Abstract
Purpose
Racial disparities in colorectal cancer (CRC) persist, despite overall reductions in morbidity and mortality. In addition, incidence is rising among individuals younger than 50 years of age. We compared the survival of young-onset CRC among non-Hispanic black (NHB), non-Hispanic white (NHW), and Hispanic individuals.
Patients and Methods
Using the National Cancer Institute’s Surveillance, Epidemiology, and End Results program data, we identified individuals between the ages of 20 and 49 years, diagnosed with CRC between 2000 and 2009. Survival rates and Cox proportional hazards models were used to compare stage-specific 5-year survival among NHBs, NHWs, and Hispanics.
Results
We identified 28,145 patients with young-onset CRC (19,497 NHW; 4,384 NHB; 4,264 Hispanic) during the 10-year study period. Overall survival at 5 years after CRC diagnosis was 54.9% among NHB, 68.1% among NHW, and 62.9% among Hispanic individuals (P < .001). NHB individuals had a significantly higher hazard of cancer-specific death compared with NHWs after adjusting for age, sex, race, stage, county-level poverty, and treatment history in cases of colon (hazard ratio [HR], 1.35; 95% CI 1.26 to 1.45) and rectum/rectosigmoid junction (HR, 1.51; 95% CI, 1.37 to 1.68) cancers, whereas there was no significant difference in survival between NHWs and Hispanics. The greatest racial disparities in cancer-specific survival were observed among NHB and NHW patients diagnosed with stage II cancers of the colon (HR, 1.69; 95% CI, 1.33 to 2.14) and stage III cancers of the rectum (HR, 1.98; 95% CI, 1.63 to 2.40).
Conclusion
Survival after CRC diagnosis at a young age is significantly worse among NHBs compared with NHWs, even among patients with early-stage disease. Further study is needed to determine whether differences in tumor biology and/or treatment are associated with racial disparities in outcomes, which would have implications for CRC treatment and prevention.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer deaths in men and women in the United States, with an estimated 136,830 people diagnosed and 50,310 deaths reported annually.1 Implementation of routine CRC screening among individuals 50 years of age and older and advances in adjuvant therapies have resulted in overall reductions in both CRC incidence and mortality in the United States.2 However, racial disparities in survival rates have grown more pronounced.3-5 CRC incidence and mortality are significantly higher among non-Hispanic blacks (NHBs) compared with non-Hispanic whites (NHWs).3,6,7 Differences in uptake of cancer screening and unequal access to high-quality treatments (surgery, adjuvant chemotherapy) have been proposed as major contributors to racial disparities in CRC incidence and mortality.8,9
Even as the absolute numbers of patients with CRC in the United States have been declining, CRC incidence rates among individuals younger than 50 years old have continued to rise by 1.5% annually since 1992.10,11 Approximately one in 10 individuals diagnosed with CRC is younger than 50 years, and the proportion of those with CRCs who are younger than 50 years is nearly two-fold higher among NHBs compared with NHWs.12 Young patients are more likely than their older counterparts to present with regional or distant disease, and although some studies have suggested that CRCs behave more aggressively in young patients,13,14 others have demonstrated improved disease-specific survival compared with individuals diagnosed at older ages.15-17
Study of patients with young-onset CRC offers an opportunity to examine differences in cancer-specific survival by race, minimizing the potential impact that routine CRC screening among individuals 50 years of age and older might have on CRC-related outcomes. The purpose of our study was to compare survival among NHB, NHW, and Hispanic individuals diagnosed with CRC at younger than 50 years of age.
PATIENTS AND METHODS
Data Sources and Patient Selection
Data were obtained from the National Cancer Institute’s SEER program, which collects cancer incidence and mortality data from 18 population-based cancer registries covering approximately 28% of the US population.18
A case listing session in SEER*Stat was run on the SEER 18 incidence data set to obtain demographic, tumor characteristic, and survival information on early-onset CRCs.19 Early-onset CRCs were defined as cancers of the colon and rectum diagnosed in individuals 20 to 49 years old at diagnosis. We restricted our analysis to include patients diagnosed during the years 2000 to 2009 with race/ethnicity classified as NHW, non-Hispanic African American or black (NHB), and Hispanic; subjects whose race was coded as Asian or Pacific Islander, American Indian or Alaskan Native, or other/unknown were not included in this analysis because of the small sample size (n = 3,595). Cancers diagnosed at autopsy, subjects who survived for < 2 months after diagnosis (n = 876), and histopathologic subtypes other than adenocarcinoma (eg, squamous cell, sarcoma, carcinoid, etc.) were excluded from this study (n = 3,141).
Clinical and demographic variables examined included sex, age at diagnosis, American Joint Committee on Cancer (AJCC) clinical stage, grade (categorized as well to moderately differentiated or poorly differentiated to undifferentiated), and first course of treatment (receipt of surgery or radiation therapy). Patients diagnosed with another cancer before their CRC diagnosis (ie, not a first primary diagnosis) were excluded from survival analyses (n = 1,405).
It has been suggested that race/ethnicity might be a proxy for socioeconomic status (SES).5 Therefore, we used an area-based measure of poverty that is available in SEER as an approximation of SES. This measure was the percentage of persons and families whose incomes are below 200% of the poverty level taken from the US Census 2008-2012 American Community Survey, county attribute table.19 On the basis of the distribution in our cohort of patients with CRC, quartiles of the percentage of persons with incomes below 200% of the poverty level were created. Quartile 1 (Q1) represents the fourth of the cohort that reside in areas where the lowest proportion of residents are low income (defined as below 200% of the poverty level), and quartile 4 (Q4) represents the highest proportion of low-income residents, divided as follows: Q1: < 20% low income; Q2: 20% to < 30% low income; Q3: 30% to < 38% low income; and Q4: ≥ 38% low income.
Tumor location was grouped by primary site, with C18.0 to C18.5 categorized as proximal colon and C18.6 to C18.9, C19.9, C20.9, and C26.0 categorized as distal colon/rectum. Surgical resection as part of first course of therapy was evaluated as a dichotomous variable (yes/no) and by type of resection (local resection, segmental resection, or segmental resection involving other organs) on the basis of the SEER program standardized coding and staging codes. Segmental resection included partial colectomy; subtotal colectomy/hemicolectomy; total colectomy; total proctocolectomy; colectomy, not otherwise specified; wedge or segmental resection; partial proctosigmoidectomy; partial proctectomy; proctectomy; and surgery, not otherwise specified. Survival time was calculated from the date of diagnosis to the last date of follow-up or until the date of death. Cancer-specific survival was classified on the basis of available death certificate information using SEER-defined variables. Follow-up for each patient is current, within 22 months of the annual submission date (November 1, 2014).
Statistical Analysis
Differences in demographic and tumor characteristics by race/ethnicity were examined by χ2 tests for categorical variables and t tests for continuous variables. The mean and median survival (months) and the proportion of patients alive at 5 years was calculated on the basis of Kaplan-Meier analysis. Log-rank tests and Cox proportional hazards models were used to assess 5-year survival. Hazard ratios (HRs) and 95% CIs were estimated for race, sex, AJCC stage, tumor location, poverty level, and the use of surgical resection and/or radiation treatment in adjusted models. Cancer-specific survival was evaluated for all patients combined and stratified by AJCC stage and tumor location. All data were analyzed using SAS version 9.4 statistical software (SAS Institute, Cary, NC). A P value of < .05 was considered to be statistically significant. We tested the proportional hazards assumption in the multivariate models by adding an interaction term with race and follow-up time to the final models. This interaction term was not significant for the overall model or for the models stratified by stage.
RESULTS
A total of 28,145 incident CRC cases diagnosed in individuals < 50 years of age with race categorized as 19,497 NHW, 4,384 NHB, and 4,264 Hispanic were identified from SEER over the 10-year study period (Table 1). Approximately one quarter of these patients with young-onset CRC (6,686 of 28,145 patients) were diagnosed before the age of 40 years. Age at cancer diagnosis differed by race, with Hispanic individuals diagnosed at a younger age than were NHBs or NHWs (41.4 years v 42.9 and 43.0 years, respectively; P < .001; Table 1). Among young CRC patients, 3,479 reported one or more additional cancer diagnoses, with NHB and Hispanic patients less likely than NHW patients to report multiple primary tumors (breast, cervical, and uterine cancers were the most commonly reported; data not shown). CRCs located in the proximal colon were significantly more frequent among NHB compared with NHW and Hispanic patients (39.9% v 30.3% and 30.7%, respectively; P < .001, .5573, and < .001, respectively) and women comprised a higher proportion of NHB patients (50.3% v 45.8% and 46.3%, respectively; P < .001). NHB patients were more likely to present with advanced stage disease (stage III/IV) with higher-grade tumors compared with NHW or Hispanic individuals.
Table 1.
Summary of Demographic and Clinical Characteristics by Race/Ethnicity in Individuals With Colorectal Cancer: SEER 18, 2000-2009
| Characteristic | NHW | NHB | H | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | NHW:NHB | NHW:H | NHB:H | |
| Total | 19,497 | 4,384 | 4,264 | ||||||
| Age at diagnosis, years | .5394 | < .001 | < .001 | ||||||
| 20-29 | 708 | 3.6 | 146 | 3.3 | 268 | 6.3 | |||
| 30-39 | 3,664 | 18.8 | 811 | 18.5 | 1,110 | 26.0 | |||
| 40-49 | 15,125 | 77.6 | 3,427 | 78.2 | 2,886 | 67.7 | |||
| Mean (SD), years | 43.0 | (5.7) | 42.9 | (5.6) | 41.4 | (6.5) | .6064 | < .001 | < .001 |
| Sex | < .001 | .5378 | < .001 | ||||||
| Female | 8,934 | 45.8 | 2,206 | 50.3 | 1,976 | 46.3 | |||
| Male | 10,563 | 54.2 | 2,178 | 49.7 | 2,288 | 53.7 | |||
| Poverty index | < .001 | < .001 | < .001 | ||||||
| Q1 | 5,464 | 28.0 | 658 | 15.0 | 618 | 14.5 | |||
| Q2 | 5,449 | 27.9 | 995 | 22.7 | 826 | 19.4 | |||
| Q3 | 4,567 | 23.4 | 1,425 | 32.5 | 1,089 | 25.5 | |||
| Q4 | 4,016 | 20.6 | 1,306 | 29.8 | 1,731 | 40.6 | |||
| Unknown | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | |||
| AJCC stage | < .001 | < .001 | .0369 | ||||||
| 0 | 499 | 2.6 | 90 | 2.1 | 93 | 2.2 | |||
| I | 3,660 | 18.8 | 619 | 14.1 | 602 | 14.1 | |||
| II | 4,169 | 21.4 | 964 | 22.0 | 982 | 23.0 | |||
| III | 5,834 | 29.9 | 1,258 | 28.7 | 1,297 | 30.4 | |||
| IV | 4,228 | 21.7 | 1,187 | 27.1 | 1,033 | 24.2 | |||
| Unknown | 1,107 | 5.7 | 266 | 6.1 | 257 | 6.0 | |||
| Tumor location | < .001 | .5573 | < .001 | ||||||
| Proximal | 5,901 | 30.3 | 1,750 | 39.9 | 1,310 | 30.7 | |||
| Distal | 13,596 | 69.7 | 2,634 | 60.1 | 2,954 | 69.3 | |||
| Grade | < .001 | .7288 | .0012 | ||||||
| I (well differentiated) | 1,606 | 8.2 | 313 | 7.1 | 362 | 8.5 | |||
| II (moderately differentiated) | 11,664 | 59.8 | 2,742 | 62.5 | 2,519 | 59.1 | |||
| III (poorly differentiated) | 3,599 | 18.5 | 710 | 16.2 | 788 | 18.5 | |||
| IV (undifferentiated) | 259 | 1.3 | 48 | 1.1 | 49 | 1.1 | |||
| Unknown | 2,369 | 12.2 | 571 | 13.0 | 546 | 12.8 | |||
| Cancer sequence | .0062 | .0378 | .9005 | ||||||
| Only 1 primary | 16,988 | 87.1 | 3,902 | 89.0 | 3,776 | 88.6 | |||
| 1st of ≥ 2 | 1,475 | 7.6 | 295 | 6.7 | 304 | 7.1 | |||
| 2nd of ≥ 2 | 943 | 4.8 | 171 | 3.9 | 169 | 4.0 | |||
| 3rd of ≥ 3 | 91 | 0.5 | 16 | 0.4 | 15 | 0.4 | |||
| Previous cancer | .0048 | .008 | .9092 | ||||||
| No | 18,463 | 94.7 | 4,197 | 95.7 | 4,080 | 95.7 | |||
| Yes | 1034 | 5.3 | 187 | 4.3 | 184 | 4.3 | |||
| Surgery | < .001 | < .001 | .142 | ||||||
| No | 1,763 | 9.0 | 567 | 12.9 | 508 | 11.9 | |||
| Yes | 17,667 | 90.6 | 3,800 | 86.7 | 3,747 | 87.9 | |||
| Unknown | 67 | 0.3 | 17 | 0.4 | 9 | 0.2 | |||
| Surgery type* | .0023 | .0062 | .9667 | ||||||
| Local resection | 1,160 | 6.5 | 192 | 5.1 | 194 | 5.2 | |||
| Segmental resection | 15,383 | 86.7 | 3,359 | 88.4 | 3,306 | 88.2 | |||
| Resection involving organs | 1,124 | 6.3 | 249 | 6.6 | 247 | 6.6 | |||
| Unknown | 67 | 0.4 | 17 | 0.4 | 9 | 0.2 | |||
| Radiation therapy | < .001 | .1016 | < .001 | ||||||
| No | 14,461 | 74.2 | 3,508 | 80.0 | 3,233 | 75.8 | |||
| Yes | 4,658 | 23.9 | 821 | 18.7 | 975 | 22.9 | |||
| Unknown | 378 | 1.9 | 55 | 1.3 | 56 | 1.3 | |||
NOTE. P value calculations do not include unknown values.
Abbreviations: AJCC, American Joint Committee on Cancer; H, Hispanic; NHW, non-Hispanic white; NHB, non-Hispanic black; Q1, < 20% low income; Q2, 20% to < 30% low income; Q3, 30% to < 38% low income; Q4, > 38% low income.
Excludes nonsurgical patients.
Mean overall and cancer-specific survival were lower among NHB patients diagnosed with CRC at younger than 50 years of age, with this survival discrepancy observed among patients with colon and rectum/rectosigmoid cancer diagnosed at stage II to IV (Table 2). Irrespective of race, > 87% of all patient deaths were attributable to the diagnosis of CRC (8,235 of 9,417 patient deaths; data not shown). HRs for overall and cancer-specific deaths by tumor site, adjusted for age, sex, race, county-level poverty, stage, surgery, and radiation therapy are presented in Table 3. For colon and rectum/rectosigmoid junction cancers, surgical intervention was associated with increased survival at every stage of disease (Table 4, Appendix Table A1, online only). Among all patients with CRC, men experienced significantly worse overall and cancer-specific survival compared with women. NHB patients demonstrated a significantly higher hazard of cancer-specific death in cases of colon (HR, 1.35; 95%, CI 1.26 to 1.45) and rectum/rectosigmoid junction (HR, 1.51; 95% CI, 1.37 to 1.68) cancers, compared with NHW patients. No increase in hazard of death was observed for Hispanics compared with NHW individuals.
Table 2.
Mean Overall and Cancer-Specific Survival Months for Patients With Colorectal Cancer by Tumor Stage and Site by Race/Ethnicity, SEER 18, 2000-2009
| Survival | Colon | Rectum and Rectosigmoid Junction | ||||
|---|---|---|---|---|---|---|
| White | Black | Hispanic | White | Black | Hispanic | |
| Overall survival | ||||||
| Stage 0-I | 58.8 | 57.9 | 52.4 | 56.7 | 54.8 | 53.6 |
| Stage II | 55.7 | 54.1 | 56.6 | 54.7 | 50.6 | 53.7 |
| Stage III | 51.4 | 48.2 | 50.1 | 53.3 | 47.2 | 50.3 |
| Stage IV | 27.3 | 22.8 | 26.1 | 28.6 | 22.6 | 26.1 |
| Cancer-specific survival | ||||||
| Stage 0-I | 59.6 | 48.2 | 53.3 | 57.2 | 55.8 | 54.0 |
| Stage II | 55.6 | 55.1 | 57.6 | 55.6 | 51.7 | 54.5 |
| Stage III | 51.9 | 49.1 | 50.9 | 53.7 | 47.7 | 50.8 |
| Stage IV | 27.9 | 23.4 | 27.0 | 29.0 | 23.2 | 27.2 |
Table 3.
Adjusted Hazard of Overall Death and Cancer-Specific Death for Patients With Colorectal Cancer by Tumor Site: SEER 18, 2000-2009
| Observational Study Estimate | Colon* | Rectum and Rectosigmoid Junction* | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Overall death | ||||
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.36 (1.27 to 1.45) | < .001 | 1.52 (1.38 to 1.68) | < .001 |
| Hispanic | 1.03 (0.95 to 1.14) | .4688 | 1.11 (1.00 to 1.22) | .0474 |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.15 (1.09 to 1.22) | < .001 | 1.20 (1.11 to 1.29) | < .001 |
| AJCC stage | ||||
| 0-I | Ref | Ref | ||
| II | 3.09 (2.52 to 3.77) | < .001 | 2.22 (1.85 to 2.65) | < .001 |
| III | 7.64 (6.34 to 9.21) | < .001 | 3.34 (2.84 to 3.91) | < .001 |
| IV | 37.33 (31.07 to 44.85) | < .001 | 15.28 (13.10 to 17.82) | < .001 |
| Cancer-specific death | ||||
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.35 (1.26 to 1.45) | < .001 | 1.51 (1.37 to 1.68) | < .001 |
| Hispanic | 1.00 (0.92 to 1.09) | .9649 | 1.09 (0.99 to 1.21) | .0927 |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.12 (1.06 to 1.19) | < .01 | 1.18 (1.09 to 1.28) | < .001 |
| AJCC stage | ||||
| 0-I | Ref | Ref | ||
| II | 5.18 (3.90 to 6.87) | < .001 | 2.42 (1.98 to 2.95) | < .001 |
| III | 15.19 (11.59 to 19.91) | < .001 | 4.05 (3.39 to 4.83) | < .001 |
| IV | 77.75 (59.50 to 101.61) | < .001 | 19.10 (16.08 to 22.68) | < .001 |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; Ref, reference.
Adjusted for age (5-year groups), county-level poverty (quartiles), race, sex, AJCC stage, surgery, and radiation.
Table 4.
Adjusted HRs of Cancer-Specific Death for Patients With Colorectal Cancer by Tumor Site and Stage
| Observational Study Estimate | AJCC Stage 0-I* | AJCC Stage II* | AJCC Stage III* | AJCC Stage IV* | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Colon | ||||||||
| Race | ||||||||
| White | Ref | Ref | Ref | Ref | ||||
| Black | 2.05 (1.08 to 3.90) | .0292 | 1.69 (1.33 to 2.14) | < .001 | 1.33 (1.16 to 1.53) | < .001 | 1.29 (1.18 to 1.41) | < .001 |
| Hispanic | 1.47 (0.69 to 3.14) | .3229 | 0.89 (0.66 to 1.22) | .4764 | 0.99 (0.84 to 1.16) | .8708 | 1.01 (0.91 to 1.12) | .8568 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.52 (0.88 to 2.64) | .1334 | 1.10 (0.90 to 1.35) | .3621 | 1.18 (1.05 to 1.32) | .0047 | 1.10 (1.03 to 1.18) | .0059 |
| Surgery | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 0.09 (0.04 to 0.18) | < .001 | 0.14 (0.08 to 0.22) | < .001 | 0.37 (0.20 to 0.68) | .0016 | 0.42 (0.39 to 0.46) | < .001 |
| Radiation | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 1.40 (0.18 to 10.65) | .7445 | 2.31 (1.65 to 3.23) | < .001 | 1.58 (1.28 to 1.95) | < .001 | 1.31 (1.14 to 1.50) | .001 |
| Rectum and rectosigmoid junction | ||||||||
| Race | ||||||||
| White | Ref | Ref | Ref | Ref | ||||
| Black | 1.46 (0.92 to 2.31) | .1062 | 1.59 (1.16 to 2.17) | .0036 | 1.98 (1.63 to 2.40) | < .001 | 1.31 (1.14 to 1.50) | .001 |
| Hispanic | 1.28 (0.79 to 2.06) | .3126 | 1.09 (0.78 to 1.53) | .6099 | 1.33 (1.09 to 1.61) | .0045 | 0.98 (0.85 to 1.12) | .7260 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.75 (1.24 to 2.46) | .0013 | 0.99 (0.78 to 1.25) | .9142 | 1.23 (1.06 to 1.43) | .0055 | 1.14 (1.03 to 1.26) | .0136 |
| Surgery | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 0.15 (0.10 to 0.22) | < .001 | 0.24 (0.18 to 0.33) | < .001 | 0.33 (0.25 to 0.44) | < .001 | 0.47 (0.42 to 0.52) | < .001 |
| Radiation | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 2.19 (1.59 to 3.01) | < .001 | 1.95 (1.41 to 2.69) | < .001 | 1.13 (0.95 to 1.34) | .1602 | 0.82 (0.74 to 0.90) | < .001 |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; Ref, reference.
Adjusted for age (5-year groups), race, poverty level (quartiles), sex, surgery, and radiation therapy.
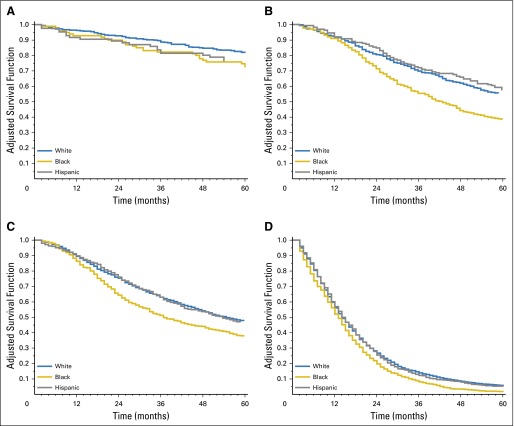
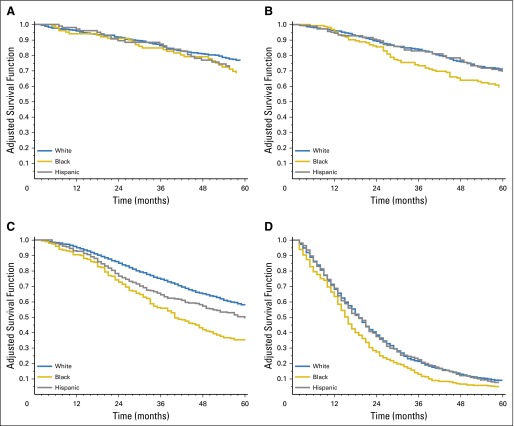
Stratification of patients by tumor stage and site (using NHW patients as the referent) revealed that NHB individuals were at an increased risk of cancer-specific death for colon cancers diagnosed at every stage, with the greatest differences observed among those diagnosed with stage II cancers (HR, 1.69; 95% CI, 1.33 to 2.14; Table 4). Among patients with rectum/rectosigmoid junction cancer, cancer-specific survival was significantly worse among NHB patients diagnosed at stages II to IV, with the largest HR of death observed among those diagnosed with stage II (HR, 1.59; 95% CI, 1.16 to 2.17) and stage III (HR, 1.98; 95% CI, 1.63 to 2.40) disease (Table 4). Adjusted survival curves stratified by stage and tumor site confirmed that the greatest racial disparities were observed among NHB stage II and III colon and rectum/rectosigmoid junction cancers (Figs 1 and 2).
Fig 1.
Adjusted overall survival curves for young-onset (A) stage 0 to I, (B) stage II, (C) stage III, and (D) stage IV colon cancers. Covariates adjusted for include age (5-year groups), county-level poverty (quartiles), sex, surgery, and radiation therapy by race/ethnicity.
Fig 2.
Adjusted overall survival curves for young-onset (A) stage 0 to I, (B) stage II, (C) stage III, and (D) stage IV rectum/rectosigmoid cancers. Covariates adjusted for include age (5-year groups), county-level poverty (quartiles), sex, surgery, and radiation therapy by race/ethnicity.
DISCUSSION
Our analysis of outcomes for 28,145 individuals with CRC diagnosed at younger than 50 years of age identified significantly worse 5-year survival for NHB compared with NHW and Hispanic patients at every disease stage, with the greatest disparities noted among individuals with stage II and III cancers of the colon and rectum, respectively. This finding is novel, because previous studies have found the most pronounced racial disparities in CRC survival among individuals with advanced stage disease.3,5,20
Implementation of CRC screening and improved treatments have contributed to significant reductions in overall CRC incidence and mortality. Unfortunately, racial disparities in CRC survival in the United States have actually worsened since 2000.3-5 NHB individuals have the highest CRC incidence and mortality compared with all other racial and ethnic groups.3,21 Reports that CRCs in NHBs tend to be diagnosed at later stages compared with CRCs in NHWs have raised the question of whether racial differences in uptake of CRC screening and delays in CRC diagnosis could explain a large part of racial disparities in survival.3,9,22 Indeed, there remains little doubt that widespread implementation of CRC screening in individuals older than 50 years of age has changed the epidemiology of CRC. Before 1980, CRC mortality was lower among black men compared with white men and similar among women of both races, but by 2007, CRC mortality was 44% higher for blacks compared with whites.3,5,23-25 It has also been proposed that inequalities in access to high-quality surgical and oncology care are responsible for the increased CRC mortality observed among NHBs compared with NHWs. Studies conducted in populations in which barriers to care are removed and treatments are standardized have demonstrated that racial differences in outcomes are diminished.26,27
However, it is important to note that most previous studies of CRC outcomes by race have analyzed data from all-comers with CRC, and few have specifically examined survival among the subgroup of individuals diagnosed at younger than 50 years of age. In their analysis of patients with advanced-stage CRC from the South Carolina Central Cancer Registry, Wallace et al4 found that African Americans had a significantly higher risk of death compared with European Americans but also noted that racial disparities in survival were significantly greater among individuals diagnosed at younger than 50 years of age when compared with those who were diagnosed at an older age. Similarly, in analyzing predictors of CRC survival among patients treated in Texas hospitals, Phatak et al20 identified an interaction between age and race, with African Americans and Hispanics exhibiting significantly worse survival at younger ages, whereas mortality among African Americans older than 70 years of age was lower compared with whites.
Our study is novel in that we focused on comparing clinical outcomes by race/ethnicity specifically among individuals diagnosed with CRC at younger than 50 years of age. Although we observed racial differences in survival at all disease stages, we found that the greatest racial disparities in outcomes were between NHB and NHW patients with local/regional disease stage at the time of diagnosis. These findings are important for several reasons. First, because of their young age (under 50 years), these patients would not have been considered candidates for routine CRC screening. By examining stage-specific survival, we were able to minimize the potential impact that use of CRC screening might have on racial disparities in survival. Second, our finding that young NHB individuals diagnosed with stage II colon cancer have a 60% to 70% higher risk of death compared with NHWs is interesting in light of existing data demonstrating that adjuvant therapy in stage II cancers is of marginal benefit (associated with improvements in overall survival of 5% or less).28 In a recent nationwide cohort study of 13,102 patients diagnosed with CRC between the ages of 18 and 49 years, Kneuertz et al15 found that young patients were significantly more likely than older patients to receive multiagent systemic chemotherapy at all disease stages, suggesting a trend toward overtreatment of young patients. Because use of adjuvant chemotherapy in stage II CRC is often at the discretion of the treating oncologist, it is possible that differences in the treatment of stage II cancers may contribute to racial disparities in survival. Unfortunately, because SEER registry data do not include information about chemotherapy treatments, it was not possible to ascertain which subjects received adjuvant chemotherapy; nor could we assess for racial differences in how treatments were administered.
Even so, our findings raise a number of questions about the possible impact that treatment factors and patient factors have with regard to differences in CRC survival by race. To date, the majority of the focus has been on differences in cancer screening and cancer care; studies have shown consistently that NHBs have the lowest rates of CRC screening, have lower SES, have less access to high-quality oncology care, and thus may be more likely to be understaged and receive nonstandard treatments.5 Although it is widely accepted that differences in quality of care contribute to racial disparities in CRC outcomes, there have been randomized clinical trials of adjuvant chemotherapy in subjects with stage III CRC that have found worse overall and recurrence-free survival for NHBs compared with NHWs.29,30 These findings suggest that equal treatments may not necessarily result in equal outcomes.
It is known that specific molecular characteristics of colorectal tumors, such as microsatellite instability and somatic mutations in KRAS and BRAF, are associated with differential responses to adjuvant therapies.31,32 Data suggest that NHB patients are at a higher risk of advanced neoplasia in the proximal colon and are more likely than NHWs to develop proximal CRCs that are microsatellite stable.33,34 Information regarding the prevalence of specific characteristics in CRC tumors and the effects of these on survival is continuing to emerge; in one of the largest studies to date, Sylvester et al35 compared CRCs diagnosed in 200 black and 172 white individuals and found a higher prevalence of KRAS mutated tumors in blacks compared with whites, with the subgroup of blacks with microsatellite stable tumors having a 73% increased risk of death compared with whites. Elevated microsatellite alterations at selected tetranucleotide repeats, which correlates with somatic loss of function of the DNA mismatch repair protein hMSH3, has been associated with worse prognosis and is more frequent in CRC tumors of NHB individuals.36 Although information about the molecular characteristics of CRC tumors diagnosed in young individuals is limited, published studies suggest that these more often have histologic features associated with adverse prognosis (such as poor differentiation and perineural and/or lymphovascular invasion) when compared with tumors from older individuals.13,37,38 Consequently, if CRC tumors that develop in young NHW patients and in NHBs tend to respond differently to standard treatments, then it is possible that young NHB individuals who develop CRC might respond less favorably to conventional therapies, which would have significant implications for the clinical management of these patients.
Although our findings suggest that racial disparities in CRC survival are amplified among patients diagnosed at a young age, we acknowledge that our study has limitations. Our analyses were conducted using SEER registry data from a large number of young patients with CRC with standardized 5-year follow-up. SEER does not record information about chemotherapy use, so we were unable to assess for differences in treatment regimens by race or to investigate whether these differences were associated with differences in survival. SEER lacks detailed information regarding patient-level characteristics, such as comorbid conditions, environmental exposures, and family history, which are known to be associated with CRC risk, and individual-level socioeconomic factors that can affect quality of care and survival. Because race/ethnicity classification in SEER is based on self-identification, it is subject to misclassification. Although comparison of mean survival months should be interpreted with caution because this could be affected by censored data, we expect that any bias should be equal across race/ethnicity groups. Lastly, SEER does not routinely collect data regarding tumor molecular phenotypes (such as microsatellite instability and somatic mutations) that are clinically relevant for CRC treatment/prognosis. Thus, although our findings raise the possibility that interactions between CRC tumor biology and treatments might contribute to racial disparities in CRC outcomes, our study does not provide any data about the tumor molecular phenotypes or treatment regimens of young patients with CRC.
Despite these limitations, our findings have important implications. We observed significantly worse survival after CRC diagnosis among NHB individuals younger than 50 years of age, compared with NHWs younger than 50 years of age, at every stage, but particularly among those diagnosed with early stage (II) cancers of the colon and stage II to III cancers of the rectum and rectosigmoid junction. We observed that racial disparities in CRC survival persisted, even after adjusting for county-level poverty, with outcomes significantly worse among NHBs compared with NHWs, whereas no differences were observed between NHWs and Hispanics who had SES similar to NHBs and presumably experienced similar barriers to oncology care. CRC incidence in individuals younger than 50 years of age is continuing to rise, and the proportion of patients with young-onset CRC is two-fold higher among NHBs compared with NHWs (10.6% v 5.5%), leading some to suggest that race be considered as a factor in CRC screening algorithms.12,39-41 Diagnosis of CRC at younger than 50 years of age remains one of the primary indications for genetic evaluation.42 However, nonwhites have been underrepresented in these studies and whether the prevalence of genetic variants associated with CRC predisposition is higher among NHB individuals remains unknown.43 Further studies of the clinical and molecular characteristics of young-onset CRCs are needed to explore potential tumor/treatment interactions associated with racial differences in survival and to refine clinical algorithms for CRC treatment and early detection.
Appendix
Table A1.
Adjusted HRs of Overall Death for Patients With Colorectal Cancer by Tumor Site and Stage
| Observational Study Estimate | AJCC Stage 0-I* | AJCC Stage II* | AJCC Stage III* | AJCC Stage IV* | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Colon | ||||||||
| Race | ||||||||
| White | Ref | Ref | Ref | Ref | ||||
| Black | 1.61 (1.02 to 2.53) | .0421 | 1.63 (1.32 to 2.02) | < .001 | 1.35 (1.18 to 1.54) | < .001 | 1.29 (1.19 to 1.41) | < .001 |
| Hispanic | 1.45 (0.87 to 2.41) | .1499 | 0.96 (0.74 to 1.25) | .7746 | 1.01 (0.86 to 1.18) | .9035 | 1.03 (0.93 to 1.14) | .5542 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.29 (0.90 to 1.86) | .1719 | 1.27 (1.06 to 1.53) | .0088 | 1.20 (1.08 to 1.34) | < .001 | 1.11 (1.04 to 1.19) | .0022 |
| Surgery | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 0.16 (0.09 to 0.28) | < .001 | 0.15 (0.09 to 0.24) | < .001 | 0.33 (0.19 to 0.58) | < .001 | 0.42 (0.39 to 0.46) | < .001 |
| Radiation | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 0.82 (0.11 to 5.98) | .8439 | 1.87 (1.36 to 2.57) | .001 | 1.58 (1.29 to 1.93) | < .001 | 1.30 (1.13 to 1.49) | < .001 |
| Rectum and rectosigmoid junction | ||||||||
| Race | ||||||||
| White | Ref | Ref | Ref | Ref | ||||
| Black | 1.36 (0.90 to 2.05) | .1464 | 1.55 (1.16 to 2.07) | .0028 | 1.95 (1.62 to 2.36) | < .001 | 1.34 (1.17 to 1.53) | < .001 |
| Hispanic | 1.20 (0.78 to 1.85) | .4143 | 1.03 (0.75 to 1.41) | .8551 | 1.31 (1.08 to 1.58) | .0053 | 1.02 (0.89 to 1.17) | .7939 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.92 (1.42 to 2.61) | < .001 | 1.06 (0.86 to 1.33) | .5782 | 1.24 (1.08 to 1.43) | .0031 | 1.13 (1.03 to 1.25) | .0149 |
| Surgery | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 0.16 (0.11 to 0.23) | < .001 | 0.24 (0.18 to 0.31) | < .001 | 0.34 (0.26 to 0.45) | < .001 | 0.47 (0.43 to 0.52) | < .001 |
| Radiation | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Yes | 2.05 (1.54 to 2.71) | < .001 | 1.71 (1.29 to 2.28) | < .001 | 1.13 (0.96 to 1.34) | .1440 | 0.84 (0.76 to 0.92) | < .001 |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; Ref, reference.
Adjusted for age (5-year groups), race, poverty level (quartiles), sex, surgery, and radiation therapy.
Footnotes
Supported by funding from the Cancer Biology Graduate Program, Wayne State University School of Medicine, to ANH; by a grant from the National Institutes of Health/National Cancer Institute (K07CA120448-05) to EMS; and, in part, by Epidemiology Core and National Institutes of Health Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Andreana N. Holowatyj, Julie J. Ruterbusch, Michele L. Cote, Elena M. Stoffel
Financial support: Michele L. Cote, Elena M. Stoffel
Administrative support: Michele L. Cote
Collection and assembly of data: Andreana N. Holowatyj, Julie J. Ruterbusch, Michele L. Cote, Elena M. Stoffel
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Andreana N. Holowatyj
No relationship to disclose
Julie J. Ruterbusch
No relationship to disclose
Laura S. Rozek
No relationship to disclose
Michele L. Cote
No relationship to disclose
Elena M. Stoffel
No relationship to disclose
REFERENCES
- 1.American Cancer Society . Colorectal Cancer Facts & Figures 2014-2016. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401–405. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 4.Wallace K, Hill EG, Lewin DN, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes Control. 2013;24:463–471. doi: 10.1007/s10552-012-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soneji S, Iyer SS, Armstrong K, et al. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010;100:1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999-2004 : An updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 8.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102:538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroy PC, III, Coe A, Chen CA, et al. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159:13–20. doi: 10.7326/0003-4819-159-1-201307020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer JE, Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: An analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:4354–4359. doi: 10.1002/cncr.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 12.Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology. 2001;120:848–856. doi: 10.1053/gast.2001.22535. [DOI] [PubMed] [Google Scholar]
- 13.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 14.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 15.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: More intense treatments with unmatched survival gains. JAMA Surg. 2015;150:402–409. doi: 10.1001/jamasurg.2014.3572. [DOI] [PubMed] [Google Scholar]
- 16.McMillan DC, McArdle CS. The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br J Cancer. 2009;101:557–560. doi: 10.1038/sj.bjc.6605222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122:929–934. doi: 10.1002/cncr.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howlader N, Krapcho M, Garshell J, et al (eds): SEER Cancer Statistics Review, 1975-2012. Bethesda, MD, National Cancer Institute. http://seer.cancer.gov/csr/1975_2012/
- 19. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. http://www.seer.cancer.gov.
- 20.Phatak UR, Kao LS, Millas SG, et al. Interaction between age and race alters predicted survival in colorectal cancer. Ann Surg Oncol. 2013;20:3363–3369. doi: 10.1245/s10434-013-3045-z. [DOI] [PubMed] [Google Scholar]
- 21.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 22.Sineshaw HM, Robbins AS, Jemal A. Disparities in survival improvement for metastatic colorectal cancer by race/ethnicity and age in the United States. Cancer Causes Control. 2014;25:419–423. doi: 10.1007/s10552-014-0344-z. [DOI] [PubMed] [Google Scholar]
- 23.DeLancey JO, Thun MJ, Jemal A, et al. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 24.Irby K, Anderson WF, Henson DE, et al. Emerging and widening colorectal carcinoma disparities between blacks and whites in the United States (1975-2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 25.Chu KC, Tarone RE, Chow WH, et al. Temporal patterns in colorectal cancer incidence, survival, and mortality from 1950 through 1990. J Natl Cancer Inst. 1994;86:997–1006. doi: 10.1093/jnci/86.13.997. [DOI] [PubMed] [Google Scholar]
- 26.Laryea JA, Siegel E, Klimberg S. Racial disparity in colorectal cancer: The role of equal treatment. Dis Colon Rectum. 2014;57:295–302. doi: 10.1097/DCR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 28.Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 29.Yothers G, Sargent DJ, Wolmark N, et al. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: An analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103:1498–1506. doi: 10.1093/jnci/djr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 31.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 32. Phipps AI, Limburg PJ, Baron JA, et al: Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology148:77-87, 2015. [DOI] [PMC free article] [PubMed]
- 33. Lieberman DA, Williams JL, Holub JL, et al: Race, ethnicity, and sex affect risk for polyps > 9 mm in average-risk individuals. Gastroenterology 147:351-8, 2014; quiz e14-5. [DOI] [PMC free article] [PubMed]
- 34. doi: 10.1371/journal.pone.0100461. Carethers JM, Murali B, Yang B, et al: Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PLoS One 9:e100461, 2014 [Erratum: PLoS One 9: e103699, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sylvester BE, Huo D, Khramtsov A, et al. Molecular analysis of colorectal tumors within a diverse patient cohort at a single institution. Clin Cancer Res. 2012;18:350–359. doi: 10.1158/1078-0432.CCR-11-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carethers JM, Koi M, Tseng-Rogenski SS. EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes (Basel) 2015;6:185–205. doi: 10.3390/genes6020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schellerer VS, Merkel S, Schumann SC, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int J Colorectal Dis. 2012;27:71–79. doi: 10.1007/s00384-011-1291-8. [DOI] [PubMed] [Google Scholar]
- 38.Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol. 2009;33:572–582. doi: 10.1097/PAS.0b013e31818afd6b. [DOI] [PubMed] [Google Scholar]
- 39. doi: 10.1111/j.1572-0241.2005.41829.x. Agrawal S, Bhupinderjit A, Bhutani MS, et al: Colorectal cancer in African Americans. Am J Gastroenterol 100:515-523; discussion 514, 2005 [Erratum: Am J Gastroenterol 100:1432, 2005] [DOI] [PubMed] [Google Scholar]
- 40.Carethers JM. Screening for colorectal cancer in African Americans: Determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015;60:711–721. doi: 10.1007/s10620-014-3443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Care Network . Colon Cancer. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [Google Scholar]
- 43.Guindalini RS, Win AK, Gulden C, et al. Mutation spectrum and risk of colorectal cancer in African American families with Lynch syndrome. Gastroenterology. 2015;149:1446–1453. doi: 10.1053/j.gastro.2015.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]