females have been limited to pregnant women, mothers of children in birth cohorts, and women undergoing evaluations in fertility clinics. It has been previously shown that a higher ratio of mono-2-ethylhexyl phthalate to mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHP/MEHHP) or MEHP to mono-(2-ethyl-5-oxohexyl) phthalate (MEHP/MEOHP), major secondary metabolites of the most common phthalate, di-2-ethylhexyl phthalate (DEHP) (Figure 1) (13, 14), is associated with a greater physiologic effect and potentially greater endocrine disrupting capacity as compared to individual metabolites (15). We investigated the association of urinary phthalate metabolites and, for the first time, the ratios of the major DEHP metabolites with body mass index (BMI), waist circumference, and serum lipid levels in a representative sample of the U.S. female population, using the data from the National Health and Nutrition Examination Survey (NHANES), 1999–2004.
Figure 1. Di-2-ethylhexyl phthalate (DEHP) metabolism.
Abbreviations: MBP - monobutyl phthalate; MBzP - Mono-benzyl phthalate; MEHP - Mono-2-ethylhexyl phthalate; MEP - Mono-ethyl phthalate; MEHHP - Mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP - Mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - Mono-(2-ethyl-5-carboxypentyl) phthalate; MOEHP - Mono-2-(1-oxoethyl)hexyl-d4 phthalate; MCMHP - mono(2-carboxymethylhexyl) phthalate; MHEHP - mono-2-(hydrohyethyl)hexyl phthalate, MHECPP - mono-e-(1-hydroxyethyl)-5-carboxypentyl phthalate, MEHCPP - mono-(2-ethyl-4-hydroxy-5-carboxypentyl) phthalate, MEOCPP - mono-(2-ethyl-4-oxo-5-carboxypentyl) phthalate; MECBP - mono-(2-ehtyl-4-carboxybutyl) phthalate, MHECBP - mono-2-(1-hydroxyethyl)-4-carboxybutyl phthalate, MECPrP - Mono(2-ethyl-3-carboxypropyl) phthalate
Methods
Study population
National Health and Nutrition Examination Survey (NHANES) is an ongoing survey conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) to assess health of the U.S. population. The survey utilizes a multistage, stratified, clustered design that selects a representative sample of the civilian, non-institutionalized U.S. population with oversampling of specific subgroups including adults older than 60, Mexican Americans, non-Hispanic blacks, and low-income persons (16). The survey data are collected through household interviews and standardized examinations at mobile examination centers throughout the United States as described in detail previously (16). Blood, serum and urine samples were collected as part of the NHANES examination. Environmental chemicals were measured in randomly selected subsamples of participants within specific age groups. All NHANES protocols were approved by the National Center for Health Statistics’(NCHS) Research Ethics Review Board and all participants signed a consent form before their participation.
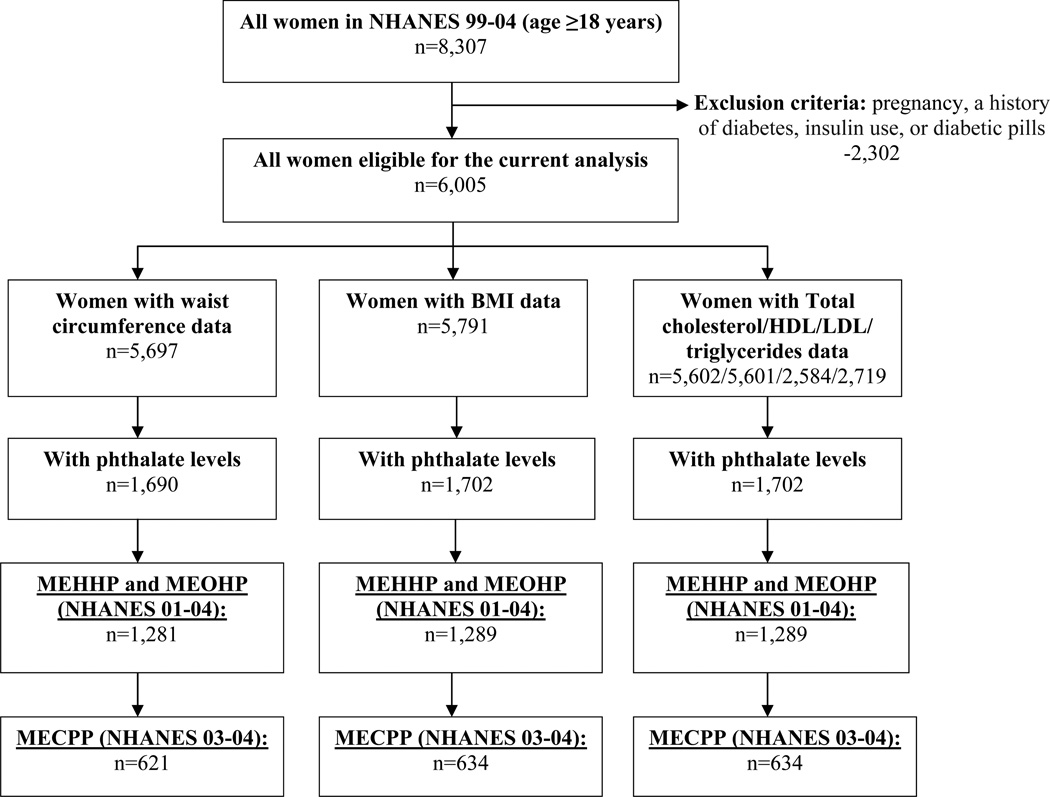
This analysis was restricted to women who were 18 and older at the time of the survey who were not pregnant and did not have a history of diabetes (72.3% of the NHANES 99-04 sample). Of 6,005 eligible women, 1,702 women had data on BMI, serum lipids, and phthalate measurements, and 1,690 women had data on waist circumference and phthalate levels (Figure 2).
Figure 2.
Participant selection flowchart
Two of the phthalate metabolites, MEHHP and MEOHP, were measured only in 2001–2002 and 2003–2004, reducing sample size for the analyses with these metabolites and their ratios to 1,289 women for analyses of BMI and serum lipids and 1,281 women for analysis of waist circumference. Mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) was measured only in 2003–2004, and, thus, the associations of MECPP and the corresponding metabolite ratios were investigated only in this subset of women (634 for analyses of BMI and serum lipids and 621 for analysis of waist circumference) (Figure 2).
Body mass index and waist circumference
Participant’s height, weight, and waist circumference were measured as a part of the physical examination. BMI was calculated as weight in kilograms divided by height in meters squared and was categorized as <18.5 kg/m2 (underweight), 18.5-<25 kg/m2 (normal weight), 25-<30 kg/m2 (overweight), and ≥30 kg/m2 (obese or morbidly obese) (17). Waist circumference was measured in centimeters (cm). In the analysis, we defined waist circumference as quartiles based on the distribution in the study sample (<81.3, 81.3-<91.6, 91.6-<102.8, and ≥102.8 cm) rather than defined cutoffs as some previous studies suggest that these cutoffs as criteria of abdominal obesity might not apply to all populations and ethnic groups (18).
Serum lipids
Sample collection and processing has been described in details in the NHANES Laboratory/Medical Technologists Procedures Manual. Briefly, fasting levels of total cholesterol and triglycerides were measured enzymatically. High-density lipoprotein (HDL) cholesterol was measured in adults using the heparin-manganese (Mn) method where apolipoprotein B containing lipoproteins were first removed by precipitation with heparin sulfate and MnCl2. Low-density lipoptotein (LDL) cholesterol levels were calculated using the Friedewald formula (19). For each lipid, the quartiles were defined based on the distribution of corresponding lipid’s levels in the study sample (total cholesterol: <172, 172-<197, 197-<226, ≥226 mg/dL; HDL: <45, 45-<55, 55-<65, ≥65 mg/dL; triglycerides: <76, 76-<108, 108-<157, ≥157 mg/dL; LDL: <94, 94-<114, 114-<139, ≥139 mg/dL).
Urinary phthalates
Urine samples were collected according to the standard protocol from participants at age 6 years and older at the time of the medical examination. Urine specimens were processed, stored at −20°C, and then shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis (20–22). Urinary phthalates were quantified with high performance liquid chromatography–tandem mass spectrometry as previously described (23–25). To standardize for the renal function and urine dilution, we adjusted absolute concentrations of phthalates by urinary creatinine. Creatinine-adjusted concentrations of phthalate metabolites (µg/g creatinine) were calculated by dividing the absolute phthalate concentration (ng/mL) by creatinine concentration (mg/dL) and multiplying it by 100 (26). In addition to creatinine-corrected phthalate concentrations, we examined the associations of obesity-related outcomes with ratios of different DEHP metabolites as follows: MEHP to MEHHP (MEHP:MEHHP), MEHHP to MECPP (MEHHP:MECPP), MEHHP to MEOHP (MEHHP:MEOHP), and MEOHP to MECPP (MEOHP:MECPP). Finally, we created a composite measure of DEHP metabolites defined as the sum of MEHP and two downstream metabolites, MEHHP and MEOHP.
Seven metabolites were measured between 1999 and 2004: monobutyl phthalate (MBP), mono-benzyl phthalate (MBzP), MEHP, mono-ethyl phthalate (MEP), MEHHP, MEOHP, and MECPP. Among these, MEHHP and MEOHP were measured as part of NHANES 2001–2004 only, and MECPP measurements were limited to NHANES 2003–2004. All phthalates with exception of MEHP were detectable in at least 99% of the samples; MEHP was detectable in 63% of the samples. The values below limit of detection (LOD) were substituted with LOD for the given analyte (specific to the NHANES cycle) divided by the square root of 2 (27).
Covariates
Information on the following variables was extracted from the NHANES questionnaires: age, race/ethnicity, poverty level, education, dietary fat and total calorie intake, menopausal status and postmenopausal hormone use, physical activity, alcohol consumption, and smoking.
Statistical Analysis
All analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC, USA). We accounted for the complex survey structure (multistage probability sample design) throughout the analyses. After weighting, our analyses included 76,075,598 women for BMI and for serum lipids and 75,799,724 women for waist circumference.
We used χ2 test to compare distributions of the following socio-demographic characteristics and other covariates across BMI categories (the primary outcome of interest): age (quintiles as 18-<25, 25-<38, 38-<50, 50-<66, and ≥66 years), race/ethnicity (Hispanic, African American, White, and other), poverty level (below, above poverty), education (≤high school, >high school), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal with no history of hormone use, postmenopausal with past hormone use, postmenopausal with current hormone use, and postmenopausal with unknown hormone use), current alcohol use (none, any), smoking status (never smoker, past smoker, current smoker), physical activity (inactive, moderately active, active), total calorie intake (quartiles as <1280, 1280-<1698, 1698-<2228, and ≥2228 Kcal), and total fat intake (quartiles as <42.16, 42.16-<62.57, 62.57-<86.89 and ≥86.89 g/day). Distribution of phthalate levels, their ratios and composite metabolite concentration measure in the study sample were presented as their medians and geometric means (standard error), adjusted for age and race. Tests for the difference in geometric means across BMI categories were performed (28).
We used ordered multivariate logistic regression to analyze the association of phthalate measures with BMI, waist circumference, and serum lipids. Each of the phthalate measures was modeled as quartiles based on its distribution in the study sample. The quartile cutoffs for individual metabolites are presented in supplemental material, table S1. The risk estimates were adjusted for age, race, education, poverty level, total calorie intake, total fat intake, physical activity, menopausal status/postmenopausal hormone use, alcohol consumption, and smoking. Additionally, the models for serum lipids were adjusted for BMI.
In a secondary analysis, we used polychotomous logistic regression to examine the associations of phthalate measures with obesity-related outcomes. In the analysis for BMI, underweight, overweight and obese women were compared to women with normal weight. In the analysis for waist circumference and serum lipids, women in the lowest quartile were used as the comparison group. However, the risk estimates were unstable in the polychotomous logistic regression analyses due to the small number of women in some of the strata, and we report the results of the ordered logistic regression analysis instead. Statistical significance was assessed at the level of 0.05 in all analyses.
Results
Characteristics of the study population (weighted N=76,075,598) stratified by BMI category are presented in Table 1. Women with BMI ≥25kg/m2 were more likely to be older (p=0.0005), to be African American or Hispanic (p<0.0001), to be postmenopausal (p<0.0001), and to be non-drinkers (p<0.0001). Greater proportion of women with BMI≥25kg/m2 had higher levels of total cholesterol, triglycerides, LDL, and lower levels of HDL, as compared to women with BMI<25. Distributions of other characteristics across BMI groups were similar. Age and race adjusted phthalate levels in the study sample by BMI category are presented in Table 2.
Table 1.
Weighted characteristics of the study population (percent and standard error of percent)
| Characteristic | All N=76,075,598 |
Underweight (BMI < 18.5) N=2,323,028 |
Normal weight (BMI 18.5– < 25) N=27,472,193 |
Overweight (BMI 25– < 30) N=20,979,698 |
Obese (BMI ≥ 30) N=25,300,679 |
|---|---|---|---|---|---|
| Age (years)* | |||||
| 18–<25 | 13.91 (1.01) | 23.67 (9.08) | 19.30 (1.90) | 8.82 (1.70) | 11.38 (1.55) |
| 25–<38 | 23.20 (1.33) | 30.41 (8.68) | 24.97 (2.17) | 21.08 (2.54) | 22.37 (2.63) |
| 38–<50 | 28.68 (1.55) | 23.41 (8.53) | 24.11 (2.35) | 33.71 (2.50) | 29.93 (2.88) |
| 50–<66 | 20.31 (0.99) | 14.02 (6.52) | 17.38 (1.71) | 19.72 (1.97) | 24.57 (1.94) |
| ≥66 | 13.90 (1.05) | 8.49 (3.66) | 14.23 (1.58) | 16.67 (1.81) | 11.74 (1.50) |
| Race* | |||||
| Hispanic | 12.49 (1.90) | 8.23 (4.76) | 9.20 (1.54) | 16.08 (2.95) | 13.46 (2.49) |
| Black | 10.38 (1.16) | 3.11 (1.77) | 6.46 (0.93) | 10.15 (1.33) | 15.51 (2.14) |
| White | 72.92 (2.07) | 82.34 (6.08) | 79.07 (2.11) | 68.37 (3.25) | 69.17 (2.91) |
| Other | 4.20 (0.61) | 6.32 (4.40) | 5.26 (1.04) | 5.40 (1.39) | 1.85 (0.62) |
| Menopausal status/hormone use** | |||||
| Pre-menopausal | 52.01 (1.55) | 56.93(8.07) | 58.75 (2.10) | 49.24 (2.71) | 46.55 (2.55) |
| Postmenopausal, no hormone history | 24.98 (1.11) | 29.01(8.75) | 20.70 (1.57) | 26.61 (1.78) | 27.90 (2.40) |
| Postmenopausal, past hormone use | 9.35 (0.77) | 0 | 7.51 (0.98) | 9.79 (1.41) | 11.82 (1.55) |
| Postmenopausal, current hormone use | 10.85 (1.08) | 12.35(6.23) | 9.42 (1.62) | 11.86 (1.69) | 11.41 (1.70) |
| Postmenopausal, unknown hormone use history | 2.82 (0.38) | 1.71 (1.29) | 3.62 (0.79) | 2.50 (0.67) | 2.31 (0.57) |
| Poverty level | |||||
| Above | 83.32 (1.50) | 80.09 (7.40) | 85.53 (1.84) | 84.63 (2.20) | 80.13 (2.35) |
| Below | 16.68 (1.50) | 19.91 (7.40) | 14.47 (1.84) | 15.37 (2.20) | 19.87 (2.35) |
| Total calories (Kcal) | |||||
| <1280 | 22.71 (1.16) | 24.03 (7.50) | 19.23 (1.91) | 26.73 (2.13) | 23.01 (2.41) |
| 1280–<1698 | 24.88 (1.47) | 20.38 (8.79) | 24.96 (2.91) | 26.88 (2.49) | 23.55 (2.13) |
| 1698–<2228 | 26.91 (1.26) | 29.12 (8.88) | 28.14 (2.31) | 23.43 (2.60) | 28.26 (2.50) |
| ≥2228 | 25.51 (1.41) | 26.47 (6.96) | 27.68 (2.47) | 22.96 (2.65) | 25.18 (1.97) |
| Total fat (g/day) | |||||
| <42.16 | 22.31 (1.35) | 32.61 (8.24) | 21.25 (1.87) | 25.53 (2.41) | 19.84 (1.80) |
| 42.16–<62.57 | 25.16 (1.27) | 23.11 (8.82) | 26.72 (2.31) | 27.46 (2.72) | 21.73 (1.73) |
| 62.57–<86.89 | 25.76 (1.47) | 16.37 (6.44) | 26.61 (2.29) | 21.92 (2.39) | 28.91 (2.75) |
| ≥86.89 | 26.77 (1.38) | 27.92 (7.86) | 25.43 (2.22) | 25.08 (2.70) | 29.53 (2.12) |
| Alcohol use (g/day) * | |||||
| 0 | 78.37 (1.78) | 61.66 (9.07) | 71.29 (2.96) | 79.50 (2.40) | 86.66 (1.64) |
| >0 | 21.63 (1.78) | 38.34 (9.07) | 28.71 (2.96) | 20.50 (2.40) | 13.34 (1.64) |
| Physical activity** | |||||
| None | 35.92 (1.78) | 40.15 (10.35) | 29.56 (2.46) | 35.26 (2.97) | 43.00 (3.14) |
| Moderate | 53.93 (1.93) | 53.39 (10.99) | 57.78 (2.49) | 57.89 (2.70) | 46.50 (3.24) |
| Active | 10.15 (0.79) | 6.46 (4.59) | 12.66 (1.64) | 6.85 (1.38) | 10.51 (1.33) |
| Education | |||||
| ≤ High school | 41.56 (1.63) | 33.95 (8.17) | 37.23 (2.70) | 45.13 (2.82) | 44.00 (2.46) |
| >High school | 58.44 (1.63) | 66.05 (8.17) | 62.77 (2.70) | 54.87 (2.82) | 56.00 (2.46) |
| Waist circumference (inches)* | |||||
| ≤81.3 | 26.86 (1.51) | 100.00 (0.00) | 61.64 (3.18) | 5.80 (1.32) | 0 |
| 81.4–91.6 | 25.36 (1.48) | 0 | 34.91 (2.88) | 43.12 (2.98) | 2.28 (0.63) |
| 91.7–102.8 | 22.90 (1.15) | 0 | 3.46 (0.82) | 44.41 (3.10) | 28.03 (2.19) |
| ≥102.9 | 24.89 (1.54) | 0 | 0 | 6.66 (1.32) | 69.69 (2.44) |
| Smoking status | |||||
| Never | 55.02 (1.80) | 51.69 (10.22) | 53.49 (3.13) | 54.87 (2.87) | 57.12 (3.05) |
| Past | 5.00 (0.50) | 8.81 (3.41) | 7.22 (1.18) | 2.87 (0.71) | 4.02 (0.67) |
| Current | 39.93 (1.89) | 39.50 (9.57) | 39.29 (3.05) | 42.26 (2.93) | 38.86 (3.02) |
| Total cholesterol (mg/dL)** | |||||
| ≤172 | 23.21 (1.62) | 44.93 (9.23) | 26.26 (2.81) | 18.70 (2.35) | 21.84 (2.48) |
| 172–<197 | 25.72 (1.68) | 31.89 (8.84) | 26.13 (2.45) | 25.81 (3.06) | 24.66 (2.77) |
| 197–<226 | 26.19 (1.47) | 6.72 (4.75) | 26.37 (2.10) | 26.94 (2.66) | 27.07 (2.19) |
| ≥ 226 | 24.88 (1.56) | 16.46 (6.95) | 21.24 (2.36) | 28.55 (3.04) | 26.43 (2.54) |
| Triglycerides (mg/dL)* | |||||
| <76 | 25.05 (1.88) | 27.14 (14.05) | 37.18 (4.23) | 21.60 (3.22) | 16.28 (2.38) |
| 76–<108 | 26.95 (2.12) | 44.70 (13.70) | 33.10 (4.07) | 23.23 (3.07) | 23.06 (2.90) |
| 108–<157 | 24.07 (1.86) | 24.18 (11.66) | 20.04 (3.38) | 23.77 (4.28) | 28.07 (2.77) |
| ≥157 | 23.92 (1.77) | 3.97 (4.06) | 9.68 (1.91) | 31.39 (3.39) | 32.59 (3.24) |
| LDL (mg/dL) | |||||
| <94 | 23.87 (2.07) | 49.56 (13.54) | 28.15 (3.20) | 17.77 (3.12) | 23.09 (3.27) |
| 94–<114 | 23.20 (1.96) | 24.65 (12.65) | 26.58 (3.52) | 24.79 (3.72) | 18.62 (2.74) |
| 114–<139 | 26.90 (2.06) | 13.65 (11.11) | 25.31 (3.12) | 27.27 (3.02) | 28.93 (3.97) |
| ≥139 | 26.04 (2.11) | 12.14 (11.40) | 19.96 (3.31) | 30.17 (2.99) | 29.36 (4.25) |
| HDL (mg/dL)* | |||||
| < 45 | 22.91 (1.48) | 16.51 (7.81) | 9.20 (1.35) | 25.75 (2.63) | 35.71 (2.98) |
| 45–<55 | 25.28 (1.52) | 14.94 (6.74) | 22.73 (2.16) | 23.72 (2.25) | 30.23 (2.98) |
| 55–<65 | 23.92 (1.39) | 26.11 (10.40) | 27.12 (2.55) | 23.44 (1.92) | 20.71 (2.24) |
| <65 | 27.90 (1.24) | 42.45 (8.45) | 40.95 (2.11) | 27.09 (2.19) | 13.34 (2.17) |
Abbreviations: BMI – body mass index, LDL – low density lipoprotein; HDL – high-density lipoprotein
p<0.001 - Difference across BMI categories is significant at 0.001 level
p<0.05 - Difference across BMI categories is significant at 0.05 level
Table 2.
Distribution of phthalates in the study sample, age and race adjusted median and geometric means (standard error)
| Phthalate measure | All Median GM (SE) |
BMI <18.5 Median GM (SE) |
BMI 18.5–< 25 Median GM (SE) |
BMI 25–< 30 Median GM (SE) |
BMI ≥ 30 Median GM (SE) |
|---|---|---|---|---|---|
| MBP | 22.14 22.48 (1.06) |
25.30 26.68 (1.21) |
23.52 23.34 (1.08) |
21.82 22.60 (1.11) |
21.40 21.33 (1.07) |
| MBzP | 8.89 9.00 (1.11) |
10.24 10.12 (1.38) |
8.77 8.70 (1.08) |
8.89 9.11 (1.16) |
9.19 9.17 (1.22) |
| MEHP* | 3.07 3.21 (1.14) |
3.83 3.26 (1.36) |
3.21 3.60 (1.20) |
3.10 3.09 (1.10) |
3.13 2.97 (1.23) |
| MEP* | 138.03 149.84 (1.24) |
90.01 99.67 (1.30) |
134.36 141.08 (1.24) |
140.02 152.42 (1.32) |
153.26 160.81 (1.27) |
| MEHHPa | 16.84 18.71 (1.13) |
16.10 14.46 (1.23) |
17.09 19.00 (1.15) |
16.40 17.35 (1.14) |
18.41 20.06 (1.17) |
| MEOHPa | 11.52 12.66 (1.12) |
11.07 9.87 (1.24) |
11.69 12.93 (1.15) |
11.14 11.79 (1.11) |
12.22 13.44 (1.15) |
| MECPPb* | 28.49 32.51 (1.10) |
20.94 18.39 (1.55) |
29.43 34.27 (1.09) |
27.83 28.77 (1.18) |
32.14 35.31 (1.25) |
| MEHP:MEHHPa* | 0.17 0.17 (1.09) |
0.18 0.21 (1.74) |
0.18 0.18 (1.15) |
0.18 0.18 (1.17) |
0.15 0.15 (1.11) |
| MEHHP:MECPPb* | 0.63 0.61 (1.11) |
0.64 0.68 (1.19) |
0.64 0.62 (1.10) |
0.60 0.56 (1.16) |
0.66 0.63 (1.10) |
| MEHHP:MEOHPa | 1.47 1.48 (1.03) |
1.51 1.46 (1.07) |
1.46 1.47 (1.05) |
1.45 1.47 (1.04) |
1.49 1.49 (1.02) |
| MEOHP: MECPPb* | 0.42 0.40 (1.12) |
0.47 0.48 (1.19) |
0.43 0.41 (1.13) |
0.40 0.37 (1.14) |
0.42 0.42 (1.09) |
| ΣDEHPa, c | 32.30 36.84 (1.12) |
32.78 30.92 (1.26) |
32.76 37.64 (1.16) |
31.42 34.71 (1.11) |
34.25 38.49 (1.18) |
Abbreviations: GM – geometric mean; SE – standard error; MBP - monobutyl phthalate; MBzP - Mono-benzyl phthalate; MEHP - Mono-2-ethylhexyl phthalate; MEP - Mono-ethyl phthalate; MEHHP - Mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP - Mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - Mono-(2-ethyl-5-carboxypentyl) phthalate; DEHP - Di-2-ethylhexyl phthalate
Limited to 2001–2004 only
Limited to 2003–2004
ΣDEHP – sum of MEHP, MEHHP, and MEOHP
p<0.001 - Difference across BMI categories is significant at 0.001 level
In multivariate logistic regression, BMI was positively associated with creatinine-adjusted concentrations of MBP (OR=1.13, 95% CI 1.03-1.23) and MEHP (OR=1.12; 95% CI 1.03-1.23) (Table 3). A positive association was also found for BMI and the ratio of MEHP to MEHHP (OR=1.21; 95% CI 1.09-1.34). Similarly, positive associations of MBP and MEHP to MEHHP ratio were found for waist circumference (OR=1.13, 95% CI 1.03-1.24 for MBP; OR=1.20, 95% CI 1.10-1.31 for MEHP:MEHHP). We did not find any significant associations of other metabolites or their ratios with either BMI or waist circumference. Serum total cholesterol, triglycerides, HDL and LDL were not associated with any of the metabolites or their ratios (Table 3).
Table 3.
Association of phthalate metabolites with Body Mass Index, waist circumference, and serum lipids in NHANES 1999–2004 (ordered logistic regression, Odds Ratios and 95% Confidence Intervals)a
| Phthalate measure | Body Mass Index | Waist circumference |
Total cholesterol | Triglycerides | HDL | LDL |
|---|---|---|---|---|---|---|
| MBP | 1.13 (1.03, 1.23) | 1.13 (1.03, 1.24) | 1.04 (0.94, 1.15) | 0.91 (0.78, 1.07) | 0.94 (0.85, 1.03) | 1.04 (0.90, 1.19) |
| MBzP | 0.95 (0.84, 1.08) | 0.93 (0.83, 1.05) | 0.97 (0.88, 1.07) | 0.95 (0.80, 1.12) | 1.01 (0.91, 1.13) | 1.00 (0.85, 1.18) |
| MEHP | 1.12 (1.03, 1.23) | 1.05 (0.96, 1.15) | 1.00 (0.90, 1.11) | 0.91 (0.78, 1.05) | 1.02 (0.91, 1.13) | 0.98 (0.85, 1.14) |
| MEP | 1.02 (0.92, 1.14) | 1.02 (0.92, 1.14) | 0.97 (0.87, 1.09) | 0.99 (0.87, 1.12) | 0.91 (0.81, 1.02) | 1.08 (0.94, 1.24) |
| MEHHPb | 0.90 (0.80, 1.00) | 0.88 (0.79, 1.00) | 1.01 (0.90, 1.14) | 0.94 (0.81, 1.09) | 1.05 (0.96, 1.15) | 1.10 (0.94, 1.29) |
| MEOHPb | 0.90 (0.80, 1.01) | 0.89 (0.78, 1.01) | 0.98 (0.88, 1.10) | 0.99 (0.84, 1.16) | 1.03 (0.94, 1.12) | 1.12 (0.96, 1.31) |
| MECPPc | 0.81 (0.66, 1.00) | 0.80 (0.64, 0.99) | 0.95 (0.80, 1.13) | 1.15 (0.88, 1.50) | 1.06 (0.93, 1.21) | 1.14 (0.95, 1.36) |
| MEHP:MEHHPb | 1.21 (1.09, 1.34) | 1.20 (1.10, 1.31) | 0.95 (0.84, 1.06) | 1.04 (0.87, 1.24) | 0.96 (0.84, 1.09) | 0.97 (0.84, 1.13) |
| MEHHP:MECPPc | 1.02 (0.83, 1.26) | 1.02 (0.86, 1.21) | 0.98 (0.83, 1.17) | 0.91 (0.68, 1.22) | 0.96 (0.79, 1.16) | 1.00 (0.72, 1.38) |
| MEOHP:MECPPc | 1.11 (0.91, 1.36) | 1.07 (0.87, 1.31) | 1.00 (0.85, 1.19) | 0.98 (0.78, 1.22) | 0.95 (0.76, 1.19) | 1.14 (0.87, 1.49) |
| MEHHP:MEOHPb | 0.97 (0.83, 1.12) | 0.95 (0.84, 1.08) | 1.02 (0.92, 1.12) | 1.00 (0.85, 1.17) | 1.06 (0.92, 1.22) | 1.09 (0.94, 1.26) |
| ΣDEHPb, d | 0.93 (0.84, 1.03) | 0.90 (0.81, 1.00) | 1.00 (0.90, 1.11) | 0.94 (0.81, 1.09) | 1.05 (0.95, 1.15) | 1.08 (0.92, 1.26) |
Abbreviations: HDL –high-density lipoprotein cholesterol; LDL – low-density lipoprotein cholesterol; MBP - monobutyl phthalate; MBzP - Mono-benzyl phthalate; MEHP - Mono-2-ethylhexyl phthalate; MEP - Mono-ethyl phthalate; MEHHP - Mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP - Mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - Mono-(2-ethyl-5-carboxypentyl) phthalate; DEHP - Di-2-ethylhexyl phthalate
Adjusted for age, race, education, poverty, total calories, total fat, physical activity, menopausal status/hormone use, alcohol consumption and smoking
Limited to 2001–2004 only
Limited to 2003–2004
ΣDEHP – sum of MEHP, MEHHP, and MEOHP
Discussion
In this study of 76,075,598 women representative of the U.S. female population, we found significant association of BMI and waist circumference with MBP, MEHP, and the ratio of MEHP to MEHHP. None of the other phthalate measures were associated with BMI, waist circumference, or serum lipids.
This is the first study to examine the association of urinary phthalate metabolite ratios, rather than only individual metabolite concentrations, with BMI, waist circumference, and serum lipids. The differences in the ratios of phthalate metabolites are reflective of inter-individual differences in oxidative metabolism of DEHP, the most common phthalate (29). Previous studies suggest that a higher ratio of MEHP to MEHHP or MEHP to MEOHP is associated with a greater physiologic effect and potentially greater endocrine disrupting capacity as compared to individual metabolites (15, 30). Our findings show a positive association of BMI with the ratio of MEHP to MEHHP, suggesting that women with greater BMI may have slower rate of oxidative metabolism of MEHP. A similar association was observed for waist circumference, a measure of abdominal obesity that has shown stronger associations with several health outcomes as compared to BMI (31). MEHHP is formed as the result of MEHP oxidation by cytochrome P450 enzymes and differences in the ratio of MEHP to MEHHP could reflect underlying genetic variation in metabolizing capacity and, as the result, higher susceptibility to the endocrine disrupting effects of MEHP (32, 33). On the other hand, these differences in the phthalate metabolism rate might occur due to the overall slower metabolic rate in obese individuals (34, 35). Further, some previous reports suggest possible decrease in activity of selected P450 enzymes among obese, which might in turn affect metabolism of xenobiotics, including phthalates (36). However, due to the cross-sectional nature of the study, the temporal sequence of the exposure and outcome cannot be determined and prospective studies are needed to explore the association of the phthalate exposure to the subsequent risk of obesity and to provide an evidence of possible causal relationship.
Our findings on the association of MBP with obesity and waist circumference are consistent with some of the previous reports (37, 38). However, unlike previous studies, we found a positive rather than an inverse association of MEHP with BMI (39). Hatch et al. found an inverse association of both MBP and MEHP with BMI and waist circumference. Our analysis included a larger sample of women by combining NHANES 1999–2004, while Hatch et al. only included data from NHANES 1999–2002 (1,702 women at age 18 or older in our study versus 1,129 women at age 20 and older in Hatch et al.). In addition, the findings from the study by Hatch et al. were limited to the subset of women aged 60–80, while our results apply to women at age 18 and older. To compare our results with the findings in Hatch et al., we conducted an additional stratified analysis for the associations of MBP and MEHP with BMI and waist circumference in women at age 20–59 years and at age 60 years and older in our study sample. We found that the associations of MBP and MEHP were similar in these two age subgroups. Further, the statistical analyses in Hatch et al. differed from those in our study. Specifically, Hatch et al. used multiple linear regression, while our study utilized ordered logistic regression because distributions of BMI and waist circumference were not normal. Finally, the risk estimates for phthalates in Hatch et al. were adjusted for age, race/ethnicity, creatinine, height, social economic status, dietary factors, hours of sedentary behavior, mets/month, smoking, and reproductive factors, while the risk estimates for creatinine-corrected phthalates in our study were adjusted for age, race, education, poverty level, total calorie intake, total fat intake, physical activity, menopausal status/postmenopausal hormone use, alcohol consumption, and smoking.
This analysis used the data from a large representative sample of the U.S. population. Nonetheless, our study has a few limitations. As mentioned previously, the cross-sectional nature of the data does not allow us to determine if phthalate exposure predicts the outcomes of interest. In addition, phthalates were measured in a single urine sample. Even though phthalates are rapidly metabolized within hours (half-life 6–12 hours) (1, 40), previous studies suggest that a single urine sample can accurately classify phthalate exposure over the previous 3 months (41, 42).
Findings of our analysis suggest an association of the metabolic conversion rate of MEHP to MEHHP with BMI and waist circumference. Nonetheless, it remains unclear whether this slower rate of MEHP metabolism could cause increase in BMI and abdominal obesity or whether it is merely a reflection of the overall slower metabolic rate in obese individuals or perhaps the influence of BMI on the activity of selected P450 enzymes responsible for oxidation of phthalates (43). Future prospective studies are needed to examine temporal relationship between phthalate exposure and obesity-related outcomes and to elucidate the causal links, if any, between phthalates and obesity.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (U54 CA 155496), the Agency for Healthcare Research and Quality (K01 HS022330) and Foundation for Barnes-Jewish Hospital.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009 Jun;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals. 2009 [Google Scholar]
- 3.Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005 May 1;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Center for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals. 2009:2009. [Google Scholar]
- 5.Grün F, Blumberg B. Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology. 2009;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtcamp W. Obesogens: an environmental link to obesity. Environ Health Perspect. 2012 Feb;120(2):a62–a68. doi: 10.1289/ehp.120-a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janesick A, Blumberg B. The Role of Environmental Obesogens in the Obesity Epidemic. In: Lustig RH, editor. Obesity Before Birth. Endocrine Updates. Vol. 30. US: Springer; 2011. pp. 383–399. [Google Scholar]
- 8.Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009 May 25;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell F. Effects of Phthalate Esters on Lipid Metabolism in Various Tissues, Cells and Organelles in Mammals. Environ Health Perspect. 1982;45:41–50. doi: 10.1289/ehp.824541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by Environmental Phthalate Monoesters. Toxicological Sciences. 2003 Aug 1;74(2):297–308. doi: 10.1093/toxsci/kfg145. 2003. [DOI] [PubMed] [Google Scholar]
- 11.Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Reviews in Endocrine and Metabolic Disorders. 2007;8(2):161–171. doi: 10.1007/s11154-007-9049-x. 2007/06/01. [DOI] [PubMed] [Google Scholar]
- 12.Goodman M, Lakind JS, Mattison DR. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol. 2014 Feb;44(2):151–175. doi: 10.3109/10408444.2013.860076. [DOI] [PubMed] [Google Scholar]
- 13.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure--an update and latest results. Int J Androl. 2006 Feb;29(1):155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 81-5. [DOI] [PubMed] [Google Scholar]
- 14.Silva MJ, Samandar E, Preau JL, Jr, Needham LL, Calafat AM. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology. 2006 Feb 15;219(1-3):22–32. doi: 10.1016/j.tox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen H, Skakkebaek NE, Andersson A-M. Metabolism of phthalates in humans. Molecular Nutrition & Food Research. 2007;51(7):899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- 16.CDC. CDC (Centers for Disease Control and Prevention) National Health and Nutrition Examination Survey; 2011b. [accessed 22 May 2014]. Available: http://www.cdc.gov/nchs/nhanes.htm. 2011b. [Google Scholar]
- 17.NHLBI. Practical Guide on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 2000. Oct, National Heart, Lung, and Blood Institute and North American Association for the Study of Obesity. NIH publication No. 00-4084. 2000. [Google Scholar]
- 18.Misra A, Wasir JS, Vikram NK. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition. 2005 Sep;21(9):969–976. doi: 10.1016/j.nut.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.CDC. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S.: Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [accessed 30 June 2014]. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) Reprint: http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 20.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey: 2003–2004. Laboratory Procedures Manual; Naional Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/lab.pdf. [Google Scholar]
- 21.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey: 1999–2000. Laboratory Procedures Manual; Naional Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/lab.pdf. [Google Scholar]
- 22.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey: 2001–2002. Laboratory Procedures Manual; Naional Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/specimen_collection_year_3.pdf. [Google Scholar]
- 23.Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, et al. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jun 15;789(2):393–404. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 24.Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000 Sep 1;72(17):4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- 25.Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr, Herbert AR, Samandar E, et al. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Jun 5;805(1):161–167. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol. 2012 Sep;22(5):468–475. doi: 10.1038/jes.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. 1990/01/01. [Google Scholar]
- 28.Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to the time since the mammogram. Cancer Epidemiol Biomarkers Prev. 2013 Jun;22(6):1110–1117. doi: 10.1158/1055-9965.EPI-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003 Jul;111(9):1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch HM, Rossbach B, Drexler H, Angerer J. Internal exposure of the general population to DEHP and other phthalates--determination of secondary and primary phthalate monoester metabolites in urine. Environ Res. 2003 Oct;93(2):177–185. doi: 10.1016/s0013-9351(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 31.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007 Apr;28(7):850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 32.Choi K, Joo H, Campbell JL, Jr, Andersen ME, Clewell Iii HJ. In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicology in Vitro. 2013;27(5):1451–1457. doi: 10.1016/j.tiv.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Walker C. Organic Pollutants: An Ecotoxicological Perspective. Taylor & Francis; 2002. [Google Scholar]
- 34.Shaikh MG, Grundy RG, Kirk JM. Reductions in basal metabolic rate and physical activity contribute to hypothalamic obesity. J Clin Endocrinol Metab. 2008 Jul;93(7):2588–2593. doi: 10.1210/jc.2007-2672. [DOI] [PubMed] [Google Scholar]
- 35.Zhang K, Sun M, Werner P, Kovera AJ, Albu J, Pi-Sunyer FX, et al. Sleeping metabolic rate in relation to body mass index and body composition. Int J Obes Relat Metab Disord. 2002 Mar;26(3):376–383. doi: 10.1038/sj.ijo.0801922. [DOI] [PubMed] [Google Scholar]
- 36.Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999 Jan;37(1):8–19. [PubMed] [Google Scholar]
- 37.James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, et al. Urinary Phthalate Metabolite Concentrations and Diabetes among Women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect. 2012 Sep;120(9):1307–1313. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang P-C, Kuo P-L, Guo Y-L, Liao P-C, Lee C-C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Human Reproduction. 2007 Oct 1;22(10):2715–2722. doi: 10.1093/humrep/dem205. 2007. [DOI] [PubMed] [Google Scholar]
- 39.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005 Nov;113(11):1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004 Dec;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002 May;110(5):515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotlyar M, Carson S. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999;37(1):8–19. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.